Primary Ovarian Leiomyosarcoma Is a Very Rare Entity: A Narrative Review of the Literature
Abstract
Simple Summary
Abstract
1. Introduction
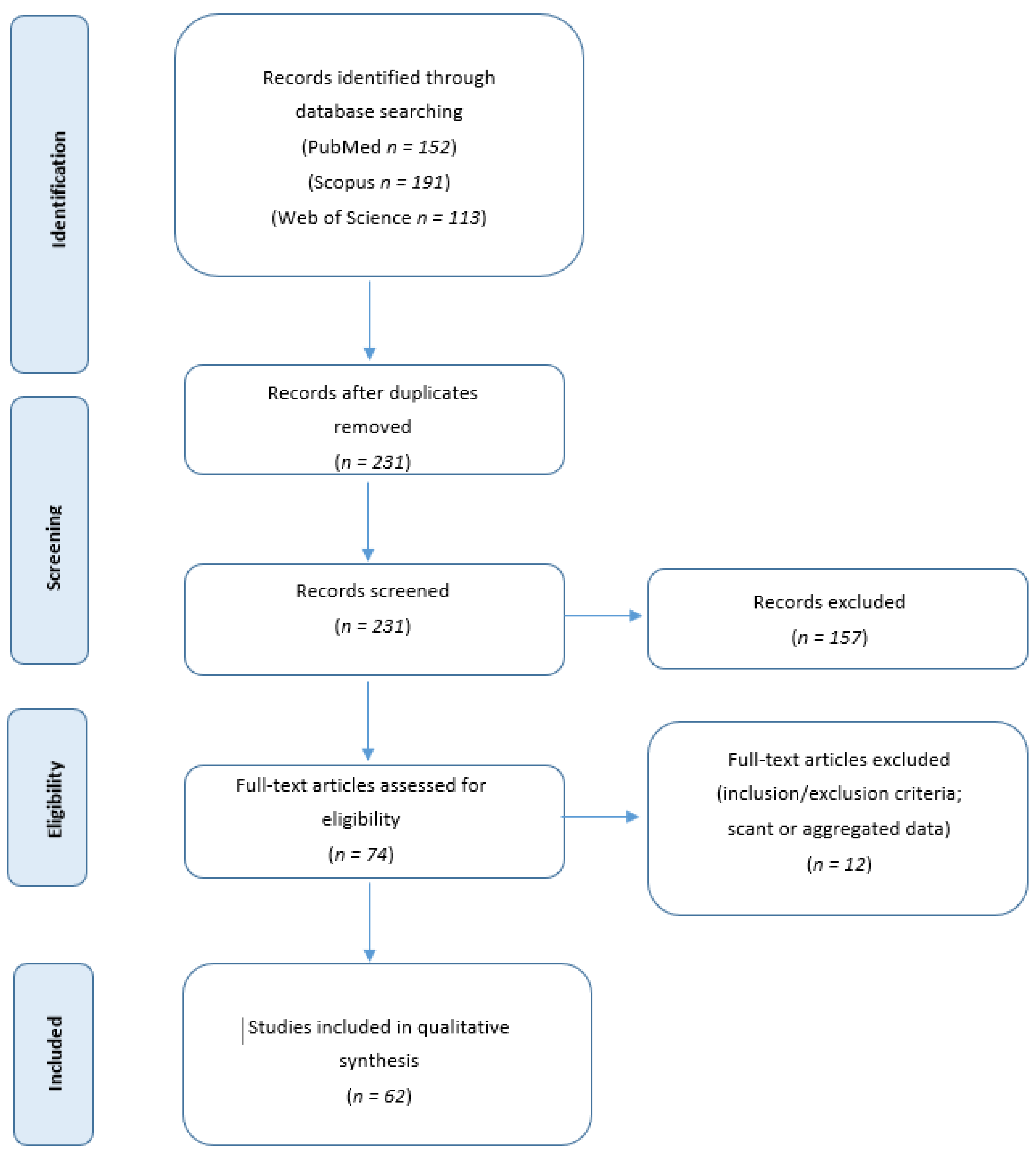
2. Methods
2.1. Systematic Review of the Literature
2.2. Statistical Analysis
3. Results
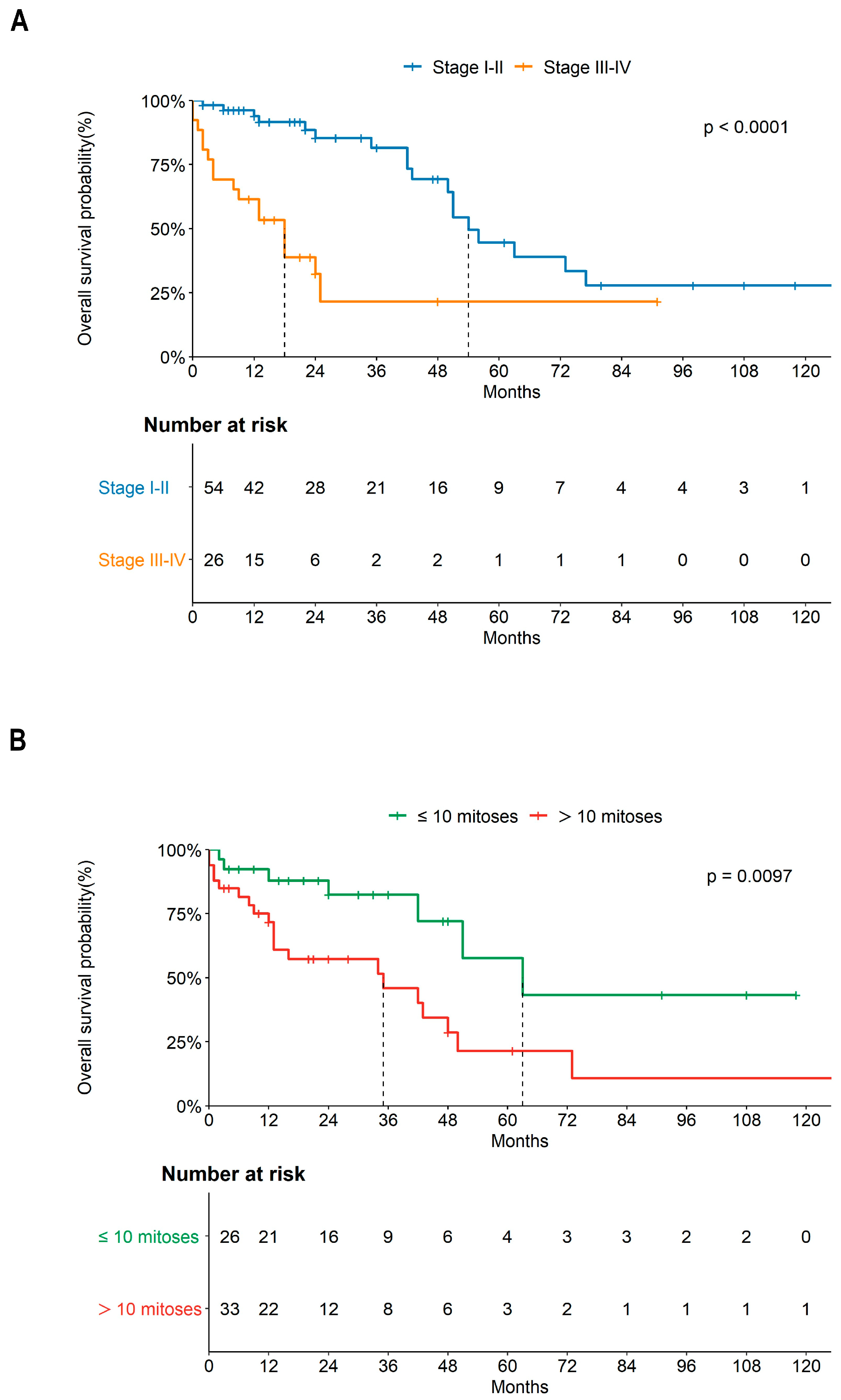
3.1. Clinical Features
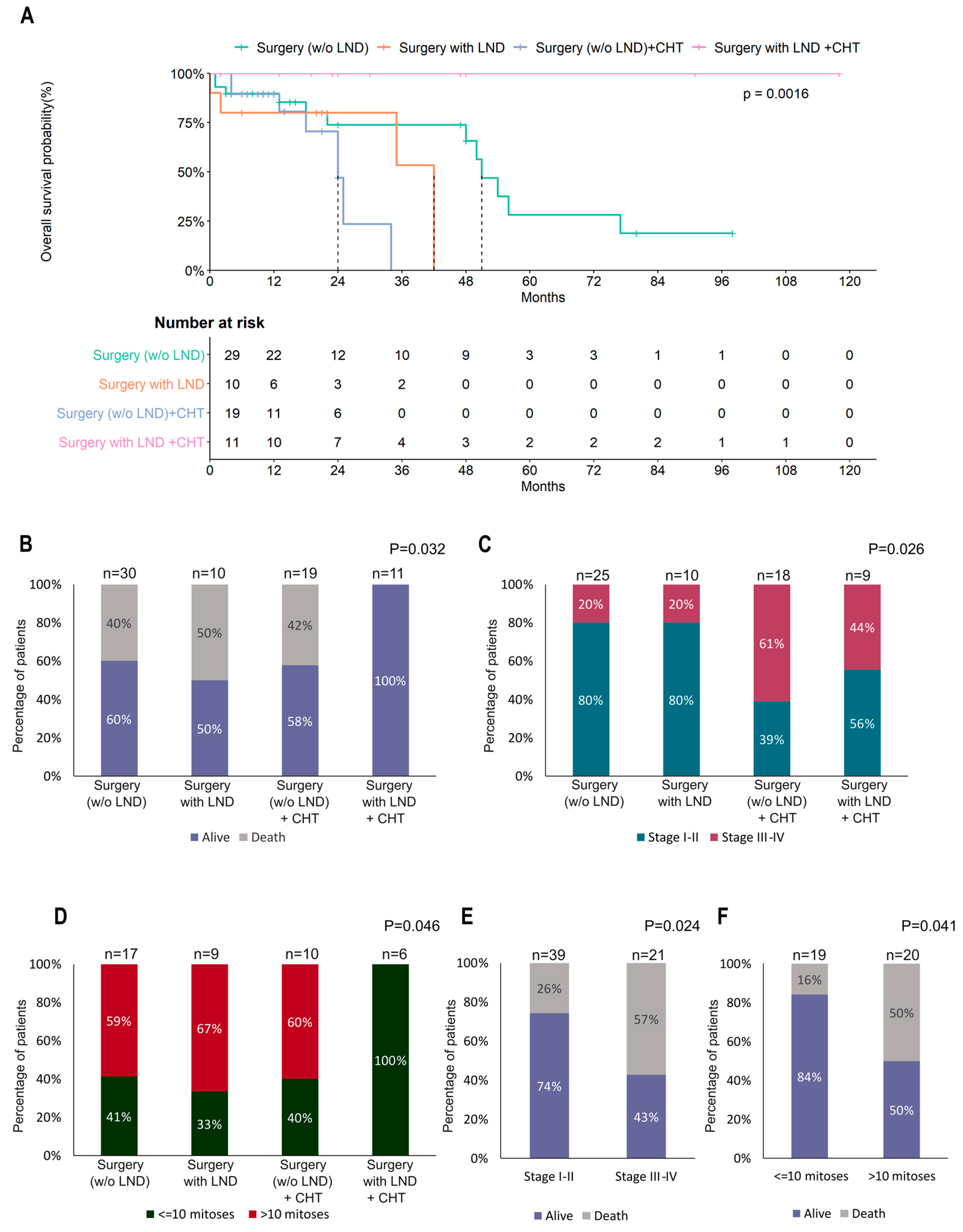
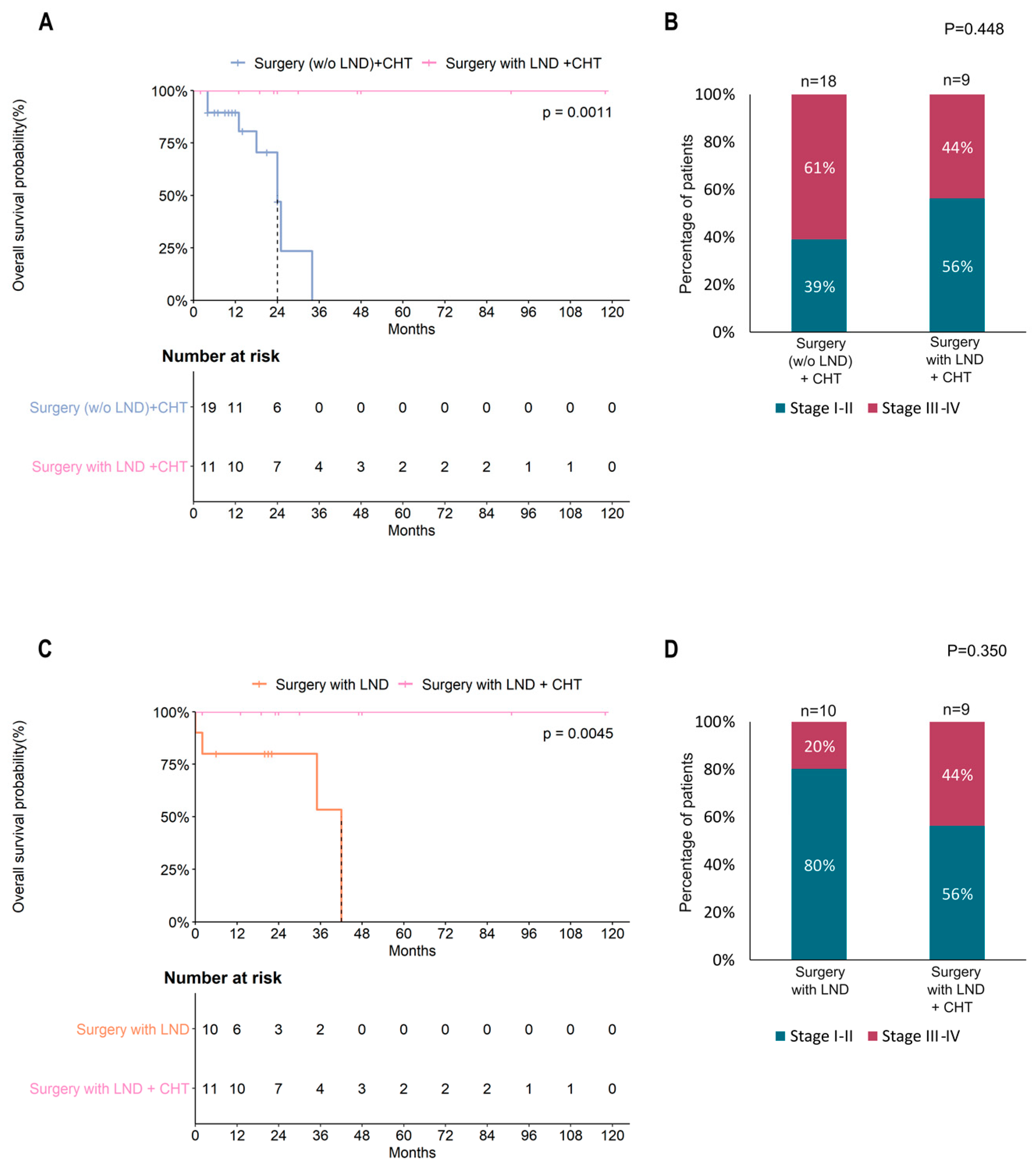
3.2. Surgery
3.3. Adjuvant Treatment
3.4. Risk Factors
3.5. Immunohistochemistry
3.6. Follow-Up Data
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Arslan, O.S.; Sumer, C.; Cihangiroğlu, G.; Kanat-Pektas, M.; Gungor, T. A rare tumor of the female genital tract: Primary ovarian leiomyosarcoma. Arch. Gynecol. Obstet. 2010, 283, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, E.; Gamage, G.P.; Butler, J.; Barton, D.P.; Thway, K.; Fisher, C.; Messiou, C.; Miah, A.B.; Zaidi, S.; Gennatas, S.; et al. Clinical management and outcomes of primary ovarian leiomyosarcoma—Experience from a sarcoma specialist unit. Gynecol. Oncol. Rep. 2021, 36, 100737. [Google Scholar] [CrossRef] [PubMed]
