The Assessment of White Matter Integrity Alteration Pattern in Patients with Brain Tumor Utilizing Diffusion Tensor Imaging: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Methodology
2.1. Guidelines
2.2. Search Chain
2.3. PICOS, Inclusion and Exclusion Criteria
2.4. Eligibility Assessment
3. Results
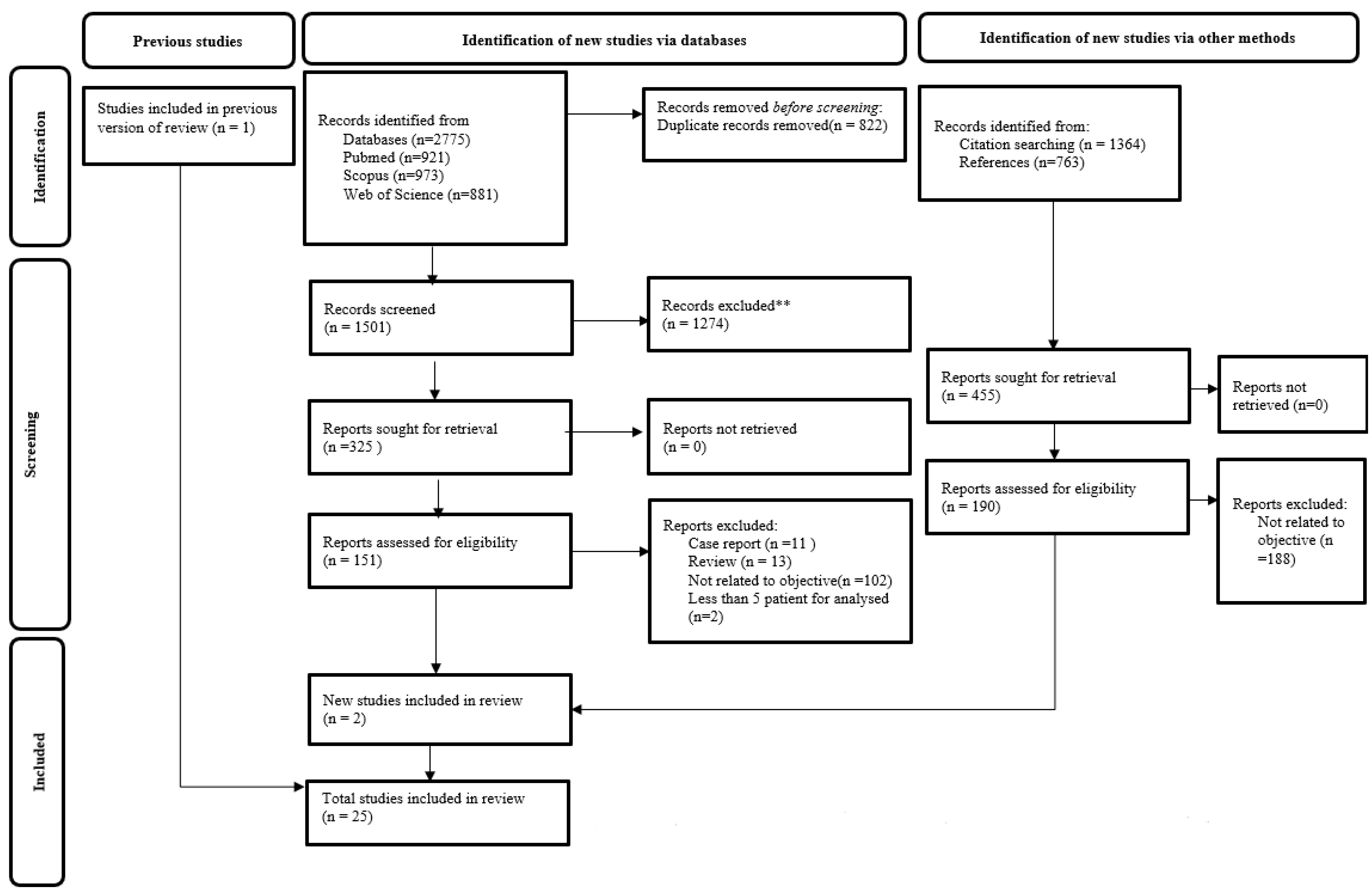
3.1. Article Selection
3.2. Extraction and Tabulation of Data
3.3. Demographic Data and Summarization of Selected Papers
| No | Author (Year) [Ref] | Patients; (Male, Female) | Range Age, (Mean) Years Old | Tumor Type | DTI Acquisition and Processing Data | White Matter Tracts Involved | Objective of the Assessment of the WMT Microstructure | |
|---|---|---|---|---|---|---|---|---|
| Scanner, Vendor, Gradient (mT/M) | Fiber Tracking Software: Visualization Method/ Type of Analysis | |||||||
| Retrospective Study | ||||||||
| 1 | Witwer et al. (2002) [1] | 9 (5 M, 4 F) | 20–66 (44 years) | Intrinsic brain tumor | 1.5 T, GE Signa, 40 mT/m | DTI analysis software toolbox (Research systems); Color-coded DTI maps/ NR | AF, CST, ILF, brainstem, OR, CC | Assessment of WMT characterization in relation to cerebral neoplasms in preoperative mapping. |
| 2 | Field et al. (2004) [21] | 13 (NR) | 20–66 (43 years) | Intracranial neoplasm | 1.5 T, GE CVi Signa, NR | IDL (Research systems); Directional color maps/ ROI | WMT fiber tracts in the vicinity of the tumor | Assessment of characterization of DTI pattern of the WMT in vicinity of tumor using DT eigenvector directional color maps. |
| 3 | Yu et al. (2005) [20] | 16 (12 M, 4 F) Control group: 24 (17 M, 7 F) | 20–72 (51.7 years) Control group: 25–68 (52.5 years) | Brainstem | 1.5 T, Siemens Sonata, NR | Siemens workstation, Leonardo; Tractography/ ROI | PT, OR, CC | Assessment the role of DTT in preoperative mapping for the surgical approach and postoperative assessment. |
| 4 | Lazar et al. (2006) [7] | 6 (NR) | 2–61 (NR) | Brain lesion | 1.5 T, GE Signa, 40 mT/m | Tensor deflection algorithm; Tractography /ROI | WMT adjacent to lesion | Assessment of WMT postoperative, comparison between preoperative and postoperative DTI criteria. |
| 5 | Chen et al. (2007) [22] | 10 (5 M, 5 F) | 16–73 (41.9 years) | Brain stem tumor | 1.5 T, Siemens Sonata, NR | BrainLab iPlan 2.5; Tractography /VOI | CST, ML | Assessment of DTI and WMT visualization for preoperative surgical planning and postoperative assessment. |
| 6 | Yen et al. (2009) [23] | 43 (19 M, 24 F) | NR | Brain lesion | NR | VOLUME-ONE; Directionally encoded color maps /ROI | Tract adjacent to tumor, CST, OR | Assessment of characterization of WMT by DTI for preoperative viability or respectability of the tumor adjacent to WMT. |
| 7 | Nievas et al. (2010) [24] | 8 (4 M, 4 F); | 16–73 (53.25 years) | Solid posterior fossa tumors | 1.5 T, Siemens Erlangen, NR | 3D software (Siemens); Tractography /ROI | Brainstem-assessed fiber, CST, traverse pontine fibers lateral and medial lemniscus. | Assessment of WMT alteration between preoperative and postoperative DTI criteria of PFT. |
| 8 | Itagiba et al. (2010) [25] | 44 (24 M, 20 F) | 3–88 (44 years) | Intracranial neoplasms | 1.5 T, Avanto, Siemens; NR | DTI Task Card (Siemens); FA Color-coded map /NR | WMT vicinity to tumor | Assessment of different patterns of characterization of WMT in brain tumor pt. using DTI, and its differential diagnosis utilization. |
| 9 | Kovanlikaya et al. (2011) [26] | 14 (7 F, 7 M); | 5–65 (28.7 years) | Brain stem tumor | 3.0 T, Philips Achieva; NR | PRIDE V4—Fiber tracking 6.1, Phillips; Tractography /VOI | CST | Assessment of DTI/DTT on CST alteration in brainstem tumor and its comparison on preoperative and postoperative. |
| 10 | Bagadia et al. (2011) [27] | 50 (33 M, 17 F); | 24–77 (48.5 years) | Intra-axial brain lesion | 1.5 T, NR, NR | Ge Healthcare workstation; Tractography /ROI | CST, Optic pathways, arcuate fasciculus | Assessment of preoperative DTI for surgical planning and postoperative prognostic. |
| 11 | Castellano et al. (2012) [28] | 73 (27 F, 46 M); | 9–70 (44.2 years) | Glioma | 3.0 T, Philips Intera, 80 mT/M. | DTI studio, version 2.4.10; Tractography /ROI | CST, IFOF, SLF | Assessment of DTI tractography in preoperative in predicting EOR in glioma resection. |
| 12 | Ibrahim et al. (2013) [29] | 32 (24 M, 8 F); | 1–74 (32.8 years) | Intracranial neoplasm | 1.5 T, Gyroscan Intera, Philips; NR | Philips medical EWS, Pride; Tractography and Color coded FA maps; /ROI | WMT involved in vicinity of the tumor | Assessment DTI characterizing of WMT in relation to brain neoplasm and its utilization in preoperative. |
| 13 | Farshidfar et al. (2014) [30] | 10 (7 M, 3 F); | 36–60 (48.6 years) | Intra-axial brain tumor/Cerebral gliomas | 1.5 T, Siemens, Espree; NR | DTI studio software Tractography /ROI | WMT tract vicinity to the tumor, CC, CST, SFC, UC | Assessment of DTI-FT in preoperative planning and in treatment strategy technique of brain tumor patient based on the WMT criteria in Iran |
