Ionizing Radiation Reduces Head and Neck Squamous Cell Carcinoma Cell Viability and Is Associated with Predictive Tumor-Specific T Cell Responses
Abstract
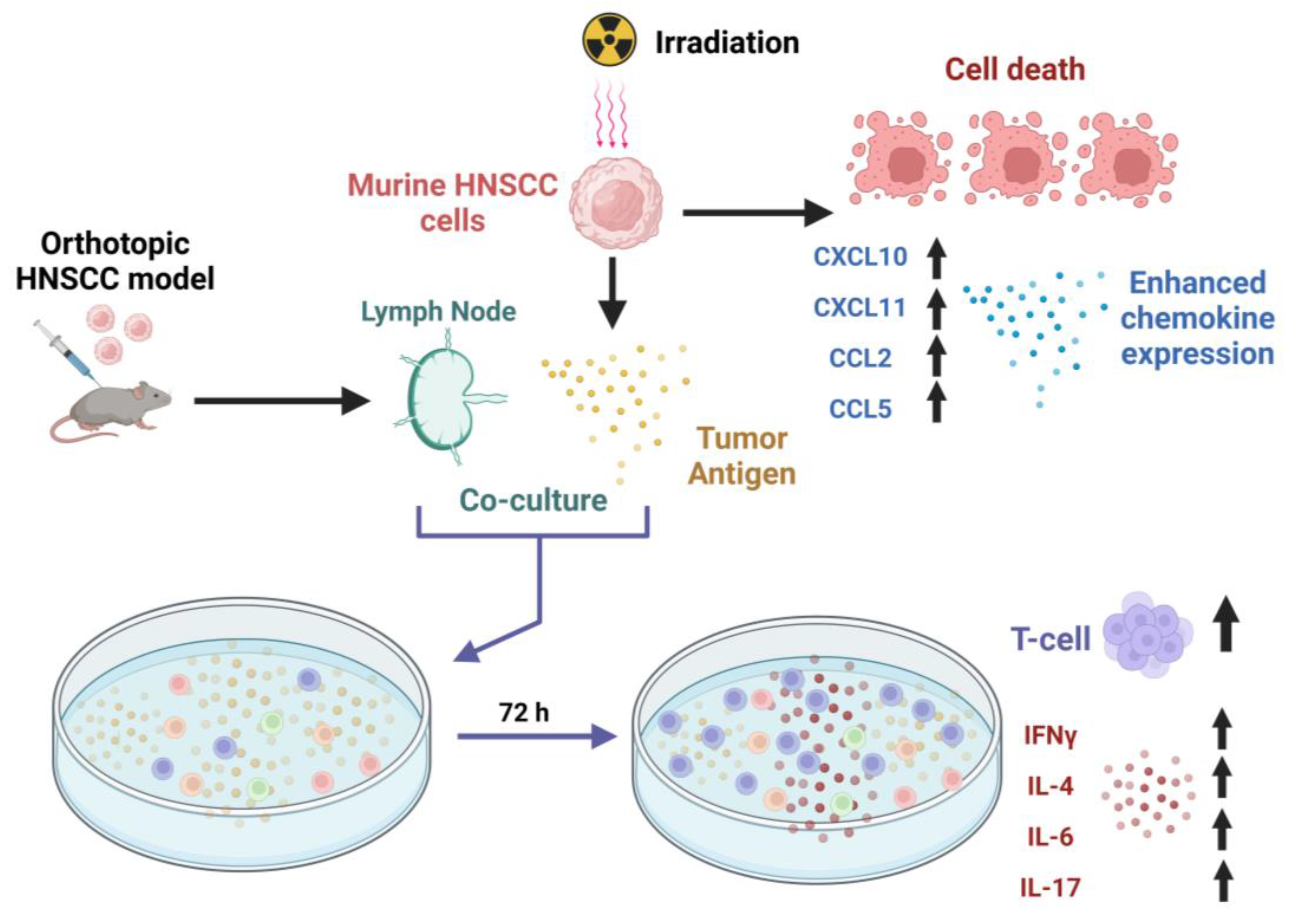
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. In Vivo Oral Cancer Cell Injections
2.3. Irradiation
2.4. Antigen Generation
2.5. T Cell Antigen Stimulation
2.6. T Cell Proliferation
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Real-Time Quantitative PCR
2.9. Statistical Analysis
3. Results
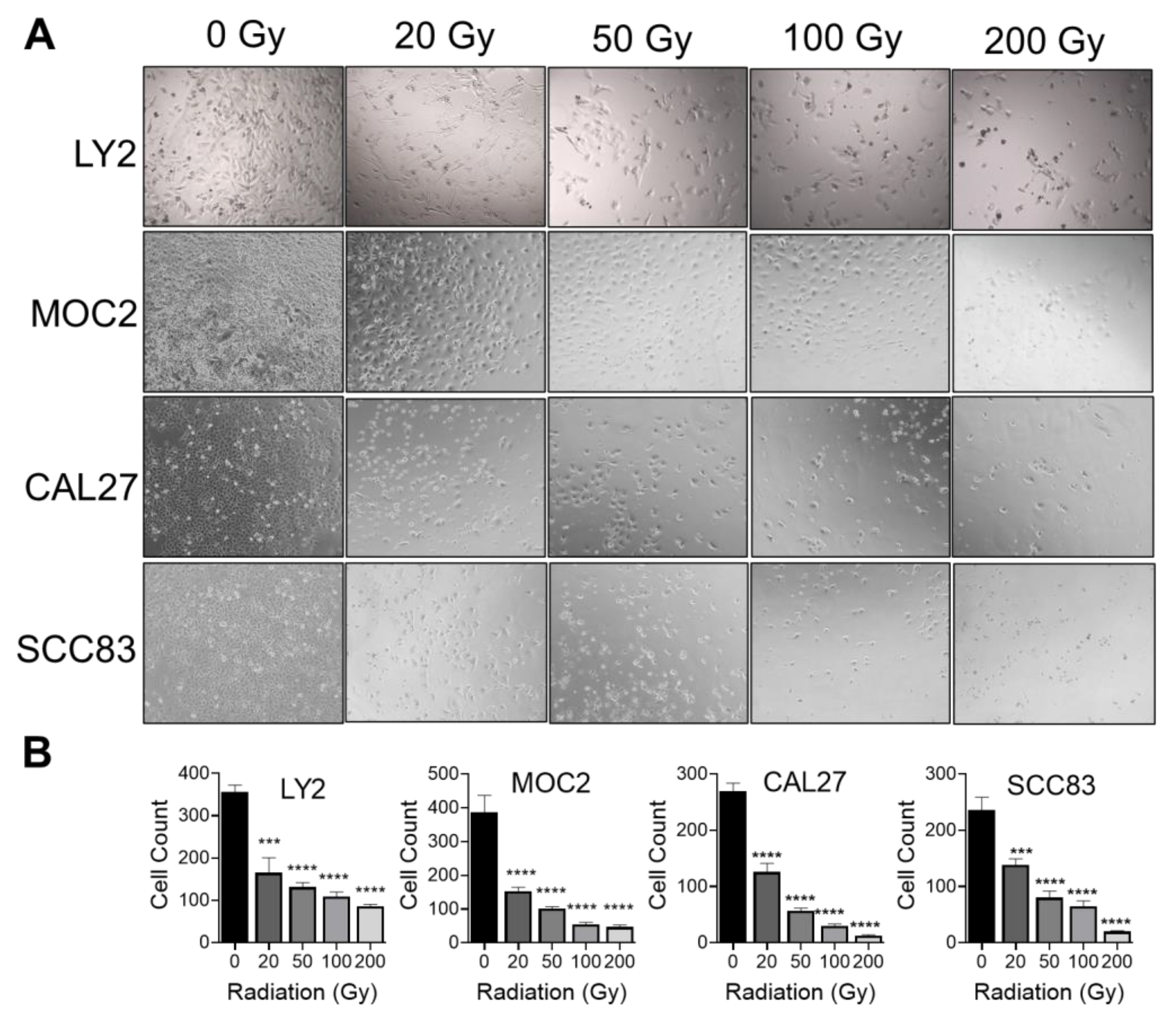
3.1. Effect of Ionizing Radiation on HNSCC Cellular Proliferation
3.2. Effect of Ionizing Radiation on Expression of T Cell Chemotactic Cytokines in HNSCC Cells
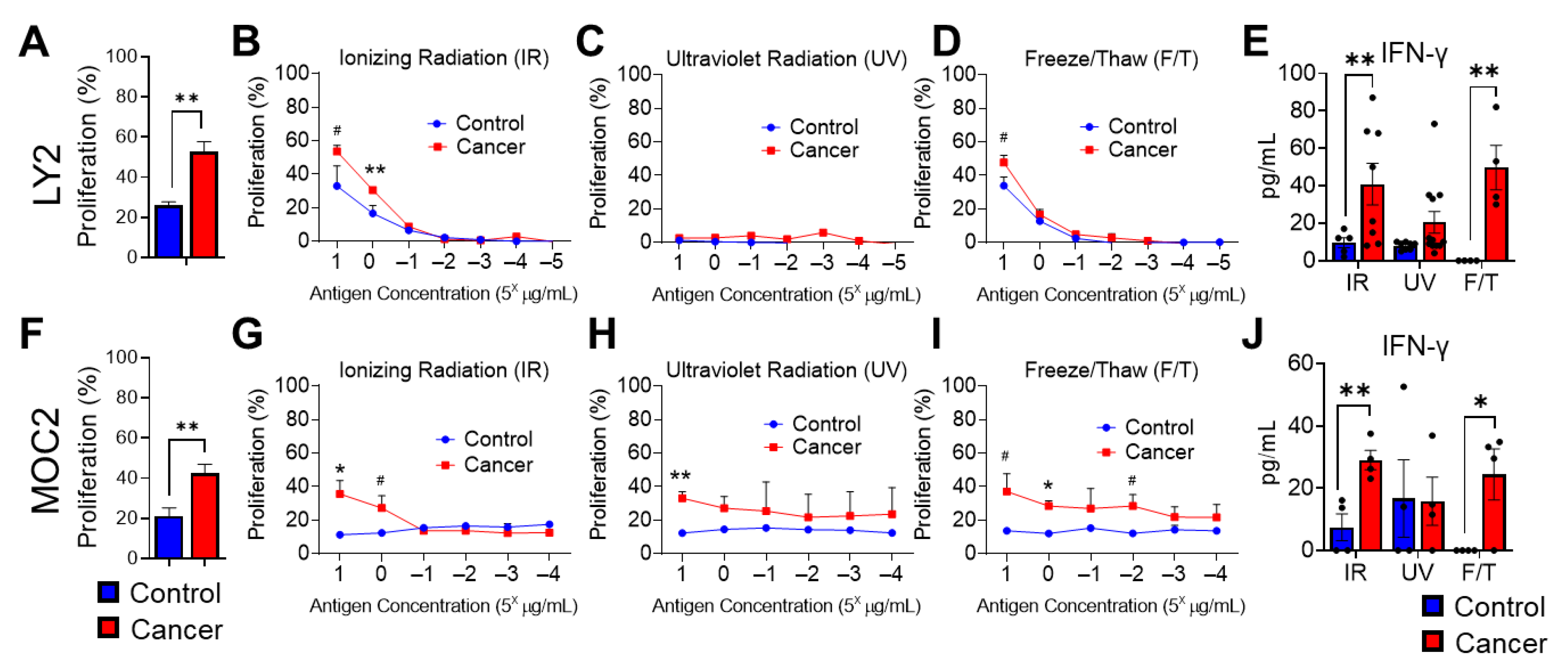
3.3. Effect of Various HNSCC Tumor Antigen Preparations on Anti-Tumor T Cell Responses
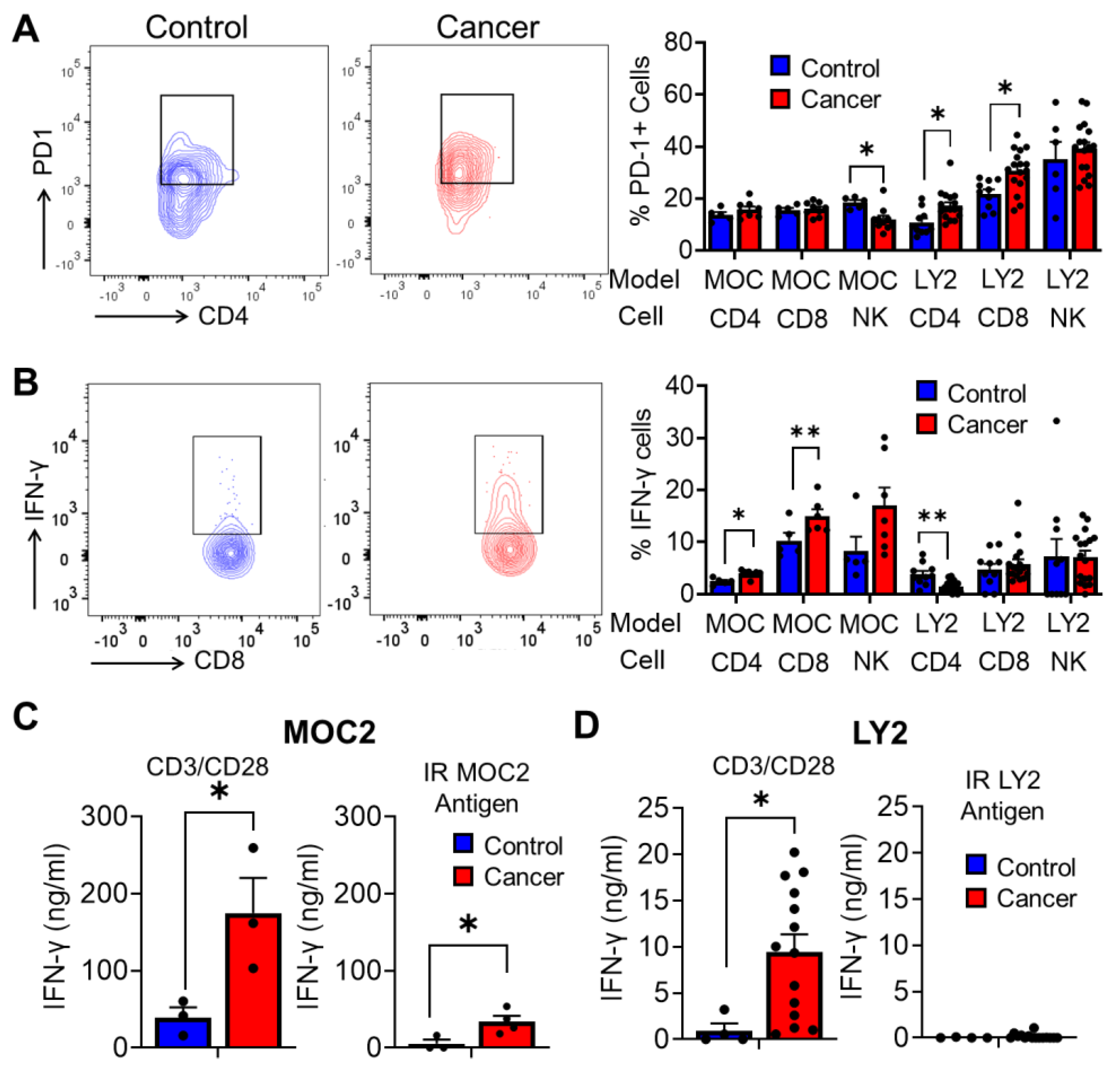
3.4. Analysis of Antitumoral T Cell Immune Responses in Tumor Bearing Mice after Stimulation with Irradiated HNSCC Tumor Antigen Ex Vivo
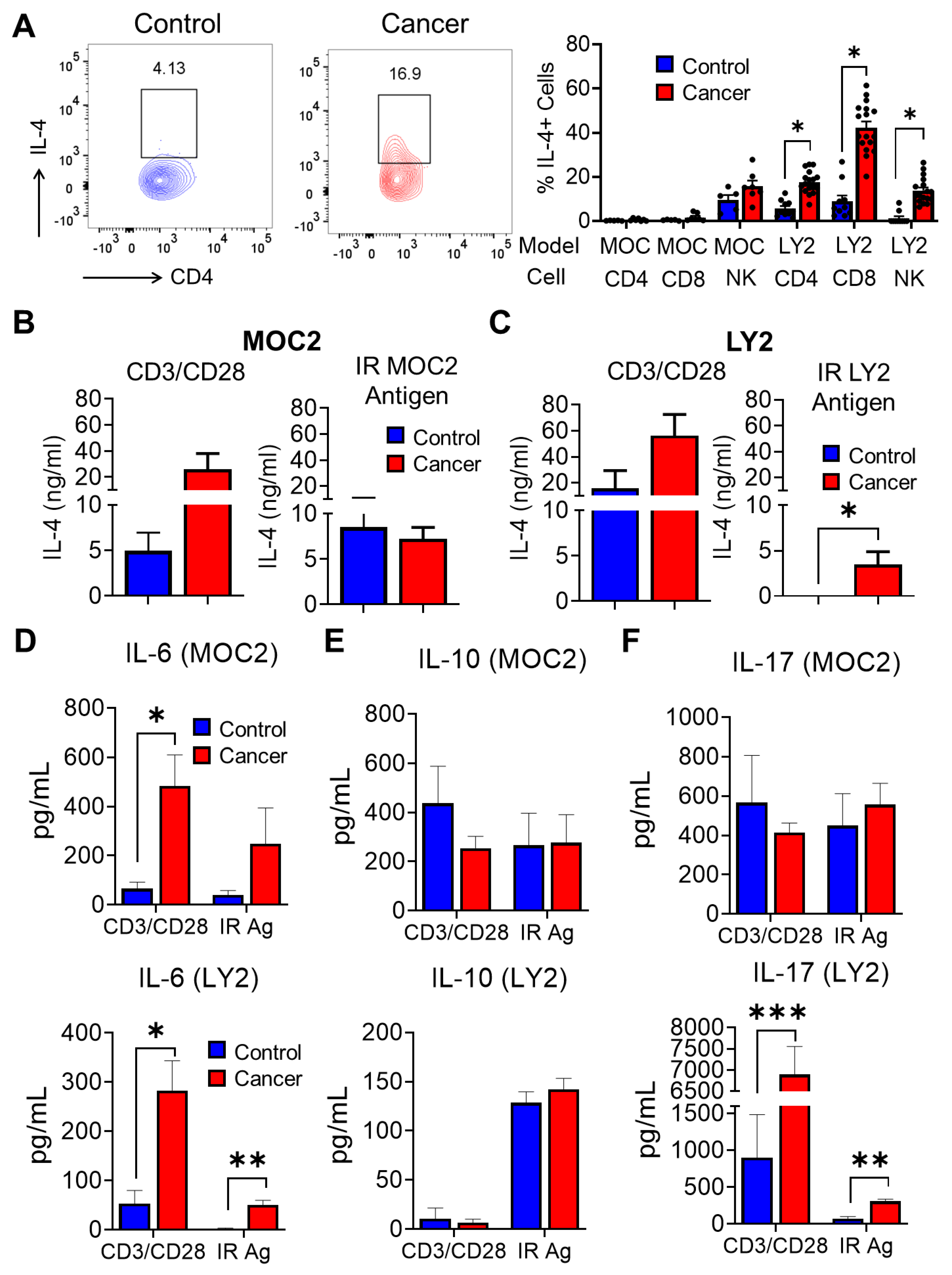
3.5. Analysis of T Cell Immune Responses Associated with HNSCC Progression after Ex Vivo Stimulation with Irradiated HNSCC Tumor Antigen
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Maier, H.; Schwetschke, O.; Weidauer, H. Decreased aspiration after extensive tumor surgical interventions in the area of the mouth cavity and pharynx by laryngeal suspension. HNO 1992, 40, 472–475. [Google Scholar] [PubMed]