- Kozłowski, M.; Nowak, K.; Kordek, A.; Cymbaluk-Płoska, A. Therapeutic Management of Rare Primary Ovarian Neoplasms: Carcinosarcoma, Leiomyosarcoma, Melanoma and Carcinoid. Int. J. Environ. Res. Public Health 2021, 18, 7819. [Google Scholar] [CrossRef] [PubMed]
- Goodall, E.J.; Madhuri, T.; Manuel, S.B. The Management Dilemma of Leiomyosarcoma of the Ovary. World J. Oncol. 2011, 2, 265–266. [Google Scholar] [CrossRef]
- Inoue, J.; Gomibuchi, H.; Minoura, S. A Case of a Primary Ovarian Leiomyosarcoma. J. Obstet. Gynaecol. Res. 2000, 26, 401–407. [Google Scholar] [CrossRef]
- Rasmussen, C.C.; Skilling, J.S.; Sorosky, J.I.; Lager, D.J.; Buller, R.E. Stage IIIC Ovarian Leiomyosarcoma in a Premenopausal Woman with Multiple Recurrences: Prolonged Survival with Surgical Therapy. Gynecol. Oncol. 1997, 66, 519–525. [Google Scholar] [CrossRef]
- Selvi, K.; Toi, P.C.; Badhe, B.A. A Rare Case of Primary Leiomyosarcoma of the Ovary in Association with Cellular Leiomyoma of the Broad Ligament and Uterus. J. Gynecol. Surg. 2014, 30, 363–366. [Google Scholar] [CrossRef]
- Gupta, N. Leiomyosarcoma. PathologyOutlines.com Website. Available online: https://www.pathologyoutlines.com/topic/ovarytumorleiomyosarcoma.html (accessed on 6 April 2023).
