Diagnostic and Therapeutic Approaches for Glioblastoma and Neuroblastoma Cancers Using Chlorotoxin Nanoparticles
Abstract
Simple Summary
Abstract
1. Introduction
2. Glioblastoma Multiforme (GB): Standard Treatments and Challenges
3. Neuroblastomas (NBs): Standard Treatments and Challenges
4. Current Challenges Associated with Drug Delivery to the Brain
5. Chlorotoxin (CTX): A Promising Natural Targeting Peptide for Cancers
5.1. Molecular Targets of CTX
5.1.1. Chloride Channels
5.1.2. Matrix Metalloproteinase-2 (MMP-2)
5.1.3. Annexin A2
5.1.4. Estrogen Receptor Alpha (ERα)-Mediated Signalling Pathway
5.1.5. Neuropilin-1 (NRP-1)
5.2. The Blood–Brain Barrier Crossing Potential of CTX
6. Nanotechnology for Cancer Applications
7. CTX-NPs with Diagnostic Potential
| Name of Nanoparticle (NP) Formulation | Imaging Modality | Size in nm (Hydrodynamic Size/Core Size) | Ref. |
|---|---|---|---|
| mPEI-CTX-99mTc/DOX | SPECT | 394.77 nm | [240] |
| CTX-PEG-Dox-Eu-Gd2O3 NRs | MRI | 116.3 nm | [231] |
| 131I-labeled BmK-Au-PENPs | SPECT/CT | 147 nm | [239] |
| 131I-labeled CTX-Au-PENPs | SPECT/CT imaging | 151 nm | [236] |
| Fe3O4;/PEG-FA–Cy5.5-CTX | MRI | <20 nm | [217] |
| 131I-I-G5.NHAc-HPAO-(PEG-BmK CT)-(mPEG) | SPECT imaging | ~4 nm | [211] |
| SPIONP-PEG-CTX | MRI | <100 nm | [243] |
| QD(Ag-In-S/ZnS)-CTX | Optical imaging | 126 nm | [222] |
| Fe3O4/MnO–Cy5.5-CTX | MRI | 25 nm | [216] |
| Ag/Ali-PNPs-CTX-99mTc | Optical imaging | 199 nm | [242] |
| NaGdF4-Ho3+-CTX | MRI/Optical imaging | 44.2 nm | [229] |
| CTX-PEG-Gd2O3 | MRI | 3.46 nm | [230] |
| Pdot-CTX | Optical imaging | ~15 nm | [223] |
| Gd-DTPA/BODIPY-dendrigraft poly-L-lysines-PEG-CTX | MRI | N/A | [233] |
| SPIONP-PEG-PEI-siRNA-CTX | Optical imaging | 7.5 nm | [244] |
| IONP-PEG-Chitosan-DNA-CTX | MRI | 48.8 nm | [62] |
| MFNP–CTX | MRI/Optical imaging | <100 nm | [210] |
| IONP-PEG-Chitosan-Cy5.5-CTX | MRI/Optical imaging | 7 nm | [209] |
| PEI-NaYF(4):Yb, Er/Ce-CTX | Optical imaging | Width: 55 nm; length: 25 nm | [226] |
| NP-MTX-CTX | MRI | 5–8 nm | [111] |
| IONP-PEG-CTX | MRI | 10–15 nm | [208] |
| SPIONP-FITC-CTX | MRI/Optical imaging | 80 nm | [245] |
| IONP-PEG-CTX | MRI/Optical imaging | 10 nm | [207] |
8. Therapeutic and Targeting Applications of CTX-NPs for GB Tumors
| Name | Therapeutic Effect | Theranostic Application | Size in nm (Hydrodynamic Size/Core Size) | Ref. |
|---|---|---|---|---|
| NP-siMGMT-CTX | The effective number of siRNAs (MGMT) delivered to tumors to sensitize both GB cells and GB stem-like cells (GSCs) to TMZ in vivo via CTX targeting | Yes | 60.97 nm | [264] |
| CTX/DOTA/LND-PANPs Lf/CTX/TPP/DOTA/LND-PANPs | Increased localization of NPs in mitochondria both in vitro and in vivo, resulting in apoptosis. Photothermal therapy (PTT) with NPs occurred using NIR | Yes | <20 nm | [126] |
| mPEI-CTX-99mTc/DOX | In vivo targeted delivery of DOX | Yes | 394.77 nm | [239] |
| CTX-PEG-Dox-Eu-Gd2O3 NRs | No significant toxicity was reported in HUVEC cells, while toxicity was reported in U251 cells owing to CTX targeting MMP-2. In vivo experiments showed the inhibition of brain tumors with no significant toxicity to normal organs | Yes | 116.3 nm | [230] |
| CTX-KRKRK-GFP-H6 and CTX-GFP-H6 | Two recombinant CTX-fluorescent protein NPs demonstrated significant cytotoxicity in cell lines U87 (over-expressing MMP2) and Hela (overexpressing Annexin 2) | No | ~12 nm | [267] |
| CTX-PMLA-LLL-ICG | Systemic IV injection into a xenogeneic mouse model carrying human U87 GB cells indicated tumor cell binding and internalization of NPs resulting in long-lasting tumor fluorescence which guided the resection of GB and significantly improved the precision of tumor removal | Yes | 11.82 nm | [159] |
| CTX and mApoE-Dox-Lip | Enhanced DOX across the BBB via CTX-liposomes | No | 184 nm | [249] |
| CTX-PLGA-Morusin | NPs resulted in enhanced inhibitory effects on U87 and GI-1 glioma cells | No | 242.9 nm | [248] |
| CTX-TMZ noisome | Enhanced TMZ delivery into the brain in vivo with less deposition in the highly perfused organs | No | 220 nm | [261] |
| M-CTX-Fc-L-Dox | Significant cytotoxicity observed with DOX loaded CTX- liposomes in U251 cells in vitro and tumor suppression in BALB/c mice bearing tumors of transplanted U251 cells in vivo | No | 100–150 nm | [247] |
| RGD-Eu-Gd2O3 NRs-CTX | Nanorods specifically target U251 cells, leading to cellular apoptosis. In vivo results show NPs could effectively inhibit early tumor growth, without any damage to normal tissues/organ | Yes | ~78 nm | [268] |
| IONP-HA-GEM-CTX | NPs effectively crossed BBB and killed GB cells, had prolonged blood circulation duration, and were excreted from the renal system | Yes | ~32 nm | [265] |
| Ag-PNP-CTX | In vitro experiments performed with different human GB cell lines showed higher uptake of Ag-PNP-CTX, with respect to non-functionalized Ag-PNP NPs, and in vivo experiments showed that Ag-NP-CTX efficiently targets the tumors | Yes | 199.1 nm | [103] |
| CTX-SNALPs-miR-21 | MiRNA-21 silencing because of tumor-targeted CTX-NPs and decreased tumor cell proliferation and enhanced apoptosis in combination with Sunitinib | No | <190 nm | [266] |
| NP-TMZ-CTX | CTX-NPs demonstrated targeting of GB cells and 2–6-fold higher uptake and 50–90% reduction of IC50 at 72 h post-treatment as compared to NPs without CTX | Yes | <100 nm | [71] |
| Ag/Ali-PNPs-CTX-99mTc | Significant tumor reduction was achieved in vivo as the result of the synergistic effects of Alisertib and NPs | Yes | 199 nm | [241] |
| AuNRs-PNPs-Cltx/Cy5.5 | NPs showed enhanced binding affinity toward GB cells in vitro using optoacoustic microscopy and PTT using laser irradiation of the cells led to cell damage | Yes | 122.5 nm | [257] |
| CTX-Lip | CTX was attached to the surface of liposomes which interacts with the MMP-2 on the surface of U87 human glioma cell line cells and A549, demonstrating targeting | No | 103.4 nm | [245] |
| CTX-IONP-siMGMT | Combination treatment of mice bearing orthotopic tumors with CTX-NP-siMGMT and TMZ led to a significant reduction of tumor growth | Yes | 37.3 nm | [263] |
| CTX-SNALPs | Targeted NP-mediated miR-21 silencing in U87 and GL261 cells resulted in increased levels of the tumor suppressors PTEN and PDCD4, caspase 3/7 activation, and decreased tumor cell proliferation | No | <180 nm | [58] |
| AgNPs-PNS-CTX | Significantly higher uptake of Ag into U87 cells compared to the non-targeted NPs. Cytotoxic effect in glioma cell lines was also reported | No | 130 nm | [240] |
| CTX-DoX-Lip | Increased cytotoxicity against U87 and U251 glioma and significant tumor growth inhibition in vivo | No | 128 nm | [113] |
| NP-DNA-CTX | Enhanced uptake specifically into glioma cells in vivo | Yes | 48.8 nm | [62] |
| IONPs-PEG-CTX and IONS-PEG-RDG | NP-CTX and NP-RGD were target-specific to integrin MMP-2 and αvβ3 integrin | Yes | ~12 nm | [269] |
| NP(ION/PEG)-CTX-Cy5.5 | NPs showed tumor-specific accumulation in vivo and no toxicity effects | Yes | 13.5 nm | [270] |
| NP–CTX-chitosan-Cy5.5 | Optimal serum half-life, biodistribution, stability, and non-toxicity were confirmed in mice | Yes | 7 nm | [208] |
| MFNP-CTX | CTX-NPs demonstrated high specific cellular uptake | Yes | <100 nm | [209] |
| NP-siRNA-CTX | Increased small interfering RNA (siRNA) internalization by targeting glioma cells and intracellular trafficking towards enhanced knockdown of targeted gene expression | Yes | 6–10 nm | [243] |
| NP-PEIb-siRNA-CTX | CTX-NPs showed long-term stability and good magnetic properties, significant cytotoxic effects, and gene silencing effects at acidic pH conditions for C6 glioma cells | Yes | ~60 nm | [256] |
| NP-AF647-CTX-DNA | Results showed low cytotoxicity because of CTX targeting and excellent gene transfection efficiency | Yes | 134.8 nm | [253] |
| NP-CTX-AF680 | The NPs enhanced cellular uptake via MMP-2 | Yes | ~11 nm | [112] |
| NPCP-Cy5.5-CTX | NPs showed cytotoxicity, sustained retention in tumors, and the ability to cross the BBB and specifically target brain tumors in vivo | Yes | 33 nm | [214] |
| NP-MTX-CTX | Increased cytotoxicity of methotrexate (MTX) in GB cells and prolonged retention of NPs was observed within tumors in vivo NPs | Yes | 5–8 nm | [111] |
9. Prospective Applications of CTX-NP Formulations
9.1. Optoacoustic Imaging Using CTX-NPs
9.2. Diagnostic and Therapeutic Potential of Biomimetic CTX-NPs
9.3. Hyperthermia Treatment Using CTX-NPs
10. CTX-like Peptides
11. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ayed, A.S.; Omran, M.A.A.A.; Nabil, Z.; Strong, P.; Newton, K.; Abdel Rahman, M. GBD 2017 US Neurological Disorders Collaborators Burden of Neurological Disorders Across the US from 1990–2017: A Global Burden of Disease Study. JAMA Neurol. 2021, 78, 165–176. [Google Scholar] [CrossRef]
- Mohammadi, E.; Ghasemi, E.; Azadnajafabad, S.; Rezaei, N.; Moghaddam, S.S.; Meimand, S.E.; Fattahi, N.; Habibi, Z.; Yarandi, K.K.; Amirjamshidi, A.; et al. A global, regional, and national survey on burden and Quality of Care Index (QCI) of brain and other central nervous system cancers; global burden of disease systematic analysis 1990–2017. PLoS ONE 2021, 16, e0247120. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Ostrom, Q.T.; Kruchko, C.; Patil, N.; Tihan, T.; Cioffi, G.; Fuchs, H.E.; Waite, K.A.; Jemal, A.; Siegel, R.L.; et al. Brain and other central nervous system tumor statistics, 2021. CA Cancer J. Clin. 2021, 71, 381–406. [Google Scholar] [CrossRef]
- Thon, N.; Tonn, J.-C.; Kreth, F.-W. The surgical perspective in precision treatment of diffuse gliomas. OTT 2019, 12, 1497–1508. [Google Scholar] [CrossRef]
- Jain, K.K. A Critical Overview of Targeted Therapies for Glioblastoma. Front. Oncol. 2018, 8, 419. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, C.; Cavalli, R.; Panciani, P.P.; Battaglia, L. Overcoming the Blood–Brain Barrier: Successes and Challenges in Developing Nanoparticle-Mediated Drug Delivery Systems for the Treatment of Brain Tumours. IJN 2020, 15, 2999–3022. [Google Scholar] [CrossRef]
- Southgate, H.E.D.; Chen, L.; Curtin, N.J.; Tweddle, D.A. Targeting the DNA Damage Response for the Treatment of High Risk Neuroblastoma. Front. Oncol. 2020, 10, 371. [Google Scholar] [CrossRef]
- Aravindan, N.; Jain, D.; Somasundaram, D.B.; Herman, T.S.; Aravindan, S. Cancer stem cells in neuroblastoma therapy resistance. Cancer Drug Resist. 2019, 2, 948–967. [Google Scholar] [CrossRef]
- Smith, V.; Foster, J. High-Risk Neuroblastoma Treatment Review. Children 2018, 5, 114. [Google Scholar] [CrossRef]
- Emadi, A.; Jones, R.J.; Brodsky, R.A. Cyclophosphamide and cancer: Golden anniversary. Nat. Rev. Clin. Oncol. 2009, 6, 638–647. [Google Scholar] [CrossRef]
- Schacht, J.; Talaska, A.E.; Rybak, L.P. Cisplatin and Aminoglycoside Antibiotics: Hearing Loss and Its Prevention. Anat. Rec. 2012, 295, 1837–1850. [Google Scholar] [CrossRef] [PubMed]
- Laverdière, C.; Cheung, N.-K.V.; Kushner, B.H.; Kramer, K.; Modak, S.; LaQuaglia, M.P.; Wolden, S.; Ness, K.K.; Gurney, J.G.; Sklar, C.A. Long-term complications in survivors of advanced stage neuroblastoma. Pediatr. Blood Cancer 2005, 45, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Trahair, T.N.; Vowels, M.R.; Johnston, K.; Cohn, R.J.; Russell, S.J.; Neville, K.A.; Carroll, S.; Marshall, G.M. Long-term outcomes in children with high-risk neuroblastoma treated with autologous stem cell transplantation. Bone Marrow Transplant. 2007, 40, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Speckhart, B.; Antony, R.; Fernandez, K.S. Long-term side effects of high-risk neuroblastoma survivors in a referral center in central Illinois. JCO 2017, 35, 129. [Google Scholar] [CrossRef]