- Gillison, M.L.; Koch, W.M.; Capone, R.B.; Spafford, M.; Westra, W.H.; Wu, L.; Zahurak, M.L.; Daniel, R.W.; Viglione, M.; Symer, D.E.; et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J. Natl. Cancer Inst. 2000, 92, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Camisasca, D.R.; Silami, M.A.; Honorato, J.; Dias, F.L.; de Faria, P.A.; Lourenço, S.e.Q. Oral squamous cell carcinoma: Clinicopathological features in patients with and without recurrence. ORL J. Otorhinolaryngol. Relat. Spec. 2011, 73, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Viet, C.T.; Schmidt, B.L. Biologic mechanisms of oral cancer pain and implications for clinical therapy. J. Dent. Res. 2012, 91, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.J.; Sharma, A.; Vuong, D.V.; Erler, J.T.; Pruschy, M.; Broggini-Tenzer, A. Ionizing radiation induces tumor cell lysyl oxidase secretion. BMC Cancer 2014, 14, 532. [Google Scholar] [CrossRef]
- Mettler, F.A.; Voelz, G.L. Major radiation exposure--what to expect and how to respond. N. Engl. J. Med. 2002, 346, 1554–1561. [Google Scholar] [CrossRef]
- Wong, T.; Wiesenfeld, D. Oral Cancer. Aust. Dent. J. 2018, 63 (Suppl. S1), S91–S99. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, C.; He, Q. Radiation therapy’s efficacy on tongue cancer: A population-based survival analysis. OncoTargets Ther. 2018, 11, 7271–7276. [Google Scholar] [CrossRef]
- Lalla, R.V.; Treister, N.; Sollecito, T.; Schmidt, B.; Patton, L.L.; Mohammadi, K.; Hodges, J.S.; Brennan, M.T.; Group, O.S. Oral complications at 6 months after radiation therapy for head and neck cancer. Oral Dis. 2017, 23, 1134–1143. [Google Scholar] [CrossRef]
- Mirzayans, R.; Andrais, B.; Scott, A.; Wang, Y.W.; Murray, D. Ionizing radiation-induced responses in human cells with differing TP53 status. Int. J. Mol. Sci. 2013, 14, 22409–22435. [Google Scholar] [CrossRef]
- Park, B.; Yee, C.; Lee, K.M. The effect of radiation on the immune response to cancers. Int. J. Mol. Sci. 2014, 15, 927–943. [Google Scholar] [CrossRef]