| 14 | Deilami et al. (2015) [31] | 12 (NR) | NR | Intracranial lesions | 1.5 T, Siemens, 45 mT/m | MedINRIA (version 19.0); Color coded maps/ ROI | Peritumoral region | Defining diagnostic cut-off for differentiating four major types of peritumoral WM involvement using FA |
| 15 | Zhukov et al. (2016) [17] | 29 (13 M, 16\W) | NR (45 years) | Supratentorial tumor | 3.0 T, NR, NR | MRI machine software; Tractography /ROI | PT | Investigation on the relationship between different tumor’s histology types and WMT |
| 16 | Dubey et al. (2018) [32] | 34 (21 M, 13 F); | 17–70 (48.3 years) | Intra-axial brain tumor | 3.0 T, Phillips Ingenia, Phillips; NR | DTI Studio; Tractography and DEC /ROI | CST and major subcortical tracts | Assessment preoperatively the integrity and location of WMT and plan surgical corridor |
| 17 | Yu et al. (2019) [33] | 17 (9 M, 8 F); | 29–72 (53.2 years) | Intracranial lesion | 3.0 T, Ingenia, Phillips, NR | Philips Extended MR Workspace; Tractography /ROI | CST | Assessment of preoperative surgical planning, the intraoperative evaluations and clinical outcome prognosis based on DTI-FT |
| 18 | Leroy et al. (2020) [34] | 11 (8 M, 3 F) | 27–68 (43 years) | Glial tumors | 1.5 T, General Electric; NR | Volume viewer 11.3 Ext 8, GE; DEC/NR | Peritumoral tracts | Assessment of the correlation between the preoperative DTI tractography and histology mainly in fiber directional and tumor related fiber destruction. |
| 19 | Schneider et al. (2021) [35] | 25 (NR) | 30–85 (57.1 years) | Space-occupying intracranial lesion. | 1.5 T, or 3.0 T GE siemens; NR | FuncTool or FiberTrak; CCM and Tractography/ROI | PT, OR | Assessment of pattern WMT using color -coded maps versus tractography. |
| 20 | Bakhshi et al. (2021) [36] | 77; (54 M, 23 F) | NR (40.7 ± 14.8 years) | Intra-axial brain tumor | 1.5 T: NR;NR | NR Tractography /ROI | Whole brain, most white matter tracts | Assessment of pattern involved WMT morphological changes by intra-axial brain tumor |
| Prospective study | ||||||||
| 21 | Zhang et al. (2017) [37] | 32 (17 M, 15 F) Control 30 (15 M, 15 F) | 35–61 (44.1± 3.6 years) 20–63 (39.2 ± 3.3 years) | Occipital neoplasm | 1.5 T, Signa Twin, GE; NR | Volume One 1.72 and diffusion Tensor Visualizer; DTT and DEC /ROI | GCT | Assessment of the disruption of GCT in different occipital neoplasm by DTI |
| 22 | Khan et al. (2019) [38] | 128 (78 M, 50 F) | 16–82 (49 years) | Intra-axial supratentorial brain tumor | 3.0 T, Philips Ingenia; NR | DTI studio; Tractography and color-coded map /ROI | WMT and fascicles involved in vicinity of the tumor | Assessment preoperative DTI planning in term of surgical corridor and outcome, tumor characterization and postoperative prediction according to the DTI criteria |
| 23 | Shalan et al. (2021) [39] | 20 (14 M, 6 F) | 20–55 (NR) | Brain gliomas | 1.5 T, GE sigma, NR | Offline workstation; Tractography and color-coded map/ROI | CST, SLF, IFOF, CC, UC | Assessment of utility of DTI tractography as image technique and neurosurgery brain gliomas planning |
| 24 | Wende et al. (2021) [40] | 14 (6 M, 8 F) | 30–70 (50.1 ± 4.0 years) | Intracerebral tumor | 3.0 T, Ingenia, Phillips, NR | MRtrix3; Tractography/ ROI | CST | Assessment of reliable FA cut-off tractography of CST |
| 25 | Camins et al. (2022) [18] | 34 (18 M, 16 F) | 22–71 (48 years) | Temporal insular tumor | 1.5 T, Phillips Intera or Achieva; NR | Phillips Intellispace portal vers 10; DEC and tractography/ROI | IFOF | Assessment of preoperative IFOF involvement predetermined predictable patterns |
3.4. The Characterization of White Matter Tract Integrity Visualized by DTI
| Pattern | Anatomical Description | Indices/Parameters Used | |||
|---|---|---|---|---|---|
| FA (Quantitative) | FA | ADC | Percentage Difference | ||
| Normal | Not affected, fiber in the correct anatomical location. | 0.163–0.286 | Normal | Normal | NR |
| Displacement (Deviated/Deformed) /Pattern 1 | Maintained normal anisotropy relative to the corresponding tract in the contralateral hemisphere but were situated in an abnormal location or with an abnormal orientation on color coded orientation maps. | 0.085–0.093 | Normal or mildly decrease | Normal or mildly increase | Nearly (<25%), for both ADC and FA |
| Edematous /Pattern 2 | Maintained normal anisotropy and orientation but demonstrated high signal intensity on T2 weighted MR images | 0.092–0.149 | Decreased | Increased | NR |
| Infiltration /Pattern 3 | Reduced anisotropy but remained identified on orientation maps | 0.050–0.059 | Decreased | Increased | NR |
| Disruption (Destroyed/Interrupted) /Pattern 4 | Anisotropy was markedly reduced such that tracts could not be identified on the orientation maps | <0.050 | Unidentified Isotropic/near isotropic | Isotropic, near isotropic | NR |