- Applebaum, M.A.; Vaksman, Z.; Lee, S.M.; Hungate, E.A.; Henderson, T.O.; London, W.B.; Pinto, N.; Volchenboum, S.L.; Park, J.R.; Naranjo, A.; et al. Neuroblastoma survivors are at increased risk for second malignancies: A report from the International Neuroblastoma Risk Group Project. Eur. J. Cancer 2017, 72, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Welch, D.R.; Hurst, D.R. Defining the Hallmarks of Metastasis. Cancer Res. 2019, 79, 3011–3027. [Google Scholar] [CrossRef]
- Tahtamouni, L.; Ahram, M.; Koblinski, J.; Rolfo, C. Molecular Regulation of Cancer Cell Migration, Invasion, and Metastasis. Anal. Cell. Pathol. 2019, 2019, e1356508. [Google Scholar] [CrossRef]
- Lah, T.T.; Novak, M.; Breznik, B. Brain malignancies: Glioblastoma and brain metastases. Semin. Cancer Biol. 2020, 60, 262–273. [Google Scholar] [CrossRef]
- Jiang, W.G.; Sanders, A.J.; Katoh, M.; Ungefroren, H.; Gieseler, F.; Prince, M.; Thompson, S.K.; Zollo, M.; Spano, D.; Dhawan, P.; et al. Tissue invasion and metastasis: Molecular, biological and clinical perspectives. Semin. Cancer Biol. 2015, 35, S244–S275. [Google Scholar] [CrossRef]
- Walker, C.; Mojares, E.; del Río Hernández, A. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018, 19, 3028. [Google Scholar] [CrossRef] [PubMed]
- Winer, A.; Adams, S.; Mignatti, P. Matrix Metalloproteinase Inhibitors in Cancer Therapy: Turning Past Failures into Future Successes. Mol. Cancer Ther. 2018, 17, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Latifi, Z.; Fattahi, A.; Ranjbaran, A.; Nejabati, H.R.; Imakawa, K. Potential roles of metalloproteinases of endometrium-derived exosomes in embryo-maternal crosstalk during implantation. J. Cell. Physiol. 2018, 233, 4530–4545. [Google Scholar] [CrossRef] [PubMed]
- Tardáguila-García, A.; García-Morales, E.; García-Alamino, J.M.; Álvaro-Afonso, F.J.; Molines-Barroso, R.J.; Lázaro-Martínez, J.L. Metalloproteinases in chronic and acute wounds: A systematic review and meta-analysis. Wound Repair Regen. 2019, 27, 415–420. [Google Scholar] [CrossRef]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef]
- Raeeszadeh-Sarmazdeh, M.; Do, L.D.; Hritz, B.G. Metalloproteinases and Their Inhibitors: Potential for the Development of New Therapeutics. Cells 2020, 9, 1313. [Google Scholar] [CrossRef]
- Conant, K.; Allen, M.; Lim, S.T. Activity dependent CAM cleavage and neurotransmission. Front. Cell. Neurosci. 2015, 9, 305. [Google Scholar] [CrossRef]
- Allen, M.; Ghosh, S.; Ahern, G.P.; Villapol, S.; Maguire-Zeiss, K.A.; Conant, K. Protease induced plasticity: Matrix metalloproteinase-1 promotes neurostructural changes through activation of protease activated receptor 1. Sci. Rep. 2016, 6, 35497. [Google Scholar] [CrossRef]
- Forsyth, P.A.; Wong, H.; Laing, T.D.; Rewcastle, N.B.; Morris, D.G.; Muzik, H.; Leco, K.J.; Johnston, R.N.; Brasher, P.M.; Sutherland, G.; et al. Gelatinase-A (MMP-2), gelatinase-B (MMP-9) and membrane type matrix metalloproteinase-1 (MT1-MMP) are involved in different aspects of the pathophysiology of malignant gliomas. Br. J. Cancer 1999, 79, 1828–1835. [Google Scholar] [CrossRef]
- Guo, P.; Imanishi, Y.; Cackowski, F.C.; Jarzynka, M.J.; Tao, H.-Q.; Nishikawa, R.; Hirose, T.; Hu, B.; Cheng, S.-Y. Up-regulation of angiopoietin-2, matrix metalloprotease-2, membrane type 1 metalloprotease, and laminin 5 gamma 2 correlates with the invasiveness of human glioma. Am. J. Pathol. 2005, 166, 877–890. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, X.; Sun, S.; Zhang, X.; Yang, W.; Zhang, J.; Zhang, X.; Jiang, Z. Increased expression of MMP-2 and MMP-9 indicates poor prognosis in glioma recurrence. Biomed. Pharmacother. 2019, 118, 109369. [Google Scholar] [CrossRef]
- Sugiura, Y.; Shimada, H.; Seeger, R.C.; Laug, W.E.; DeClerck, Y.A. Matrix metalloproteinases-2 and -9 are expressed in human neuroblastoma: Contribution of stromal cells to their production and correlation with metastasis. Cancer Res. 1998, 58, 2209–2216. [Google Scholar]
- Ribatti, D.; Surico, G.; Vacca, A.; De Leonardis, F.; Lastilla, G.; Montaldo, P.G.; Rigillo, N.; Ponzoni, M. Angiogenesis extent and expression of matrix metalloproteinase-2 and -9 correlate with progression in human neuroblastoma. Life Sci. 2001, 68, 1161–1168. [Google Scholar] [CrossRef]
- Hall, M.K.; Whitman, A.A.; Weidner, D.A.; Schwalbe, R.A. Knockdown of N-Acetylglucosaminyltransferase-II Reduces Matrix Metalloproteinase 2 Activity and Suppresses Tumorigenicity in Neuroblastoma Cell Line. Biology 2020, 9, 71. [Google Scholar] [CrossRef]
- Yu, C.-F.; Chen, F.-H.; Lu, M.-H.; Hong, J.-H.; Chiang, C.-S. Dual roles of tumour cells-derived matrix metalloproteinase 2 on brain tumour growth and invasion. Br. J. Cancer 2017, 117, 1828–1836. [Google Scholar] [CrossRef]
- Habela, C.W.; Olsen, M.L.; Sontheimer, H. ClC3 is a critical regulator of the cell cycle in normal and malignant glial cells. J. Neurosci. 2008, 28, 9205–9217. [Google Scholar] [CrossRef]
- Wang, B.; Xie, J.; He, H.-Y.; Huang, E.-W.; Cao, Q.-H.; Luo, L.; Liao, Y.-S.; Guo, Y. Suppression of CLC-3 chloride channel reduces the aggressiveness of glioma through inhibiting nuclear factor-κB pathway. Oncotarget 2017, 8, 63788–63798. [Google Scholar] [CrossRef]
- McFerrin, M.B.; Sontheimer, H. A role for ion channels in glioma cell invasion. Neuron Glia Biol. 2006, 2, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Staquicini, D.I.; Rangel, R.; Guzman-Rojas, L.; Staquicini, F.I.; Dobroff, A.S.; Tarleton, C.A.; Ozbun, M.A.; Kolonin, M.G.; Gelovani, J.G.; Marchiò, S.; et al. Intracellular targeting of annexin A2 inhibits tumor cell adhesion, migration, and in vivo grafting. Sci. Rep. 2017, 7, 4243. [Google Scholar] [CrossRef]
- Valls, M.D.; Soldado, M.; Arasa, J.; Perez-Aso, M.; Williams, A.J.; Cronstein, B.N.; Noguera, M.A.; Terencio, M.C.; Montesinos, M.C. Annexin A2-Mediated Plasminogen Activation in Endothelial Cells Contributes to the Proangiogenic Effect of Adenosine A2A Receptors. Front. Pharmacol. 2021, 12, 709. [Google Scholar] [CrossRef]
- Chen, L.; Lin, L.; Xian, N.; Zheng, Z. Annexin A2 regulates glioma cell proliferation through the STAT3-cyclin D1 pathway. Oncol. Rep. 2019, 42, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Nie, S.; Lv, Z.; Ma, L.; Song, Y.; Hu, Z.; Hu, X.; Liu, Z.; Zhou, G.; Dai, Z.; et al. Overexpression of Annexin A2 promotes proliferation by forming a Glypican 1/c-Myc positive feedback loop: Prognostic significance in human glioma. Cell Death Dis. 2021, 12, 261. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, K.; Cai, Y.; Cai, Y.; Yuan, X.; Wang, L.; Wu, Z.; Wu, Y. Annexin A2 could enhance multidrug resistance by regulating NF-κB signaling pathway in pediatric neuroblastoma. J. Exp. Clin. Cancer Res. 2017, 36, 111. [Google Scholar] [CrossRef] [PubMed]
- Khanyile, S.; Masamba, P.; Oyinloye, B.E.; Mbatha, L.S.; Kappo, A.P. Current Biochemical Applications and Future Prospects of Chlorotoxin in Cancer Diagnostics and Therapeutics. Adv. Pharm. Bull. 2019, 9, 510–520. [Google Scholar] [CrossRef]
- Soroceanu, L.; Gillespie, Y.; Khazaeli, M.B.; Sontheimer, H. Use of chlorotoxin for targeting of primary brain tumors. Cancer Res. 1998, 58, 4871–4879. [Google Scholar] [PubMed]
- Soroceanu, L.; Manning, T.J.; Sontheimer, H. Modulation of glioma cell migration and invasion using Cl− and K+ ion channel blockers. J. Neurosci. 1999, 19, 5942–5954. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, J.; Qiao, W.; Chen, K. Recent advances in diagnosis and treatment of gliomas using chlorotoxin-based bioconjugates. Am. J. Nucl. Med. Mol. Imaging 2014, 4, 385–405. [Google Scholar]
- Deshane, J.; Garner, C.C.; Sontheimer, H. Chlorotoxin Inhibits Glioma Cell Invasion via Matrix Metalloproteinase-2 *. J. Biol. Chem. 2003, 278, 4135–4144. [Google Scholar] [CrossRef]
- El-Ghlban, S.; Kasai, T.; Shigehiro, T.; Yin, H.X.; Sekhar, S.; Ida, M.; Sanchez, A.; Mizutani, A.; Kudoh, T.; Murakami, H.; et al. Chlorotoxin-Fc Fusion Inhibits Release of MMP-2 from Pancreatic Cancer Cells. BioMed Res. Int. 2014, 2014, e152659. [Google Scholar] [CrossRef]
- Ayomide, S.O.; Oko, G.E.; Chukwu, C.C.; Vo, K.T.K.; Okoi, E.P. Effects of Chlorotoxin on Matrix Metalloproteinase-2 (MMP-2) in Melanoma and Breast Cancer Cell Lines. J. Adv. Med. Pharm. Sci. 2018, 17, 1–11. [Google Scholar] [CrossRef]
- Kesavan, K.; Ratliff, J.; Johnson, E.W.; Dahlberg, W.; Asara, J.M.; Misra, P.; Frangioni, J.V.; Jacoby, D.B. Annexin A2 is a molecular target for TM601, a peptide with tumor-targeting and anti-angiogenic effects. J. Biol. Chem. 2010, 285, 4366–4374. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-M.; Luo, X.; Guo, Z.-Y. Recombinant expression and downstream processing of the disulfide-rich tumor-targeting peptide chlorotoxin. Exp. Ther. Med. 2013, 6, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, K.; Han, S.; Tian, Y.; Hu, P.; Xu, X.; He, Y.; Pan, W.; Gao, Y.; Zhang, Z.; et al. Chlorotoxin targets ERα/VASP signaling pathway to combat breast cancer. Cancer Med. 2019, 8, 1679–1693. [Google Scholar] [CrossRef] [PubMed]
- McGonigle, S.; Majumder, U.; Kolber-Simonds, D.; Wu, J.; Hart, A.; Noland, T.; TenDyke, K.; Custar, D.; Li, D.; Du, H.; et al. Neuropilin-1 drives tumor-specific uptake of chlorotoxin. Cell Commun. Signal. CCS 2019, 17, 67. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Braga, C.B.; Chen, K.-E.; Jia, X.; Ramanujam, V.; Collins, B.M.; Rittner, R.; Mobli, M. Structural basis for the binding of the cancer targeting scorpion toxin, ClTx, to the vascular endothelia growth factor receptor neuropilin-1. Curr. Res. Struct. Biol. 2021, 3, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, V.; Mekky, G.; van der Meer, S.B.; Seeds, M.C.; Atala, A.J.; Epple, M. Transport of ultrasmall gold nanoparticles (2 nm) across the blood–brain barrier in a six-cell brain spheroid model. Sci. Rep. 2020, 10, 18033. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Costa, P.M.; Cardoso, A.L.; Mendonça, L.S.; Serani, A.; Custódia, C.; Conceição, M.; Simões, S.; Moreira, J.N.; Pereira de Almeida, L.; Pedroso de Lima, M.C. Tumor-targeted Chlorotoxin-coupled Nanoparticles for Nucleic Acid Delivery to Glioblastoma Cells: A Promising System for Glioblastoma Treatment. Mol. Ther. Nucleic Acids 2013, 2, e100. [Google Scholar] [CrossRef]
- Dardevet, L.; Rani, D.; Abd El Aziz, T.; Bazin, I.; Sabatier, J.-M.; Fadl, M.; Brambilla, E.; De Waard, M. Chlorotoxin: A Helpful Natural Scorpion Peptide to Diagnose Glioma and Fight Tumor Invasion. Toxins 2015, 7, 1079–1101. [Google Scholar] [CrossRef]
- Ojeda, P.G.; Wang, C.K.; Craik, D.J. Chlorotoxin: Structure, activity, and potential uses in cancer therapy. Biopolymers 2016, 106, 25–36. [Google Scholar] [CrossRef]
- Cohen, G.; Burks, S.R.; Frank, J.A. Chlorotoxin-A Multimodal Imaging Platform for Targeting Glioma Tumors. Toxins 2018, 10, 496. [Google Scholar] [CrossRef] [PubMed]
- Kievit, F.M.; Veiseh, O.; Fang, C.; Bhattarai, N.; Lee, D.; Ellenbogen, R.G.; Zhang, M. Chlorotoxin Labeled Magnetic Nanovectors for Targeted Gene Delivery to Glioma. ACS Nano 2010, 4, 4587–4594. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Shi, X.; Zhao, J. Chlorotoxin-conjugated nanoparticles for targeted imaging and therapy of glioma. Curr. Top. Med. Chem. 2015, 15, 1196–1208. [Google Scholar] [CrossRef] [PubMed]
- Patil, C.G.; Walker, D.G.; Miller, D.M.; Butte, P.; Morrison, B.; Kittle, D.S.; Hansen, S.J.; Nufer, K.L.; Byrnes-Blake, K.A.; Yamada, M.; et al. Phase 1 Safety, Pharmacokinetics, and Fluorescence Imaging Study of Tozuleristide (BLZ-100) in Adults with Newly Diagnosed or Recurrent Gliomas. Neurosurgery 2019, 85, E641–E649. [Google Scholar] [CrossRef]
- Yamada, M.; Miller, D.M.; Lowe, M.; Rowe, C.; Wood, D.; Soyer, H.P.; Byrnes-Blake, K.; Parrish-Novak, J.; Ishak, L.; Olson, J.M.; et al. A first-in-human study of BLZ-100 (tozuleristide) demonstrates tolerability and safety in skin cancer patients. Contemp. Clin. Trials Commun. 2021, 23, 100830. [Google Scholar] [CrossRef]