- Dull, A.B.; Wilsker, D.; Hollingshead, M.; Mazcko, C.; Annunziata, C.M.; LeBlanc, A.K.; Doroshow, J.H.; Kinders, R.J.; Parchment, R.E. Development of a quantitative pharmacodynamic assay for apoptosis in fixed tumor tissue and its application in distinguishing cytotoxic drug-induced DNA double strand breaks from DNA double strand breaks associated with apoptosis. Oncotarget 2018, 9, 17104–17116. [Google Scholar] [CrossRef]
- Hu, B.; Jin, C.; Li, H.B.; Tong, J.; Ouyang, X.; Cetinbas, N.M.; Zhu, S.; Strowig, T.; Lam, F.C.; Zhao, C.; et al. The DNA-sensing AIM2 inflammasome controls radiation-induced cell death and tissue injury. Science 2016, 354, 765–768. [Google Scholar] [CrossRef]
- Lei, G.; Zhang, Y.; Koppula, P.; Liu, X.; Zhang, J.; Lin, S.H.; Ajani, J.A.; Xiao, Q.; Liao, Z.; Wang, H.; et al. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 2020, 30, 146–162. [Google Scholar] [CrossRef]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Meng, X.; Feng, R.; Yang, L.; Xing, L.; Yu, J. The Role of Radiation Oncology in Immuno-Oncology. Oncologist 2019, 24, S42–S52. [Google Scholar] [CrossRef]
- Punnanitinont, A.; Kannisto, E.D.; Matsuzaki, J.; Odunsi, K.; Yendamuri, S.; Singh, A.K.; Patnaik, S.K. Sublethal Radiation Affects Antigen Processing and Presentation Genes to Enhance Immunogenicity of Cancer Cells. Int. J. Mol. Sci. 2020, 21, 2573. [Google Scholar] [CrossRef]
- Wennerberg, E.; Vanpouille-Box, C.; Bornstein, S.; Yamazaki, T.; Demaria, S.; Galluzzi, L. Immune recognition of irradiated cancer cells. Immunol. Rev. 2017, 280, 220–230. [Google Scholar] [CrossRef]