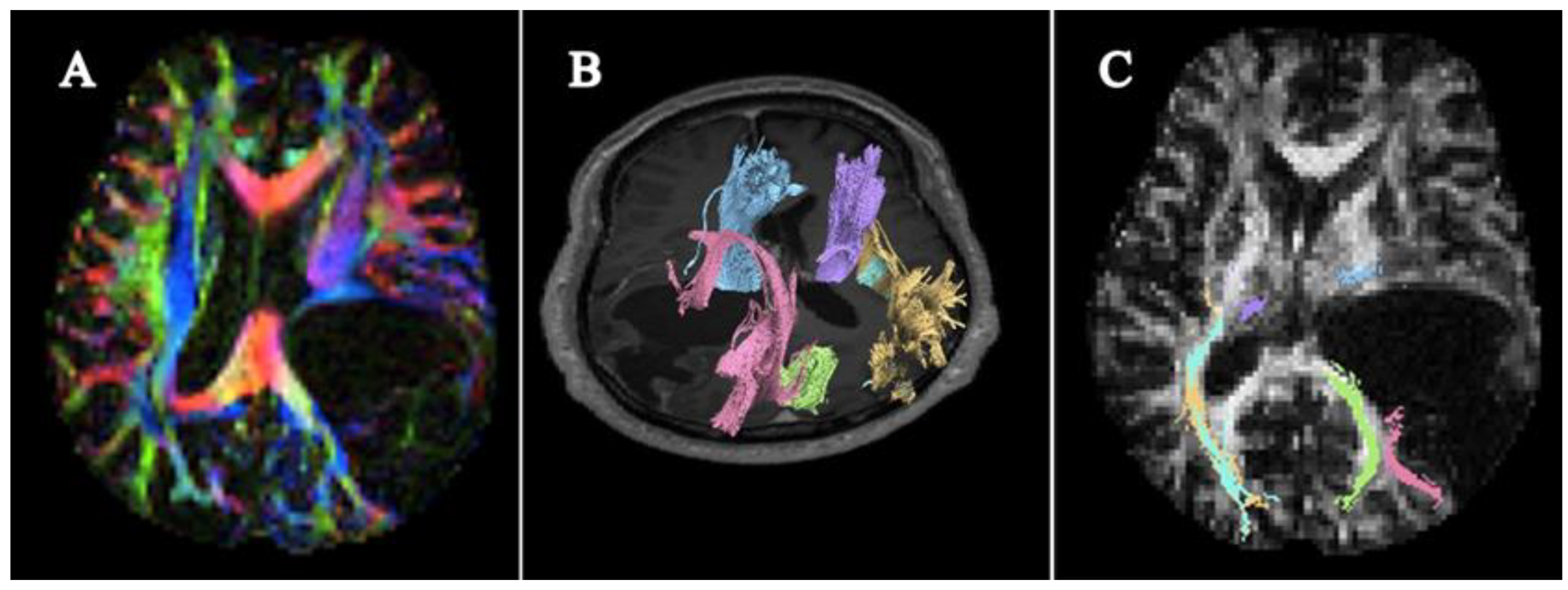
3.5. Morphological Assessment of White Matter Tract Integrity with DTI DEC FA Map and Tractography
3.5.1. Evaluation of the Individual Patients
| (a) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Author (Year) | Characterization Assessment of White Matter Tracts (n = Number of Patient) | |||||||||
| Patient (n) | Normal/Non-Affected | Displaced/Deformed/Deviated | Edema | Infiltrated | Disrupted/Destroyed | Others/More That 2 Characteristics | |||||
| 1 | Witwer et al. (2002) [1] | 9 | - | Oligodendroglioma II (3), Malignant Oligoastrocytoma III (1) | Metastasis (1) | Oligodendroglioma III (1) Oligodendroglioma II (1) | - | Displaced and Disrupted: Pilocytic astrocytoma I (1) Edema and Disrupted: Astrocytoma IV (1) | |||
| 2 | Yen et al. (2009) [23] | 28 | - | Meningioma (4), Metastasis (1), Pontine glioma arachnoid cyst (1), GBM IV (2), Acoustic neuroma (2), PNET (1) | Metastasis (2), Gliomatosis cerebri (1) | Meningioma (1), GBM (2), Trigeminal neuroma (1), Gliomatosis cerebri (2) | Metastasis (2), Pontine glioma arachnoid cyst (1), Pilocytic astrocytoma (1), GBM (1), Oligodendroglioma (1), Ependymoma (1), Gliosis (1) | - | |||
| 3 | Itagiba et al. (2010) [25] | 44 | - | LGG (14) | - | - | - | Displaced, Infiltrated, and Disrupted GBM (12) Anaplastic astrocytoma (8/9) Displaced and Edema Metastasis (9) | |||
| 4 | Farshidfar et al. (2014) [30] | 9 | - | Astrocytoma I (1), Astrocytoma II (1), Oligodendrioma I (2) Oligodendrioma II (1), | - | - | GBM IV (1) | Displaced and Edema Oligodendrioma II (1) Infiltrated and Disrupted Oligodendrioma II (1), Astrocytoma II (1) | |||
| 5 | Lazar et al. (2006) [7] | 4 | - | Pilocytic astrocytoma I (1), Ganglioglioma I (1), | - | - | - | Deviated and Infiltrated Astrocytoma III (1) Astrocytoma IV(1) | |||
| 6 | Chen et al. (2007) [22] | 3 | - | Astrocytoma II (1), | - | - | - | Deviated and Disrupted Astrocytoma II (1), Interrupted and Infiltrated Astrocytoma II (1) | |||
| 7 | Kovanlikaya et al. (2011) [26] | 7 | Hemangio blastoma (1) | Diffuse fibrillary astrocytoma II (1) Pilocytic astrocytoma I (1) Astrocytoma II (1) Mixed neuronal glial I (1) Metastasis (1) | - | - | Displaced and Disrupted Anaplastic astrocytoma III (1) | ||||
| 8 | Nievas et al. (2015) [24] | 6 | - | - | - | - | Deviated deformed, thinning interrupted Meningioma (2), Metastasis (2) Deviated, deformed, thinning Neurinoma (1) Metastasis (2) Deviated thinning Neurioma (1) | ||||
| 9 | Bagadia et al. (2011) [27] | 44 | - | Oligodendroglioma (10) Metastasis (3) GBM IV (7) Anaplastic astrocytoma (4) Diffuse fibrillary astrocytoma (1) Pleomorphic xanthoastrocytoma (1) Pilocytic astrocytoma (1) | GBM IV (1) | GBM IV (3) Other (1) | Oligodendroma (1) GBM IV (1) | Displaced and Edema GBM IV (1) Metastasis (1) Displaced and infiltrated GBM IV (6) Oligodendroglioma (1) Infiltrated and destroyed GBM IV(1) | |||
| 10 | Yu et al. (2005) [20] | 20 (3 Tracts) | - | OR Astrocytoma II (1), OR Astrocytoma III (1), PT GBM IV (1), OR Metastasis (1) | - | - | PT Astrocytoma II (1), CC Oligodendroglioma II (1), CC GBM IV (2) PT Metastasis (1) CC Metastasis (1) | Displaced and disrupted PT Astrocytoma III (1) PT Oligoastrocytoma III (3), CC Oligoastrocytoma III (2), PT GBM IV (2), PT Metastasis (2) | |||
| 11 | Deilami et al. (2015) [31] | 12, (100 ROI) | - | LGG (7), HGG (15) | HGG (9) | LGG (15) HGG (36) | LGG (1), HGG (14) | ||||
| 12 | Zhukov et al. (2016) [17] | 29 | Glioma II (5), Glioma III (3), Glioma IV (3) Metastases (1) | Glioma I (2), Glioma II (1), Glioma IV (3) Metastases (1) | - | Glioma II (2), Glioma III (1), Glioma IV (5) Metastases (2) | - | ||||
| 13 | Khan et al. (2019) [38] | 128 | - | HGG (9), LGG (36), Metastasis (3) | - | - | - | Infiltrated and Disrupted: HGG (57), LGG (12), Metastasis (11) | |||
| 14 | Dubey et al. (2018) [32] | 34 | - | HGG (5), LGG (9), Metastasis (4) | - | - | - | Infiltrated and Disrupted HGG (12), LGG (3), Metastasis (1) | |||
| 15 | Zhang et al. (2017) [37] | 32 | - | Meningioma (6), Metastases (10) | - | - | - | Displaced and Infiltrated Glioma II (2) Infiltrated and Disrupted Glioma III and IV (7) Displaced and Disrupted Metastases (7) | |||
| 16 | Leroy et al (2020) [34] | 11 | - | - | - | GBM IV (1) Anaplastic oligodendroglioma III (3) Astrocytoma II (2) | GBM IV (1) | Infiltrated and Disrupted Astrocytoma II (1) GBM IV (2) Anaplastic oligodendroglioma III (1) | |||
| 17 | Shalan et al. (2021) [39] | 44 | LGG (2) | HGG (10), LGG (5) | HGG (7), LGG (4) | HGG (11), LGG (1) | HGG (4) | - | |||