- Boltman, T. The Development of Targeting Nanosystems for the Treatment of Glioblastoma and Neuroblastoma Tumours. Ph.D. Thesis, University of the Western Cape, Bellville, South Africa, 2022. [Google Scholar]
- Bastiancich, C.; Da Silva, A.; Estève, M.-A. Photothermal Therapy for the Treatment of Glioblastoma: Potential and Preclinical Challenges. Front. Oncol. 2021, 10, 610356. [Google Scholar] [CrossRef]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.M.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients with Glioblastoma: A Randomized Clinical Trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef]
- Tan, A.C.; Ashley, D.M.; López, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of glioblastoma: State of the art and future directions. CA. Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef]
- Singh, N.; Miner, A.; Hennis, L.; Mittal, S. Mechanisms of temozolomide resistance in glioblastoma—A comprehensive review. Cancer Drug Resist. 2021, 4, 17–43. [Google Scholar] [CrossRef]
- Fang, C.; Wang, K.; Stephen, Z.R.; Mu, Q.; Kievit, F.M.; Chiu, D.T.; Press, O.W.; Zhang, M. Temozolomide nanoparticles for targeted glioblastoma therapy. ACS Appl. Mater. Interfaces 2015, 7, 6674–6682. [Google Scholar] [CrossRef]
- Chien, C.-H.; Hsueh, W.-T.; Chuang, J.-Y.; Chang, K.-Y. Dissecting the mechanism of temozolomide resistance and its association with the regulatory roles of intracellular reactive oxygen species in glioblastoma. J. Biomed. Sci. 2021, 28, 18. [Google Scholar] [CrossRef] [PubMed]
- Hatoum, A.; Mohammed, R.; Zakieh, O. The unique invasiveness of glioblastoma and possible drug targets on extracellular matrix. Cancer Manag. Res. 2019, 11, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Prager, B.C.; Wu, Q.; Kim, L.J.Y.; Gimple, R.C.; Shi, Y.; Yang, K.; Morton, A.R.; Zhou, W.; Zhu, Z.; et al. Reciprocal Signaling between Glioblastoma Stem Cells and Differentiated Tumor Cells Promotes Malignant Progression. Cell Stem Cell 2018, 22, 514–528.E5. [Google Scholar] [CrossRef] [PubMed]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and Radiation Therapy: Current Advances and Future Directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef]
- Mohan, J.C.; Praveen, G.; Chennazhi, K.P.; Jayakumar, R.; Nair, S.V. Functionalised gold nanoparticles for selective induction of in vitro apoptosis among human cancer cell lines. J. Exp. Nanosci. 2013, 8, 32–45. [Google Scholar] [CrossRef]
- Harrison, E.; Nicol, J.R.; Macias-Montero, M.; Burke, G.A.; Coulter, J.A.; Meenan, B.J.; Dixon, D. A comparison of gold nanoparticle surface co-functionalization approaches using Polyethylene Glycol (PEG) and the effect on stability, non-specific protein adsorption and internalization. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 62, 710–718. [Google Scholar] [CrossRef]
- Luksch, R.; Castellani, M.R.; Collini, P.; De Bernardi, B.; Conte, M.; Gambini, C.; Gandola, L.; Garaventa, A.; Biasoni, D.; Podda, M.; et al. Neuroblastoma (Peripheral neuroblastic tumours). Crit. Rev. Oncol. Hematol. 2016, 107, 163–181. [Google Scholar] [CrossRef] [PubMed]
- Rohrer, T.; Trachsel, D.; Engelcke, G.; Hammer, J. Congenital central hypoventilation syndrome associated with Hirschsprung’s disease and neuroblastoma: Case of multiple neurocristopathies. Pediatr. Pulmonol. 2002, 33, 71–76. [Google Scholar] [CrossRef]
- Swift, C.C.; Eklund, M.J.; Kraveka, J.M.; Alazraki, A.L. Updates in Diagnosis, Management, and Treatment of Neuroblastoma. RadioGraphics 2018, 38, 566–580. [Google Scholar] [CrossRef]
- Pudela, C.; Balyasny, S.; Applebaum, M.A. Nervous system: Embryonal tumors: Neuroblastoma. Atlas Genet. Cytogenet. Oncol. Haematol. 2020, 24, 284–290. [Google Scholar] [CrossRef]
- Chu, C.M.; Rasalkar, D.D.; Hu, Y.J.; Cheng, F.W.T.; Li, C.K.; Chu, W.C.W. Clinical presentations and imaging findings of neuroblastoma beyond abdominal mass and a review of imaging algorithm. BJR 2011, 84, 81–91. [Google Scholar] [CrossRef]
- Rifatbegovic, F.; Frech, C.; Abbasi, M.R.; Taschner-Mandl, S.; Weiss, T.; Schmidt, W.M.; Schmidt, I.; Ladenstein, R.; Ambros, I.M.; Ambros, P.F. Neuroblastoma cells undergo transcriptomic alterations upon dissemination into the bone marrow and subsequent tumor progression. Int. J. Cancer 2018, 142, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Lee, J.-S.; Guo, F.; Shin, J.; Perez-Atayde, A.R.; Kutok, J.L.; Rodig, S.J.; Neuberg, D.S.; Helman, D.; Feng, H.; et al. Activated ALK Collaborates with MYCN in Neuroblastoma Pathogenesis. Cancer Cell 2012, 21, 362–373. [Google Scholar] [CrossRef]
- Mallepalli, S.; Gupta, M.K.; Vadde, R. Neuroblastoma: An Updated Review on Biology and Treatment. Curr. Drug Metab. 2019, 20, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Fati, F.; Pulvirenti, R.; Paraboschi, I.; Martucciello, G. Surgical Approaches to Neuroblastoma: Review of the Operative Techniques. Children 2021, 8, 446. [Google Scholar] [CrossRef] [PubMed]
- Strother, D.R.; London, W.B.; Schmidt, M.L.; Brodeur, G.M.; Shimada, H.; Thorner, P.; Collins, M.H.; Tagge, E.; Adkins, S.; Reynolds, C.P.; et al. Outcome After Surgery Alone or with Restricted Use of Chemotherapy for Patients with Low-Risk Neuroblastoma: Results of Children’s Oncology Group Study P9641. J. Clin. Oncol. 2012, 30, 1842–1848. [Google Scholar] [CrossRef] [PubMed]
- Veschi, V.; Verona, F.; Thiele, C.J. Cancer Stem Cells and Neuroblastoma: Characteristics and Therapeutic Targeting Options. Front. Endocrinol. 2019, 10, 782. [Google Scholar] [CrossRef]
- Kumar, S.K.; Lacy, M.Q.; Dispenzieri, A.; Buadi, F.K.; Hayman, S.R.; Dingli, D.; Gay, F.; Sinha, S.; Leung, N.; Hogan, W.; et al. Early versus delayed autologous transplantation after immunomodulatory agents-based induction therapy in patients with newly diagnosed multiple myeloma. Cancer 2012, 118, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- Shohet, J.; Foster, J. Neuroblastoma. BMJ 2017, 357, j1863. [Google Scholar] [CrossRef] [PubMed]
- Hudson, M.M. Reproductive Outcomes for Survivors of Childhood Cancer. Obstet. Gynecol. 2010, 116, 1171–1183. [Google Scholar] [CrossRef]
- Friedman, D.N.; Henderson, T.O. Late Effects and Survivorship Issues in Patients with Neuroblastoma. Children 2018, 5, 107. [Google Scholar] [CrossRef] [PubMed]
- Brodeur, G.M. Spontaneous regression of neuroblastoma. Cell Tissue Res. 2018, 372, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yao, W.; Li, K. Applications and prospects of targeted therapy for neuroblastoma. World J. Pediatr. Surg. 2020, 3, e000164. [Google Scholar] [CrossRef] [PubMed]
- Lampron, A.; Elali, A.; Rivest, S. Innate immunity in the CNS: Redefining the relationship between the CNS and Its environment. Neuron 2013, 78, 214–232. [Google Scholar] [CrossRef] [PubMed]
- Castro Dias, M.; Mapunda, J.A.; Vladymyrov, M.; Engelhardt, B. Structure and Junctional Complexes of Endothelial, Epithelial and Glial Brain Barriers. Int. J. Mol. Sci. 2019, 20, 5372. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Drug transport across the blood–brain barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef]
- Belykh, E.; Shaffer, K.V.; Lin, C.; Byvaltsev, V.A.; Preul, M.C.; Chen, L. Blood-Brain Barrier, Blood-Brain Tumor Barrier, and Fluorescence-Guided Neurosurgical Oncology: Delivering Optical Labels to Brain Tumors. Front. Oncol. 2020, 10, 739. [Google Scholar] [CrossRef]
- Thuerauf, N.; Fromm, M.F. The role of the transporter P-glycoprotein for disposition and effects of centrally acting drugs and for the pathogenesis of CNS diseases. Eur. Arch. Psychiatry Clin. Neurosci. 2006, 256, 281–286. [Google Scholar] [CrossRef]
- Groothuis, D.R. The blood-brain and blood-tumor barriers: A review of strategies for increasing drug delivery. Neuro. Oncol. 2000, 2, 45–59. [Google Scholar] [CrossRef]
- Sarin, H.; Kanevsky, A.S.; Wu, H.; Brimacombe, K.R.; Fung, S.H.; Sousa, A.A.; Auh, S.; Wilson, C.M.; Sharma, K.; Aronova, M.A.; et al. Effective transvascular delivery of nanoparticles across the blood-brain tumor barrier into malignant glioma cells. J. Transl. Med. 2008, 6, 80. [Google Scholar] [CrossRef]
- Dubois, L.G.; Campanati, L.; Righy, C.; D’Andrea-Meira, I.; de Sampaio E Spohr, T.C.L.; Porto-Carreiro, I.; Pereira, C.M.; Balça-Silva, J.; Kahn, S.A.; DosSantos, M.F.; et al. Gliomas and the vascular fragility of the blood brain barrier. Front. Cell. Neurosci. 2014, 8, 418. [Google Scholar] [CrossRef] [PubMed]
- Tamborini, M.; Locatelli, E.; Rasile, M.; Monaco, I.; Rodighiero, S.; Corradini, I.; Franchini, M.C.; Passoni, L.; Matteoli, M. A Combined Approach Employing Chlorotoxin-Nanovectors and Low Dose Radiation to Reach Infiltrating Tumor Niches in Glioblastoma. ACS Nano 2016, 10, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Liu, Y.; Zhang, Y.; Meng, L.; Wei, J.; Wang, B.; Wang, H.; Xin, Y.; Dong, L.; Jiang, X. Role and toxicity of radiation therapy in neuroblastoma patients: A literature review. Crit. Rev. Oncol. Hematol. 2020, 149, 102924. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; Barresi, V.; Castellano, A.; Tabouret, E.; Pasqualetti, F.; Salvalaggio, A.; Cerretti, G.; Caccese, M.; Padovan, M.; Zagonel, V.; et al. Clinical Management of Diffuse Low-Grade Gliomas. Cancers 2020, 12, 3008. [Google Scholar] [CrossRef]
- Sbeih, A.H.; Salami, K.; Morabito, F.; Saleh, H. Epidemiological and Clinical Data in Low and Intermediate Risk Neuroblastoma: A Single Institution Experience and Survival Outcomes in Jerusalem. Asian Pac. J. Cancer Care 2020, 5, 139–144. [Google Scholar] [CrossRef]
- McCutcheon, I.E.; Preul, M.C. Historical Perspective on Surgery and Survival with Glioblastoma: How Far Have We Come? World Neurosurg. 2021, 149, 148–168. [Google Scholar] [CrossRef]
- Cohen-Inbar, O.; Zaaroor, M. Glioblastoma multiforme targeted therapy: The Chlorotoxin story. J. Clin. Neurosci. 2016, 33, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Pennington, M.W.; Czerwinski, A.; Norton, R.S. Peptide therapeutics from venom: Current status and potential. Bioorg. Med. Chem. 2018, 26, 2738–2758. [Google Scholar] [CrossRef]
- Lyons, S.A.; O’Neal, J.; Sontheimer, H. Chlorotoxin, a scorpion-derived peptide, specifically binds to gliomas and tumors of neuroectodermal origin. Glia 2002, 39, 162–173. [Google Scholar] [CrossRef]
- Sun, C.; Fang, C.; Stephen, Z.; Veiseh, O.; Hansen, S.; Lee, D.; Ellenbogen, R.G.; Olson, J.; Zhang, M. Tumor-targeted drug delivery and MRI contrast enhancement by chlorotoxin-conjugated iron oxide nanoparticles. Nanomedicine 2008, 3, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Gunn, J.W.; Kievit, F.M.; Sun, C.; Fang, C.; Lee, J.S.H.; Zhang, M. Inhibition of tumor-cell invasion with chlorotoxin-bound superparamagnetic nanoparticles. Small 2009, 5, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Liang, L.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. Chloride channel-mediated brain glioma targeting of chlorotoxin-modified doxorubicine-loaded liposomes. J. Control Release 2011, 152, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, D.B.; Dyskin, E.; Yalcin, M.; Kesavan, K.; Dahlberg, W.; Ratliff, J.; Johnson, E.W.; Mousa, S.A. Potent Pleiotropic Anti-angiogenic Effects of TM601, a Synthetic Chlorotoxin Peptide. Anticancer Res. 2010, 30, 39–46. [Google Scholar] [PubMed]
- Veiseh, M.; Gabikian, P.; Bahrami, S.-B.; Veiseh, O.; Zhang, M.; Hackman, R.C.; Ravanpay, A.C.; Stroud, M.R.; Kusuma, Y.; Hansen, S.J.; et al. Tumor Paint: A Chlorotoxin: Cy5.5 Bioconjugate for Intraoperative Visualization of Cancer Foci. Cancer Res. 2007, 67, 6882–6888. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; He, B.; Dai, W.; Zhang, H.; Wang, X.; Wang, J.; Zhang, X.; Wang, G.; Yin, L.; Zhang, Q. Inhibition of metastatic tumor growth and metastasis via targeting metastatic breast cancer by chlorotoxin-modified liposomes. Mol. Pharm. 2014, 11, 3233–3241. [Google Scholar] [CrossRef]