- Ryan, N.; Anderson, K.; Volpedo, G.; Hamza, O.; Varikuti, S.; Satoskar, A.R.; Oghumu, S. STAT1 inhibits T-cell exhaustion and myeloid derived suppressor cell accumulation to promote antitumor immune responses in head and neck squamous cell carcinoma. Int. J. Cancer 2020, 146, 1717–1729. [Google Scholar] [CrossRef]
- Anderson, K.; Ryan, N.; Alkhimovitch, A.; Siddiqui, A.; Oghumu, S. Inhibition of PI3K Isoform p110γ Increases Both Anti-Tumor and Immunosuppressive Responses to Aggressive Murine Head and Neck Squamous Cell Carcinoma with Low Immunogenicity. Cancers 2021, 13, 953. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Ryan, N.; Nedungadi, D.; Lamenza, F.; Swingler, M.; Siddiqui, A.; Satoskar, A.; Upadhaya, P.; Pietrzak, M.; Oghumu, S. STAT1 is regulated by TRIM24 and promotes immunosuppression in head and neck squamous carcinoma cells, but enhances T cell antitumour immunity in the tumour microenvironment. Br. J. Cancer 2022, 127, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Farhood, B.; Najafi, M.; Mortezaee, K. CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: A review. J. Cell Physiol. 2019, 234, 8509–8521. [Google Scholar] [CrossRef] [PubMed]
- Kohli, K.; Pillarisetty, V.G.; Kim, T.S. Key chemokines direct migration of immune cells in solid tumors. Cancer Gene Ther. 2022, 29, 10–21. [Google Scholar] [CrossRef]
- Oghumu, S.; Terrazas, C.A.; Varikuti, S.; Kimble, J.; Vadia, S.; Yu, L.; Seveau, S.; Satoskar, A.R. CXCR3 expression defines a novel subset of innate CD8+ T cells that enhance immunity against bacterial infection and cancer upon stimulation with IL-15. FASEB J. 2015, 29, 1019–1028. [Google Scholar] [CrossRef]