| 18 | Bakhshi et al. (2021) [36] | 77 | Astrocytoma I (1), Metastasis (1) | Astrocytoma I (1), Astrocytoma II (1), Astrocytoma III (2), GBM IV (7), Oligoastrocytoma II (1), Oligoastracytoma III (2), Oligodendroglioma II (2), Oligodendroglioma III (2), Others (4) | - | GBM IV (17), Metastasis (2), Oligoastrocytoma II (1), Oligoastracytoma III (1), Oligodendroglioma II (10), Oligodendroglioma III (9), Others (5) | Astrocytoma I (1), GBM IV (2), Oligoastrocytoma II (1), Oligodendroglioma II (1), Oligodendroglioma III (1), Metastases (1) Others (1) | - | |||
| 19 | Schneider et al. (2021) [35] | 25 (both DEC and TG) | Metastasis (1) DEC Glioma II (1) | Benign (2) Metastasis (3) Glioma II (2) Glioma III (2) TG Glioma IV (3) | - | - | Metastases (3) | Displaced and Edema TG Glioma IV (1) Displaced and Disrupted Metastasis (2) Gliomas IV (7) Displaced and infiltrated Glioma III (1) DEC Glioma IV (1) Infiltrated and Disrupted Glioma III (1) Glioma IV (1) Edema and Disrupted TG Glioma III (1) Edema and Infiltrated DEC Glioma III (1) | |||
| 20 | Camins et al. (2022) [18] | 34 | Pleomorphic xanthoastrocytoma II (1) | Ganglioglioma I (1), Diffuse astrocytoma II (1), Anaplastic astrocytoma III (1), GBM IV (2) | Anaplastic astrocytoma III (2), Anaplastic oligodendroglioma III (1) GBM IV (4) | Edema and Infiltrated Diffuse astrocytoma II (3), Anaplastic astrocytoma III (6), Gliosarcoma IV (1), Anaplastic oligodendroglioma III (4), GBM IV (7) | |||||
| (b) | |||||||||||
| Type of Tumor | Tumor Grade | ||||||||||
| Pattern | Oligodendroglioma | Astrocytoma | Oligo astrocytoma | GBM | Meningioma | Ganglioma | Metastasis | LGG | HGG | ||
| Normal | 3.5% (3/86) | 6% (10/180) | 2% (6/353) | ||||||||
| Displacement | 36% (20/55) | 54% (22/41) | 36% (4/11) | 29% (19/65) | 77% (10/13) | (2/2) 100% | 31% (27/86) | 56% (101/180) | 19% (66/353) | ||
| Edematous | 2% (1/65) | 3.5% (3/86) | 2% (4/180) | 5% (17/353) | |||||||
| Infiltration | 43% (24/55) | 5% (2/41) | 18% (2/11) | 35% (23/65) | 8% (1/13) | 7% (6/86) | 18% (32/180) | 25% (88/353) | |||
| Disruption | 13% (7/55) | 10% (4/41) | 9% (1/11) | 15% (10/65) | 13% (11/86) | 3% (6/180) | 9% (32/353) | ||||
| Non-exclusive | |||||||||||
| Displacement + edema | 2% (1/55) | 2% (1/65) | 12% (10/86) | <1% (1/180) | <1% (1/353) | ||||||
| Displacement + infiltration | 2% (1/55) | 7% (3/41) | 9% (6/65) | 1% (1/86) | 2% (3/180) | 1% (4/353) | |||||
| Displacement + disruption | 10% (4/41) | 36% (4/11) | 3% (2/65) | 15% (2/13) | 13% (11/86) | 1% (2/180) | 5% (16/353) | ||||
| Infiltration + edema | 2% (3/180) | 5% (19/353) | |||||||||
| Infiltration + disruption | 2% (1/55) | 12% (5/41) | 5% (3/65) | 14% (12/86) | 10% (18/180) | 23% (81/353) | |||||
| Disruption + edema | 2% (1/55) | 2% (1/41) | <1% (2/353) | ||||||||
| Others | 2% (2/86) | 6% (21/353) | |||||||||
3.5.2. Evaluation of the Patients in Groups
| Author (Year) [Ref] | Assessment of White Matter Tracts Characterizations and Microstructure Integrity |
|---|---|
| Tumor Type and Grade | |
| Ibrahim et al. (2018) [29] | Prevalence of disruption was higher in malignant compared to benign tumor group. Prevalence of displacement was higher in benign tumors compared to malignant tumors. |
| Deilami et al. (2019) [31] | Infiltration was the major pattern for HGG and LGG. Edematous comprised the minority. |
| Khan et al. (2019) [38] | Sig. correlations of HGG associated with disruption or infiltration, as well as metastasis. LGG mainly with displaced fibers |
| Dubey et al. (2019) [32] | HGG have showed to be more infiltrated or disrupted, meanwhile the LGG and metastasis have more association with displacement. |
| Leroy et al. (2020) [32] | Higher grade glioblastoma has higher proportional of destruction WMT |
| Shalan et al. (2021) [39] | HGG showed higher percentage of infiltration and disruption pattern than LGG |
| Schneider et al. (2021) [35] | Difference of pattern visualize to be compared between DEC and tractography was showed in the 6 of the studies, one case showed no useful in tractography. There is no correlation between pathology of the tumor in the method of visualization of WMT pattern using DEC and TG. |
| Bakhshi et al. (2021) [36] | High grade astrocytoma cause infiltration of WMT, low grade astrocytoma caused displacement, and oligodendroglioma tumor type often infiltrated WMT. The involvement of WMT in oligodendroglioma was not associate with the grade tumor. |
| Tumor location | |
| Yu et al. (2005) [20] | Location of the tumor in vicinity to different type of WMT. Nature of pyramidal tract having longer fiber and larger range, resulting in more displayed of displacement and disruption. Corpus callosum have short fiber and small range of movement, resulting displayed disruption pattern |
| Yu et al. (2019) [33] | Partial interruption was evidence in lesion close to cortex, in the range of 0–5 mm. WMT closest to the proximity to the tumor was found displaced in all patients in the range of 2–21 mm. |
