Sustaining the Activation of EGFR Signal by Inflammatory Cytokine IL17A Prompts Cell Proliferation and EGFR-TKI Resistance in Lung Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection from Bioinformatics Analyses
2.2. Cell Lines and Cell Culture
2.3. Reagents, Chemical Inhibitors, and Antibodies
2.4. DNA Transient Transfection
2.5. Western Blot Assay
2.6. Dot Blot Assay
2.7. Cell Proliferation Assay
2.8. Lentiviral Infection
2.9. Human Phospho-Receptor Tyrosine Kinase (RTK) and Phospho-Kinase Arrays
2.10. Flow Cytometric Analysis
2.11. IF and Confocal Microscopic Analysis
2.12. Statistical Analysis
3. Results
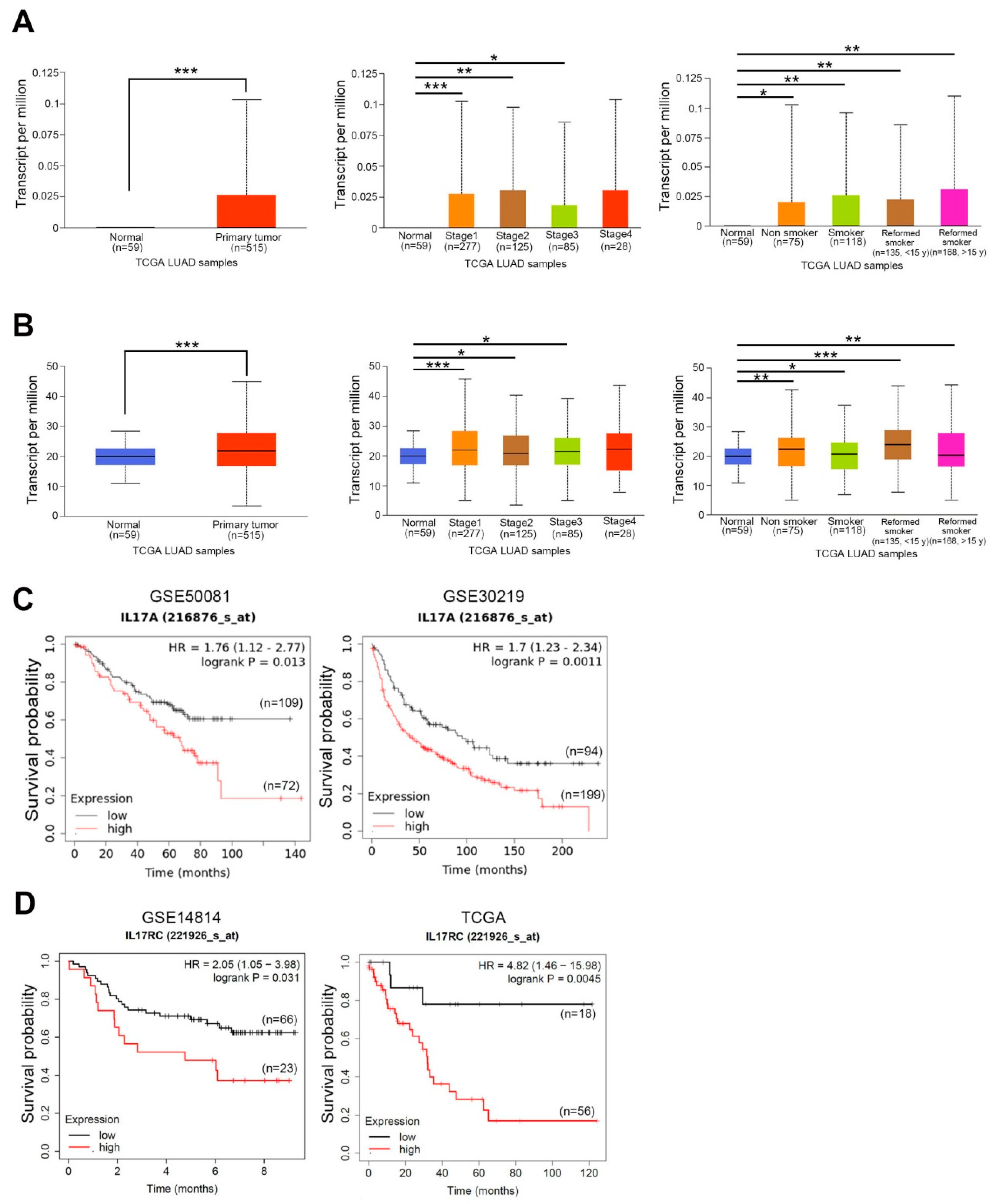
3.1. Expression Levels of IL-17A and IL-17RC and Their Prognostic Potential in NSCLC
3.2. IL-17A Induces Cell Proliferation of NSCLC Cells with Wild-Type (WT) or Mutant EGFR
3.3. IL-17A Enhances EGFR and MET Activation and Confers In Vitro Resistance to EGFR-Targeted Therapies in NSCLC Cells with Mutant EGFR
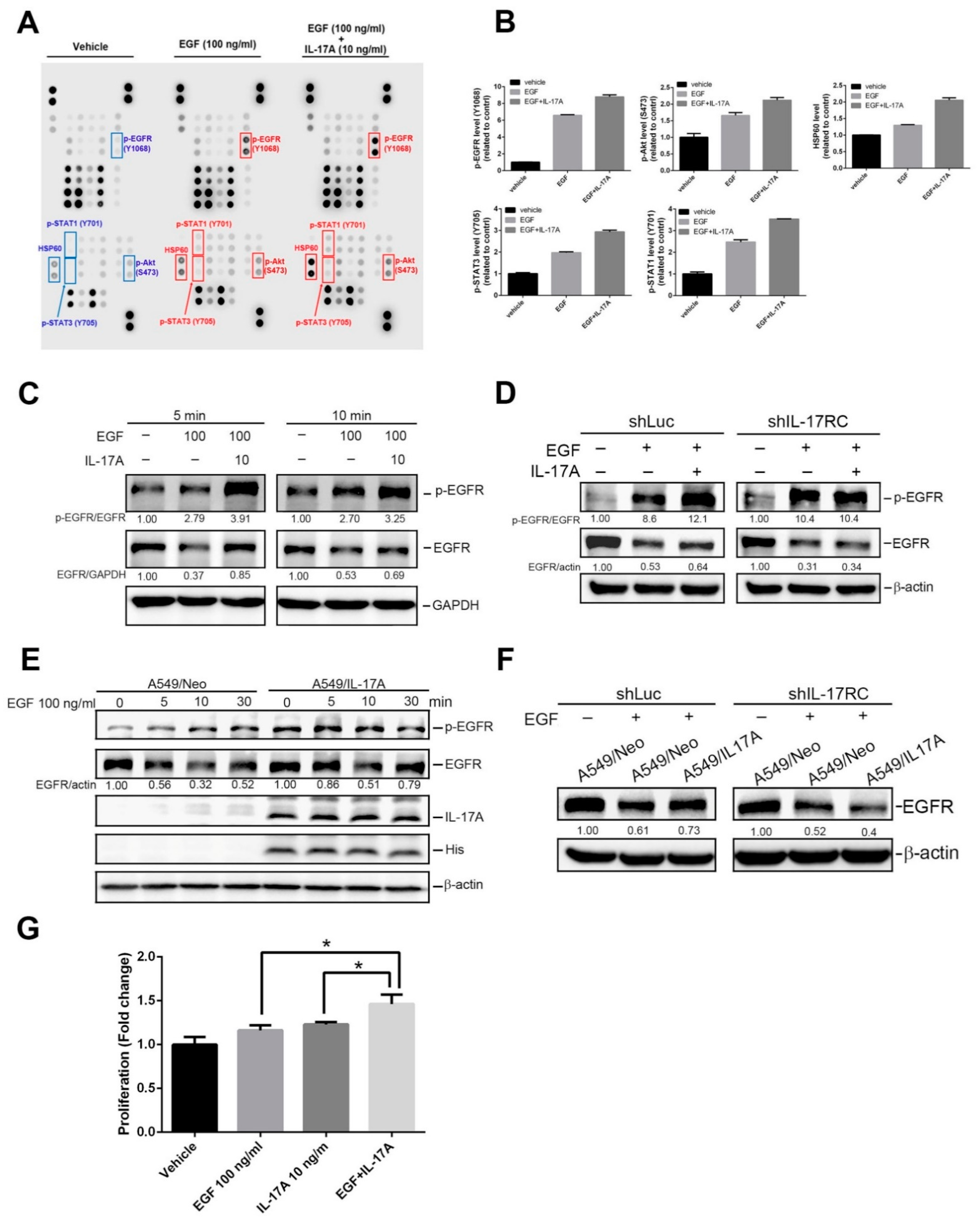
3.4. IL-17A Enhances EGF-Induced EGFR Activation and Cell Proliferation through Causing Prevention of EGF-Mediated EGFR Degradation
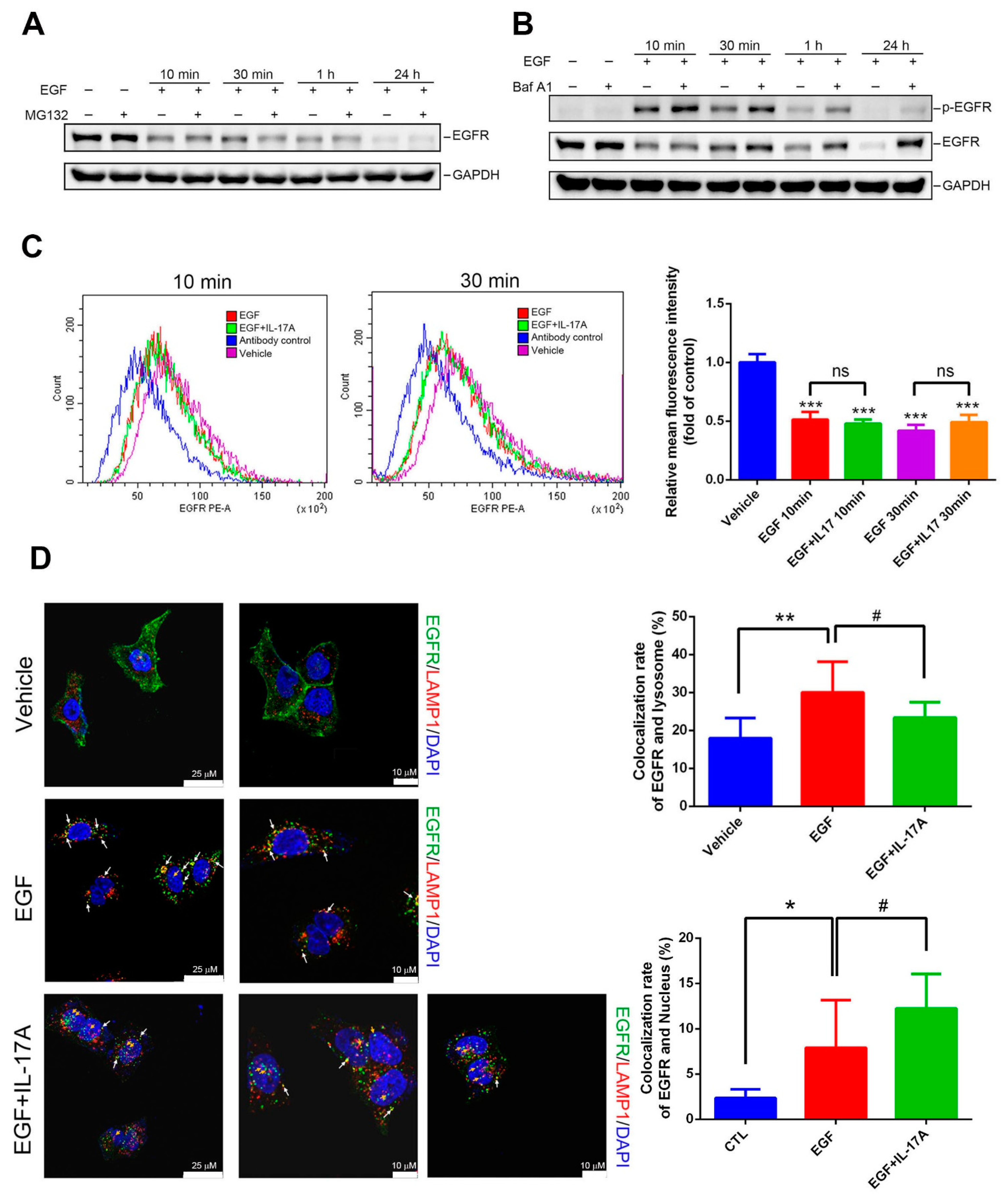
3.5. IL-17A Prevents EGF-Induced EGFR Downregulation through Causing Impairment of the Lysosomal Degradation of EGFR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taniguchi, K.; Karin, M. NF-κB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [PubMed]
- Zhao, J.; Chen, X.; Herjan, T.; Li, X. The role of interleukin-17 in tumor development and progression. J. Exp. Med. 2020, 217, e20190297. [Google Scholar] [PubMed]
- Vitiello, G.A.; Miller, G. Targeting the interleukin-17 immune axis for cancer immunotherapy. J. Exp. Med. 2020, 217, e20190456. [Google Scholar]