- Divya, N.S.; Srinivasamurthy, V. Myxoid Leiomyosarcoma of Ovary—A Rare Case Report. J. Clin. Diagn. Res. 2014, 8, FD05–FD06. [Google Scholar]
- Vishwanath; Vyas, N.M.; Goripally, S.; Rai, S. A Rare Case of Primary Leiomyosarcoma of the Ovary. J. Clin. Diagn. Res. 2018, 12, QD01–QD02. [Google Scholar] [CrossRef]
- Doubeni, C.A.; Doubeni, A.R.; Myers, A.E. Diagnosis and Management of Ovarian Cancer. Am. Fam. Physician 2016, 93, 937–944. [Google Scholar]
- Hensley, M.L.; Barrette, B.A.; Baumann, K.; Gaffney, D.; Hamilton, A.L.; Kim, J.-W.; Maenpaa, J.U.; Pautier, P.; Siddiqui, N.A.; Westermann, A.M.; et al. Gynecologic Cancer InterGroup (GCIG) Consensus Review: Uterine and Ovarian Leiomyosarcomas. Int. J. Gynecol. Cancer 2014, 24 (Suppl. 3), S61–S66. [Google Scholar] [CrossRef] [PubMed]
- Pongsuvareeyakul, T.; Sukpan, K.; Chaicharoen, S.; Khunamornpong, S. Leiomyosarcoma and Squamous Cell Carcinoma Arising in Mature Cystic Teratoma of the Ovary. Case Rep. Pathol. 2017, 2017, 7907359. [Google Scholar] [CrossRef]
- He, M.; Deng, Y.-J.; Zhao, D.-Y.; Zhang, Y.; Wu, T. Synchronous leiomyosarcoma and fibroma in a single ovary: A case report and review of the literature. Oncol. Lett. 2016, 11, 2510–2514. [Google Scholar] [CrossRef] [PubMed]
- Taşkn, S.; Taşkn, E.A.; Üzüm, N.; Ataoğlu; Ortaç, F. Primary Ovarian Leiomyosarcoma: A Review of the Clinical and Immunohistochemical Features of the Rare Tumor. Obstet. Gynecol. Surv. 2007, 62, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Pu, T.; Fan, L.; Wang, L.; Li, L.; Zeng, H. Recurrent primary ovarian leiomyosarcoma preconception, pregnancy, delivery, and puerperal management: A case report and literature review. J. Obstet. Gynaecol. Res. 2022, 48, 1489–1494. [Google Scholar] [CrossRef]
- Istre, B. Sarcoma of the ovary. J. Oslo. City Hosp. 1951, 1, 231–237. [Google Scholar]
- Balasz, M.; Laszo, J. Das leiomyosarcoma des ovar. Zentralbl. Gynaekol. 1965, 87, 633–638. [Google Scholar]
- Kelley, R.R.; Scully, R.E. Cancer developing in dermoid cysts of the ovary. A report of 8 cases, including a carcinoid and a leiomyosarcoma. Cancer 1961, 14, 989–1000. [Google Scholar] [CrossRef]
- von Numers, V.; Mikkonen, R. Leiomyosarcoma arising in serous cystadenoma of ovary: Report of a case. Ann. Chir. Gynaecol. Fenn. 1960, 49, 240–244. [Google Scholar]
- De Gribble, M.G.; Blair, J.S. Haemoperitoneum complicating malignant disease. Br. J. Surg. 1962, 49, 432–435. [Google Scholar] [CrossRef]
- Nieminen, U.; Von Numers, C.; Purola, E. Primary Sarcoma of the Ovary. Acta Obstet. Gynecol. Scand. 1969, 48, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Azoury, R.S.; Woodruff, J.D. Primary ovarian sarcomas. Obstet. Gynecol. 1971, 37, 920. [Google Scholar] [PubMed]
- Connor, E.; Aydinel, O.; Lee, J.; Homayouni, M. Leiomyosarcoma of the ovary. In Diagnosis and Treatment of Ovarian Neoplastic Alterations; Excerpta Medica: Amsterdam, The Netherlands, 1975; pp. 47–50. [Google Scholar]
- Walts, A.E.; Lichtenstein, I. Primary leiomyosarcoma associated with serous cystadenocarcinoma of the ovary. Gynecol. Oncol. 1977, 5, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.A.; Poon, T.P.; Ramaswamy, G.; Tchertkoff, V. Leiomyosarcoma of the ovary. N. Y. State J. Med. 1985, 85, 218–220. [Google Scholar] [PubMed]
- Ansaldi, E.; Cravero, S.; Zamburlini, S. Primary leiomyosarcoma in preserved ovary after unilateral oophorectomy. Eur. J. Gynaecol. Oncol. 1986, 7, 133–134. [Google Scholar]
- Cortés, J.; Cuartero, M.L.; Rosselló, J.J.; Torrecabota, J.; Yarnoz, M.C.; Llompart, M. Ovarian pure leiomyosarcoma: Case report. Eur. J. Gynaecol. Oncol. 1987, 8, 19–22. [Google Scholar]
- Shakfeh, S.M.; Woodruff, J.D. Primary ovarian sarcomas: Report of 46 cases and review of the literature. Obstet. Gynecol. Surv. 1987, 42, 331–349. [Google Scholar] [CrossRef]
- Anderson, B.; Turner, D.A.; Benda, J. Ovarian sarcoma. Gynecol. Oncol. 1987, 26, 183–192. [Google Scholar] [CrossRef]
- Balaton, A.; Vaury, P.; Imbert, M.; Mussy, M. Primary leiomyosarcoma of the ovary: A histological and immunocytochemical study. Gynecol. Oncol. 1987, 28, 116–120. [Google Scholar] [CrossRef]
- Friedman, H.D.; Mazur, M.T. Primary ovarian leiomyosarcoma. An immunohistochemical and ultrastructural study. Arch. Pathol. Lab. Med. 1991, 115, 941–945. [Google Scholar]
- Nogales, F.F.; Ayala, A.; Ruiz-Avila, I.; Sirvent, J.J. Myxoid leiomyosarcoma of the ovary: Analysis of three cases. Hum. Pathol. 1991, 22, 1268–1273. [Google Scholar] [CrossRef] [PubMed]
- Monk, B.J.; Nieberg, R.; Berek, J.S. Primary Leiomyosarcoma of the Ovary in a Perimenarchal Female. Gynecol. Oncol. 1993, 48, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Dixit, S.; Singhal, S.; Baboo, H.A.; Vyas, R.K.; Neema, J.P.; Murthy, R.; Sooryanaraya, U. Leiomyosarcoma of the ovary. J. Postgrad. Med. 1993, 39, 151. [Google Scholar] [PubMed]
- Piura, B.; Rabinovich, A.; Yanai-Inbar, I.; Cohen, Y.; Glezerman, M. Primary sarcoma of the ovary: Report of five cases and review of the literature. Eur. J. Gynaecol. Oncol. 1998, 19, 257–261. [Google Scholar]
- O’Sullivan, S.G.; Das Narla, L.; Ferraro, E. Primary ovarian leiomyosarcoma in an adolescent following radiation for medulloblastoma. Pediatr. Radiol. 1998, 28, 468–470. [Google Scholar] [CrossRef]
- Nasu, M.; Inoue, J.; Matsui, M.; Minoura, S.; Matsubara, O. Ovarian leiomyosarcoma: An autopsy case report. Pathol. Int. 2000, 50, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Rampaul, R.; Barrow, S.; Naraynsingh, V. A Primary Ovarian Leiomyosarcoma with Micro-Invasive Features (Stage I): Is Surgical Excision Enough? Gynecol. Oncol. 1999, 73, 464–467. [Google Scholar] [CrossRef]
- Seracchioli, R.; Colombo, F.M.; Bagnoli, A.; Trengia, V.; Venturoli, S. Primary ovarian leiomyosarcoma as a new component in the nevoid basal cell carcinoma syndrome: A case report. Am. J. Obstet. Gynecol. 2003, 188, 1093–1095. [Google Scholar] [CrossRef]
