Efficacy of a HER2-Targeted Thorium-227 Conjugate in a HER2-Positive Breast Cancer Bone Metastasis Model
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Compounds
2.2. Cell Culture
2.3. Synthesis and Characterization of HER2-TTC
2.4. In Vitro Cytotoxicity of HER2-TTC
2.5. In Vitro Mode-of-Action of HER2-TTC
2.6. In Vivo Efficacy of HER2-TTC in Various Xenograft Models
2.7. In Vivo Mode-of-Action Analyses in the Calu-3 Model
2.8. Radiography and Micro-CT Analyses in the BT-474 Model
2.9. Alpha Camera Imaging and Gamma Counting in the BT-474 Model
2.10. Histology and Histomorphometry Analyses in the BT-474 Model
2.11. Biochemical Marker Analysis in the BT-474 Model
2.12. Detection of HER2 Expression in Patient Samples
2.13. Statistical Analyses
3. Results
3.1. HER2-TTC Shows Specific and Potent Binding in Various HER2-Expressing Cancer Cells
3.2. HER2-TTC Reduces Cell Viability and Induces DNA Double Strand Break Formation and Cell Cycle Arrest In Vitro
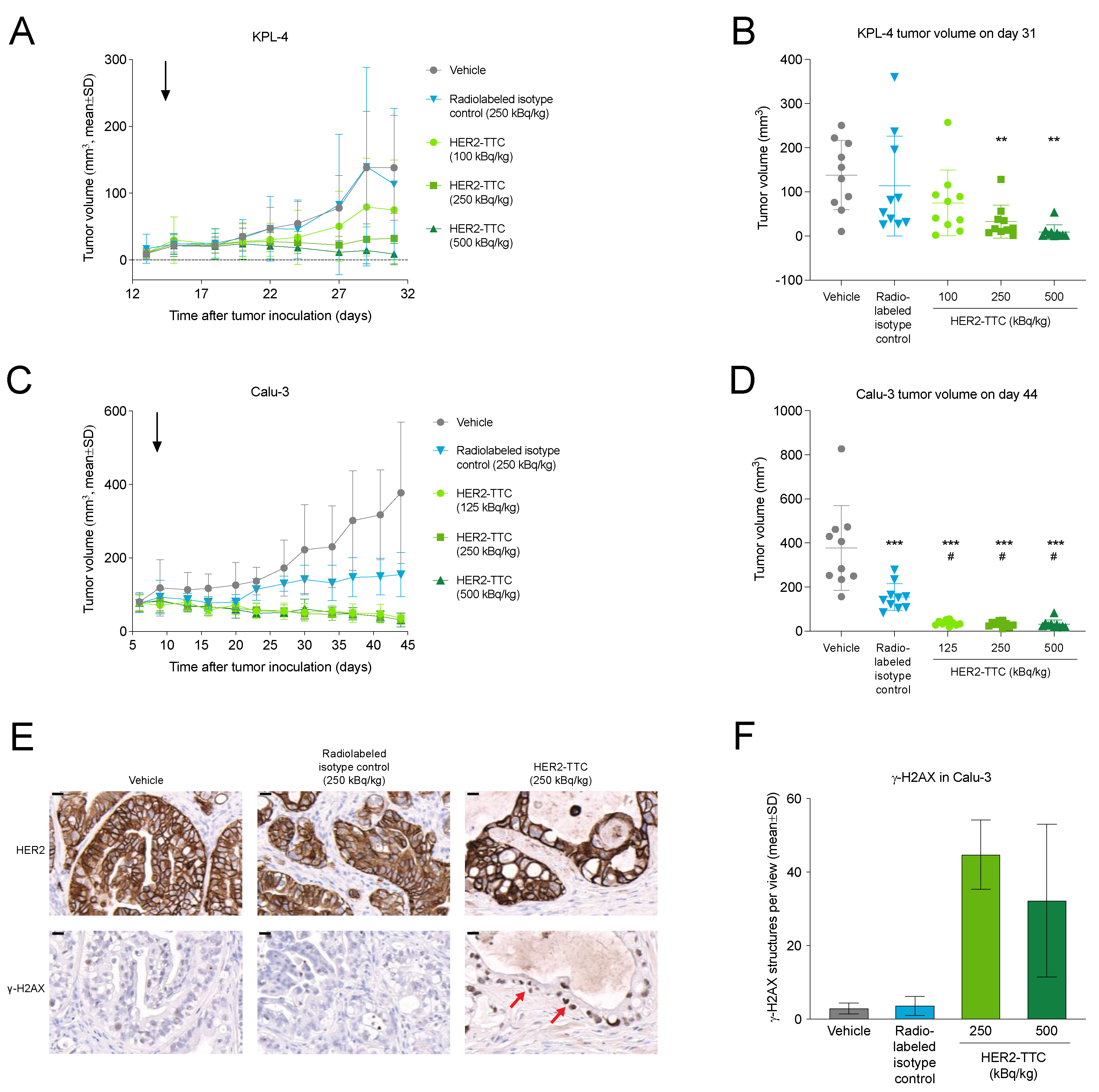
3.3. HER2-TTC Shows Dose-Dependent Antitumor Efficacy in Subcutaneous KPL-4 and Calu-3 Xenograft Models
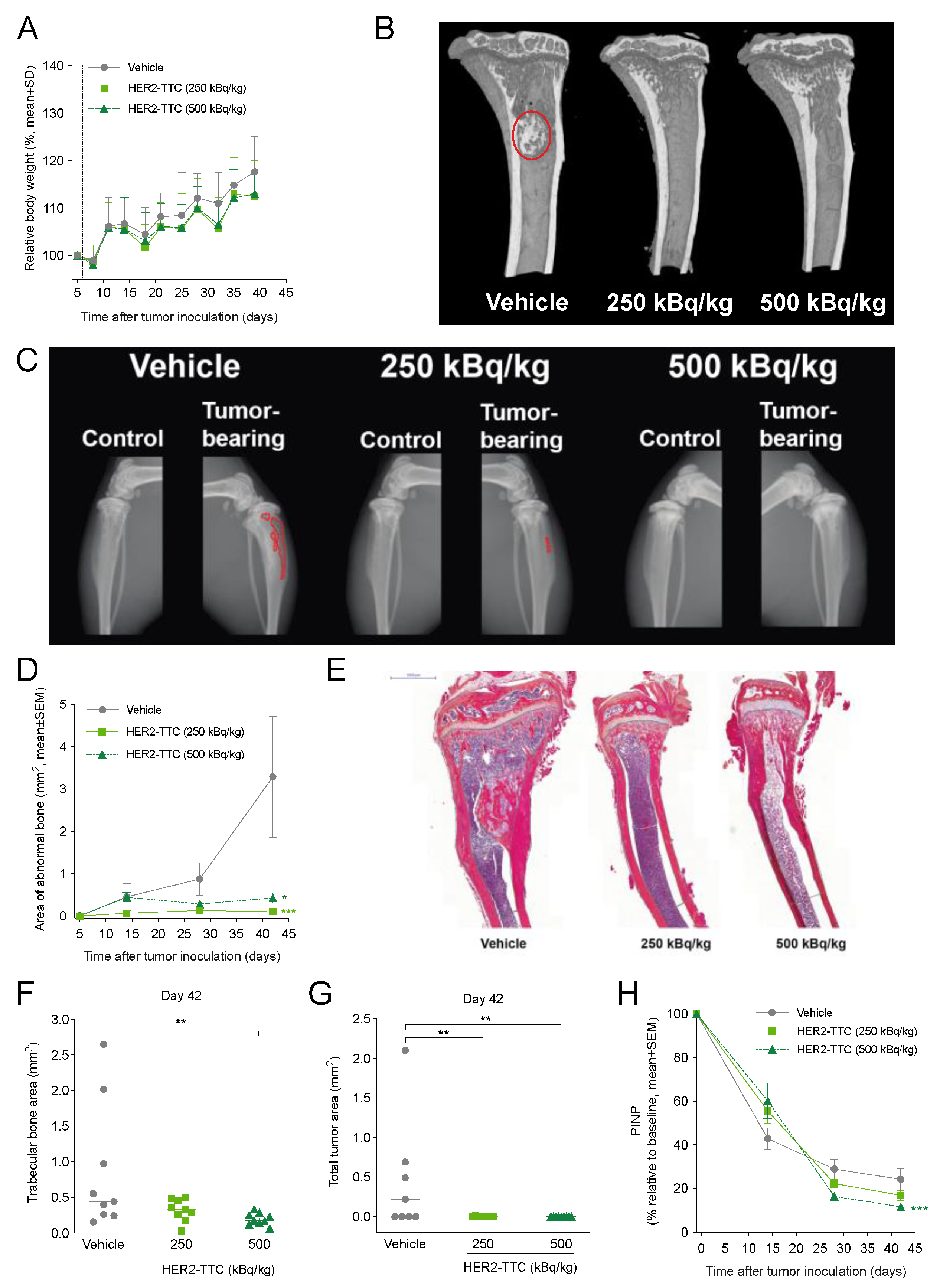
3.4. HER2-TTC Inhibits Intratibial Tumor Growth and Tumor-Induced Abnormal Bone Formation in a BT-474 Mouse Model Mimicking Breast Cancer Metastasized to Bone
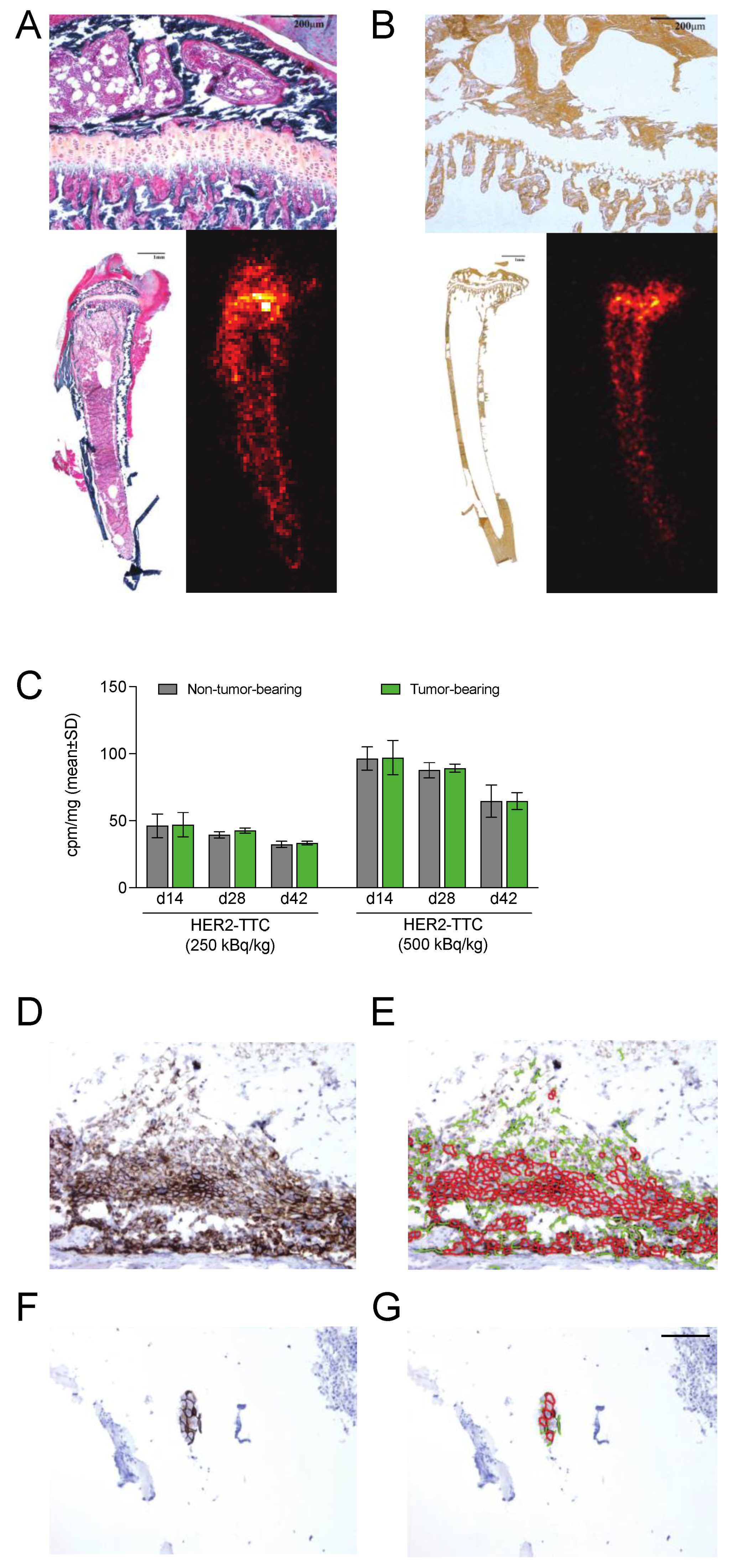
3.5. HER2-TTC Accumulates in Bone at Sites of Active Bone Turnover
3.6. HER2 Is Expressed in Samples from the BT-474 Mouse Model Mimicking Bone Metastases
3.7. HER2 Expression Is Detected in Bone Metastasis Samples from HER2-Positive Breast Cancer Patients
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, B.J.; Huang, C.Y.; Clarke, R.A. Targeted alpha anticancer therapies: Update and future prospects. Biologics 2014, 8, 255–267. [Google Scholar] [CrossRef]
- Hagemann, U.B.; Wickstroem, K.; Wang, E.; Shea, A.O.; Sponheim, K.; Karlsson, J.; Bjerke, R.M.; Ryan, O.B.; Cuthbertson, A.S. In Vitro and In Vivo Efficacy of a Novel CD33-Targeted Thorium-227 Conjugate for the Treatment of Acute Myeloid Leukemia. Mol. Cancer Ther. 2016, 15, 2422–2431. [Google Scholar] [CrossRef]