- Al Omar, S.; Flanagan, B.F.; Almehmadi, M.; Christmas, S.E. The effects of IL-17 upon human natural killer cells. Cytokine 2013, 62, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Yu, J. The role and metabolic adaptations of neutrophils in premetastatic niches. Biomark. Res. 2023, 11, 50. [Google Scholar] [PubMed]
- Li, X.; Bechara, R.; Zhao, J.; McGeachy, M.J.; Gaffen, S.L. IL-17 receptor-based signaling and implications for disease. Nat. Immunol. 2019, 20, 1594–1602. [Google Scholar] [PubMed]
- Miossec, P.; Kolls, J.K. Targeting IL-17 and TH17 cells in chronic inflammation. Nat. Rev. Drug Discov. 2012, 11, 763–776. [Google Scholar]
- Cochaud, S.; Giustiniani, J.; Thomas, C.; Laprevotte, E.; Garbar, C.; Savoye, A.M.; Curé, H.; Mascaux, C.; Alberici, G.; Bonnefoy, N.; et al. IL-17A is produced by breast cancer TILs and promotes chemoresistance and proliferation through ERK1/2. Sci. Rep. 2013, 3, 3456. [Google Scholar]
- Chen, J.; Ye, X.; Pitmon, E.; Lu, M.; Wan, J.; Jellison, E.R.; Adler, A.J.; Vella, A.T.; Wang, K. IL-17 inhibits CXCL9/10-mediated recruitment of CD8+ cytotoxic T cells and regulatory T cells to colorectal tumors. J. Immunother. Cancer 2019, 7, 324. [Google Scholar]
- Wu, X.; Yang, T.; Liu, X.; Guo, J.N.; Xie, T.; Ding, Y.; Lin, M.; Yang, H. IL-17 promotes tumor angiogenesis through Stat3 pathway mediated upregulation of VEGF in gastric cancer. Tumor Biol. 2016, 37, 5493–5501. [Google Scholar] [CrossRef]