- Mayerhofer, K.; Lozanov, P.; Bodner, K.; Bodner-Adler, B.; Mayerhofer-Gallenbacher, N.; Hudelist, G.; Czerwenka, K. Immunohistochemical analysis of a primary ovarian leiomyosarcoma. Case report. Anticancer. Res. 2003, 23, 3433–3436. [Google Scholar]
- Kafalh, H.; Yakın, K.; Turan, T.; Engin, Y.; Ortaç, F.; Dünder, I. Primary ovarian leiomyosarcoma—Case report optimal. Tip. Dergisi. 2003, 16, 25–27. [Google Scholar]
- Lerwill, M.F.; Sung, R.; Oliva, E.; Prat, J.; Young, R.H. Smooth Muscle Tumors of the Ovary: A clinicopathologic study of 54 cases emphasizing prognostic criteria, histologic variants, and differential diagnosis. Am. J. Surg. Pathol. 2004, 28, 1436–1451. [Google Scholar] [CrossRef] [PubMed]
- Nicòtina, P.A.; Antico, F.; Caruso, C.; Triolo, O. Primary ovarian leiomyosarcoma. Proliferation rate and survival. Eur. J. Gynaecol. Oncol. 2004, 25, 515. [Google Scholar]
- Bouie, S.M.; Cracchiolo, B.; Heller, D. Epithelioid leiomyosarcoma of the ovary. Gynecol. Oncol. 2005, 97, 697–699. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Schuetze, S.M.; Conrad, I.E.U., 3rd; Swisshelm, K.L.; Norwood, T.H.; Rubin, B.P. So-Called “Inflammatory Leiomyosarcoma”: A Series of 3 Cases Providing Additional Insights into a Rare Entity. Int. J. Surg. Pathol. 2005, 13, 185–195. [Google Scholar] [CrossRef]
- Kuscu, E.; Erkanli, S.; Haberal, A.; Ergin, T.; Ozdemir, H.; Demirhan, B. Primary ovarian leiomyosarcoma: A case report. Eur. J. Gynaecol. Oncol. 2005, 26, 120–122. [Google Scholar]
- Li, Y.; Feng, L.D.; Ma, A.G.; Lu, H.J. Primary ovarian leiomyosarcoma associated with Brenner tumor. Eur. J. Gynaecol. Oncol. 2009, 30, 440–442. [Google Scholar] [PubMed]
- Khizar, S.; Decruze, S.B.; Kirwan, J. Ovarian leiomyosarcoma with co-existing fibroid. J. Obstet. Gynaecol. 2007, 27, 100–101. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Shen, K.; Lang, J.-H.; Huang, H.-F.; Pan, L.-Y.; Wu, M.; Yang, J.-X.; Zhong, D.-R. Primary sarcoma of the ovary: Clinicopathological characteristics, prognostic factors and evaluation of therapy. Chin. Med. J. 2011, 124, 1316–1321. [Google Scholar] [PubMed]
- Zygouris, D.; Androutsopoulos, G.; Grigoriadis, C.; Arnogiannaki, N.; Terzakis, E. Primary ovarian leiomyosarcoma. Eur. J. Gynaecol. Oncol. 2012, 33, 331–333. [Google Scholar]
- Pankaj, S. High Grade Pleomorphic Leiomyosarcoma of Ovary in young female: A case. World J. Surg. Res. 2013, 7, 7–11. [Google Scholar]
- Rivas, G.; Bonilla, C.; Rubiano, J.; Arango, N. Primary Leiomyosarcoma of the Ovary: A Case Report. Case Rep. Clin. Med. 2014, 3, 192–196. [Google Scholar] [CrossRef]
- Sunita, S.; Divya, S.; Shivani, M.; Hemant, Y.; Sonia, S. Ovarian Leiomyosarcoma in a Young Woman. J. Gynecol. Surg. 2014, 30, 297–300. [Google Scholar] [CrossRef]
- Kumar, J.V.; Khurana, A.; Kaur, P.; Chuahan, A.K.; Singh, S. A rare presentation of primary leiomyosarcoma of ovary in a young woman. Ecancermedicalscience 2015, 9, 524. [Google Scholar] [CrossRef] [PubMed]
- Nazneen, S.; Kumari, A.; Choudhary, V.; Kumari, S.; Pankaj, S. Prolonged Survival of a Young Female with High Grade Pleomorphic Leiomyosarcoma of Ovary Without Recurrence. J. Obstet. Gynecol. India 2015, 66 (Suppl. 2), S639–S641. [Google Scholar] [CrossRef] [PubMed]
- Thyagaraju, C.; Durairaj, J.; Venkataraman, S. Leiomyosarcoma of the ovary associated with leiomyoma of uterus: A case report. Int. J. Reprod. Contracept. Obstet. Gynecol. 2015, 4, 837–839. [Google Scholar] [CrossRef]
- Mamta, G.; Ramavatar, B.; SeemA, M.; Devendra, B.; Manju, S.; Jayanti, M. A rare case of primary ovarian leiomyosarcoma during pregnancy in 27 year old patient with 38 weeks gestational age. Indian J. Basic Appl. Med. Res. 2015, 5, 528–531. [Google Scholar]
- Na Lee, B.; Ouh, Y.T.; Choi, H.J.; Yang, S.Y.; Lee, J.K.; Hong, J.H. Leiomyosarcoma of the Ovary Mimicking Gastrointestinal Stromal Tumor Originating from Small Bowel: A Case Report and Literature Review. Gynecol. Obstet. 2016, 6, 1000359. [Google Scholar] [CrossRef]
- Furutake, Y.; Fukagawa, T.; Suga, Y.; Nagasawa, T.; Sato, S.; Omi, H.; Kagabu, M.; Chiba, A.; Shoji, T.; Takeuchi, S.; et al. Gemcitabine and docetaxel in a patient with primary ovarian leiomyosarcoma: A case report and review of literature. Int. Cancer Conf. J. 2018, 7, 11–15. [Google Scholar] [CrossRef]
- Tanaka, A.; Miyoshi, A.; Kanao, S.; Takeda, M.; Mimura, M.; Nagamatsu, M.; Yokoi, T. Case Report of a Primary Ovarian Leiomyosarcoma Diagnosed by H-Caldesmon Staining. J. Clin. Gynecol. Obstet. N. Am. 2018, 7, 26–29. [Google Scholar] [CrossRef]
- Sukgen, G.; Altunkol, A. Primary leimyosarcoma of the ovary. Cukurova Med. J. 2018, 43, 1038–1041. [Google Scholar] [CrossRef]
- Shafiee, M.N.; Teik, C.K.; Zain, R.R.; Kampan, N. Ovarian and uterine leiomyosarcoma: Which one is the primary? Horm. Mol. Biol. Clin. Investig. 2019, 41, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Fischetti, A.; Rutigliani, M.; Romano, N.; Marinaro, E.L.; Puppo, C.; Gorlero, F.; Melani, E.F.; Rollandi, G.A. Imaging findings of ovarian leiomyosarcoma with histopathologic correlations. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 236, 261–262. [Google Scholar] [CrossRef]
- Yuksel, D.; Cakir, C.; Kilic, C.; Karalok, A.; Kimyon, G.; Çöteli, S.; Boyraz, G.; Tekin, M.; Turan, T. Primary leiomyosarcoma of the ovary: A report of three cases and a systematic review of literature. J. Gynecol. Obstet. Hum. Reprod. 2020, 50, 101825. [Google Scholar] [CrossRef]
- Khadjetou, V.; Cheikh, T.E.; Haiba, M.V.A.; Cheikh, M.A.A.; Mouhamed, B.N.; Abdi, A.B. Primary perimenarcheal ovarian leiomyosarcoma: A case report and review of the literature. Int. J. Surg. Case Rep. 2022, 94, 107094. [Google Scholar] [CrossRef]
- Bahadur, A.; Mundhra, R.; Verma, P.; Phulware, R.H. Primary ovarian leiomyosarcoma in a woman with uterovaginal prolapse. BMJ Case Rep. 2022, 15, e251733. [Google Scholar] [CrossRef] [PubMed]