- Suominen, M.I.; Rissanen, J.P.; Kakonen, R.; Fagerlund, K.M.; Alhoniemi, E.; Mumberg, D.; Ziegelbauer, K.; Halleen, J.M.; Kakonen, S.M.; Scholz, A. Survival benefit with radium-223 dichloride in a mouse model of breast cancer bone metastasis. J. Natl. Cancer Inst. 2013, 105, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Supiot, S.; Faivre-Chauvet, A.; Couturier, O.; Heymann, M.F.; Robillard, N.; Kraeber-Bodere, F.; Morandeau, L.; Mahe, M.A.; Cherel, M. Comparison of the biologic effects of MA5 and B-B4 monoclonal antibody labeled with iodine-131 and bismuth-213 on multiple myeloma. Cancer 2002, 94, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Latif, Z.; Watters, A.D.; Dunn, I.; Grigor, K.M.; Underwood, M.A.; Bartlett, J.M. HER2/neu overexpression in the development of muscle-invasive transitional cell carcinoma of the bladder. Br. J. Cancer 2003, 89, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Haro, K.J.; Scott, A.C.; Scheinberg, D.A. Mechanisms of resistance to high and low linear energy transfer radiation in myeloid leukemia cells. Blood 2012, 120, 2087–2097. [Google Scholar] [CrossRef] [PubMed]
- Sgouros, G.; Roeske, J.C.; McDevitt, M.R.; Palm, S.; Allen, B.J.; Fisher, D.R.; Brill, A.B.; Song, H.; Howell, R.W.; Akabani, G.; et al. MIRD Pamphlet No. 22 (abridged): Radiobiology and dosimetry of alpha-particle emitters for targeted radionuclide therapy. J. Nucl. Med. 2010, 51, 311–328. [Google Scholar] [CrossRef]
- Baidoo, K.E.; Yong, K.; Brechbiel, M.W. Molecular pathways: Targeted alpha-particle radiation therapy. Clin. Cancer Res. 2013, 19, 530–537. [Google Scholar] [CrossRef]
- Frankenberg-Schwager, M.; Frankenberg, D.; Harbich, R. Repair of DNA double-strand breaks as a determinant of RBE of alpha particles. Br. J. Cancer Suppl. 1984, 6, 169–173. [Google Scholar]
- Hagemann, U.B.; Wickstroem, K.; Hammer, S.; Bjerke, R.M.; Zitzmann-Kolbe, S.; Ryan, O.B.; Karlsson, J.; Scholz, A.; Hennekes, H.; Mumberg, D.; et al. Advances in Precision Oncology: Targeted Thorium-227 Conjugates As a New Modality in Targeted Alpha Therapy. Cancer Biother. Radiopharm. 2020, 35, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, U.B.; Sommer, A.; Kristian, A.; Wang, E.; Larsen, A.; Wirnitzer, U.; Ellinger-Ziegelbauer, H.; Sandmann, S.; Poethko, T.; Karlsson, J.; et al. Abstract 5199: Preclinical activity of the FGFR2-targeted thorium-227 conjugate in preclinical models of colorectal, gastric and triple-negative breast cancer. Cancer Res. 2017, 77, 5199. [Google Scholar] [CrossRef]