- Oghumu, S.; Dong, R.; Varikuti, S.; Shawler, T.; Kampfrath, T.; Terrazas, C.A.; Lezama-Davila, C.; Ahmer, B.M.; Whitacre, C.C.; Rajagopalan, S.; et al. Distinct populations of innate CD8+ T cells revealed in a CXCR3 reporter mouse. J. Immunol. 2013, 190, 2229–2240. [Google Scholar] [CrossRef]
- Dangaj, D.; Bruand, M.; Grimm, A.J.; Ronet, C.; Barras, D.; Duttagupta, P.A.; Lanitis, E.; Duraiswamy, J.; Tanyi, J.L.; Benencia, F.; et al. Cooperation between Constitutive and Inducible Chemokines Enables T Cell Engraftment and Immune Attack in Solid Tumors. Cancer Cell 2019, 35, 885–900.e10. [Google Scholar] [CrossRef]
- Schröter, P.; Hartmann, L.; Osen, W.; Baumann, D.; Offringa, R.; Eisel, D.; Debus, J.; Eichmüller, S.B.; Rieken, S. Radiation-induced alterations in immunogenicity of a murine pancreatic ductal adenocarcinoma cell line. Sci. Rep. 2020, 10, 686. [Google Scholar] [CrossRef]
- Shehadul Islam, M.; Aryasomayajula, A.; Selvaganapathy, P.R. A Review on Macroscale and Microscale Cell Lysis Methods. Micromachines 2017, 8, 83. [Google Scholar] [CrossRef]
- Panikkanvalappil, S.R.; Hira, S.M.; El-Sayed, M.A. Elucidation of ultraviolet radiation-induced cell responses and intracellular biomolecular dynamics in mammalian cells using surface-enhanced Raman spectroscopy. Chem. Sci. 2016, 7, 1133–1141. [Google Scholar] [CrossRef]
- Arnold, K.M.; Flynn, N.J.; Raben, A.; Romak, L.; Yu, Y.; Dicker, A.P.; Mourtada, F.; Sims-Mourtada, J. The Impact of Radiation on the Tumor Microenvironment: Effect of Dose and Fractionation Schedules. Cancer Growth Metastasis 2018, 11, 1179064418761639. [Google Scholar] [CrossRef]
- Ravichandran, K.S. Beginnings of a good apoptotic meal: The find-me and eat-me signaling pathways. Immunity 2011, 35, 445–455. [Google Scholar] [CrossRef]