- Liu, J.; Duan, Y.; Cheng, X.; Chen, X.; Xie, W.; Long, H.; Lin, Z.; Zhu, B. IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochem. Biophys. Res. Commun. 2011, 407, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ma, Y.; Wang, X.; Jiang, J.; Zhang, C.; Wang, X.; Jiang, Y.; Huang, H.; Hong, L. IL-17A Increases Multiple Myeloma Cell Viability by Positively Regulating Syk Expression. Transl. Oncol. 2019, 12, 1086–1091. [Google Scholar] [CrossRef]
- Gomes, M.; Teixeira, A.L.; Coelho, A.; Araújo, A.; Medeiros, R. The role of inflammation in lung cancer. Adv. Exp. Med. Biol. 2014, 816, 1–23. [Google Scholar]
- Youlden, D.R.; Cramb, S.M.; Baade, P.D. The International Epidemiology of Lung Cancer: Geographical distribution and secular trends. J. Thorac. Oncol. 2008, 3, 819–831. [Google Scholar] [PubMed]
- Miller, V.A.; Hirsh, V.; Cadranel, J.; Chen, Y.M.; Park, K.; Kim, S.W.; Zhou, C.; Su, W.C.; Wang, M.; Sun, Y.; et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): A phase 2b/3 randomised trial. Lancet Oncol. 2012, 13, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.T.; Yeh, K.C.; Lee, C.H.; Chen, Y.Y.; Ho, P.J.; Chang, K.Y.; Chen, C.H.; Lai, Y.K.; Chen, C.T. Molecular profiling of afatinib-resistant non-small cell lung cancer cells in vivo derived from mice. Pharmacol. Res. 2020, 161, 105183. [Google Scholar]
- Nukaga, S.; Yasuda, H.; Tsuchihara, K.; Hamamoto, J.; Masuzawa, K.; Kawada, I.; Naoki, K.; Matsumoto, S.; Mimaki, S.; Ikemura, S.; et al. Amplification of EGFR Wild-Type Alleles in Non-Small Cell Lung Cancer Cells Confers Acquired Resistance to Mutation-Selective EGFR Tyrosine Kinase Inhibitors. Cancer Res. 2017, 77, 2078–2089. [Google Scholar]
- Chen, X.; Cai, G.; Liu, C.; Zhao, J.; Gu, C.; Wu, L.; Hamilton, T.A.; Zhang, C.J.; Ko, J.; Zhu, L.; et al. IL-17R-EGFR axis links wound healing to tumorigenesis in Lrig1+ stem cells. J. Exp. Med. 2019, 216, 195–214. [Google Scholar]