- Berg-Larsen, A.; Mobergslien, A.; Moen, I.; Petros, G.; Kristian, A.; Gunvaldsen, K.S.; Cruciani, V.; Wickstroem, K.; Bjerke, R.M.; Karlsson, J.; et al. Tumor growth inhibition and immune system activation following treatment with thorium-227 conjugates and PD-1 check-point inhibition in the MC-38 murine model. Front. Med. 2022, 9, 1033303. [Google Scholar] [CrossRef]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fossa, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, G.; Fisher, D.R.; Roeske, J.C.; Bruland, O.S.; Larsen, R.H. Targeting of osseous sites with alpha-emitting 223Ra: Comparison with the beta-emitter 89Sr in mice. J. Nucl. Med. 2003, 44, 252–259. [Google Scholar]
- Suominen, M.I.; Wilson, T.; Kakonen, S.M.; Scholz, A. The Mode-of-Action of Targeted Alpha Therapy Radium-223 as an Enabler for Novel Combinations to Treat Patients with Bone Metastasis. Int. J. Mol. Sci. 2019, 20, 3899. [Google Scholar] [CrossRef]
- Coleman, R. Treatment of Metastatic Bone Disease and the Emerging Role of Radium-223. Semin. Nucl. Med. 2016, 46, 99–104. [Google Scholar] [CrossRef]
- Makvandi, M.; Dupis, E.; Engle, J.W.; Nortier, F.M.; Fassbender, M.E.; Simon, S.; Birnbaum, E.R.; Atcher, R.W.; John, K.D.; Rixe, O.; et al. Alpha-Emitters and Targeted Alpha Therapy in Oncology: From Basic Science to Clinical Investigations. Target. Oncol. 2018, 13, 189–203. [Google Scholar] [CrossRef]
- Frantellizzi, V.; Cosma, L.; Brunotti, G.; Pani, A.; Spanu, A.; Nuvoli, S.; De Cristofaro, F.; Civitelli, L.; De Vincentis, G. Targeted Alpha Therapy with Thorium-227. Cancer Biother. Radiopharm. 2020, 35, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, J.; Schatz, C.A.; Wengner, A.M.; Hammer, S.; Scholz, A.; Cuthbertson, A.; Wagner, V.; Hennekes, H.; Jardine, V.; Hagemann, U.B. Targeted thorium-227 conjugates as treatment options in oncology. Front. Med. 2023, 9, 1071086. [Google Scholar] [CrossRef] [PubMed]
- Ramdahl, T.; Bonge-Hansen, H.T.; Ryan, O.B.; Larsen, S.; Herstad, G.; Sandberg, M.; Bjerke, R.M.; Grant, D.; Brevik, E.M.; Cuthbertson, A.S. An efficient chelator for complexation of thorium-227. Bioorg. Med. Chem. Lett. 2016, 26, 4318–4321. [Google Scholar] [CrossRef]