- Charkhchi, P.; Cybulski, C.; Gronwald, J.; Wong, F.O.; Narod, S.A.; Akbari, M.R. CA125 and Ovarian Cancer: A Comprehensive Review. Cancers 2020, 12, 3730. [Google Scholar] [CrossRef]
- Paik, E.S.; Kang, J.H.; Kim, J.; Lee, Y.-J.; Choi, C.H.; Kim, T.-J.; Kim, B.-G.; Bae, D.-S.; Lee, J.-W. Prognostic factors for recurrence and survival in uterine leiomyosarcoma: Korean single center experience with 50 cases. Obstet. Gynecol. Sci. 2019, 62, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Yang, X.; Zeng, Z.; Wei, X.; Li, K.-Z.; Xu, X.-Y. Outcomes of patients with pelvic leiomyosarcoma treated by surgery and relevant auxiliary diagnosis. World J. Clin. Cases 2020, 8, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Lertkhachonsuk, A.; Buranawongtrakoon, S.; Lekskul, N.; Rermluk, N.; Wee-Stekly, W.; Charakorn, C. Serum CA19 -9, CA -125 and CEA as tumor markers for mucinous ovarian tumors. J. Obstet. Gynaecol. Res. 2020, 46, 2287–2291. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Y.; Quan, Q.; Meng, Y.; Mu, X. Diagnostic Value of Preoperative CA125, LDH and HE4 for Leiomyosarcoma of the Female Reproductive System. Cancer Manag. Res. 2021, 13, 4657–4664. [Google Scholar] [CrossRef]
- Cabasag, C.J.; Fagan, P.J.; Ferlay, J.; Vignat, J.; Laversanne, M.; Liu, L.; van der Aa, M.A.; Bray, F.; Soerjomataram, I. Ovarian cancer today and tomorrow: A global assessment by world region and Human Development Index using GLOBOCAN 2020. Int. J. Cancer 2022, 151, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Cree, I.A.; Tan, P.H.; Travis, W.D.; Wesseling, P.; Yagi, Y.; White, V.A.; Lokuhetty, D.; Scolyer, R.A. Counting mitoses: SI(ze) matters! Mod. Pathol. 2021, 34, 1651–1657. [Google Scholar] [CrossRef]
- Maroof, N.; Khan, A.; Qureshi, S.A.; Rehman, A.U.; Khalil, R.K.; Shim, S.-O. Mitosis detection in breast cancer histopathology images using hybrid feature space. Photodiagnosis Photodyn. Ther. 2020, 31, 101885. [Google Scholar] [CrossRef] [PubMed]
- Campora, M.; Paudice, M.; Gambella, A.; Comandini, D.; Parente, P.; Sbaraglia, M.; Tos, A.P.D.; Grillo, F.; Mastracci, L. Counting mitoses in gastrointestinal stromal tumours (GISTs): Variable practices in the real-world setting and their clinical implications. Virchows Arch. 2023, 482, 589–594. [Google Scholar] [CrossRef]
- Kros, J.M.; Rushing, E.; Uwimana, A.L.; Hernández-Laín, A.; Michotte, A.; Al-Hussaini, M.; Bielle, F.; Mawrin, C.; Marucci, G.; Tesileanu, C.M.S.; et al. Mitotic count is prognostic in IDH mutant astrocytoma without homozygous deletion of CDKN2A/B. Results of consensus panel review of EORTC trial 26053 (CATNON) and EORTC trial 22033-26033. Neuro-Oncology 2022, 26, noac282. [Google Scholar] [CrossRef] [PubMed]
- Chapel, D.B.; Sharma, A.; Lastra, R.R.; Maccio, L.; Bragantini, E.; Zannoni, G.F.; George, S.; Quade, B.J.; Parra-Herran, C.; Nucci, M.R. A novel morphology-based risk stratification model for stage I uterine leiomyosarcoma: An analysis of 203 cases. Mod. Pathol. 2022, 35, 794–807. [Google Scholar] [CrossRef]
- Bodner, K.; Bodner-Adler, B.; Kimberger, O.; Czerwenka, K.; Leodolter, S.; Mayerhofer, K. Evaluating prognostic parameters in women with uterine leiomyosarcoma. A clinicopathologic study. J. Reprod. Med. 2003, 48, 95–100. [Google Scholar] [PubMed]
- Kaloni, D.; Diepstraten, S.T.; Strasser, A.; Kelly, G.L. BCL-2 protein family: Attractive targets for cancer therapy. Apoptosis 2022, 28, 20–38. [Google Scholar] [CrossRef]
- Ha, H.I.; Cho, S.-H.; Lim, J.; Lee, Y.J.; Yoo, C.W.; Won, Y.-J.; Lim, M.C. Incidence and treatment outcomes of ovarian sarcoma compared to epithelial ovarian cancer from the national cancer registry. Gynecol. Oncol. 2021, 163, 506–510. [Google Scholar] [CrossRef]
- Bacalbasa, N.; Balescu, I.; Dima, S.; Popescu, I. Ovarian sarcoma carries a poorer prognosis than ovarian epithelial cancer throughout all FIGO stages: A single-center case-control matched study. Anticancer. Res. 2014, 34, 7303–7308. [Google Scholar]
- del Carmen, M.G.; Birrer, M.; Schorge, J.O. Carcinosarcoma of the ovary: A review of the literature. Gynecol. Oncol. 2012, 125, 271–277. [Google Scholar] [CrossRef]
- van der Hel, O.; Timmermans, M.; van Altena, A.; Kruitwagen, R.; Slangen, B.; Sonke, G.; van de Vijver, K.; van der Aa, M. Overview of non-epithelial ovarian tumours: Incidence and survival in the Netherlands, 1989–2015. Eur. J. Cancer 2019, 118, 97–104. [Google Scholar] [CrossRef]
- Mangone, L.; Marinelli, F.; Bisceglia, I.; Braghiroli, M.B.; Mastrofilippo, V.; Cerullo, L.; Pellegri, C.; Zambelli, A.; Aguzzoli, L.; Mandato, V.D. Ovarian Cancer in a Northern Italian Province and the Multidisciplinary Team. Cancers 2022, 15, 299. [Google Scholar] [CrossRef] [PubMed]
- Mandato, V.D.; Torricelli, F.; Uccella, S.; Pirillo, D.; Ciarlini, G.; Ruffo, G.; Annunziata, G.; Manzotti, G.; Pignata, S.; Aguzzoli, L. An Italian National Survey on Ovarian Cancer Treatment at first diagnosis. There’s None so Deaf as those who will not Hear. J. Cancer 2021, 12, 4443–4454. [Google Scholar] [CrossRef] [PubMed]
- Mandato, V.D.; Abrate, M.; De Iaco, P.; Pirillo, D.; Ciarlini, G.; Leoni, M.; Comerci, G.; Ventura, A.; Lenzi, B.; Amadori, A.; et al. Gynecological Oncology Network of Emilia Romagna Region. Clinical governance network for clinical audit to improve quality in epithelial ovarian cancer management. J. Ovarian Res. 2013, 6, 19. [Google Scholar] [CrossRef] [PubMed]

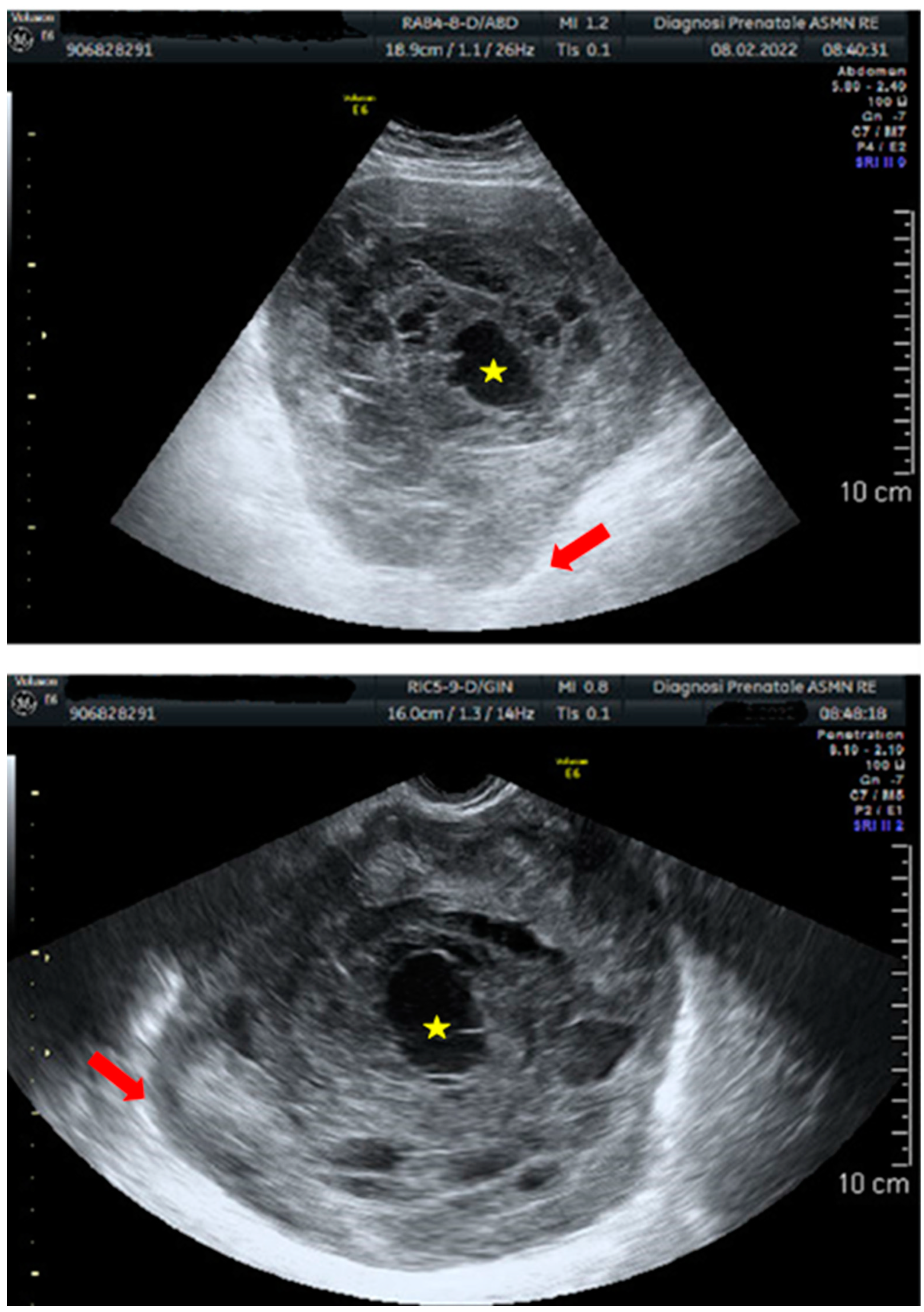


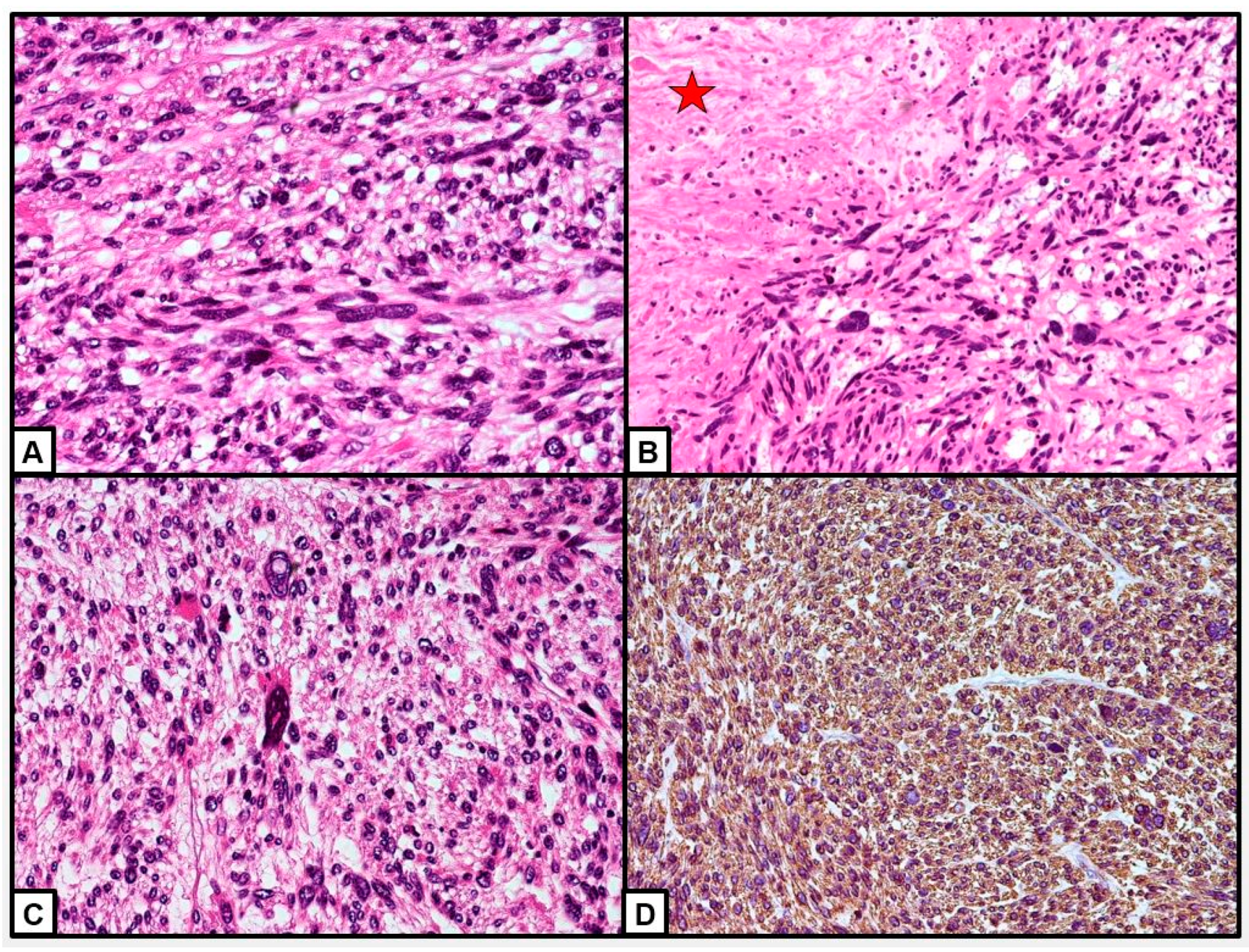
| FIRST AUTHOR | YEAR | AGE | ETHNICITY | SYMPTOMS | SITE | SIZE (mm) | CA 125 | TREATMENT | ADJUVANT THERAPY | STAGE | RECURRENCE | TIME RECURRENCE | TREATMENT RECURRENCE | THERAPY RECURRENCE | FOLLOW-UP (months from first diagnosis) | STATE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Istre B. [17] | 1951 | 84 | C | - | RO | - | - | - | - | - | Local metastasis | - | - | - | 0 | DOD |