- Wedekind, H.; Walz, K.; Buchbender, M.; Rieckmann, T.; Strasser, E.; Grottker, F.; Fietkau, R.; Frey, B.; Gaipl, U.S.; Ruckert, M. Head and neck tumor cells treated with hypofractionated irradiation die via apoptosis and are better taken up by M1-like macrophages. Strahlenther. Onkol. 2022, 198, 171–182. [Google Scholar] [CrossRef]
- Oweida, A.; Lennon, S.; Calame, D.; Korpela, S.; Bhatia, S.; Sharma, J.; Graham, C.; Binder, D.; Serkova, N.; Raben, D.; et al. Ionizing radiation sensitizes tumors to PD-L1 immune checkpoint blockade in orthotopic murine head and neck squamous cell carcinoma. Oncoimmunology 2017, 6, e1356153. [Google Scholar] [CrossRef]
- Allen, C.T.; Judd, N.P.; Bui, J.D.; Uppaluri, R. The clinical implications of antitumor immunity in head and neck cancer. Laryngoscope 2012, 122, 144–157. [Google Scholar] [CrossRef]
- Nishimura, T.; Nakui, M.; Sato, M.; Iwakabe, K.; Kitamura, H.; Sekimoto, M.; Ohta, A.; Koda, T.; Nishimura, S. The critical role of Th1-dominant immunity in tumor immunology. Cancer Chemother. Pharmacol. 2000, 46, S52–S61. [Google Scholar] [CrossRef]
- Zolkind, P.; Przybylski, D.; Marjanovic, N.; Nguyen, L.; Lin, T.; Johanns, T.; Alexandrov, A.; Zhou, L.; Allen, C.T.; Miceli, A.P.; et al. Cancer immunogenomic approach to neoantigen discovery in a checkpoint blockade responsive murine model of oral cavity squamous cell carcinoma. Oncotarget 2018, 9, 4109–4119. [Google Scholar] [CrossRef]
- Kono, M.; Saito, S.; Egloff, A.M.; Allen, C.T.; Uppaluri, R. The mouse oral carcinoma (MOC) model: A 10-year retrospective on model development and head and neck cancer investigations. Oral Oncol. 2022, 132, 106012. [Google Scholar] [CrossRef]