- Deblonde, G.J.; Lohrey, T.D.; Booth, C.H.; Carter, K.P.; Parker, B.F.; Larsen, A.; Smeets, R.; Ryan, O.B.; Cuthbertson, A.S.; Abergel, R.J. Solution Thermodynamics and Kinetics of Metal Complexation with a Hydroxypyridinone Chelator Designed for Thorium-227 Targeted Alpha Therapy. Inorg. Chem. 2018, 57, 14337–14346. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, U.B.; Ellingsen, C.; Schuhmacher, J.; Kristian, A.; Mobergslien, A.; Cruciani, V.; Wickstroem, K.; Schatz, C.A.; Kneip, C.; Golfier, S.; et al. Mesothelin-Targeted Thorium-227 Conjugate (MSLN-TTC): Preclinical Evaluation of a New Targeted Alpha Therapy for Mesothelin-Positive Cancers. Clin. Cancer Res. 2019, 25, 4723–4734. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, U.B.; Mihaylova, D.; Uran, S.R.; Borrebaek, J.; Grant, D.; Bjerke, R.M.; Karlsson, J.; Cuthbertson, A.S. Targeted alpha therapy using a novel CD70 targeted thorium-227 conjugate in in vitro and in vivo models of renal cell carcinoma. Oncotarget 2017, 8, 56311–56326. [Google Scholar] [CrossRef] [PubMed]
- Hammer, S.; Hagemann, U.B.; Zitzmann-Kolbe, S.; Larsen, A.; Ellingsen, C.; Geraudie, S.; Grant, D.; Indrevoll, B.; Smeets, R.; von Ahsen, O.; et al. Preclinical efficacy of a PSMA-targeted thorium-227 conjugate (PSMA-TTC), a targeted alpha therapy for prostate cancer. Clin. Cancer Res. 2019, 26, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
- Hammer, S.; Schlicker, A.; Zitzmann-Kolbe, S.; Baumgart, S.; Hagemann, U.B.; Scholz, A.; Haendler, B.; Lejeune, P.; Karlsson, J.; Ellingsen, C.; et al. Darolutamide Potentiates the Antitumor Efficacy of a PSMA-targeted Thorium-227 Conjugate by a Dual Mode of Action in Prostate Cancer Models. Clin. Cancer Res. 2021, 27, 4367–4378. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, J.; Hagemann, U.B.; Schatz, C.; Grant, D.; Kristian, A.; Ellingsen, C.; Mihaylova, D.; Geraudie, S.; Indrevoll, B.; Wirnitzer, U.; et al. Abstract 5859: HER2-targeted thorium-227 conjugate (HER2-TTC): Efficacy in preclinical models of trastuzumab and T-DM1 resistance. Cancer Res. 2017, 77, 5859. [Google Scholar] [CrossRef]
- Lejeune, P.; Cruciani, V.; Berg-Larsen, A.; Schlicker, A.; Mobergslien, A.; Bartnitzky, L.; Berndt, S.; Zitzmann-Kolbe, S.; Kamfenkel, C.; Stargard, S.; et al. Immunostimulatory effects of targeted thorium-227 conjugates as single agent and in combination with anti-PD-L1 therapy. J. Immunother. Cancer 2021, 9, e002387. [Google Scholar] [CrossRef]