- Merrouche, Y.; Fabre, J.; Cure, H.; Garbar, C.; Fuselier, C.; Bastid, J.; Antonicelli, F.; Al-Daccak, R.; Bensussan, A.; Giustiniani, J. IL-17E synergizes with EGF and confers in vitro resistance to EGFR-targeted therapies in TNBC cells. Oncotarget 2016, 7, 53350–53361. [Google Scholar] [CrossRef]
- Wang, R.; Yang, L.; Zhang, C.; Wang, R.; Zhang, Z.; He, Q.; Chen, X.; Zhang, B.; Qin, Z.; Wang, L.; et al. Th17 cell-derived IL-17A promoted tumor progression via STAT3/NF-κB/Notch1 signaling in non-small cell lung cancer. Oncoimmunology 2018, 7, e1461303. [Google Scholar] [CrossRef]
- Wu, Z.; He, D.; Zhao, S.; Wang, H. IL-17A/IL-17RA promotes invasion and activates MMP-2 and MMP-9 expression via p38 MAPK signaling pathway in non-small cell lung cancer. Mol. Cell Biochem. 2019, 455, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Ke, X.; Shang, W.; Liu, S.; Fu, X.; Wang, T.; Jin, S. Distribution and Clinical Significance of IL-17A in Tumor-Infiltrating Lymphocytes of Non-Small Cell Lung Cancer Patients. Pathol. Oncol. Res. 2022, 28, 1610384. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wan, J.; Liu, J.; Xie, W.; Diao, X.; Xu, J.; Zhu, B.; Chen, Z. Increased IL-17-producing cells correlate with poor survival and lymphangiogenesis in NSCLC patients. Lung Cancer 2010, 69, 348–354. [Google Scholar]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.; Varambally, S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [PubMed]
- Chien, M.H.; Lee, W.J.; Yang, Y.C.; Tan, P.; Pan, K.F.; Liu, Y.C.; Tsai, H.C.; Hsu, C.H.; Wen, Y.C.; Hsiao, M.; et al. N-α-acetyltransferase 10 protein promotes metastasis by stabilizing matrix metalloproteinase-2 protein in human osteosarcomas. Cancer Lett. 