- Barbi, J.; Oghumu, S.; Lezama-Davila, C.M.; Satoskar, A.R. IFN-gamma and STAT1 are required for efficient induction of CXC chemokine receptor 3 (CXCR3) on CD4+ but not CD8+ T cells. Blood 2007, 110, 2215–2216. [Google Scholar] [CrossRef]
- Meissl, K.; Macho-Maschler, S.; Muller, M.; Strobl, B. The good and the bad faces of STAT1 in solid tumours. Cytokine 2017, 89, 12–20. [Google Scholar] [CrossRef]
- Karam, S.D.; Raben, D. Radioimmunotherapy for the treatment of head and neck cancer. Lancet Oncol. 2019, 20, e404–e416. [Google Scholar] [CrossRef] [PubMed]
- Biau, J.; Bourhis, J. Combining immunotherapy and radiotherapy in head and neck squamous cell cancers: Which perspectives? Curr. Opin. Oncol. 2020, 32, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Feller, G.; Khammissa, R.A.G.; Nemutandani, M.S.; Feller, L. Biological consequences of cancer radiotherapy in the context of oral squamous cell carcinoma. Head Face Med. 2021, 17, 35. [Google Scholar] [CrossRef]
- Hayman, T.J.; Bhatia, A.K.; Jethwa, K.R.; Young, M.R.; Park, H.S. Combinations of immunotherapy and radiation therapy in head and neck squamous cell carcinoma: A narrative review. Transl. Cancer Res. 2021, 10, 2571–2585. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Liang, H.; Xu, M.; Yang, X.; Burnette, B.; Arina, A.; Li, X.D.; Mauceri, H.; Beckett, M.; Darga, T.; et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity 2014, 41, 843–852. [Google Scholar] [CrossRef]
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, C.N.; Formenti, S.C.; Demaria, S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 2017, 8, 15618. [Google Scholar] [CrossRef]
- Qian, J.M.; Schoenfeld, J.D. Radiotherapy and Immunotherapy for Head and Neck Cancer: Current Evidence and Challenges. Front. Oncol. 2020, 10, 608772. [Google Scholar] [CrossRef]