| Camins et al. (2022) [18] | Displacement patterns depended on main tumor location in temporal lobe, and insular involvement. All tumor showed superior displacement pattern. Lateral tumors tend to displace medially, medial tumor to laterally, and insular tumor tend to have medial displacement. |
| DTI parameter (cut-off points) | |
| Yen et al. (2009) [23] | Significant ΔFA (FA changes) between affected hemisphere and control contralateral WMT only in disruption Edematous and disruption ΔFA are sig. less than displacement ΔFA Positive percentage of ΔFA was associated with edematous and displacement, 0% to −30%, is likely associated with displacement or infiltration, and the percentage less that −30%, was associated with WMT disruption |
| Deilami et al. (2015) [31] | ΔFA% was more than −35 for displaced and edematous fiber and less than −35 for the majority of disrupted, but infiltration fibers scattered distribution. Mean ΔFA was the least for disruption, infiltration, edematous, and displaced parts. Disruption the most several presumptive cut-off points. |
| Wende et al. (2021) [40] | FA cut off in infiltrated fiber bundles, a difference of 0.1 value should be considered. Infiltrate CST trigger vigilance and may require lower cut-offs. |
| Author [Ref] | Summarization of the Outcome to the WMT Tract Morphology Alteration |
|---|---|
| Yu et al. [20] | Surgical approach different based on the pattern of WMT. Maximizing the resection in simple displacement, to enlarge the extent of tumor resection while preservation of displaced part in displaced with disruption and maximized tumor while preserved the residual part in disrupted tracts. |
| Bagadia et al. [27] | Pt. with pure displacement had the best postoperative prediction outcome, while those with infiltration had a poorer outcome |
| Castellano et al. [28] | In preoperative tumor volume less than 100 cm3, intact fascicles higher probability of total resection. In preoperative tumor volume more than 100 cm3, infiltration or displaced fascicles predicted partial resection or subtotal. |
| Dubey et al. [32] | Total resection achieved in 61.2% of displacement pattern tract and 31.2 % in infiltration or disruption pattern of the tract. DTI have given crucial information of the infiltration and displacement course. |
| Khan et al. [38] | Respectability of maximum safe resection was higher in pt. with displaced fibers and lower in those with disrupted/infiltrated fibers, statistically sig. Fewer pt. had neurologic deterioration in displaced, compared to disruption or infiltration |
| Leroy et al. [34] | LGG have higher preservation of subcortical fiber tracts. DTI sensitivity and specificity to predict disrupted fiber tracts were, respectively, of 89% and 90%. |
| Schneider et al. [35] | Postoperative improvement of the pattern was seen mainly in the disruption DEC showed more improvement of WMT to be compared with tractography, postoperatively |
| Bakshi et al. [36] | Infiltration tumors have poor functional outcome, low KPS score |
| Shalan et al. [39] | Postoperative evaluation of WMT pattern were improved in displaced and edematous meanwhile not the case for infiltration and disruptive pattern HGG and pattern disrupted have the higher subtotal resection |
| Wende et al. [40] | FA cut-off value of 0.15 for tractography for neurosurgery and shorten the TG workflow. |
| Camins et al. [18] | IFOF displacement pattern are reproducible |
3.6. Preoperative and Clinical Outcome of the WMT Characterization Assessment
4. Discussion
Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Witwer, B.P.; Moftakhar, R.; Hasan, K.M.; Deshmukh, P.; Haughton, V.; Field, A.; Arfanakis, K.; Noyes, J.; Moritz, C.H.; Meyerand, M.E.; et al. Diffusion-tensor imaging of white matter tracts in patients with cerebral neoplasm. J. Neurosurg. 2002, 97, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Jellison, B.J.; Field, A.S.; Medow, J.; Lazar, M.; Salamat, M.S.; Alexander, A.L. Diffusion Tensor Imaging of Cerebral White Matter: A Pictorial Review of Physics, Fiber Tract Anatomy, and Tumor Imaging Patterns. Am. J. Neuroradiol. 2004, 25, 356–369. [Google Scholar] [PubMed]
- Essayed, W.I.; Zhang, F.; Unadkat, P.; Cosgrove, G.R.; Golby, A.J.; O’Donnell, L.J. White matter tractography for neurosurgical planning: A topography-based review of the current state of the art. NeuroImage Clin. 2017, 15, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.M.; Marques, P.; Alves, V.; Sousa, N. A hitchhiker’s guide to diffusion tensor imaging. Front. Neurosci. 2013, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, G.; Cikla, U.; el Tecle, N.; Kulkarni, N.; Pierson, M.; Mercier, P.; Kemp, J.; Coppens, J.; Mahmoud, S.; Sehi, M.; et al. The Value of White Matter Tractography by Diffusion Tensor Imaging in Altering a Neurosurgeon’s Operative Plan. World Neurosurg. 2019, 132, e305–e313. [Google Scholar] [CrossRef]