| Istre B. [17] | 1951 | 61 | C | - | RO | - | - | - | - | - | Local metastasis | - | - | - | 0 | DOD |
| Istre B. [17] | 1951 | 44 | C | - | Bilateral | - | - | - | - | - | Local metastasis | - | - | - | 0 | DOD |
| Balasz M. et al. [18] | 1960 | 60 | C | - | RO | - | - | - | - | - | Local metastasis | 0 | DOD | |||
| Kelley RR. et al. [19] | 1960 | 70 | C | AP, nocturia | RO | 106 | - | BSO | NO | - | Abdomen | 1 | NO | NO | 1 | DOD |
| Numers Cv. et al. [20] | 1960 | 70 | C | Abdominal enlargement | RO | 125 | - | Supravaginal hysterectomy, remove mass, BSO and appendectomy | RT | - | - | - | ||||
| De Gribble MG. et al. [21] | 1962 | 46 | C | AP | RO | 120 | - | no | NO | - | NO | 0 | DOD | |||
| Nieminen U. et al. [22] | 1969 | 70 | C | - | LO | 50 | - | Subtotal hysterectomy and BSO and appendectomy | RT | I | NO | 80 | NED | |||
| Azoury RS. et al. [23] | 1971 | 66 | - | - | - | - | - | - | - | - | - | 1 | DOD | |||
| Azoury RS. et al. [23] | 1971 | 67 | - | - | RO | - | - | - | - | - | Abdomen | 24 | DOD | |||
| Azoury RS. et al. [23] | 1971 | 61 | - | - | LO | 160 | - | - | - | - | Abdomen | 16 | DOD | |||
| Connor EJ. et al. [24] | 1975 | 17 | - | - | - | 150 | - | - | - | - | NO | 12 | NED | |||
| Walts AE. et al. [25] | 1977 | 56 | C | AP | LO | 110 | - | TAH, BSO | CT(NA) and RT | I | NO | 7 | NED | |||
| Reddy SA. et al. [26] | 1985 | 48 | C | AP | Bilateral | 100 | - | TAH, BSO and omentectomy | NO | III | NO | 16 | NED | |||
| Ansaldi E. et al. [27] | 1986 | 60 | C | AP, urinary frequency | RO | 210 | - | RSO, partial omentectomy | CT (adriamycin and Lomustine) | I | NO | 10 | NED | |||
| Cortes J. et al. [28] | 1987 | 81 | C | AP and constipation | LO | 350 | - | TAH, BSO | NO | I | NO | 10 | NED | |||
| Shakfen SM. et al. [29] | 1987 | NA | C | - | - | - | - | NA | NA | - | NO | 12 | DOD | |||
| Shakfen SM. et al. [29] | 1987 | 24 | C | - | - | - | - | TAH, BSO | RT | - | NO | 48 | DOD | |||
| Anderson B. et al. [30] | 1987 | 59 | C | - | - | - | - | BSO | CT (NA) | III | Local metastasis | 18 | 18 | DOD | ||
| Anderson B. et al. [30] | 1987 | 45 | C | - | - | - | - | USO | NO | I | Chest | 36 | 54 | DOD | ||
| Balaton A. et al. [31] | 1987 | 35 | C | AP | RO | 120 | - | TAH, BSO, omentectomy | CT (Adriblastine, vincristine, fluorouracil, cisplatin) | III | Peritoneum | 7 | RT | 21 | NED | |
| Friedman HD. et al. [32] | 1991 | 58 | C | Lower AP and nausea | RO | 260 | - | TAH, BSO, PW | CT (dacarbazine, doxorubicin hydrochloride and cisplatin) | I | NO | 9 | NED | |||
| Nogales FF. et al. [33] | 1991 | 32 | C | AP | LO | - | - | no | NO | I | NO | 36 | NED | |||
| Nogales FF. et al. [33] | 1991 | 48 | C | Metrorrhagia and pain | LO | - | - | TAH, LSO, cytoreductive surgery, omentectomy | CT (ifosfamide and cisplatin) | III | Vagina and abdomen | 9 | 24 | DOD | ||
| Nogales FF. et al. [33] | 1991 | 68 | C | - | - | - | TAH, BSO, omentectomy | CT (cyclophosphamide, actinomycin D and vincristine) | III | NO | 13 | DOD | ||||
| Monk BJ. et al. [34] | 1992 | 12 | C | Satiety, malaise, abdominal and back pain | LO | 170 | Normal | Exploratory laparoromy and BSO | Hormone therapy | I | NO | - | ||||
| Dixit S. et al. [35] | 1993 | 60 | AA | Generalised weakness | RO | - | - | Excision of mass with TAH and BSO | RT on pelvis | IV | Lung | 6 | NO | NO | 18 | DOD |
| Rasmussen CC. et al. [6] | 1996 | 32 | C | Abdominal fullness | RO | 130 | 35 | TAH, BSO, partial ometectomy, PPLND, PW | CT (ifosfamide and mesna) | IIIC | Omentum, transverse colon | 41 | Optimally cytoreduced | NO | 91 | NED |
| Piura B. et al. [36] | 1997 | 78 | C | - | LO | 150 | - | NO | NO | IA | NO | 21 | Dead due to myocardial infarction | |||
| O’Sullivan SG. et al. [37] | 1998 | 12 | C | AP, malaise, fever, anorexia | RO | 105 | - | Laparotomy: RSO, omental biopsy, right iliac node biopsy, appendectomy | RT | - | YES | 18 | - | |||
| Nasu M. et al. [38] | 1999 | 73 | As | Difficulty in defecating | LO | 170 | Normal | TAH, BSO, omentectomy, dissection of regional lymph nodes | NO | I | Retroperitoneal | 18 | 42 | DOD | ||
| Rampaul RS. et al. [39] | 1999 | 50 | AA | AP | LO | 150 | - | TAH, BSO | NO | I | NO | 24 | NED | |||
| Inoue J. et al. [5] | 2000 | 73 | As | Constipation | - | 170 | Normal | TAH, BSO, PLND and omentectomy | NO | IIC | Liver, left kidney | 16 | 42 | DOD | ||
| Seracchioli R. et al. [40] | 2002 | 20 | C | NO | LO | 80 | Normal | LSO and multiple biposies | NO | - | NO | 12 | NED | |||
| Mayerhofer K. et al. [41] | 2003 | 71 | C | AP | LO | 240 | 122 | BSO and omentectomy | CT (cisplatin and ifosfamide) | IIIC | NO | 14 | Dead due to apoplexy | |||
| Kafah H. et al. [42] | 2003 | 75 | C | - | - | - | - | TAH, BSO, PPLND, omentectomy and appendectomy | CT | III | NO | 24 | NED | |||
| Lerwill MF. et al. [43] | 2004 | 82 | C | - | - | 150 | IA | NO | , | DOD | ||||||
| Lerwill MF. et al. [43] | 2004 | 54 | C | - | - | 150 | IA | Preauricular lymph node | 24 | - | ||||||
| Lerwill MF. et al. [43] | 2004 | 66 | C | - | - | 48 | IA | NO | 28 | NED | ||||||
| Lerwill MF. et al. [43] | 2004 | 56 | C | - | - | 112 | IA | NO | 33 | NED | ||||||
| Lerwill MF. et al. [43] | 2004 | 55 | C | - | - | 220 | IA | Lung and vertebrae | 51 | DOD | ||||||
| Lerwill MF. et al. [43] | 2004 | 70 | C | - | - | 95 | IA | Pelvic | 47 | 63 | DOD | |||||
| Lerwill MF. et al. [43] | 2004 | 25 | C | - | - | 80 | IA | NO | 48 | NED | ||||||
| Lerwill MF. et al. [43] | 2004 | 67 | C | - | - | 145 | IA | Liver | 49 | 73 | DOD | |||||
| Lerwill MF. et al. [43] | 2004 | 42 | C | - | - | 80 | IA | - | 61 | NED | ||||||
| Lerwill MF. et al. [43] | 2004 | 69 | C | - | - | 300 | IA | Pelvic | 67 | - | ||||||
| Lerwill MF. et al. [43] | 2004 | 78 | C | - | - | 160 | IA | NO | 144 | NED | ||||||