2018, 433, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.Y.; Lee, W.J.; Cheng, G.Z.; Tsai, C.H.; Yang, Y.C.; Lai, T.C.; Chen, J.Q.; Chung, C.L.; Chang, J.H.; Chien, M.H. Blocking MMP-12-modulated epithelial-mesenchymal transition by repurposing penfluridol restrains lung adenocarcinoma metastasis via uPA/uPAR/TGF-β/Akt pathway. Cell. Oncol. 2021, 44, 1087–1103. [Google Scholar]
- Sholl, L.M.; Aisner, D.L.; Varella-Garcia, M.; Berry, L.D.; Dias-Santagata, D.; Wistuba, I.I.; Chen, H.; Fujimoto, J.; Kugler, K.; Franklin, W.A.; et al. Multi-institutional Oncogenic Driver Mutation Analysis in Lung Adenocarcinoma: The Lung Cancer Mutation Consortium Experience. J. Thorac. Oncol. 2015, 10, 768–777. [Google Scholar]
- Sebastian, S.; Settleman, J.; Reshkin, S.J.; Azzariti, A.; Bellizzi, A.; Paradiso, A. The complexity of targeting EGFR signalling in cancer: From expression to turnover. Biochim. Biophys. Acta 2006, 1766, 120–139. [Google Scholar]
- van der Wekken, A.J.; Saber, A.; Hiltermann, T.J.; Kok, K.; van den Berg, A.; Groen, H.J. Resistance mechanisms after tyrosine kinase inhibitors afatinib and crizotinib in non-small cell lung cancer, a review of the literature. Crit. Rev. Oncol. Hematol. 2016, 100, 107–116. [Google Scholar]
- Arteaga, C.L.; Engelman, J.A. ERBB receptors: From oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell 2014, 25, 282–303. [Google Scholar]
- Caldieri, G.; Malabarba, M.G.; Di Fiore, P.P.; Sigismund, S. EGFR Trafficking in Physiology and Cancer. Prog. Mol. Subcell. Biol. 2018, 57, 235–272. [Google Scholar] [PubMed]
- Mimnaugh, E.G.; Chavany, C.; Neckers, L. Polyubiquitination and proteasomal degradation of the p185c-erbB-2 receptor protein-tyrosine kinase induced by geldanamycin. J. Biol. Chem. 1996, 271, 22796–22801. [Google Scholar] [CrossRef] [PubMed]