- Manan, A.A.; Yahya, N.; Idris, Z.; Manan, H.A. The Utilization of Diffusion Tensor Imaging as an Image-Guided Tool in Brain Tumor Resection Surgery: A Systematic Review. Cancers 2022, 14, 2466. [Google Scholar] [CrossRef]
- Lazar, M.; Alexander, A.; Thottakara, P.; Badie, B.; Field, A.; Tractography, W.M. Resection of Brain Tumors and Vascular. Am. J. Neuroradiol. 2006, 27, 1258–1271. [Google Scholar]
- Manan, H.A.; Yahya, N.; Han, P.; Hummel, T. A systematic review of olfactory-related brain structural changes in patients with congenital or acquired anosmia. Brain Struct. Funct. 2021, 227, 177–202. [Google Scholar] [CrossRef]
- Manan, H.A.; Yahya, N. Ageing and Olfactory Dysfunction in Trisomy 21: A Systematic Review. Brain Sci. 2021, 11, 952. [Google Scholar] [CrossRef]
- Manan, H.A.; Franz, E.A.; Yahya, N. The utilisation of resting-state fMRI as a pre-operative mapping tool in patients with brain tumours in comparison to task-based fMRI and intraoperative mapping: A systematic review. Eur. J. Cancer Care 2021, 30, e13428. [Google Scholar] [CrossRef]
- Manan, H.A.; Franz, E.A.; Yahya, N. Functional connectivity changes in patients with brain tumours—A systematic review on resting state-fMRI. Neurol. Psychiatry Brain Res. 2020, 36, 73–82. [Google Scholar] [CrossRef]
- Yahya, N.; Manan, H.A. Neurocognitive impairment following proton therapy for paediatric brain tumour: A systematic review of post-therapy assessments. Support. Care Cancer 2020, 29, 3035–3047. [Google Scholar] [CrossRef]
- Yahya, N.; Manan, H.A. Diffusion tensor imaging indices to predict cognitive changes following adult radiotherapy. Eur. J. Cancer Care 2020, 30, e13329. [Google Scholar] [CrossRef]
- Yahya, N.; Manan, H.A. Utilisation of Diffusion Tensor Imaging in Intracranial Radiotherapy and Radiosurgery Planning for White Matter Dose Optimization: A Systematic Review. World Neurosurg. 2019, 130, e188–e198. [Google Scholar] [CrossRef]
- Wei, C.W.; Guo, G.; Mikulis, D.J. Tumor effects on cerebral white matter as characterized by diffusion tensor tractography. Can. J. Neurol. Sci. 2007, 34, 62–68. [Google Scholar] [CrossRef]
- Laundre, B.J.; Jellison, B.J.; Badie, B.; Alexander, A.L.; Field, A.S. Diffusion Tensor Imaging of the Corticospinal Tract before and after Mass Resection as Correlated with Clinical Motor Findings: Preliminary Data. Am. J. Neuroradiol. 2005, 26, 791–796. [Google Scholar]
- Zhukov, V.Y.; Goryaynov, S.A.; Ogurtsova, A.A.; Ageev, I.S.; Protskiy, S.V.; Pronin, I.N.; Tonoyan, A.S.; Kobyakov, G.L.; Nenashev, E.A.; Smirnov, A.S.; et al. Diffusion tensor imaging tractography and intraoperative neurophysiological monitoring in surgery of intracranial tumors located near the pyramidal tract. Vopr. Neirokhirurgii Im. N.N. Burdenko 2016, 80, 15–18. [Google Scholar] [CrossRef]
- Camins, À.; Naval-Baudin, P.; Majós, C.; Sierpowska, J.; Sanmillan, J.L.; Cos, M.; Rodriguez-Fornells, A.; Gabarrós, A. Inferior fronto-occipital fascicle displacement in temporoinsular gliomas using diffusion tensor imaging. J. Neuroimaging 2022, 32, 638–646. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Yu, C.S.; Li, K.C.; Xuan, Y.; Ji, X.M.; Qin, W. Diffusion tensor tractography in patients with cerebral tumors: A helpful technique for neurosurgical planning and postoperative assessment. Eur. J. Radiol. 2005, 56, 197–204. [Google Scholar] [CrossRef]
- Field, A.S.; Alexander, A.L.; Wu, Y.-C.; Hasan, K.M.; Witwer, B.; Badie, B. Diffusion tensor eigenvector directional color imaging patterns in the evaluation of cerebral white matter tracts altered by tumor. J. Magn. Reson. Imaging 2004, 20, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Weigel, D.; Ganslandt, O.; Buchfelder, M.; Nimsky, C. Diffusion tensor imaging and white matter tractography in patients with brainstem lesions. Acta Neurochir. 2007, 149, 1117–1131. [Google Scholar] [CrossRef] [PubMed]
- Yen, P.S.; Teo, B.T.; Chiu, C.H.; Chen, S.C.; Chiu, T.L.; Su, C.F. White matter tract involvement in brain tumors: A diffusion tensor imaging analysis. Surg. Neurol. 2009, 72, 464–469. [Google Scholar] [CrossRef]
- Nievas, M.N.C.Y.; Hoellerhage, H.-G.; Drahten, C. White matter tract alterations assessed with diffusion tensor imaging and tractography in patients with solid posterior fossa tumors. Neurol. India 2010, 58, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Itagiba, V.G.A.; Borges, R.; da Cruz, L.C.H.; Furtado, A.D.; Domingues, R.C.; Gasparetto, E.L. Use of diffusion tensor magnetic resonance imaging in the assessment of patterns of white matter involvement in patients with brain tumors: Is it useful in the differential diagnosis? Radiol. Bras. 2010, 43, 362–368. [Google Scholar] [CrossRef]
- Kovanlikaya, I.; Firat, Z.; Kovanlikaya, A.; Uluğ, A.M.; Cihangiroglu, M.M.; John, M.; Bingol, C.A.; Ture, U. Assessment of the corticospinal tract alterations before and after resection of brainstem lesions using Diffusion Tensor Imaging (DTI) and tractography at 3T. Eur. J. Radiol. 2011, 77, 383–391. [Google Scholar] [CrossRef]