| Lerwill MF. et al. [43] | 2004 | 53 | C | - | - | 140 | IA | - | - | |||||||
| Lerwill MF. et al. [43] | 2004 | 38 | C | - | - | 180 | IA | Mediastinum, vertebrae | 10 | 13 | DOD | |||||
| Lerwill MF. et al. [43] | 2004 | 61 | C | - | - | 40 | IA | NO | 108 | NED | ||||||
| Lerwill MF. et al. [43] | 2004 | 65 | C | - | - | 130 | IC | - | - | |||||||
| Lerwill MF. et al. [43] | 2004 | 48 | C | - | - | 160 | IIA | NO | 12 | DOD | ||||||
| Lerwill MF. et al. [43] | 2004 | 70 | C | - | - | - | IIB | Pelvic | 4 | 6 | DOD | |||||
| Lerwill MF. et al. [43] | 2004 | 70 | C | - | - | 90 | IIB | NO | 2 | DOD | ||||||
| Lerwill MF. et al. [43] | 2004 | 69 | C | - | - | - | IIB | Lung | 6 | 43 | DOD | |||||
| Lerwill MF. et al. [43] | 2004 | 68 | C | - | Bilateral | 350 | TAH, BSO and omentectomy | III | NO | 13 | DOD | |||||
| Lerwill MF. et al. [43] | 2004 | 47 | C | - | Bilateral | 130 | TAH, BSO and omentectomy | IIIB | NO | 3 | DOD | |||||
| Lerwill MF. et al. [43] | 2004 | 61 | C | - | - | 180 | IIIC | Pelvic | 2 | 9 | DOD | |||||
| Lerwill MF. et al. [43] | 2004 | 29 | C | - | - | - | IIIC | NO | 8 | DOD | ||||||
| Lerwill MF. et al. [43] | 2004 | 51 | C | - | - | 130 | IIIC | - | - | |||||||
| Lerwill MF. et al. [43] | 2004 | 49 | C | - | - | 175 | IIIC | - | - | |||||||
| Lerwill MF. et al. [43] | 2004 | 62 | C | - | - | 200 | IIIC | NO | 0 | DOD | ||||||
| Nicotina PA. et al. [44] | 2004 | 66 | C | AP and abdominal enlargement | RO | 140 | - | TAH, BSO | CT (NA) | IIA | Liver, lung | 18 | NO | NO | 24 | DOD |
| Bouie SM. et al. [45] | 2005 | 42 | C | - | LO | 180 | - | Exploratory laparotomy and TAH with omentectomy | CT (NA) | I | NO | 24 | NED | |||
| Chang A. et al. [46] | 2005 | 25 | C | Fever, anorexia | RO | 110 | - | Excision of the mass | CT (doxorubicin, ifosfamide) | - | Large bowel | 1 | - | - | 34 | DOD |
| Kuscu E. et al. [47] | 2005 | 62 | C | Nocturia, Incontinence | RO | 33 | Normal | Laparoscopy: BSO. TAH, PPLNS | NO | I | NO | 20 | NED | |||
| Taskin S. et al. [15] | 2007 | 68 | C | NO | RO | 120 | Normal | TAH, BSO, PPLND, omentectomy and appendectomy | CT (ifosfamide and mesna) | IA | NO | 118 | NED | |||
| Taskin S. et al. [15] | 2007 | 52 | C | Abdominal distension | RO | - | - | TAH, BSO, PPLND, omentectomy and appendectomy | CT (ifosfamide and mesna) | IA | NO | 19 | NED | |||
| LI Y. et al. [48] | 2008 | 71 | As | AP and weight loss | LO | 150 | 120.2 | TAH, BSO, omentectomy, PLNS, peritoneal and pelvic wall biopsy | NO | III | NO | 0 | DOD | |||
| Khizar S. et al. [49] | 2009 | 73 | C | Painless | LO | 140 | Normal | BSO and omentectomy | CT, RT | - | - | - | ||||
| Arslan OS. et al. [1] | 2010 | 52 | C | Inguinal pain | RO | 80 | 139 | TAH, BSO, infracolic omentectomy, appendectomy, bilateral PPLND | NO | IA | NO | 6 | NED | |||
| Dai Y. et al. [50] | 2011 | - | As | - | - | - | 39.4 | Optimal debulking | CT (cisplatin, VP-16/vincristine and bleomycin combination) | III | NO | 4 | DOD | |||
| Dai Y. et al. [50] | 2011 | - | As | - | - | - | 39.4 | Suboptimaldebulking | CT (cisplatin, VP-16/vincristine and bleomycin combination) | III | NO | 4 | DOD | |||
| Goodall EJ. et al. [4] | 2011 | 60 | C | Left loin pain, loss of appetite, sterile pyuria | LO | - | 20 | RH, BSO, omental biopsy, left PLNS | NO | IA | NO | 22 | NED | |||
| Zygouris D. et al. [51] | 2011 | 58 | C | AP | RO | 250 | 63.4 | Exploratory laparotomy:BSO, omentectomy, PLND | NO | IA | NO | 21 | NED | |||
| Pankaj S. et al. [52] | 2013 | 27 | AA | AP | RO | 97 | 5.2 | TAH, BSO, omentectomy, PPLND | CT (docetaxel and Gemcitabina) | - | NO | 30 | NED | |||
| Divya NS. et al. [9] | 2014 | 26 | AA | AP | RO | - | - | TAH, RSO | - | - | - | - | ||||
| Rivas G. et al. [53] | 2014 | 65 | AA | AP and urinary retention | LO | 150 | Normal | TAH, BSO, PLND | CT (doxorubicin, ifosfamide and mesna) | IC | NO | 24 | NED | |||
| Sunita S. et al. [54] | 2014 | 30 | AA | AP | RO | 150 | 69.9 | Excision and omentectomy | CT (NA) | 4 | AWD | |||||
| He M. et al. [14] | 2015 | 46 | As | AP | LO | 50 | 27.73 | Exploratory laparotomy: TAH, BSO | NO | IC | Pelvis, lung | 11 | Cytoreductive surgery | CT (docetaxel and gemcitabine (1 cycle) and gemcitabine only (2 cycles) | 50 | DOD |
| Kumar V. et al. [55] | 2015 | 30 | AA | AP | RO | 147 | 69.9 | Laparotomy: TAH, BSO, PW | CT (NA) | IA | NO | 6 | NED | |||
| Nazneen S. et al. [56] | 2015 | 27 | AA | AP and distension | RO | 97 | 5.2 | Laparotomy: TAH, BSO, omentectomy, PPLND | CT (docetaxel and gemcitabine) | - | NO | 47 | NED | |||
| Thyagaraju C. et al. [57] | 2015 | 55 | AA | AP and distension | RO | 280 | Normal | TAH, BSO, partial omentectomy | NO | I | NO | 21 | NED | |||
| Mamta G. et al. [58] | 2015 | 27 | AA | AP | RO | 180 | - | RSO, after TAH and LSO | NO | IA | NO | 4 | NED | |||
| Na Lee B. et al. [59] | 2016 | 67 | As | Gynecological checkup | RO | 90 | Normal | Adhesiolysis, TAH, BSO | NO | - | NO | 3 | NED | |||
| Pongsuvareeyakul T. et al. [13] | 2017 | 65 | As | AP | RO | 242 | 202.5 | Exploratory laparotomy: TAH, BSO, omentectomy | NO | IIIC | NO | 1 | DOD | |||
| Furutake Y. et al. [60] | 2017 | 40 | As | AP | LO | 120 | - | LSO | NO | - | LO, pelvic bone, liver | 9 | NO | CT (gemcitabine and docetaxel) | 24 | NED |
| Vishwanath. et al. [10] | 2018 | 55 | AA | AP, distension, loss of appetite | RO | 150 | 150 | TAH, BSO, PLND, omental biopsy | CT (docetaxel, gemcitabine) | IC | NO | 2 | NED | |||
| Tanaka A. et al. [61] | 2018 | 64 | As | Incontinence | LO | 170 | - | TAH, BSO, PLND, omentectomy | NO | IC2 | Vaginal stump | 7 | CT (gemcitabine hydrochloride and docetaxel hydrate and then paclitaxel and carboplatin) | 35 | DOD | |
| Sukgen G. et al. [62] | 2018 | 59 | C | AP and swelling | LO | 350 | Normal | TAH, BSO, BPPLND, omentectomy, PW, appendectomy | NO | IA | NO | 6 | NED | |||