- Wickstroem, K.; Hagemann, U.B.; Cruciani, V.; Wengner, A.M.; Kristian, A.; Ellingsen, C.; Siemeister, G.; Bjerke, R.M.; Karlsson, J.; Ryan, O.B.; et al. Synergistic Effect of a Mesothelin-Targeted (227)Th Conjugate in Combination with DNA Damage Response Inhibitors in Ovarian Cancer Xenograft Models. J. Nucl. Med. 2019, 60, 1293–1300. [Google Scholar] [CrossRef]
- Wickstroem, K.; Karlsson, J.; Ellingsen, C.; Cruciani, V.; Kristian, A.; Hagemann, U.B.; Bjerke, R.M.; Ryan, O.B.; Linden, L.; Mumberg, D.; et al. Synergistic Effect of a HER2 Targeted Thorium-227 Conjugate in Combination with Olaparib in a BRCA2 Deficient Xenograft Model. Pharmaceuticals 2019, 12, 155. [Google Scholar] [CrossRef]
- Iqbal, N.; Iqbal, N. Human Epidermal Growth Factor Receptor 2 (HER2) in Cancers: Overexpression and Therapeutic Implications. Mol. Biol. Int. 2014, 2014, 852748. [Google Scholar] [CrossRef]
- Loibl, S.; Gianni, L. HER2-positive breast cancer. Lancet 2017, 389, 2415–2429. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Porter, K.; Rosenzweig, M.Q. Current and Emerging Therapies for HER2-Positive Women With Metastatic Breast Cancer. J. Adv. Pract. Oncol. 2017, 8, 164–168. [Google Scholar] [PubMed]
- Cesca, M.G.; Vian, L.; Cristovao-Ferreira, S.; Ponde, N.; de Azambuja, E. HER2-positive advanced breast cancer treatment in 2020. Cancer Treat. Rev. 2020, 88, 102033. [Google Scholar] [CrossRef]
- Fabi, A.; Malaguti, P.; Vari, S.; Cognetti, F. First-line therapy in HER2 positive metastatic breast cancer: Is the mosaic fully completed or are we missing additional pieces? J. Exp. Clin. Cancer Res. 2016, 35, 104. [Google Scholar] [CrossRef] [PubMed]
- Celik, A.; Berg, T.; Nielsen, L.B.; Jensen, M.B.; Ejlertsen, B.; Knoop, A.; Andersson, M. First-Line Treatment of HER2-Positive Metastatic Breast Cancer With Dual Blockade Including Biosimilar Trastuzumab (SB3): Population-Based Real-World Data From the DBCG. Breast Cancer 2022, 16, 11782234221086992. [Google Scholar] [CrossRef]
- Kennecke, H.; Yerushalmi, R.; Woods, R.; Cheang, M.C.; Voduc, D.; Speers, C.H.; Nielsen, T.O.; Gelmon, K. Metastatic behavior of breast cancer subtypes. J. Clin. Oncol. 2010, 28, 3271–3277. [Google Scholar] [CrossRef]