- Bagadia, A.; Purandare, H.; Misra, B.K.; Gupta, S. Application of magnetic resonance tractography in the perioperative planning of patients with eloquent region intra-axial brain lesions. J. Clin. Neurosci. 2011, 18, 633–639. [Google Scholar] [CrossRef]
- Castellano, A.; Bello, L.; Michelozzi, C.; Gallucci, M.; Fava, E.; Iadanza, A.; Riva, M.; Casaceli, G.; Falini, A. Role of diffusion tensor magnetic resonance tractography in predicting the extent of resection in glioma surgery. Neuro-Oncology 2012, 14, 192–202. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Gomaa, M.; Sakr, H.; Elzaher, Y.A. Role of diffusion tensor imaging in characterization and pre-operative planning of brain neoplasms. Egypt. J. Radiol. Nucl. Med. 2013, 44, 297–307. [Google Scholar] [CrossRef]
- Farshidfar, Z.; Faeghi, F.; Mohseni, M.; Seddighi, A.; Kharrazi, H.H.; Abdolmohammadi, J. Diffusion Tensor Tractography in the Presurgical Assessment of Cerebral Gliomas. Neuroradiol. J. 2014, 27, 75–84. [Google Scholar] [CrossRef]
- Deilami, T.; Kharrazi, H.H.; Seddighi, A.S.; Tanzifi, P.; Tayebivaljouzi, R.; Zamani, F.; Tafti, A.C. Evaluating the Possibility of Defining Cut-Off Points for ΔFA% in Order to Differentiate Four Major Types of Peri-Tumoral White Matter Tract Involvement. Iran. J. Radiol. 2015, 12, e9567. [Google Scholar] [CrossRef]
- Dubey, A.; Kataria, R.; Sinha, V.D. Role of diffusion tensor imaging in brain tumor surgery. Asian J. Neurosurg. 2018, 13, 302–306. [Google Scholar] [CrossRef]
- Yu, Q.; Lin, K.; Liu, Y.; Li, X. Clinical Uses of Diffusion Tensor Imaging Fiber Tracking Merged Neuronavigation with Lesions Adjacent to Corticospinal Tract: A Retrospective Cohort Study. J. Korean Neurosurg. Soc. 2020, 63, 248–260. [Google Scholar] [CrossRef]
- Leroy, H.-A.; Lacoste, M.; Maurage, C.-A.; Derré, B.; Baroncini, M.; Reyns, N.; Delmaire, C. Anatomo-radiological correlation between diffusion tensor imaging and histologic analyses of glial tumors: A preliminary study. Acta Neurochir. 2020, 162, 1663–1672. [Google Scholar] [CrossRef]
- Schneider, J.R.; Raval, A.B.; Black, K.; Schulder, M. Diffusion Tensor Imaging Color-Coded Maps: An Alternative to Tractography. Ster. Funct. Neurosurg. 2021, 99, 295–304. [Google Scholar] [CrossRef]
- Bakhshi, S.K.; Quddusi, A.; Mahmood, S.D.; Waqas, M.; Shamim, M.S.; Mubarak, F.; Enam, S.A. Diagnostic Implications of White Matter Tract Involvement by Intra-axial Brain Tumors. Cureus 2021, 13, e19355. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, S.; Wen, G.; Zhang, X. The Disruption of Geniculocalcarine Tract in Occipital Neoplasm: A Diffusion Tensor Imaging Study. Radiol. Res. Pract. 2016, 2016, 8213076. [Google Scholar] [CrossRef]
- Alam Khan, K.; Jain, S.K.; Sinha, V.D.; Sinha, J. Preoperative Diffusion Tensor Imaging: A Landmark Modality for Predicting the Outcome and Characterization of Supratentorial Intra-Axial Brain Tumors. World Neurosurg. 2019, 124, e540–e551. [Google Scholar] [CrossRef]
- Shalan, M.E.; Soliman, A.Y.; Nassar, I.A.; Alarabawy, R.A. Surgical planning in patients with brain glioma using diffusion tensor MR imaging and tractography. Egypt. J. Radiol. Nucl. Med. 2021, 52, 110. [Google Scholar] [CrossRef]
- Wende, T.; Kasper, J.; Wilhelmy, F.; Dietel, E.; Hamerla, G.; Scherlach, C.; Meixensberger, J.; Fehrenbach, M.K. Assessment of a Reliable Fractional Anisotropy Cutoff in Tractography of the Corticospinal Tract for Neurosurgical Patients. Brain Sci. 2021, 11, 650. [Google Scholar] [CrossRef]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007, 114, 547. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Costabile, J.D.; Alaswad, E.; D’Souza, S.; Thompson, J.A.; Ormond, D.R. Current Applications of Diffusion Tensor Imaging and Tractography in Intracranial Tumor Resection. Front. Oncol. 2019, 9, 426. [Google Scholar] [CrossRef] [PubMed]
- Faust, K.; Vajkoczy, P. Distinct displacements of the optic radiation based on tumor location revealed using pre-operative diffusion tensor imaging. J. Neurosurg. 2016, 124, 1343–1352. [Google Scholar] [CrossRef]
- Yao, Y.; Ulrich, N.H.; Guggenberger, R.; Alzarhani, Y.A.; Bertalanffy, H.; Kollias, S.S. Quantification of Corticospinal Tracts with Diffusion Tensor Imaging in Brainstem Surgery: Prognostic Value in 14 Consecutive Cases at 3T Magnetic Resonance Imaging. World Neurosurg. 2015, 83, 1006–1014. [Google Scholar] [CrossRef]
- White, M.; Zhang, Y.; Yu, F.; Kazmi, S. Diffusion Tensor MR Imaging of Cerebral Gliomas: Evaluating Fractional Anisotropy Characteristics. Am. J. Neuroradiol. 2011, 32, 374–381. [Google Scholar] [CrossRef]