- Worm, D.J.; Els-Heindl, S.; Beck-Sickinger, A.G. Targeting of peptide-binding receptors on cancer cells with peptide-drug conjugates. Pept. Sci. 2020, 112, e24171. [Google Scholar] [CrossRef]
- DeBin, J.A.; Strichartz, G.R. Chloride channel inhibition by the venom of the scorpion Leiurus quinquestriatus. Toxicon 1991, 29, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- DeBin, J.A.; Maggio, J.E.; Strichartz, G.R. Purification and characterization of chlorotoxin, a chloride channel ligand from the venom of the scorpion. Am. J. Physiol. 1993, 264, C361–C369. [Google Scholar] [CrossRef]
- Ullrich, N.; Gillespie, G.Y.; Sontheimer, H. Human astrocytoma cells express a unique chloride current. Neuroreport 1996, 7, 1020–1024. [Google Scholar] [CrossRef]
- Ullrich, N.; Sontheimer, H. Biophysical and pharmacological characterization of chloride currents in human astrocytoma cells. Am. J. Physiol. 1996, 270, C1511–C1521. [Google Scholar] [CrossRef]
- Ullrich, N.; Sontheimer, H. Cell cycle-dependent expression of a glioma-specific chloride current: Proposed link to cytoskeletal changes. Am. J. Physiol. 1997, 273, C1290–C1297. [Google Scholar] [CrossRef]
- Ullrich, N.; Bordey, A.; Gillespie, G.Y.; Sontheimer, H. Expression of voltage-activated chloride currents in acute slices of human gliomas. Neuroscience 1998, 83, 1161–1173. [Google Scholar] [CrossRef] [PubMed]
- Lui, V.C.H.; Lung, S.S.S.; Pu, J.K.S.; Hung, K.N.; Leung, G.K.K. Invasion of human glioma cells is regulated by multiple chloride channels including ClC-3. Anticancer Res. 2010, 30, 4515–4524. [Google Scholar] [PubMed]
- Rizvanovic, H.; Pinheiro, A.D.; Kim, K.; Thomas, J. Chlorotoxin Conjugated with Saporin Reduces Viability of ML-1 Thyroid Cancer Cells In Vitro. bioRxiv 2019. [Google Scholar] [CrossRef]
- Pandey, A.; Singh, K.; Subramanian, S.; Korde, A.; Singh, R.; Sawant, K. Heterogeneous surface architectured pH responsive Metal-Drug Nano-conjugates for mitochondria targeted therapy of Glioblastomas: A multimodal intranasal approach. Chem. Eng. J. 2020, 394, 124419. [Google Scholar] [CrossRef]
- Ransom, C.B.; O’Neal, J.T.; Sontheimer, H. Volume-Activated Chloride Currents Contribute to the Resting Conductance and Invasive Migration of Human Glioma Cells. J. Neurosci. 2001, 21, 7674–7683. [Google Scholar] [CrossRef]
- Wei, C.; Wang, X.; Zheng, M.; Cheng, H. Calcium gradients underlying cell migration. Curr. Opin. Cell Biol. 2012, 24, 254–261. [Google Scholar] [CrossRef]
- Turner, K.L.; Honasoge, A.; Robert, S.M.; McFerrin, M.M.; Sontheimer, H. A pro-invasive role for the Ca2+-activated K+ channel KCa3.1 in malignant glioma. Glia 2014, 62, 971–981. [Google Scholar] [CrossRef]
- Olsen, M.L.; Schade, S.; Lyons, S.A.; Amaral, M.D.; Sontheimer, H. Expression of Voltage-Gated Chloride Channels in Human Glioma Cells. J. Neurosci. 2003, 23, 5572–5582. [Google Scholar] [CrossRef]
- Turner, K.L.; Sontheimer, H. Cl− and K+ channels and their role in primary brain tumour biology. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130095. [Google Scholar] [CrossRef]
- Habela, C.W.; Ernest, N.J.; Swindall, A.F.; Sontheimer, H. Chloride accumulation drives volume dynamics underlying cell proliferation and migration. J. Neurophysiol. 2009, 101, 750–757. [Google Scholar] [CrossRef]
- Maertens, C.; Wei, L.; Tytgat, J.; Droogmans, G.; Nilius, B. Chlorotoxin does not inhibit volume-regulated, calcium-activated and cyclic AMP-activated chloride channels. Br. J. Pharmacol. 2000, 129, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Dalton, S.; Gerzanich, V.; Chen, M.; Dong, Y.; Shuba, Y.; Simard, J.M. Chlorotoxin-sensitive Ca2+-activated Cl− channel in type R2 reactive astrocytes from adult rat brain. Glia 2003, 42, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Avila, G.; Sommer, B.; Mendoza-Posada, D.A.; Ramos, C.; Garcia-Hernandez, A.A.; Falfan-Valencia, R. Matrix metalloproteinases participation in the metastatic process and their diagnostic and therapeutic applications in cancer. Crit. Rev. Oncol. Hematol. 2019, 137, 57–83. [Google Scholar] [CrossRef]
- Siddhartha, R.; Garg, M. Molecular and clinical insights of matrix metalloproteinases into cancer spread and potential therapeutic interventions. Toxicol. Appl. Pharmacol. 2021, 426, 115593. [Google Scholar] [CrossRef] [PubMed]
- Ara, T.; Fukuzawa, M.; Kusafuka, T.; Komoto, Y.; Oue, T.; Inoue, M.; Okada, A. Immunohistochemical expression of MMP-2, MMP-9, and TIMP-2 in neuroblastoma: Association with tumor progression and clinical outcome. J. Pediatr. Surg. 1998, 33, 1272–1278. [Google Scholar] [CrossRef]
- Noujaim, D.; van Golen, C.M.; van Golen, K.L.; Grauman, A.; Feldman, E.L. N-Myc and Bcl-2 coexpression induces MMP-2 secretion and activation in human neuroblastoma cells. Oncogene 2002, 21, 4549–4557. [Google Scholar] [CrossRef] [PubMed]
- Roomi, M.W.; Kalinovsky, T.; Rath, M.; Niedzwiecki, A. Modulation of MMP-2 and MMP-9 secretion by cytokines, inducers and inhibitors in human glioblastoma T-98G cells. Oncol. Rep. 2017, 37, 1907–1913. [Google Scholar] [CrossRef] [PubMed]
- Pullen, N.A.; Pickford, A.R.; Perry, M.M.; Jaworski, D.M.; Loveson, K.F.; Arthur, D.J.; Holliday, J.R.; Meter, T.E.V.; Peckham, R.; Younas, W.; et al. Current insights into matrix metalloproteinases and glioma progression: Transcending the degradation boundary. Met. Med. 2018, 5, 13–30. [Google Scholar] [CrossRef]
- Roomi, M.W.; Ivanov, V.; Kalinovsky, T.; Niedzwiecki, A.; Rath, M. Inhibition of glioma cell line A-172 MMP activity and cell invasion in vitro by a nutrient mixture. Med. Oncol. 2007, 24, 231–238. [Google Scholar] [CrossRef]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular Matrix Degradation and Remodeling in Development and Disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef] [PubMed]
- Chintala, S.K.; Tonn, J.C.; Rao, J.S. Matrix metalloproteinases and their biological function in human gliomas. Int. J. Dev. Neurosci. 1999, 17, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Haas, T.L.; Madri, J.A. Extracellular Matrix-Driven Matrix Metalloproteinase Production in Endothelial Cells: Implications for Angiogenesis. Trends Cardiovasc. Med. 1999, 9, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Dearling, J.L.J.; Barnes, J.W.; Panigrahy, D.; Zimmerman, R.E.; Fahey, F.; Treves, S.T.; Morrison, M.S.; Kieran, M.W.; Packard, A.B. Specific uptake of 99mTc-NC100692, an αvβ3-targeted imaging probe, in subcutaneous and orthotopic tumors. Nucl. Med. Biol. 2013, 40, 788–794. [Google Scholar] [CrossRef]
- Othman, H.; Wieninger, S.A.; ElAyeb, M.; Nilges, M.; Srairi-Abid, N. In Silico prediction of the molecular basis of ClTx and AaCTx interaction with matrix metalloproteinase-2 (MMP-2) to inhibit glioma cell invasion. J. Biomol. Struct. Dyn. 2017, 35, 2815–2829. [Google Scholar] [CrossRef]
- Alam, M.; Ali, S.A.; Abbasi, A.; Kalbacher, H.; Voelter, W. Design and Synthesis of a Peptidyl-FRET Substrate for Tumor Marker Enzyme human Matrix Metalloprotease-2 (hMMP-2). Int. J. Pept. Res. Ther. 2012, 18, 207–215. [Google Scholar] [CrossRef]
- Kovar, J.L.; Curtis, E.; Othman, S.F.; Simpson, M.A.; Michael Olive, D. Characterization of IRDye 800CW chlorotoxin as a targeting agent for brain tumors. Anal. Biochem. 2013, 440, 212–219. [Google Scholar] [CrossRef]
- Lizarbe, M.A.; Barrasa, J.I.; Olmo, N.; Gavilanes, F.; Turnay, J. Annexin-Phospholipid Interactions. Functional Implications. Int. J. Mol. Sci. 2013, 14, 2652–2683. [Google Scholar] [CrossRef]
- Miwa, N.; Uebi, T.; Kawamura, S. S100–annexin complexes—Biology of conditional association. FEBS J. 2008, 275, 4945–4955. [Google Scholar] [CrossRef]
- Zhang, Q.; Ye, Z.; Yang, Q.; He, X.; Wang, H.; Zhao, Z. Upregulated expression of Annexin II is a prognostic marker for patients with gastric cancer. World J. Surg. Oncol. 2012, 10, 103. [Google Scholar] [CrossRef]
- Lauritzen, S.P.; Boye, T.L.; Nylandsted, J. Annexins are instrumental for efficient plasma membrane repair in cancer cells. Semin. Cell Dev. Biol. 2015, 45, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Grewal, T. Annexins in cell migration and adhesion. Cell Adhes. Migr. 2017, 11, 245–246. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Deng, L.; Zhuang, H.; Liu, J.; Liu, D.; Li, X.; Jin, S.; Zhu, L.; Wang, H.; Lin, B. Interaction of HE4 and ANXA2 exists in various malignant cells—HE4–ANXA2–MMP2 protein complex promotes cell migration. Cancer Cell Int. 2019, 19, 161. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, H. Clinical significance of Annexin A2 expression in oral squamous cell carcinoma and its influence on cell proliferation, migration and invasion. Sci. Rep. 2021, 11, 5033. [Google Scholar] [CrossRef] [PubMed]
- Seckinger, A.; Meissner, T.; Moreaux, J.; Depeweg, D.; Hillengass, J.; Hose, K.; Rème, T.; Rösen-Wolff, A.; Jauch, A.; Schnettler, R.; et al. Clinical and prognostic role of annexin A2 in multiple myeloma. Blood 2012, 120, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Hockaday, D.C.; Shen, S.; Fiveash, J.; Raubitschek, A.; Colcher, D.; Liu, A.; Alvarez, V.; Mamelak, A.N. Imaging glioma extent with 131I-TM-601. J. Nucl. Med. 2005, 46, 580–586. [Google Scholar]
- Tatenhorst, L.; Rescher, U.; Gerke, V.; Paulus, W. Knockdown of annexin 2 decreases migration of human glioma cells in vitro. Neuropathol. Appl. Neurobiol. 2006, 32, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Díaz, R.; Sanchez-Garcia, L.; Serna, N.; Sánchez-Chardi, A.; Cano Garrido, O.; Sanchez, J.; Unzueta, U.; Vazquez, E.; Villaverde, A. Engineering a recombinant chlorotoxin as cell-targeted cytotoxic nanoparticles. Sci. China Mater. 2019, 62, 892–898. [Google Scholar] [CrossRef]
- Rothenberger, N.J.; Somasundaram, A.; Stabile, L.P. The Role of the Estrogen Pathway in the Tumor Microenvironment. Int. J. Mol. Sci. 2018, 19, 611. [Google Scholar] [CrossRef]
- Saha Roy, S.; Vadlamudi, R.K. Role of estrogen receptor signaling in breast cancer metastasis. Int. J. Breast Cancer 2012, 2012, 654698. [Google Scholar] [CrossRef]
- Hua, H.; Zhang, H.; Kong, Q.; Jiang, Y. Mechanisms for estrogen receptor expression in human cancer. Exp. Hematol. Oncol. 2018, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, A.P.G.; Cavalheiro, R.P.; Porto, C.S.; Vicente, C.M. Estrogen Receptor Signaling Pathways Involved in Invasion and Colony Formation of Androgen-Independent Prostate Cancer Cells PC-3. Int. J. Mol. Sci. 2021, 22, 1153. [Google Scholar] [CrossRef] [PubMed]
- Kolber-Simonds, D.; Wu, J.; Majumder, U.; Custar, D.; Li, D.; Du, H.; Postema, M.H.; Noland, T.; Hart, A.; Lai, G.; et al. Abstract 3961: Role for neuropilin1 in mode of action of chlorotoxin. Cancer Res. 2018, 78, 3961. [Google Scholar] [CrossRef]
- Roche, J.; Drabkin, H.; Brambilla, E. Neuropilin and Its Ligands in Normal Lung and Cancer; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Roy, S.; Bag, A.K.; Singh, R.K.; Talmadge, J.E.; Batra, S.K.; Datta, K. Multifaceted Role of Neuropilins in the Immune System: Potential Targets for Immunotherapy. Front. Immunol. 2017, 8, 1228. [Google Scholar] [CrossRef]
- Dumond, A.; Pagès, G. Neuropilins, as Relevant Oncology Target: Their Role in the Tumoral Microenvironment. Front. Cell Dev. Biol. 2020, 8, 662. [Google Scholar] [CrossRef]
- Liu, S.-D.; Zhong, L.-P.; He, J.; Zhao, Y.-X. Targeting neuropilin-1 interactions is a promising anti-tumor strategy. Chin. Med. J. 2021, 134, 508–517. [Google Scholar] [CrossRef]
- Abdellatief, A.; Omran, M.A.; Nabil, Z.; Strong, P.; Newton, K.; Abdel Rahman, M. C-Terminal Amidation of Chlorotoxin Does Not Affect Tumour Cell Proliferation and Has No Effect on Toxin Cytotoxicity. Int. J. Pept. Res. Ther. 2021, 27, 659–667. [Google Scholar] [CrossRef]