- Brook, N.; Brook, E.; Dharmarajan, A.; Dass, C.R.; Chan, A. Breast cancer bone metastases: Pathogenesis and therapeutic targets. Int. J. Biochem. Cell Biol. 2018, 96, 63–78. [Google Scholar] [CrossRef]
- Macedo, F.; Ladeira, K.; Pinho, F.; Saraiva, N.; Bonito, N.; Pinto, L.; Goncalves, F. Bone Metastases: An Overview. Oncol. Rev. 2017, 11, 321. [Google Scholar] [CrossRef]
- Abbas, N.; Heyerdahl, H.; Bruland, O.S.; Borrebaek, J.; Nesland, J.; Dahle, J. Experimental alpha-particle radioimmunotherapy of breast cancer using 227Th-labeled p-benzyl-DOTA-trastuzumab. EJNMMI Res. 2011, 1, 18. [Google Scholar] [CrossRef]
- Lindmo, T.; Boven, E.; Cuttitta, F.; Fedorko, J.; Bunn, P.A., Jr. Determination of the immunoreactive fraction of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess. J. Immunol. Methods 1984, 72, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.; Ong, G.L.; Behr, T.M.; Sharkey, R.M.; Goldenberg, D.M.; Mattes, M.J. Rapid blood clearance of mouse IgG2a and human IgG1 in many nude and nu/+ mouse strains is due to low IgG2a serum concentrations. Cancer Immunol. Immunother. 1998, 46, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, C.; Bellahcene, A.; Bonnelye, E.; Gasser, J.A.; Castronovo, V.; Green, J.; Zimmermann, J.; Clezardin, P. A cathepsin K inhibitor reduces breast cancer induced osteolysis and skeletal tumor burden. Cancer Res. 2007, 67, 9894–9902. [Google Scholar] [CrossRef]
- Suominen, M.I.; Hagemann, U.B.; Konkol, Y.; Bernoulli, J.; Fagerlund, K.M.; Bjerke, R.M.; Karlsson, J.; Halleen, J.M.; Cuthbertson, A. Abstract 640: New models of breast and lung cancer bone metastases for preclinical efficacy testing. Cancer Res. 2016, 76, 640. [Google Scholar] [CrossRef]
- Suominen, M.I.; Fagerlund, K.M.; Rissanen, J.P.; Konkol, Y.M.; Morko, J.P.; Peng, Z.; Alhoniemi, E.J.; Laine, S.K.; Corey, E.; Mumberg, D.; et al. Radium-223 Inhibits Osseous Prostate Cancer Growth by Dual Targeting of Cancer Cells and Bone Microenvironment in Mouse Models. Clin. Cancer Res. 2017, 23, 4335–4346. [Google Scholar] [CrossRef] [PubMed]
- ASCO CAP 2018 HER2 Testing for Breast Cancer Guidelines—Recommendations for Practice in Australasia. Available online: https://www.rcpa.edu.au/getattachment/fecd094c-aaf4-416b-9ed5-4a61f5ac1a93/ASCO-CAP-2018-HER2-Testing-for-Breast-Cancer-Guide.aspx (accessed on 28 September 2020).