| Shafiee MN. et al. [63] | 2019 | 39 | As | Heavy mestrual bleeding with clots and flooding, abdominal cramps and back pain | RO | 200 | 40 | TAH and BSO | CT (mesna, ifosfamide, doxorubicin and cisplatin) | IIIB | Lung | 4 | NO | CT (gemcitabine and paclitaxel) | 24 | NED |
| Fischetti A. et al. [64] | 2019 | 61 | C | Right colic hypochondrial pain | RO | 90 | - | Surgical resection | - | - | - | - | ||||
| Yuksel D. et al. [65] | 2020 | 34 | C | - | RO | - | - | LSO, total omentectomy, PPLND, right ovarian resection and after RSO, TAH | CT (doxorubicin) | IA | NO | 13 | NED | |||
| Yuksel D. et al. [65] | 2020 | 68 | C | Pelvic pain, fever, sweats and rectal hemorrhage | RO | 86 | 28 | TAH, BSO, PPLND, omentectomy | CT (doxorubicin) | IIIC | NO | 48 | NED | |||
| Yuksel D. et al. [65] | 2020 | 52 | C | AP, distension | LO | - | - | PLND, omentectomy, tumor debulking, rectosigmoid resection | NO | IIIC | Early pelvic recurrence | 1 | NO | NO | 2 | DOD |
| Cojocaru E. et al. [2] | 2021 | - | C | - | - | - | - | TAH, BSO | Palliative CT | IVB | YES | NO | CT (ifosfamide nd doxorubicin) | 25 | DOD | |
| Cojocaru E. et al. [2] | 2021 | - | C | - | - | - | - | TAH, BSO | NO | IA | NO | NO | 15 | NED | ||
| Cojocaru E. et al. [2] | 2021 | - | C | - | - | - | - | TAH, BSO | NO | IA | YES | 14 | NO | CT (doxorubicin) | 51 | DOD |
| Cojocaru E. et al. [2] | 2021 | - | C | - | - | - | - | TAH, BSO | NO | IA | YES | 31 | NO | CT (gemcitabine, docetaxel) | 77 | DOD |
| Cojocaru E. et al. [2] | 2021 | - | C | - | - | - | - | no | Palliative CT (doxorubicin) | IVB | YES | - | NO | CT (doxorubicin) | 18 | DOD |
| Cojocaru E. et al. [2] | 2021 | - | C | - | - | - | - | RSO | NO | IA | YES | 16 | NO | NO | 98 | AWD |
| Cojocaru E. et al. [2] | 2021 | - | C | - | - | - | - | TAH, BSO | NO | IA | YES | 15 | NO | CT (gemcitabine, docetaxel) | 56 | DOD |
| Cojocaru E. et al. [2] | 2021 | - | C | - | - | - | - | TAH, BSO | NO | IA | YES | 3 | NO | CT (carboplatin and gemcitabine) | 22 | DOD |
| Cojocaru E. et al. [2] | 2021 | - | C | - | - | - | - | LSO | NO | IA | NO | NO | 8 | NED | ||
| Cojocaru E. et al. [2] | 2021 | - | C | - | - | - | - | BSO | CT (gemcitabine, docetaxel) | IIB | YES | 11 | NO | CT (doxorubicin) | 12 | NED |
| Cojocaru E. et al. [2] | 2021 | - | C | - | - | - | - | NO | NO | IVB | NO | NO | 2 | DOD | ||
| Cojocaru E. et al. [2] | 2021 | - | C | - | - | - | - | BSO | NO | IA | YES | 40 | no | NO | 47 | AWD |
| Cojocaru E. et al. [2] | 2021 | - | C | - | - | - | - | LSO | NO | IA | NO | 15 | NED | |||
| Cojocaru E. et al. [2] | 2021 | - | C | - | - | - | - | TAH, BSO | NO | IIB | NO | 13 | NED | |||
| Cojocaru E. et al. [2] | 2021 | - | C | - | - | - | - | TAH, BSO | CT (gemcitabine, docetaxel) | IIIA2 | YES | 7 | NO | CT (Epirubicin-carboplatin) | 11 | AWD |
| Pu T. et al. [16] | 2022 | 29 | As | Abdominal distension | RO | 215 | 68.33 | Exploratory laparotomy (fertility sparing) | NO | IA | LO | 20 | TAH, omentum resection, pelvic LA | CT (paclitaxel and carboplatin) | 48 | NED |
| Khadjetou V. et al. [66] | 2022 | 16 | AA | Pelvic pain | RO | 150 | Normal | TAH, BSO, PPLND | CT | IIIC | NO | 23 | NED | |||
| Bahadur A. et al. [67] | 2022 | As | Vaginal prolapse, lower abdominal pain | RO | 150 | 55.6 | TAH, BSO, omentectomy, small bowel resection, metastatectomy | CT (adriamycin and ifosfamide) | IIIC | - | - | - | - | - | - |
| Overall (n = 113) | |
|---|---|
| AGE | |
| Mean (SD) | 53 (12–84) |
| NA | 18 |
| ETHNICITY | |
| African | 3 (2.8%) |
| Asian | 22 (20.2%) |
| Caucasian | 84 (77.1%) |
| NA | 4 |
| SITE | |
| Left Ovary | 22 (34.9%) |
| Right Ovary | 37 (58.7%) |
| Bilateral | 4 (6.3%) |
| NA | 50 |
| SIZE (mm) | |
| Mean (SD) | 151.2 (68.6) |
| NA | 40 |
| Treatment | |
| None | 3 (3.8%) |
| Chemotherapy only | 1 (1.2%) |
| Surgery | 34 (42.5%) |
| Surgery with LND | 10 (12.5%) |
| Surgery + CHT | 21 (26.2%) |
| Surgery with LND + CHT | 11 (13.8%) |
| NA | 33 |
| Schedule of chemotherapy | |
| Sarcoma-like | 17 (70.8%) |
| Sarcoma-like +Platinum | 5 (20.8%) |
| Dysgerminoma-like | 2 (8.4%) |
| NA | 9 |
| Adjuvant treatment | |
| None | 39 (50.0%) |
| Hormone therapy | 1 (1.3%) |
| Radiotherapy | 5 (6.4%) |
| Chemotherapy | 31 (39.7%) |
| Chemotherapy + radiotherapy | 2 (2.6%) |
| NA | 35 |
| STAGE | |
| I | 52 (58.4%) |
| II | 8 (9.0%) |
| III | 25 (28.1%) |
| IV | 4 (4.5%) |
| NA | 24 |
| Recurrence | |
| No | 58 (56.9%) |
| Yes | 44 (43.1%) |
| NA | 11 |
| Odds Ratio | C.I. 95% | p Value | |
|---|---|---|---|
| Surgery with LND | 1.3 | 0.8–1.6 | 0.284 |
| Surgery + CHT | 0.8 | 0.51–1.24 | 0.327 |
| Surgery with LND + CHT | 0.8 | 0.45–1.35 | 0.391 |
| Stage III-IV | 1.6 | 1.1–2.2 | 0.022 |
| Mitotic count > 10 | 1.2 | 0.8–1.7 | 0.413 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandato, V.D.; Torricelli, F.; Mastrofilippo, V.; Palicelli, A.; Costagliola, L.; Aguzzoli, L. Primary Ovarian Leiomyosarcoma Is a Very Rare Entity: A Narrative Review of the Literature. Cancers 2023, 15, 2953. https://doi.org/10.3390/cancers15112953
Mandato VD, Torricelli F, Mastrofilippo V, Palicelli A, Costagliola L, Aguzzoli L. Primary Ovarian Leiomyosarcoma Is a Very Rare Entity: A Narrative Review of the Literature. Cancers. 2023; 15(11):2953. https://doi.org/10.3390/cancers15112953
Chicago/Turabian StyleMandato, Vincenzo Dario, Federica Torricelli, Valentina Mastrofilippo, Andrea Palicelli, Luigi Costagliola, and Lorenzo Aguzzoli. 2023. "Primary Ovarian Leiomyosarcoma Is a Very Rare Entity: A Narrative Review of the Literature" Cancers 15, no. 11: 2953. https://doi.org/10.3390/cancers15112953
APA StyleMandato, V. D., Torricelli, F., Mastrofilippo, V., Palicelli, A., Costagliola, L., & Aguzzoli, L. (2023). Primary Ovarian Leiomyosarcoma Is a Very Rare Entity: A Narrative Review of the Literature. Cancers, 15(11), 2953. https://doi.org/10.3390/cancers15112953