- Dastpeyman, M.; Giacomin, P.; Wilson, D.; Nolan, M.J.; Bansal, P.S.; Daly, N.L. A C-Terminal Fragment of Chlorotoxin Retains Bioactivity and Inhibits Cell Migration. Front. Pharmacol. 2019, 10, 250. [Google Scholar] [CrossRef]
- Xiang, Y.; Wu, Q.; Liang, L.; Wang, X.; Wang, J.; Zhang, X.; Pu, X.; Zhang, Q. Chlorotoxin-modified stealth liposomes encapsulating levodopa for the targeting delivery against Parkinson’s disease in the MPTP-induced mice model. J. Drug Target. 2012, 20, 67–75. [Google Scholar] [CrossRef]
- Fu, Y.; An, N.; Li, K.; Zheng, Y.; Liang, A. Chlorotoxin-conjugated nanoparticles as potential glioma-targeted drugs. J. Neurooncol. 2012, 107, 457–462. [Google Scholar] [CrossRef]
- Mamelak, A.N.; Rosenfeld, S.; Bucholz, R.; Raubitschek, A.; Nabors, L.B.; Fiveash, J.B.; Shen, S.; Khazaeli, M.B.; Colcher, D.; Liu, A.; et al. Phase I single-dose study of intracavitary-administered iodine-131-TM-601 in adults with recurrent high-grade glioma. J. Clin. Oncol. 2006, 24, 3644–3650. [Google Scholar] [CrossRef] [PubMed]
- Butte, P.V.; Mamelak, A.; Parrish-Novak, J.; Drazin, D.; Shweikeh, F.; Gangalum, P.R.; Chesnokova, A.; Ljubimova, J.Y.; Black, K. Near-infrared imaging of brain tumors using the Tumor Paint BLZ-100 to achieve near-complete resection of brain tumors. Neurosurg. Focus 2014, 36, E1. [Google Scholar] [CrossRef] [PubMed]
- Baik, F.M.; Hansen, S.; Knoblaugh, S.E.; Sahetya, D.; Mitchell, R.M.; Xu, C.; Olson, J.M.; Parrish-Novak, J.; Méndez, E. Fluorescence Identification of Head and Neck Squamous Cell Carcinoma and High-Risk Oral Dysplasia with BLZ-100, a Chlorotoxin-Indocyanine Green Conjugate. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Fidel, J.; Kennedy, K.C.; Dernell, W.S.; Hansen, S.; Wiss, V.; Stroud, M.R.; Molho, J.I.; Knoblaugh, S.E.; Meganck, J.; Olson, J.M.; et al. Preclinical validation of the utility of BLZ-100 in providing fluorescence contrast for imaging canine spontaneous solid tumors. Cancer Res. 2015, 75, 4283–4291. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, M.; Eslamifar, M.; Khezri, K.; Dizaj, S.M. Applications of nanotechnology in drug delivery to the central nervous system. Biomed. Pharmacother. 2019, 111, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Opris, I.; Lebedev, M.A.; Pulgar, V.M.; Vidu, R.; Enachescu, M.; Casanova, M.F. Editorial: Nanotechnologies in Neuroscience and Neuroengineering. Front. Neurosci. 2020, 14, 33. [Google Scholar] [CrossRef]
- Mendes, M.; Sousa, J.J.; Pais, A.; Vitorino, C. Targeted Theranostic Nanoparticles for Brain Tumor Treatment. Pharmaceutics 2018, 10, 181. [Google Scholar] [CrossRef]
- Bayón-Cordero, L.; Alkorta, I.; Arana, L. Application of Solid Lipid Nanoparticles to Improve the Efficiency of Anticancer Drugs. Nanomaterials 2019, 9, 474. [Google Scholar] [CrossRef]
- Prabhakar, U.; Maeda, H.; Jain, R.K.; Sevick-Muraca, E.M.; Zamboni, W.; Farokhzad, O.C.; Barry, S.T.; Gabizon, A.; Grodzinski, P.; Blakey, D.C. Challenges and Key Considerations of the Enhanced Permeability and Retention Effect for Nanomedicine Drug Delivery in Oncology. Cancer Res. 2013, 73, 2412–2417. [Google Scholar] [CrossRef]
- Huang, D.; Sun, L.; Huang, L.; Chen, Y. Nanodrug Delivery Systems Modulate Tumor Vessels to Increase the Enhanced Permeability and Retention Effect. J. Pers. Med. 2021, 11, 124. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Sánchez, R.; Saavedra, E.; Gallardo-Pérez, J.C.; Rumjanek, F.D.; Rodríguez-Enríquez, S. Understanding the cancer cell phenotype beyond the limitations of current omics analyses. FEBS J. 2016, 283, 54–73. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, H.; Baluk, P.; Morikawa, S.; McLean, J.W.; Thurston, G.; Roberge, S.; Jain, R.K.; McDonald, D.M. Openings between Defective Endothelial Cells Explain Tumor Vessel Leakiness. Am. J. Pathol. 2000, 156, 1363–1380. [Google Scholar] [CrossRef] [PubMed]
- Haley, B.; Frenkel, E. Nanoparticles for drug delivery in cancer treatment. Urol. Oncol. 2008, 26, 57–64. [Google Scholar] [CrossRef]
- Ohta, S.; Kikuchi, E.; Ishijima, A.; Azuma, T.; Sakuma, I.; Ito, T. Investigating the optimum size of nanoparticles for their delivery into the brain assisted by focused ultrasound-induced blood–brain barrier opening. Sci. Rep. 2020, 10, 18220. [Google Scholar] [CrossRef]
- Agarwal, S.; Muniyandi, P.; Maekawa, T.; Kumar, D.S. Vesicular systems employing natural substances as promising drug candidates for MMP inhibition in glioblastoma: A nanotechnological approach. Int. J. Pharm. 2018, 551, 339–361. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef]
- Li, S.-D.; Huang, L. Nanoparticles evading the reticuloendothelial system: Role of the supported bilayer. Biochim. Biophys. Acta BBA Biomembr. 2009, 1788, 2259–2266. [Google Scholar] [CrossRef]
- Choi, C.H.J.; Zuckerman, J.E.; Webster, P.; Davis, M.E. Targeting kidney mesangium by nanoparticles of defined size. Proc. Natl. Acad. Sci. USA 2011, 108, 6656–6661. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Shi, Y.; Qi, T.; Qiu, S.; Huang, Y.; Zhao, X.; Sun, Q.; Lin, G. Precise design strategies of nanomedicine for improving cancer therapeutic efficacy using subcellular targeting. Signal Transduct. Target. Ther. 2020, 5, 262. [Google Scholar] [CrossRef] [PubMed]
- Marqus, S.; Pirogova, E.; Piva, T.J. Evaluation of the use of therapeutic peptides for cancer treatment. J. Biomed. Sci. 2017, 24, 21. [Google Scholar] [CrossRef] [PubMed]
- Haggag, Y.; El-Gizawy, S.; Osman, M. Peptides as Drug Candidates: Limitations and Recent Development Perspectives. BioMed Res. Int. 2018, 8, 001694. [Google Scholar] [CrossRef]
- Ganesan, A.; Ahmed, M.; Okoye, I.; Arutyunova, E.; Babu, D.; Turnbull, W.L.; Kundu, J.K.; Shields, J.; Agopsowicz, K.C.; Xu, L.; et al. Comprehensive in vitro characterization of PD-L1 small molecule inhibitors. Sci. Rep. 2019, 9, 12392. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Fang, X.; Yang, Y.; Wang, C. Peptide-Enabled Targeted Delivery Systems for Therapeutic Applications. Front. Bioeng. Biotechnol. 2021, 9, 577. [Google Scholar] [CrossRef]
- Accardo, A.; Tesauro, D.; Morelli, G. Peptide-based targeting strategies for simultaneous imaging and therapy with nanovectors. Polym. J. 2013, 45, 481–493. [Google Scholar] [CrossRef]
- Jeong, W.; Bu, J.; Kubiatowicz, L.J.; Chen, S.S.; Kim, Y.; Hong, S. Peptide–nanoparticle conjugates: A next generation of diagnostic and therapeutic platforms? Nano Converg. 2018, 5, 38. [Google Scholar] [CrossRef]
- Rachael Goddard, Z.; Marín, M.J.; Russell, D.A.; Searcey, M. Active targeting of gold nanoparticles as cancer therapeutics. Chem. Soc. Rev. 2020, 49, 8774–8789. [Google Scholar] [CrossRef]
- Vlieghe, P.; Lisowski, V.; Martinez, J.; Khrestchatisky, M. Synthetic therapeutic peptides: Science and market. Drug Discov. Today 2010, 15, 40–56. [Google Scholar] [CrossRef]
- Sasikumar, P.G.; Ramachandra, M. Small-Molecule Immune Checkpoint Inhibitors Targeting PD-1/PD-L1 and Other Emerging Checkpoint Pathways. BioDrugs 2018, 32, 481–497. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Hoang Thi, T.T.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S.; Park, K.D.; Truong, N.P. The Importance of Poly(ethylene glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation. Polymers 2020, 12, 298. [Google Scholar] [CrossRef]
- Beh, C.Y.; Prajnamitra, R.P.; Chen, L.-L.; Hsieh, P.C.-H. Advances in Biomimetic Nanoparticles for Targeted Cancer Therapy and Diagnosis. Molecules 2021, 26, 5052. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Sun, C.; Gunn, J.; Kohler, N.; Gabikian, P.; Lee, D.; Bhattarai, N.; Ellenbogen, R.; Sze, R.; Hallahan, A.; et al. Optical and MRI Multifunctional Nanoprobe for Targeting Gliomas. Nano Lett. 2005, 5, 1003–1008. [Google Scholar] [CrossRef]
- Sun, C.; Veiseh, O.; Gunn, J.; Fang, C.; Hansen, S.; Lee, D.; Sze, R.; Ellenbogen, R.G.; Olson, J.; Zhang, M. In vivo MRI detection of gliomas by chlorotoxin-conjugated superparamagnetic nanoprobes. Small 2008, 4, 372–379. [Google Scholar] [CrossRef]
- Lee, M.J.-E.; Veiseh, O.; Bhattarai, N.; Sun, C.; Hansen, S.J.; Ditzler, S.; Knoblaugh, S.; Lee, D.; Ellenbogen, R.; Zhang, M.; et al. Rapid pharmacokinetic and biodistribution studies using cholorotoxin-conjugated iron oxide nanoparticles: A novel non-radioactive method. PLoS ONE 2010, 5, e9536. [Google Scholar] [CrossRef]
- Wan, J.; Meng, X.; Liu, E.; Chen, K. Incorporation of magnetite nanoparticle clusters in fluorescent silica nanoparticles for high-performance brain tumor delineation. Nanotechnology 2010, 21, 235104. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhu, J.; Zhao, L.; Xiong, Z.; Tang, Y.; Liu, C.; Guo, L.; Qiao, W.; Shi, X.; Zhao, J. 131I-labeled multifunctional dendrimers modified with BmK CT for targeted SPECT imaging and radiotherapy of gliomas. Nanomedicine 2016, 11, 1253–1266. [Google Scholar] [CrossRef]
- Sangaiya, P.; Jayaprakash, R. A Review on Iron Oxide Nanoparticles and Their Biomedical Applications. J. Supercond. Nov. Magn. 2018, 31, 3397–3413. [Google Scholar] [CrossRef]
- Thorek, D.L.J.; Chen, A.K.; Czupryna, J.; Tsourkas, A. Superparamagnetic iron oxide nanoparticle probes for molecular imaging. Ann. Biomed. Eng. 2006, 34, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, J.; Chen, X.; Du, X.-S.; Zhang, J.-L.; Liu, G.; Zhang, W.-G. Application of iron oxide nanoparticles in glioma imaging and therapy: From bench to bedside. Nanoscale 2016, 8, 7808–7826. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Sun, C.; Fang, C.; Bhattarai, N.; Gunn, J.; Kievit, F.; Du, K.; Pullar, B.; Lee, D.; Ellenbogen, R.G.; et al. Specific Targeting of Brain Tumors with an Optical/Magnetic Resonance Imaging Nanoprobe across the Blood-Brain Barrier. Cancer Res. 2009, 69, 6200–6207. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shao, C.; Gu, W.; Wang, R.; Zhang, J.; Lai, J.; Li, H.; Ye, L. Targeted imaging of brain gliomas using multifunctional Fe3O4/MnO nanoparticles. RSC Adv. 2015, 5, 33639–33645. [Google Scholar] [CrossRef]
- Ai, P.; Wang, H.; Liu, K.; Wang, T.; Gu, W.; Ye, L.; Yan, C. The relative length of dual-target conjugated on iron oxide nanoparticles plays a role in brain glioma targeting. RSC Adv. 2017, 7, 19954–19959. [Google Scholar] [CrossRef]
- Jha, S.; Mathur, P.; Ramteke, S.; Jain, N.K. Pharmaceutical potential of quantum dots. Artificial Cells. Nano-Med. Biotechnol. 2018, 46, 57–65. [Google Scholar] [CrossRef]
- Tarantini, A.; Wegner, K.D.; Dussert, F.; Sarret, G.; Beal, D.; Mattera, L.; Lincheneau, C.; Proux, O.; Truffier-Boutry, D.; Moriscot, C.; et al. Physicochemical Alterations and Toxicity of InP Alloyed Quantum Dots Aged in Environmental Conditions: A Safer by Design Evaluation. NanoImpact 2019, 14, 100168. [Google Scholar] [CrossRef]
- Chou, K.F.; Dennis, A.M. Förster Resonance Energy Transfer between Quantum Dot Donors and Quantum Dot Acceptors. Sensors 2015, 15, 13288–13325. [Google Scholar] [CrossRef]
- Liang, Z.; Khawar, M.B.; Liang, J.; Sun, H. Bio-Conjugated Quantum Dots for Cancer Research: Detection and Imaging. Front. Oncol. 2021, 11, 4300. [Google Scholar] [CrossRef]
- Chen, S.; Ahmadiantehrani, M.; Publicover, N.G.; Hunter, K.W.; Zhu, X. Thermal decomposition based synthesis of Ag-In-S/ZnS quantum dots and their chlorotoxin-modified micelles for brain tumor cell targeting. RSC Adv. 2015, 5, 60612–60620. [Google Scholar] [CrossRef]
- Wu, C.; Hansen, S.J.; Hou, Q.; Yu, J.; Zeigler, M.; Jin, Y.; Burnham, D.R.; McNeill, J.D.; Olson, J.M.; Chiu, D.T. Design of highly emissive polymer dot bioconjugates for in vivo tumor targeting. Angew. Chem. Int. Ed. Engl. 2011, 50, 3430–3434. [Google Scholar] [CrossRef]