- Jiang, R.; Du, F.-Z.; He, C.; Gu, M.; Ke, Z.-W.; Li, J.-H. The Value of Diffusion Tensor Imaging in Differentiating High-Grade Gliomas from Brain Metastases: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e112550. [Google Scholar] [CrossRef]
- Suh, C.; Kim, H.; Jung, S.; Kim, S. Diffusion-Weighted Imaging and Diffusion Tensor Imaging for Differentiating High-Grade Glioma from Solitary Brain Metastasis: A Systematic Review and Meta-Analysis. Am. J. Neuroradiol. 2018, 39, 1208–1214. [Google Scholar] [CrossRef]
- Zakaria, R.; Das, K.; Bhojak, M.; Radon, M.; Walker, C.; Jenkinson, M.D. The role of magnetic resonance imaging in the management of brain metastases: Diagnosis to prognosis. Cancer Imaging 2014, 14, 8. [Google Scholar]
- De Belder, F.E.; Oot, A.R.; Van Hecke, W.; Venstermans, C.; Menovsky, T.; Van Marck, V.; Van Goethem, J.; Hauwe, L.V.D.; Vandekerckhove, M.; Parizel, P.M. Diffusion Tensor Imaging Provides an Insight Into the Microstructure of Meningiomas, High-Grade Gliomas, and Peritumoral Edema. J. Comput. Assist. Tomogr. 2012, 36, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, W. Quantitative evaluation of diffusion tensor imaging for clinical management of glioma. Neurosurg. Rev. 2020, 43, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Celtikci, P.; Fernandes-Cabral, D.T.; Yeh, F.-C.; Panesar, S.S.; Fernandez-Miranda, J.C. Generalized q-sampling imaging fiber tractography reveals displacement and infiltration of fiber tracts in low-grade gliomas. Neuroradiology 2018, 60, 267–280. [Google Scholar] [CrossRef]
- Drummond, K.J. Oligodendroglioma. In Brain Tumors; Elsevier: Amsterdam, The Netherlands, 2012; pp. 408–423. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780443069673000223 (accessed on 12 July 2019).
- Sarkar, A.; Chiocca, E.A. Glioblastoma and Malignant Astrocytoma. In Brain Tumors; Elsevier: Amsterdam, The Netherlands, 2012; pp. 384–407. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780443069673000211 (accessed on 12 July 2019).
- Kim, M.S.; Chung, C.K.; Jung, H.-W.; Park, C.-K.; Kim, C.H.; Kim, J.S. Preoperative Weakness and Demyelination of the Corticospinal Tract in Meningioma Patients: Changes in Diffusion Parameters Using Diffusion Tensor Imaging. J. Korean Neurosurg. Soc. 2014, 55, 267–272. [Google Scholar] [CrossRef]
- Schonberg, T.; Pianka, P.; Hendler, T.; Pasternak, O.; Assaf, Y. Characterization of displaced white matter by brain tumors using combined DTI and fMRI. Neuroimage 2006, 30, 1100–1111. [Google Scholar] [CrossRef]
- Goebell, E.; Fiehler, J.; Ding, X.-Q.; Paustenbach, S.; Nietz, S.; Heese, O.; Kucinski, T.; Hagel, C.; Westphal, M.; Zeumer, H. Disarrangement of Fiber Tracts and Decline of Neuronal Density Correlate in Glioma Patients—A Combined Diffusion Tensor Imaging and 1H-MR Spectroscopy Study. Am. J. Neuroradiol. 2006, 27, 1426–1431. [Google Scholar]
- Abdalla, G.; Dixon, L.; Sanverdi, E.; Machado, P.; Kwong, J.S.W.; Panovska-Griffiths, J.; Rojas-Garcia, A.; Yoneoka, D.; Veraart, J.; Van Cauter, S.; et al. The diagnostic role of diffusional kurtosis imaging in glioma grading and differentiation of gliomas from other intra-axial brain tumours: A systematic review with critical appraisal and meta-analysis. Neuroradiology 2020, 62, 791–802. [Google Scholar] [CrossRef]
| PICOS | Criteria |
|---|---|
| Patient/Population | Adult Brain tumor |
| Intervention | Undergone diffusion tensor imaging |
| Comparison | Different Factors: Tumor types, tumor grades, DTI indices and parameter measurement |
| Outcome | Characterization on the morphological changes in the pattern of the involved white matter tracts integrity in relation of the tumor |
| Study Design | Original clinical published article |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manan, A.A.; Yahya, N.A.; Taib, N.H.M.; Idris, Z.; Manan, H.A. The Assessment of White Matter Integrity Alteration Pattern in Patients with Brain Tumor Utilizing Diffusion Tensor Imaging: A Systematic Review. Cancers 2023, 15, 3326. https://doi.org/10.3390/cancers15133326
Manan AA, Yahya NA, Taib NHM, Idris Z, Manan HA. The Assessment of White Matter Integrity Alteration Pattern in Patients with Brain Tumor Utilizing Diffusion Tensor Imaging: A Systematic Review. Cancers. 2023; 15(13):3326. https://doi.org/10.3390/cancers15133326
Chicago/Turabian StyleManan, Aiman Abdul, Noorazrul Azmie Yahya, Nur Hartini Mohd Taib, Zamzuri Idris, and Hanani Abdul Manan. 2023. "The Assessment of White Matter Integrity Alteration Pattern in Patients with Brain Tumor Utilizing Diffusion Tensor Imaging: A Systematic Review" Cancers 15, no. 13: 3326. https://doi.org/10.3390/cancers15133326
APA StyleManan, A. A., Yahya, N. A., Taib, N. H. M., Idris, Z., & Manan, H. A. (2023). The Assessment of White Matter Integrity Alteration Pattern in Patients with Brain Tumor Utilizing Diffusion Tensor Imaging: A Systematic Review. Cancers, 15(13), 3326. https://doi.org/10.3390/cancers15133326