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 20 October 2016).
- Zhang, Y. The root cause of drug resistance in HER2-positive breast cancer and the therapeutic approaches to overcoming the resistance. Pharmacol. Ther. 2020, 218, 107677. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. ENHERTU® (Fam-Trastuzumab Deruxtecan-Nxki) for Injection, for Intravenous Use. Initial U.S. Approval: 2019. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761139s000lbl.pdf (accessed on 28 March 2023).
- US Food and Drug Administration. MARGENZATM (Margetuximab-Cmkb) Injection, for Intravenous Use Initial U.S. Approval: 2020. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761150s000lbl.pdf (accessed on 28 March 2023).
- Savci-Heijink, C.D.; Halfwerk, H.; Hooijer, G.K.; Horlings, H.M.; Wesseling, J.; van de Vijver, M.J. Retrospective analysis of metastatic behaviour of breast cancer subtypes. Breast Cancer Res. Treat. 2015, 150, 547–557. [Google Scholar] [CrossRef]
- D’Oronzo, S.; Coleman, R.; Brown, J.; Silvestris, F. Metastatic bone disease: Pathogenesis and therapeutic options: Up-date on bone metastasis management. J. Bone Oncol. 2019, 15, 100205. [Google Scholar] [CrossRef]
- Xiong, Z.; Deng, G.; Huang, X.; Li, X.; Xie, X.; Wang, J.; Shuang, Z.; Wang, X. Bone metastasis pattern in initial metastatic breast cancer: A population-based study. Cancer Manag. Res. 2018, 10, 287–295. [Google Scholar] [CrossRef]
- Witton, C.J.; Reeves, J.R.; Going, J.J.; Cooke, T.G.; Bartlett, J.M. Expression of the HER1-4 family of receptor tyrosine kinases in breast cancer. J. Pathol. 2003, 200, 290–297. [Google Scholar] [CrossRef]
- Choritz, H.; Büsche, G.; Kreipe, H. Quality assessment of HER2 testing by monitoring of positivity rates. Virchows Arch. 2011, 459, 283–289. [Google Scholar] [CrossRef]
- Houssami, N.; Macaskill, P.; Balleine, R.L.; Bilous, M.; Pegram, M.D. HER2 discordance between primary breast cancer and its paired metastasis: Tumor biology or test artefact? Insights through meta-analysis. Breast Cancer Res. Treat. 2011, 129, 659–674. [Google Scholar] [CrossRef]
- Aurilio, G.; Monfardini, L.; Rizzo, S.; Sciandivasci, A.; Preda, L.; Bagnardi, V.; Disalvatore, D.; Pruneri, G.; Munzone, E.; Della Vigna, P.; et al. Discordant hormone receptor and human epidermal growth factor receptor 2 status in bone metastases compared to primary breast cancer. Acta Oncol. 2013, 52, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Rack, B.; Zombirt, E.; Trapp, E.; Jückstock, J.; Andergassen, U.; Neugebauer, J.; Kost, B.; Weissenbacher, T.; Jeschke, U.; Schindlbeck, C.; et al. Comparison of HER2 Expression in Primary Tumor and Disseminated Tumor Cells in the Bone Marrow of Breast Cancer Patients. Oncology 2016, 90, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Paik, S.; Kim, C.; Wolmark, N. HER2 status and benefit from adjuvant trastuzumab in breast cancer. N. Engl. J. Med. 2008, 358, 1409–1411. [Google Scholar] [CrossRef]
- Henry, K.E.; Ulaner, G.A.; Lewis, J.S. Human Epidermal Growth Factor Receptor 2-Targeted PET/Single- Photon Emission Computed Tomography Imaging of Breast Cancer: Noninvasive Measurement of a Biomarker Integral to Tumor Treatment and Prognosis. PET Clin. 2017, 12, 269–288. [Google Scholar] [CrossRef]