- Reshma, V.G.; Mohanan, P.V. Quantum dots: Applications and safety consequences. J. Lumin. 2019, 205, 287–298. [Google Scholar] [CrossRef]
- Liu, N.; Tang, M. Toxicity of different types of quantum dots to mammalian cells in vitro: An update review. J. Hazard. Mater. 2020, 399, 122606. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-F.; Sun, Z.; Li, M.; Xiang, Y.; Wang, Q.-Q.; Tang, F.; Wu, Y.; Cao, Z.; Li, W. Neurotoxin-conjugated upconversion nanoprobes for direct visualization of tumors under near-infrared irradiation. Biomaterials 2010, 31, 8724–8731. [Google Scholar] [CrossRef] [PubMed]
- Stroud, M.R.; Hansen, S.J.; Olson, J.M. In Vivo Bio-imaging Using Chlorotoxin-based Conjugates. Curr. Pharm. Des. 2011, 17, 4362–4371. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Shi, L.; Ling, X.; Chen, Z.; Mei, Q.; Wang, F. Near-infrared photon-excited energy transfer in platinum(II)-based supramolecular polymers assisted by upconverting nanoparticles. Chem. Commun. 2021, 57, 1927–1930. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, H.; Gu, W.; Li, S.; Xiao, N.; Shao, C.; Xu, Q.; Ye, L. Ho3+ doped NaGdF4 nanoparticles as MRI/optical probes for brain glioma imaging. J. Mater. Chem. B 2014, 2, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Song, G.; Li, S.; Shao, C.; Yan, C.; Ye, L. Chlorotoxin-conjugated, PEGylated Gd2O3 nanoparticles as a glioma-specific magnetic resonance imaging contrast agent. RSC Adv. 2014, 4, 50254–50260. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, Z.; Pu, X.; Chen, X.; Yin, G.; Wang, L.; Zhang, F.; Gao, F. Fabrication of doxorubicin and chlorotoxin-linked Eu-Gd2O3 nanorods with dual-model imaging and targeted therapy of brain tumor. Chin. Chem. Lett. 2020, 31, 285–291. [Google Scholar] [CrossRef]
- Sk, U.H.; Kojima, C. Dendrimers for theranostic applications. Biomol. Concepts 2015, 6, 205–217. [Google Scholar] [CrossRef]
- Huang, R.; Han, L.; Li, J.; Liu, S.; Shao, K.; Kuang, Y.; Hu, X.; Wang, X.; Lei, H.; Jiang, C. Chlorotoxin-modified macromolecular contrast agent for MRI tumor diagnosis. Biomaterials 2011, 32, 5177–5186. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-S.; Jian, X.-C.; Yin, B.; He, Z.-J. Development of the research on the application of chlorotoxin in imaging diagnostics and targeted therapies for tumors. Chin. J. Cancer 2010, 29, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Zhao, L.; Qiao, W.; Xing, Y.; Zhao, J. BmK CT and 125I-BmK CT suppress the invasion of glioma cells in vitro via matrix metalloproteinase-2. Mol. Med. Rep. 2017, 15, 2703–2708. [Google Scholar] [CrossRef]
- Zhao, L.; Li, Y.; Zhu, J.; Sun, N.; Song, N.; Xing, Y.; Huang, H.; Zhao, J. Chlorotoxin peptide-functionalized polyethylenimine-entrapped gold nanoparticles for glioma SPECT/CT imaging and radionuclide therapy. J. Nanobiotechnol. 2019, 17, 30. [Google Scholar] [CrossRef] [PubMed]
- Staderini, M.; Megia-Fernandez, A.; Dhaliwal, K.; Bradley, M. Peptides for optical medical imaging and steps towards therapy. Bioorg. Med. Chem. 2018, 26, 2816–2826. [Google Scholar] [CrossRef]
- Zhao, L.; Zhu, J.; Cheng, Y.; Xiong, Z.; Tang, Y.; Guo, L.; Shi, X.; Zhao, J. Chlorotoxin-Conjugated Multifunctional Dendrimers Labeled with Radionuclide 131I for Single Photon Emission Computed Tomography Imaging and Radiotherapy of Gliomas. ACS Appl. Mater. Interfaces 2015, 7, 19798–19808. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Zhao, L.; Zhu, J.; Li, Y.; Song, N.; Xing, Y.; Qiao, W.; Huang, H.; Zhao, J. 131I-labeled polyethylenimine-entrapped gold nanoparticles for targeted tumor SPECT/CT imaging and radionuclide therapy. Int. J. Nanomed. 2019, 14, 4367–4381. [Google Scholar] [CrossRef]
- Zhao, L.; Zhu, J.; Gong, J.; Song, N.; Wu, S.; Qiao, W.; Yang, J.; Zhu, M.; Zhao, J. Polyethylenimine-based theranostic nanoplatform for glioma-targeting single-photon emission computed tomography imaging and anticancer drug delivery. J. Nanobiotechnol. 2020, 18, 143. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, E.; Broggi, F.; Ponti, J.; Marmorato, P.; Franchini, F.; Lena, S.; Franchini, M.C. Lipophilic silver nanoparticles and their polymeric entrapment into targeted-PEG-based micelles for the treatment of glioblastoma. Adv. Healthc. Mater. 2012, 1, 342–347. [Google Scholar] [CrossRef]
- Locatelli, E.; Naddaka, M.; Uboldi, C.; Loudos, G.; Fragogeorgi, E.; Molinari, V.; Pucci, A.; Tsotakos, T.; Psimadas, D.; Ponti, J.; et al. Targeted delivery of silver nanoparticles and alisertib: In vitro and in vivo synergistic effect against glioblastoma. Nanomedicine 2014, 9, 839–849. [Google Scholar] [CrossRef]
- Chen, Z.; Xiao, E.-H.; Kang, Z.; Zeng, W.-B.; Tan, H.-L.; Li, H.-B.; Bian, D.-J.; Shang, Q.-L. In vitro and in vivo magnetic resonance imaging with chlorotoxin-conjugated superparamagnetic nanoprobes for targeting hepatocarcinoma. Oncol. Rep. 2016, 35, 3059–3067. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Kievit, F.M.; Fang, C.; Mu, N.; Jana, S.; Leung, M.C.; Mok, H.; Ellenbogen, R.G.; Park, J.O.; Zhang, M. Chlorotoxin bound magnetic nanovector tailored for cancer cell targeting, imaging, and siRNA delivery. Biomaterials 2010, 31, 8032–8042. [Google Scholar] [CrossRef]
- Meng, X.; Wan, J.; Jing, M.; Zhao, S.; Cai, W.; Liu, E. Specific targeting of gliomas with multifunctional superparamagnetic iron oxide nanoparticle optical and magnetic resonance imaging contrast agents. Acta Pharmacol. Sin. 2007, 28, 2019–2026. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; He, B.; Dai, W.; Lin, Z.; Zhang, H.; Wang, X.; Wang, J.; Zhang, X.; Wang, G.; Yin, L.; et al. The impact of a chlorotoxin-modified liposome system on receptor MMP-2 and the receptor-associated protein ClC-3. Biomaterials 2014, 35, 5908–5920. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Z. Chlorotoxin-conjugated onconase as a potential anti-glioma drug. Oncol. Lett. 2015, 9, 1337–1342. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, H.; Kasai, T.; Khayrani, A.C.; Asakura, M.; Oo, A.K.K.; Du, J.; Vaidyanath, A.; El-Ghlban, S.; Mizutani, A.; Seno, A.; et al. Targeting Glioblastoma Cells Expressing CD44 with Liposomes Encapsulating Doxorubicin and Displaying Chlorotoxin-IgG Fc Fusion Protein. Int. J. Mol. Sci. 2018, 19, 659. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Mohamed, M.S.; Mizuki, T.; Maekawa, T.; Kumar, D.S. Chlorotoxin modified morusin–PLGA nanoparticles for targeted glioblastoma therapy. J. Mater. Chem. B 2019, 7, 5896–5919. [Google Scholar] [CrossRef]
- Formicola, B.; Dal Magro, R.; Montefusco-Pereira, C.V.; Lehr, C.-M.; Koch, M.; Russo, L.; Grasso, G.; Deriu, M.A.; Danani, A.; Bourdoulous, S.; et al. The synergistic effect of chlorotoxin-mApoE in boosting drug-loaded liposomes across the BBB. J. Nanobiotechnol. 2019, 17, 115. [Google Scholar] [CrossRef]
- Fang, C.; Veiseh, O.; Kievit, F.; Bhattarai, N.; Wang, F.; Stephen, Z.; Li, C.; Lee, D.; Ellenbogen, R.G.; Zhang, M. Functionalization of iron oxide magnetic nanoparticles with targeting ligands: Their physicochemical properties and in vivo behavior. Nanomedicine 2010, 5, 1357–1369. [Google Scholar] [CrossRef]
- Yue, P.; He, L.; Qiu, S.; Li, Y.; Liao, Y.; Li, X.; Xie, D.; Peng, Y. OX26/CTX-conjugated PEGylated liposome as a dual-targeting gene delivery system for brain glioma. Mol. Cancer 2014, 13, 191. [Google Scholar] [CrossRef]
- Zhao, M.; van Straten, D.; Broekman, M.L.D.; Préat, V.; Schiffelers, R.M. Nanocarrier-based drug combination therapy for glioblastoma. Theranostics 2020, 10, 1355–1372. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Kievit, F.M.; Gunn, J.W.; Ratner, B.D.; Zhang, M. A ligand-mediated nanovector for targeted gene delivery and transfection in cancer cells. Biomaterials 2009, 30, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Roma-Rodrigues, C.; Rivas-García, L.; Baptista, P.V.; Fernandes, A.R. Gene Therapy in Cancer Treatment: Why Go Nano? Pharmaceutics 2020, 12, 233. [Google Scholar] [CrossRef] [PubMed]
- Alavian, F.; Ghasemi, S. The Effectiveness of Nanoparticles on Gene Therapy for Glioblastoma Cells Apoptosis: A Systematic Review. Curr. Gene Ther. 2021, 21, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Mok, H.; Veiseh, O.; Fang, C.; Kievit, F.M.; Wang, F.Y.; Park, J.O.; Zhang, M. pH-Sensitive siRNA nanovector for targeted gene silencing and cytotoxic effect in cancer cells. Mol. Pharm. 2010, 7, 1930–1939. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, E.; Bost, W.; Fournelle, M.; Llop, J.; Gil, L.; Arena, F.; Lorusso, V.; Comes Franchini, M. Targeted polymeric nanoparticles containing gold nanorods: A therapeutic approach against glioblastoma. J. Nanopart. Res. 2014, 16, 2304. [Google Scholar] [CrossRef]
- Bartelds, R.; Nematollahi, M.H.; Pols, T.; Stuart, M.C.A.; Pardakhty, A.; Asadikaram, G.; Poolman, B. Niosomes, an alternative for liposomal delivery. PLoS ONE 2018, 13, e0194179. [Google Scholar] [CrossRef]
- Su, S.; Kang, P.M. Recent Advances in Nanocarrier-Assisted Therapeutics Delivery Systems. Pharmaceutics 2020, 12, 837. [Google Scholar] [CrossRef]
- Duan, Y.; Dhar, A.; Patel, C.; Khimani, M.; Neogi, S.; Sharma, P.; Kumar, N.S.; Vekariya, R.L. A brief review on solid lipid nanoparticles: Part and parcel of contemporary drug delivery systems. RSC Adv. 2020, 10, 26777–26791. [Google Scholar] [CrossRef]
- De, A.; Venkatesh, N.; Senthil, M.; Sanapalli, B.K.R.; Shanmugham, R.; Karri, V.V.S.R. Smart niosomes of temozolomide for enhancement of brain targeting. Nanobiomedicine 2018, 5, 1849543518805355. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.-Z.; Huang, G.; Guo, M.; Zhang, X.; Wang, H.; Deng, S.; Li, Y.; Xiang, W.; Chen, Z.; Pan, J.; et al. Acquired temozolomide resistance in MGMT-deficient glioblastoma cells is associated with regulation of DNA repair by DHC2. Brain J. Neurol. 2019, 142, 2352–2366. [Google Scholar] [CrossRef]
- Yoo, B.; Ifediba, M.A.; Ghosh, S.; Medarova, Z.; Moore, A. Combination treatment with theranostic nanoparticles for glioblastoma sensitization to TMZ. Mol. Imaging Biol. 2014, 16, 680–689. [Google Scholar] [CrossRef]
- Wang, K.; Kievit, F.M.; Chiarelli, P.A.; Stephen, Z.R.; Lin, G.; Silber, J.R.; Ellenbogen, R.G.; Zhang, M. siRNA Nanoparticle Suppresses Drug-Resistant Gene and Prolongs Survival in an Orthotopic Glioblastoma Xenograft Mouse Model. Adv. Funct. Mater. 2021, 31, 2007166. [Google Scholar] [CrossRef]
- Mu, Q.; Lin, G.; Patton, V.K.; Wang, K.; Press, O.W.; Zhang, M. Gemcitabine and Chlorotoxin Conjugated Iron Oxide Nanoparticles for Glioblastoma Therapy. J. Mater. Chem. B Mater. Biol. Med. 2016, 4, 32–36. [Google Scholar] [CrossRef]
- Costa, P.M.; Cardoso, A.L.; Custódia, C.; Cunha, P.; Pereira de Almeida, L.; Pedroso de Lima, M.C. MiRNA-21 silencing mediated by tumor-targeted nanoparticles combined with sunitinib: A new multimodal gene therapy approach for glioblastoma. J. Control. Release 2015, 207, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.; Galstyan, A.; Sun, T.; Shatalova, E.S.; Butte, P.; Mamelak, A.N.; Carico, C.; Kittle, D.S.; Grodzinski, Z.B.; Chiechi, A.; et al. Polymalic acid chlorotoxin nanoconjugate for near-infrared fluorescence guided resection of glioblastoma multiforme. Biomaterials 2019, 206, 146–159. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, Z.; Yin, G.; Cai, B.; Wang, L.; Gao, F. RGD/CTX-conjugated multifunctional Eu–Gd2O3 NRs for targeting detection and inhibition of early tumor. J. Mater. Chem. B 2017, 5, 4863–4875. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Du, K.; Fang, C.; Bhattarai, N.; Veiseh, O.; Kivit, F.; Stephen, Z.; Lee, D.; Ellenbogen, R.G.; Ratner, B.; et al. PEG-Mediated Synthesis of Highly Dispersive Multifunctional Superparamagnetic Nanoparticles: Their Physicochemical Properties and Function In Vivo. ACS Nano 2010, 4, 2402–2410. [Google Scholar] [CrossRef] [PubMed]