- Xing, D.T.; Khor, R.; Gan, H.; Wada, M.; Ermongkonchai, T.; Ng, S.P. Recent Research on Combination of Radiotherapy with Targeted Therapy or Immunotherapy in Head and Neck Squamous Cell Carcinoma: A Review for Radiation Oncologists. Cancers 2021, 13, 5716. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Upadhaya, P.; Ryan, N.; Roth, P.; Pero, T.; Lamenza, F.; Springer, A.; Jordanides, P.; Pracha, H.; Mitchell, D.; Oghumu, S. Ionizing Radiation Reduces Head and Neck Squamous Cell Carcinoma Cell Viability and Is Associated with Predictive Tumor-Specific T Cell Responses. Cancers 2023, 15, 3334. https://doi.org/10.3390/cancers15133334
Upadhaya P, Ryan N, Roth P, Pero T, Lamenza F, Springer A, Jordanides P, Pracha H, Mitchell D, Oghumu S. Ionizing Radiation Reduces Head and Neck Squamous Cell Carcinoma Cell Viability and Is Associated with Predictive Tumor-Specific T Cell Responses. Cancers. 2023; 15(13):3334. https://doi.org/10.3390/cancers15133334
Chicago/Turabian StyleUpadhaya, Puja, Nathan Ryan, Peyton Roth, Travis Pero, Felipe Lamenza, Anna Springer, Pete Jordanides, Hasan Pracha, Darrion Mitchell, and Steve Oghumu. 2023. "Ionizing Radiation Reduces Head and Neck Squamous Cell Carcinoma Cell Viability and Is Associated with Predictive Tumor-Specific T Cell Responses" Cancers 15, no. 13: 3334. https://doi.org/10.3390/cancers15133334
APA StyleUpadhaya, P., Ryan, N., Roth, P., Pero, T., Lamenza, F., Springer, A., Jordanides, P., Pracha, H., Mitchell, D., & Oghumu, S. (2023). Ionizing Radiation Reduces Head and Neck Squamous Cell Carcinoma Cell Viability and Is Associated with Predictive Tumor-Specific T Cell Responses. Cancers, 15(13), 3334. https://doi.org/10.3390/cancers15133334