- Coleman, R.; Aksnes, A.K.; Naume, B.; Garcia, C.; Jerusalem, G.; Piccart, M.; Vobecky, N.; Thuresson, M.; Flamen, P. A phase IIa, nonrandomized study of radium-223 dichloride in advanced breast cancer patients with bone-dominant disease. Breast Cancer Res. Treat. 2014, 145, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Ueno, N.T.; Tahara, R.K.; Fujii, T.; Reuben, J.M.; Gao, H.; Saigal, B.; Lucci, A.; Iwase, T.; Ibrahim, N.K.; Damodaran, S.; et al. Phase II study of Radium-223 dichloride combined with hormonal therapy for hormone receptor-positive, bone-dominant metastatic breast cancer. Cancer Med. 2020, 9, 1025–1032. [Google Scholar] [CrossRef]



| Cell Line | HER2 Expression Level (ABC) | In Vitro Binding EC50 (nM, Mean ± SD) | In Vitro Cytotoxicity | ||
|---|---|---|---|---|---|
| HER2 mAb | HER2- ACC | Specific Activity (kBq/µg) | IC50, HER2-TTC (kBq/mL, Mean ± SD) | ||
| BT-474 | 550,000 | 2.7 | 2.1 | 40 | 1.8 ± 1.2 |
| 20 | 0.8 | ||||
| SK-BR-3 | 500,000 | 2.4 | 2.4 | 40 | 0.2 ± 0.1 |
| 10 | 0.1 | ||||
| Calu-3 | 420,000 | 3.9 ± 0.6 | 5.0 ± 0.4 | 40 | 0.03 ± 0.02 |
| 10 | 0.02 | ||||
| KPL-4 | 280,000 | 2.0 ± 0.7 | 2.5 ± 0.4 | 40 | 0.2 ± 0.1 |
| 20 | 0.2 ± 0.1 | ||||
| 10 | 0.3 ± 0.1 | ||||
| Patient Number | Sample Type | HER2 Score (1–3) | Focal (F)/Homogeneous (H) Expression |
|---|---|---|---|
| 1 | Breast cancer | 2 | F |
| Bone metastasis | 3 | F | |
| 2 | Breast cancer | 1 | F |
| Bone metastasis | 1 | H | |
| 3 | Breast cancer | 2 | H |
| Bone metastasis | 2 | F | |
| 4 | Breast cancer | 1 | H |
| Bone metastasis | 1 | F | |
| 5 | Breast cancer | 3 | H |
| Bone metastasis | 3 | F | |
| 6 | Breast cancer | 3 | F |
| Bone metastasis | 0 | H | |
| 7 | Breast cancer | 3 | H |
| Bone metastasis | 2 | F | |
| 8 | Breast cancer | n.a. | n.a. |
| Bone metastasis | 1 | F |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karlsson, J.; Hagemann, U.B.; Cruciani, V.; Schatz, C.A.; Grant, D.; Ellingsen, C.; Kristian, A.; Katoozi, S.; Mihaylova, D.; Uran, S.R.; et al. Efficacy of a HER2-Targeted Thorium-227 Conjugate in a HER2-Positive Breast Cancer Bone Metastasis Model. Cancers 2023, 15, 3419. https://doi.org/10.3390/cancers15133419
Karlsson J, Hagemann UB, Cruciani V, Schatz CA, Grant D, Ellingsen C, Kristian A, Katoozi S, Mihaylova D, Uran SR, et al. Efficacy of a HER2-Targeted Thorium-227 Conjugate in a HER2-Positive Breast Cancer Bone Metastasis Model. Cancers. 2023; 15(13):3419. https://doi.org/10.3390/cancers15133419
Chicago/Turabian StyleKarlsson, Jenny, Urs B. Hagemann, Véronique Cruciani, Christoph A. Schatz, Derek Grant, Christine Ellingsen, Alexander Kristian, Shirin Katoozi, Dessislava Mihaylova, Steinar R. Uran, and et al. 2023. "Efficacy of a HER2-Targeted Thorium-227 Conjugate in a HER2-Positive Breast Cancer Bone Metastasis Model" Cancers 15, no. 13: 3419. https://doi.org/10.3390/cancers15133419
APA StyleKarlsson, J., Hagemann, U. B., Cruciani, V., Schatz, C. A., Grant, D., Ellingsen, C., Kristian, A., Katoozi, S., Mihaylova, D., Uran, S. R., Suominen, M., Bjerke, R. M., Ryan, O. B., & Cuthbertson, A. (2023). Efficacy of a HER2-Targeted Thorium-227 Conjugate in a HER2-Positive Breast Cancer Bone Metastasis Model. Cancers, 15(13), 3419. https://doi.org/10.3390/cancers15133419