- Bost, W.; Fournelle, M. Molecular imaging of glioblastoma cells using functionalized nanorods and a high resolution optoacoustic microscope. In Proceedings of the 2013 IEEE International Ultrasonics Symposium (IUS), Prague, Czech Republic, 21–25 July 2013; pp. 120–123. [Google Scholar]
- Kim, C.; Qin, R.; Xu, J.S.; Wang, L.V.; Xu, R. Multifunctional microbubbles and nanobubbles for photoacoustic and ultrasound imaging. J. Biomed. Opt. 2010, 15, 010510. [Google Scholar] [CrossRef] [PubMed]
- Endo-Takahashi, Y.; Negishi, Y. Microbubbles and Nanobubbles with Ultrasound for Systemic Gene Delivery. Pharmaceutics 2020, 12, 964. [Google Scholar] [CrossRef] [PubMed]
- Goertz, D.E. An overview of the influence of therapeutic ultrasound exposures on the vasculature: High intensity ultrasound and microbubble-mediated bioeffects. Int. J. Hyperth. 2015, 31, 134–144. [Google Scholar] [CrossRef]
- Rapoport, N.; Nam, K.-H.; Gupta, R.; Gao, Z.; Mohan, P.; Payne, A.; Todd, N.; Liu, X.; Kim, T.; Shea, J.; et al. Ultrasound-mediated tumor imaging and nanotherapy using drug loaded, block copolymer stabilized perfluorocarbon nanoemulsions. J. Control. Release 2011, 153, 4–15. [Google Scholar] [CrossRef]
- Bull, J.L. The application of microbubbles for targeted drug delivery. Expert Opin. Drug Deliv. 2007, 4, 475–493. [Google Scholar] [CrossRef]
- Hernot, S.; Klibanov, A.L. Microbubbles in ultrasound-triggered drug and gene delivery. Adv. Drug Deliv. Rev. 2008, 60, 1153–1166. [Google Scholar] [CrossRef] [PubMed]
- Kooiman, K.; Roovers, S.; Langeveld, S.A.G.; Kleven, R.T.; Dewitte, H.; O’Reilly, M.A.; Escoffre, J.-M.; Bouakaz, A.; Verweij, M.D.; Hynynen, K.; et al. Ultrasound-Responsive Cavitation Nuclei for Therapy and Drug Delivery. Ultrasound Med. Biol. 2020, 46, 1296–1325. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Ren, X.; Nie, F.; Li, T.; Lv, W.; Li, H.; Zhang, Y. Current advances in ultrasound-combined nanobubbles for cancer-targeted therapy: A review of the current status and future perspectives. RSC Adv. 2021, 11, 12915–12928. [Google Scholar] [CrossRef] [PubMed]
- Sirsi, S.R.; Borden, M.A. Advances in Ultrasound Mediated Gene Therapy Using Microbubble Contrast Agents. Theranostics 2012, 2, 1208–1222. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, R.-K. Ultrasound-targeted microbubble destruction in gene therapy: A new tool to cure human diseases. Genes Dis. 2017, 4, 64–74. [Google Scholar] [CrossRef]
- Yildirim, A.; Blum, N.T.; Goodwin, A.P. Colloids, nanoparticles, and materials for imaging, delivery, ablation, and theranostics by focused ultrasound (FUS). Theranostics 2019, 9, 2572–2594. [Google Scholar] [CrossRef]
- Zhu, L.; Zhong, Y.; Wu, S.; Yan, M.; Cao, Y.; Mou, N.; Wang, G.; Sun, D.; Wu, W. Cell membrane camouflaged biomimetic nanoparticles: Focusing on tumor theranostics. Mater. Today Bio 2022, 14, 100228. [Google Scholar] [CrossRef]
- Li, J.; Zeng, H.; You, Y.; Wang, R.; Tan, T.; Wang, W.; Yin, L.; Zeng, Z.; Zeng, Y.; Xie, T. Active targeting of orthotopic glioma using biomimetic liposomes co-loaded elemene and cabazitaxel modified by transferritin. J. Nanobiotechnol. 2021, 19, 289. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Wang, X.; Li, F.; Wang, S.; Zhao, J.; Wang, J.; Liu, J.; Lyu, C.; Ye, P.; Tan, H.; et al. Engineered biomimetic nanoparticles achieve targeted delivery and efficient metabolism-based synergistic therapy against glioblastoma. Nat. Commun. 2022, 13, 4214. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Yang, W.; Li, Y.; Wang, Y.; He, W.; Wang, J.; Muhammad, P.; Chaston, T.B.; Rehman, F.U.; Zheng, M.; et al. Biomimetic Dp44mT-nanoparticles selectively induce apoptosis in Cu-loaded glioblastoma resulting in potent growth inhibition. Biomaterials 2022, 289, 121760. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Wang, X.; Hu, D.; Wang, P.; Liu, Q.; Zhang, X.; Jiang, J.; Liu, X.; Sheng, Z.; Liu, B.; et al. Phototheranostics: Active Targeting of Orthotopic Glioma Using Biomimetic Proteolipid Nanoparticles. ACS Nano 2019, 13, 386–398. [Google Scholar] [CrossRef]
- Wang, G.; Hu, W.; Chen, H.; Shou, X.; Ye, T.; Xu, Y. Cocktail Strategy Based on NK Cell-Derived Exosomes and Their Biomimetic Nanoparticles for Dual Tumor Therapy. Cancers 2019, 11, 1560. [Google Scholar] [CrossRef]
- Pinel, S.; Thomas, N.; Boura, C.; Barberi-Heyob, M. Approaches to physical stimulation of metallic nanoparticles for glioblastoma treatment. Adv. Drug Deliv. Rev. 2019, 138, 344–357. [Google Scholar] [CrossRef]
- Rajan, A.; Sahu, N.K. Review on magnetic nanoparticle-mediated hyperthermia for cancer therapy. J. Nanopart. Res. 2020, 22, 319. [Google Scholar] [CrossRef]
- Bettaieb, A.; Averill-Bates, D.A. Thermotolerance induced at a mild temperature of 40 °C alleviates heat shock-induced ER stress and apoptosis in HeLa cells. Biochim. Biophys. Acta 2015, 1853, 52–62. [Google Scholar] [CrossRef]
- Lin, F.-C.; Hsu, C.-H.; Lin, Y.-Y. Nano-therapeutic cancer immunotherapy using hyperthermia-induced heat shock proteins: Insights from mathematical modeling. Int. J. Nanomed. 2018, 13, 3529–3539. [Google Scholar] [CrossRef]
- Datta, N.R.; Ordóñez, S.G.; Gaipl, U.S.; Paulides, M.M.; Crezee, H.; Gellermann, J.; Marder, D.; Puric, E.; Bodis, S. Local hyperthermia combined with radiotherapy and-/or chemotherapy: Recent advances and promises for the future. Cancer Treat. Rev. 2015, 41, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, K.; Bouras, A.; Bozec, D.; Ivkov, R.; Hadjipanayis, C. Magnetic hyperthermia therapy for the treatment of glioblastoma: A review of the therapy’s history, efficacy and application in humans. Int. J. Hyperth. 2018, 34, 1316–1328. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, E.S.; Sankey, E.W.; Grabowski, M.M.; Chongsathidkiet, P.; Fecci, P.E. The intersection between immunotherapy and laser interstitial thermal therapy: A multipronged future of neuro-oncology. Int. J. Hyperth. 2020, 37, 27–34. [Google Scholar] [CrossRef]
- Habra, K.; McArdle, S.E.B.; Morris, R.H.; Cave, G.W.V. Synthesis and Functionalisation of Superparamagnetic Nano-Rods towards the Treatment of Glioblastoma Brain Tumours. Nanomaterials 2021, 11, 2157. [Google Scholar] [CrossRef]
- Skandalakis, G.P.; Rivera, D.R.; Rizea, C.D.; Bouras, A.; Raj, J.G.J.; Bozec, D.; Hadjipanayis, C.G. Hyperthermia treatment advances for brain tumors. Int. J. Hyperth. 2020, 37, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, I.; Shokrollahi, H.; Amiri, S. Ferrite-based magnetic nanofluids used in hyperthermia applications. J. Magn. Magn. Mater. 2012, 324, 903–915. [Google Scholar] [CrossRef]
- Mazario, E.; Menéndez, N.; Herrasti, P.; Cañete, M.; Connord, V.; Carrey, J. Magnetic Hyperthermia Properties of Electrosynthesized Cobalt Ferrite Nanoparticles. J. Phys. Chem. C 2013, 117, 11405–11411. [Google Scholar] [CrossRef]
- Chen, R.; Christiansen, M.G.; Anikeeva, P. Maximizing Hysteretic Losses in Magnetic Ferrite Nanoparticles via Model-Driven Synthesis and Materials Optimization. ACS Nano 2013, 7, 8990–9000. [Google Scholar] [CrossRef]
- Abenojar, E.C.; Wickramasinghe, S.; Bas-Concepcion, J.; Samia, A.C.S. Structural effects on the magnetic hyperthermia properties of iron oxide nanoparticles. Prog. Nat. Sci. Mater. Int. 2016, 26, 440–448. [Google Scholar] [CrossRef]
- Bauer, L.M.; Situ, S.F.; Griswold, M.A.; Samia, A.C.S. High-performance iron oxide nanoparticles for magnetic particle imaging—Guided hyperthermia (hMPI). Nanoscale 2016, 8, 12162–12169. [Google Scholar] [CrossRef]
- Dalal, M.; Greneche, J.-M.; Satpati, B.; Ghzaiel, T.B.; Mazaleyrat, F.; Ningthoujam, R.S.; Chakrabarti, P.K. Microwave Absorption and the Magnetic Hyperthermia Applications of Li0.3Zn0.3Co0.1Fe2.3O4 Nanoparticles in Multiwalled Carbon Nanotube Matrix. ACS Appl. Mater. Interfaces 2017, 9, 40831–40845. [Google Scholar] [CrossRef] [PubMed]
- Kotoulas, A.; Dendrinou-Samara, C.; Sarafidis, C.; Kehagias, T.; Arvanitidis, J.; Vourlias, G.; Angelakeris, M.; Kalogirou, O. Carbon-encapsulated cobalt nanoparticles: Synthesis, properties, and magnetic particle hyperthermia efficiency. J. Nanopart. Res. 2017, 19, 399. [Google Scholar] [CrossRef]
- Hirosawa, F.; Iwasaki, T.; Watano, S. Synthesis and magnetic induction heating properties of Gd-substituted Mg–Zn ferrite nanoparticles. Appl. Nanosci. 2017, 7, 209–214. [Google Scholar] [CrossRef]
- Sengul, A.B.; Asmatulu, E. Toxicity of metal and metal oxide nanoparticles: A review. Environ. Chem. Lett. 2020, 18, 1659–1683. [Google Scholar] [CrossRef]
- Martinelli, C.; Pucci, C.; Ciofani, G. Nanostructured carriers as innovative tools for cancer diagnosis and therapy. APL Bioeng. 2019, 3, 011502. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, C.; Cuschieri, A.; Wang, L. The cytotoxicity of polycationic iron oxide nanoparticles: Common endpoint assays and alternative approaches for improved understanding of cellular response mechanism. J. Nanobiotechnol. 2012, 10, 15. [Google Scholar] [CrossRef]
- Wu, L.; Wen, W.; Wang, X.; Huang, D.; Cao, J.; Qi, X.; Shen, S. Ultrasmall iron oxide nanoparticles cause significant toxicity by specifically inducing acute oxidative stress to multiple organs. Part. Fibre Toxicol. 2022, 19, 24. [Google Scholar] [CrossRef]
- Armijo, L.M.; Brandt, Y.I.; Mathew, D.; Yadav, S.; Maestas, S.; Rivera, A.C.; Cook, N.C.; Withers, N.J.; Smolyakov, G.A.; Adolphi, N.L.; et al. Iron Oxide Nanocrystals for Magnetic Hyperthermia Applications. Nanomaterials 2012, 2, 134–146. [Google Scholar] [CrossRef]
- Estelrich, J.; Escribano, E.; Queralt, J.; Busquets, M.A. Iron oxide nanoparticles for magnetically-guided and magnetically-responsive drug delivery. Int. J. Mol. Sci. 2015, 16, 8070–8101. [Google Scholar] [CrossRef]
- Chatterjee, D.K.; Diagaradjane, P.; Krishnan, S. Nanoparticle-mediated hyperthermia in cancer therapy. Ther. Deliv. 2011, 2, 1001–1014. [Google Scholar] [CrossRef]
- Stigliano, R.V.; Shubitidze, F.; Petryk, J.D.; Shoshiashvili, L.; Petryk, A.A.; Hoopes, P.J. Mitigation of eddy current heating during magnetic nanoparticle hyperthermia therapy. Int. J. Hyperth. 2016, 32, 735–748. [Google Scholar] [CrossRef]
- Zhao, R.; Xiang, J.; Wang, B.; Chen, L.; Tan, S. Recent Advances in the Development of Noble Metal NPs for Cancer Therapy. Bioinorg. Chem. Appl. 2022, 2022, e2444516. [Google Scholar] [CrossRef] [PubMed]
- Boldoo, T.; Ham, J.; Kim, E.; Cho, H. Review of the Photothermal Energy Conversion Performance of Nanofluids, Their Applications, and Recent Advances. Energies 2020, 13, 5748. [Google Scholar] [CrossRef]
- Gobin, A.M.; Lee, M.H.; Halas, N.J.; James, W.D.; Drezek, R.A.; West, J.L. Near-infrared resonant nanoshells for combined optical imaging and photothermal cancer therapy. Nano Lett. 2007, 7, 1929–1934. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, H.; Khoshgard, K.; Akbarzadeh, F. In vitro outlook of gold nanoparticles in photo-thermal therapy: A literature review. Lasers Med. Sci. 2018, 33, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Moros, M.; Lewinska, A.; Merola, F.; Ferraro, P.; Wnuk, M.; Tino, A.; Tortiglione, C. Gold Nanorods and Nanoprisms Mediate Different Photothermal Cell Death Mechanisms In Vitro and In Vivo. ACS Appl. Mater. Interfaces 2020, 12, 13718–13730. [Google Scholar] [CrossRef]
- Loza, K.; Heggen, M.; Epple, M. Synthesis, Structure, Properties, and Applications of Bimetallic Nanoparticles of Noble Metals. Adv. Funct. Mater. 2020, 30, 1909260. [Google Scholar] [CrossRef]
- Tang, J.; Jiang, X.; Wang, L.; Zhang, H.; Hu, Z.; Liu, Y.; Wu, X.; Chen, C. Au@Pt nanostructures: A novel photothermal conversion agent for cancer therapy. Nanoscale 2014, 6, 3670–3678. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Shi, Q.; Zhu, C.; Luo, Y.; Lu, Q.; Li, H.; Ye, R.; Du, D.; Lin, Y. Mitochondrial Reactive Oxygen Species Burst for Cancer Therapy Triggered by Near-Infrared Light. Nanoscale 2017, 9, 15813–15824. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, X.; Zhu, M.; Lin, G.; Liu, J.; Zhou, Z.; Tian, X.; Pan, Y. PEGylated Au@Pt Nanodendrites as Novel Theranostic Agents for Computed Tomography Imaging and Photothermal/Radiation Synergistic Therapy. ACS Appl. Mater. Interfaces 2017, 9, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Peng, J.; Xiao, Y.; Li, W.; Tan, L.; Xu, X.; Qian, Z. Porous Au@Pt Nanoparticles: Therapeutic Platform for Tumor Chemo-Photothermal Co-Therapy and Alleviating Doxorubicin-Induced Oxidative Damage. ACS Appl. Mater. Interfaces 2018, 10, 150–164. [Google Scholar] [CrossRef]
- Depciuch, J.; Stec, M.; Klebowski, B.; Baran, J.; Parlinska-Wojtan, M. Platinum–gold nanoraspberries as effective photosensitizer in anticancer photothermal therapy. J. Nanobiotechnol. 2019, 17, 107. [Google Scholar] [CrossRef] [PubMed]
- Salado-Leza, D.; Traore, A.; Porcel, E.; Dragoe, D.; Muñoz, A.; Remita, H.; García, G.; Lacombe, S. Radio-Enhancing Properties of Bimetallic Au:Pt Nanoparticles: Experimental and Theoretical Evidence. Int. J. Mol. Sci. 2019, 20, 5648. [Google Scholar] [CrossRef] [PubMed]
- Fathima, R.; Mujeeb, A. Enhanced nonlinear and thermo optical properties of laser synthesized surfactant-free Au-Pt bimetallic nanoparticles. J. Mol. Liq. 2021, 343, 117711. [Google Scholar] [CrossRef]
- Song, Y.; Qu, Z.; Li, J.; Shi, L.; Zhao, W.; Wang, H.; Sun, T.; Jia, T.; Sun, Y. Fabrication of the biomimetic DOX/Au@Pt nanoparticles hybrid nanostructures for the combinational chemo/photothermal cancer therapy. J. Alloys Compd. 2021, 881, 160592. [Google Scholar] [CrossRef]
- Liu, H.; Li, C.; Qian, Y.; Hu, L.; Fang, J.; Tong, W.; Nie, R.; Chen, Q.; Wang, H. Magnetic-induced graphene quantum dots for imaging-guided photothermal therapy in the second near-infrared window. Biomaterials 2020, 232, 119700. [Google Scholar] [CrossRef] [PubMed]
- Perini, G.; Rosa, E.; Friggeri, G.; Di Pietro, L.; Barba, M.; Parolini, O.; Ciasca, G.; Moriconi, C.; Papi, M.; De Spirito, M.; et al. INSIDIA 2.0 High-Throughput Analysis of 3D Cancer Models: Multiparametric Quantification of Graphene Quantum Dots Photothermal Therapy for Glioblastoma and Pancreatic Cancer. Int. J. Mol. Sci. 2022, 23, 3217. [Google Scholar] [CrossRef] [PubMed]
- Perini, G.; Palmieri, V.; Friggeri, G.; Augello, A.; De Spirito, M.; Papi, M. Carboxylated graphene quantum dots-mediated photothermal therapy enhances drug-membrane permeability, ROS production, and the immune system recruitment on 3D glioblastoma models. Cancer Nanotechnol. 2023, 14, 13. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Chen, Y.; Tsai, Y.-H.; Tseng, S.-H.; Lin, K.-S. In vivo imaging of neuroblastomas using GD2-targeting graphene quantum dots. J. Pediatr. Surg. 2021, 56, 1227–1232. [Google Scholar] [CrossRef]
- Vasyukova, I.A.; Zakharova, O.V.; Kuznetsov, D.V.; Gusev, A.A. Synthesis, Toxicity Assessment, Environmental and Biomedical Applications of MXenes: A Review. Nanomaterials 2022, 12, 1797. [Google Scholar] [CrossRef]
- Shao, J.; Zhang, J.; Jiang, C.; Lin, J.; Huang, P. Biodegradable titanium nitride MXene quantum dots for cancer phototheranostics in NIR-I/II biowindows. Chem. Eng. J. 2020, 400, 126009. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, D.; Dong, C.; Huang, H.; Feng, G.; Chen, Q.; Zheng, Y.; Tang, H.; Chen, Y.; Jing, X. Two-Dimensional MXene-Originated In Situ Nanosonosensitizer Generation for Augmented and Synergistic Sonodynamic Tumor Nanotherapy. ACS Nano 2022, 16, 9938–9952. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Liu, L.-H.; Wang, P. Sonodynamic Therapy (SDT) for Cancer Treatment: Advanced Sensitizers by Ultrasound Activation to Injury Tumor. ACS Appl. Bio Mater. 2020, 3, 3456–3475. [Google Scholar] [CrossRef] [PubMed]
- Henderson, T.A.; Morries, L.D. Near-infrared photonic energy penetration: Can infrared phototherapy effectively reach the human brain? Neuropsychiatr. Dis. Treat. 2015, 11, 2191–2208. [Google Scholar] [CrossRef] [PubMed]
- Erdreich, L.S.; Klauenberg, B.J. Radio frequency radiation exposure standards: Considerations for harmonization. Health Phys. 2001, 80, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Raoof, M.; Curley, S.A. Non-invasive radiofrequency-induced targeted hyperthermia for the treatment of hepatocellular carcinoma. Int. J. Hepatol. 2011, 2011, 676957. [Google Scholar] [CrossRef]
- Raoof, M.; Corr, S.J.; Kaluarachchi, W.D.; Massey, K.L.; Briggs, K.; Zhu, C.; Cheney, M.A.; Wilson, L.J.; Curley, S.A. Stability of antibody-conjugated gold nanoparticles in the endolysosomal nanoenvironment: Implications for noninvasive radiofrequency-based cancer therapy. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 1096–1105. [Google Scholar] [CrossRef]
- Nasseri, B.; Yilmaz, M.; Turk, M.; Kocum, I.C.; Piskin, E. Antenna-type radiofrequency generator in nanoparticle-mediated hyperthermia. RSC Adv. 2016, 6, 48427–48434. [Google Scholar] [CrossRef]
- Röschmann, P. Radiofrequency penetration and absorption in the human body: Limitations to high-field whole-body nuclear magnetic resonance imaging. Med. Phys. 1987, 14, 922–931. [Google Scholar] [CrossRef]
- Day, E.S.; Morton, J.G.; West, J.L. Nanoparticles for thermal cancer therapy. J. Biomech. Eng. 2009, 131, 074001. [Google Scholar] [CrossRef]
- Porcel, E.; Liehn, S.; Remita, H.; Usami, N.; Kobayashi, K.; Furusawa, Y.; Le Sech, C.; Lacombe, S. Platinum nanoparticles: A promising material for future cancer therapy? Nanotechnology 2010, 21, 85103. [Google Scholar] [CrossRef]
- Manikandan, M.; Hasan, N.; Wu, H.-F. Platinum nanoparticles for the photothermal treatment of Neuro 2A cancer cells. Biomaterials 2013, 34, 5833–5842. [Google Scholar] [CrossRef] [PubMed]
- San, B.H.; Moh, S.H.; Kim, K.K. Investigation of the heating properties of platinum nanoparticles under a radiofrequency current. Int. J. Hyperth. 2013, 29, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.R.; Rogers, S.; Ordóñez, S.G.; Puric, E.; Bodis, S. Hyperthermia and radiotherapy in the management of head and neck cancers: A systematic review and meta-analysis. Int. J. Hyperth. 2016, 32, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Oei, A.L.; Kok, H.P.; Oei, S.B.; Horsman, M.R.; Stalpers, L.J.A.; Franken, N.A.P.; Crezee, J. Molecular and biological rationale of hyperthermia as radio- and chemosensitizer. Adv. Drug Deliv. Rev. 2020, 163–164, 84–97. [Google Scholar] [CrossRef]
- Estelrich, J.; Busquets, M.A. Iron Oxide Nanoparticles in Photothermal Therapy. Molecules 2018, 23, 1567. [Google Scholar] [CrossRef]
- Moros, M.; Idiago-López, J.; Asín, L.; Moreno-Antolín, E.; Beola, L.; Grazú, V.; Fratila, R.M.; Gutiérrez, L.; de la Fuente, J.M. Triggering antitumoural drug release and gene expression by magnetic hyperthermia. Adv. Drug Deliv. Rev. 2019, 138, 326–343. [Google Scholar] [CrossRef]
- Arzamasov, A.A.; Vasilevskiĭ, A.A.; Grishin, E.V. [Chlorotoxin and related peptides are short insect toxins from scorpion venom]. Bioorg. Khim. 2014, 40, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhu, J.; Wang, T.; Liu, C.; Song, N.; Wu, S.; Qiao, W.; Yang, J.; Zhu, M.; Zhao, J. A novel Buthus martensii Karsch chlorotoxin derivative for glioma SPECT imaging. New J. Chem. 2020, 44, 14947–14952. [Google Scholar] [CrossRef]
- Rosso, J.P.; Rochat, H. Characterization of ten proteins from the venom of the Moroccan scorpion Androctonus mauretanicus mauretanicus, six of which are toxic to the mouse. Toxicon 1985, 23, 113–125. [Google Scholar] [CrossRef]
- Ali, S.A.; Stoeva, S.; Schütz, J.; Kayed, R.; Abassi, A.; Zaidi, Z.H.; Voelter, W. Purification and primary structure of low molecular mass peptides from scorpion (Buthus sindicus) venom. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 1998, 121, 323–332. [Google Scholar] [CrossRef]
- Rjeibi, I.; Mabrouk, K.; Mosrati, H.; Berenguer, C.; Mejdoub, H.; Villard, C.; Laffitte, D.; Bertin, D.; Ouafik, L.; Luis, J.; et al. Purification, synthesis and characterization of AaCtx, the first chlorotoxin-like peptide from Androctonus australis scorpion venom. Peptides 2011, 32, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Yin, L.; Wang, W.; Chai, B.; Liang, A. Synthesis, Expression and Purification of a Type of Chlorotoxin-like Peptide from the Scorpion, Buthus martensii Karsch, and its Acute Toxicity Analysis. Biotechnol. Lett. 2005, 27, 1597–1603. [Google Scholar] [CrossRef]
- Fan, S.; Sun, Z.; Jiang, D.; Dai, C.; Ma, Y.; Zhao, Z.; Liu, H.; Wu, Y.; Cao, Z.; Li, W. BmKCT toxin inhibits glioma proliferation and tumor metastasis. Cancer Lett. 2010, 291, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Fuller, M.D.; Thompson, C.H.; Zhang, Z.-R.; Freeman, C.S.; Schay, E.; Szakács, G.; Bakos, E.; Sarkadi, B.; McMaster, D.; French, R.J.; et al. State-dependent inhibition of cystic fibrosis transmembrane conductance regulator chloride channels by a novel peptide toxin. J. Biol. Chem. 2007, 282, 37545–37555. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.H.; Olivetti, P.R.; Fuller, M.D.; Freeman, C.S.; McMaster, D.; French, R.J.; Pohl, J.; Kubanek, J.; McCarty, N.A. Isolation and characterization of a high affinity peptide inhibitor of ClC-2 chloride channels. J. Biol. Chem. 2009, 284, 26051–26062. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.-J.; Yin, L.-T.; Liang, A.-H.; Zhang, C.-F.; Wang, W.; Chai, B.-F.; Yang, J.-Y.; Fan, X.-J. Therapeutic potential of chlorotoxin-like neurotoxin from the Chinese scorpion for human gliomas. Neurosci. Lett. 2007, 412, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.-J.; An, N.; Chan, K.-G.; Wu, Y.-B.; Zheng, S.-H.; Liang, A.-H. A model of BmK CT in inhibiting glioma cell migration via matrix metalloproteinase-2 from experimental and molecular dynamics simulation study. Biotechnol. Lett. 2011, 33, 1309–1317. [Google Scholar] [CrossRef]
- Qiao, W.; Zhao, L.; Wu, S.; Liu, C.; Guo, L.; Xing, Y.; Zhao, J. SPECT imaging and radionuclide therapy of glioma using 131I labeled Buthus martensii Karsch chlorotoxin. J. Neurooncol. 2017, 133, 287–295. [Google Scholar] [CrossRef]
- Ali, S.A.; Alam, M.; Abbasi, A.; Undheim, E.A.B.; Fry, B.G.; Kalbacher, H.; Voelter, W. Structure-Activity Relationship of Chlorotoxin-Like Peptides. Toxins 2016, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Fan, Z.; Li, W.; Dietel, B.; Wu, Y.; Beckmann, M.W.; Wrosch, J.K.; Buchfelder, M.; Eyupoglu, I.Y.; Cao, Z.; et al. Identification of two novel Chlorotoxin derivatives CA4 and CTX-23 with chemotherapeutic and anti-angiogenic potential. Sci. Rep. 2016, 6, 19799. [Google Scholar] [CrossRef]
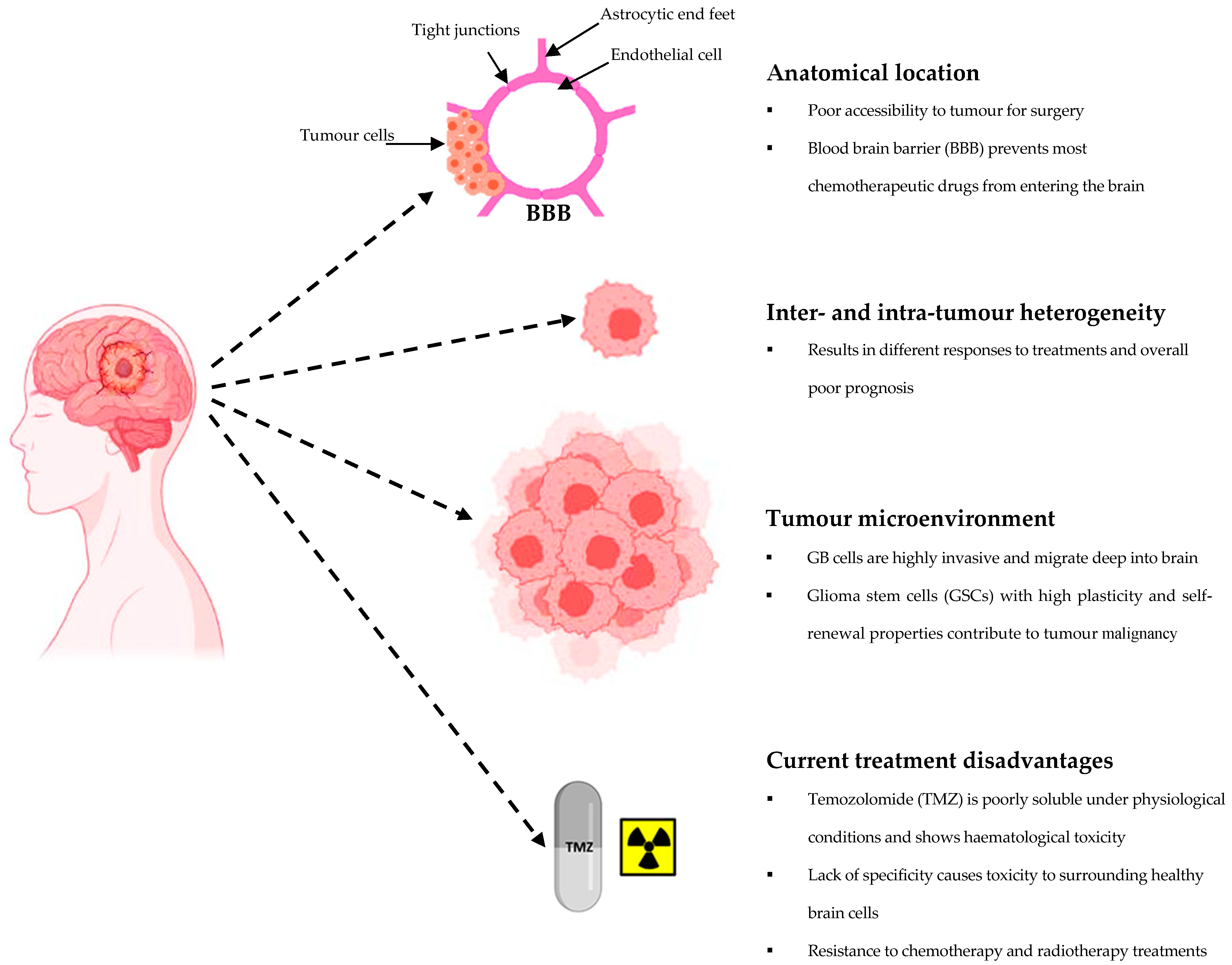

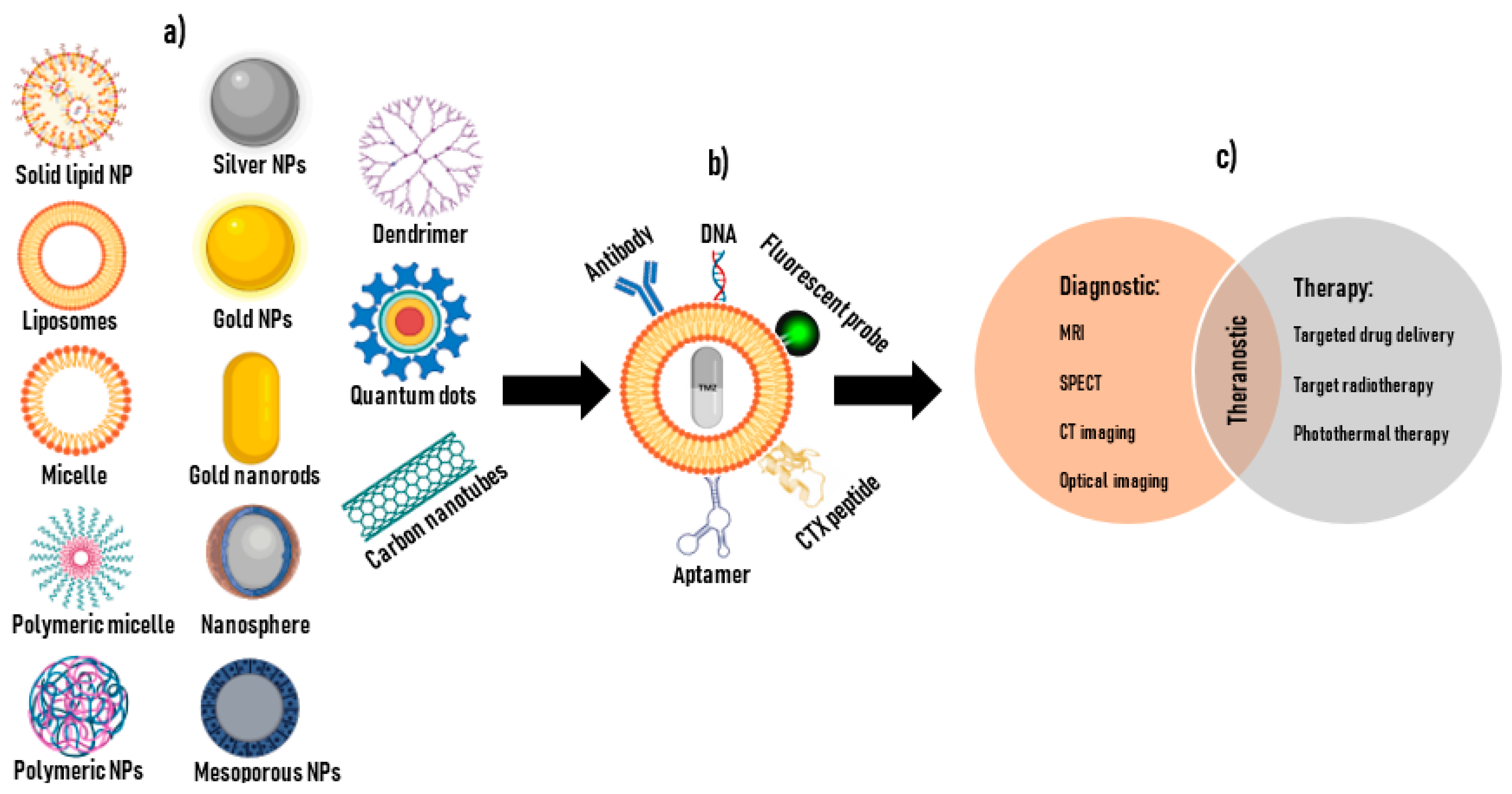
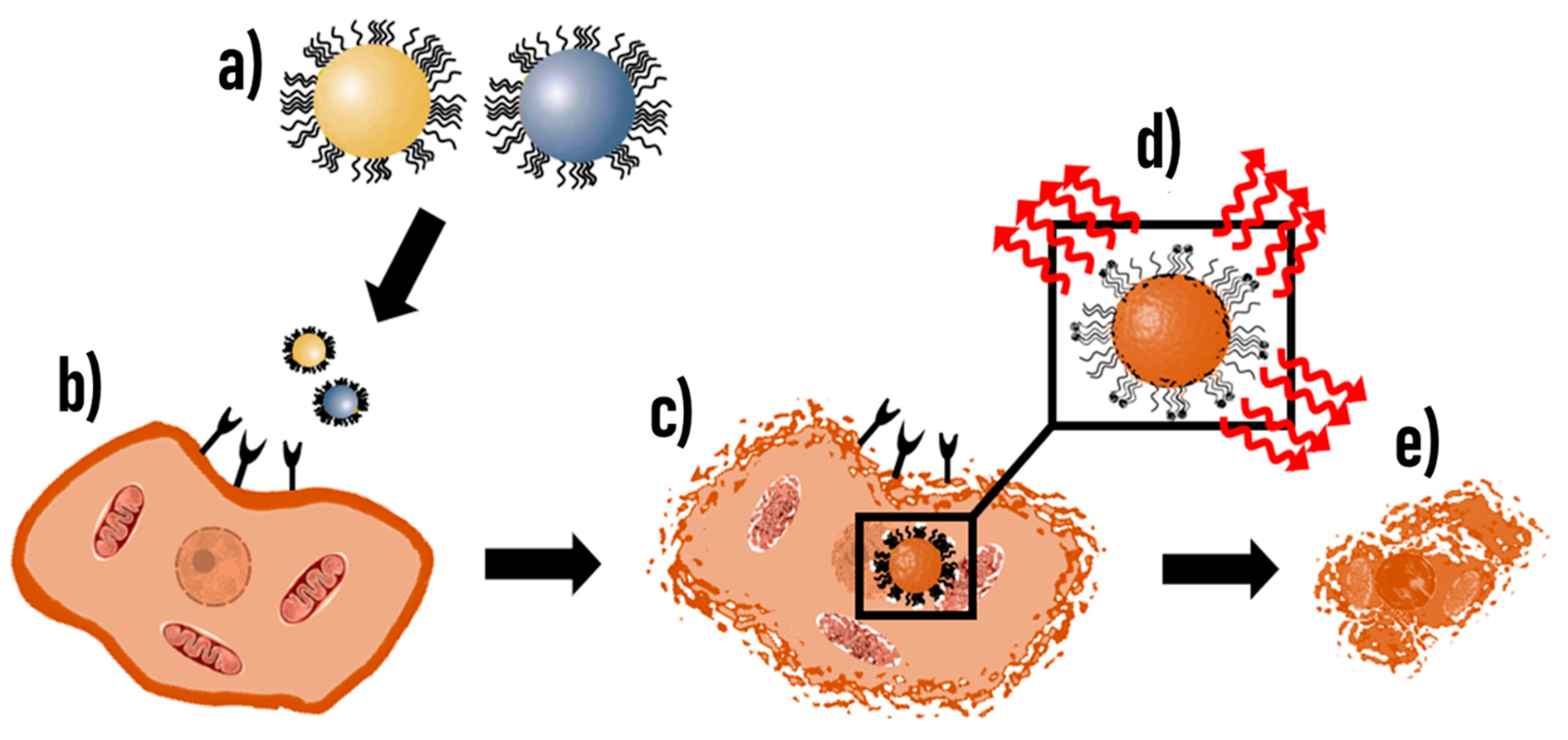
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boltman, T.; Meyer, M.; Ekpo, O. Diagnostic and Therapeutic Approaches for Glioblastoma and Neuroblastoma Cancers Using Chlorotoxin Nanoparticles. Cancers 2023, 15, 3388. https://doi.org/10.3390/cancers15133388
Boltman T, Meyer M, Ekpo O. Diagnostic and Therapeutic Approaches for Glioblastoma and Neuroblastoma Cancers Using Chlorotoxin Nanoparticles. Cancers. 2023; 15(13):3388. https://doi.org/10.3390/cancers15133388
Chicago/Turabian StyleBoltman, Taahirah, Mervin Meyer, and Okobi Ekpo. 2023. "Diagnostic and Therapeutic Approaches for Glioblastoma and Neuroblastoma Cancers Using Chlorotoxin Nanoparticles" Cancers 15, no. 13: 3388. https://doi.org/10.3390/cancers15133388
APA StyleBoltman, T., Meyer, M., & Ekpo, O. (2023). Diagnostic and Therapeutic Approaches for Glioblastoma and Neuroblastoma Cancers Using Chlorotoxin Nanoparticles. Cancers, 15(13), 3388. https://doi.org/10.3390/cancers15133388