- Alwan, H.A.; van Zoelen, E.J.; van Leeuwen, J.E. Ligand-induced lysosomal epidermal growth factor receptor (EGFR) degradation is preceded by proteasome-dependent EGFR de-ubiquitination. J. Biol. Chem. 2003, 278, 35781–35790. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Dai, Q.; Wei, J.; Shao, G.; Sun, A.; Yang, W.; Lin, Q. Stress-induced endocytosis and degradation of epidermal growth factor receptor are two independent processes. Cancer Cell Int. 2016, 16, 25. [Google Scholar] [CrossRef]
- Xu, C.; Hao, K.; Yu, L.; Zhang, X. Serum interleukin-17 as a diagnostic and prognostic marker for non-small cell lung cancer. Biomarkers 2014, 19, 287–290. [Google Scholar] [CrossRef]
- Pan, B.; Shen, J.; Cao, J.; Zhou, Y.; Shang, L.; Jin, S.; Cao, S.; Che, D.; Liu, F.; Yu, Y. Interleukin-17 promotes angiogenesis by stimulating VEGF production of cancer cells via the STAT3/GIV signaling pathway in non-small-cell lung cancer. Sci. Rep. 2015, 5, 16053. [Google Scholar] [CrossRef]
- Yang, B.; Kang, H.; Fung, A.; Zhao, H.; Wang, T.; Ma, D. The role of interleukin 17 in tumour proliferation, angiogenesis, and metastasis. Mediat. Inflamm. 2014, 2014, 623759. [Google Scholar] [CrossRef]
- Prabhala, R.H.; Pelluru, D.; Fulciniti, M.; Prabhala, H.K.; Nanjappa, P.; Song, W.; Pai, C.; Amin, S.; Tai, Y.T.; Richardson, P.G.; et al. Elevated IL-17 produced by TH17 cells promotes myeloma cell growth and inhibits immune function in multiple myeloma. Blood 2010, 115, 5385–5392. [Google Scholar] [CrossRef]
- Hassanein, S.S.; Ibrahim, S.A.; Abdel-Mawgood, A.L. Cell Behavior of Non-Small Cell Lung Cancer Is at EGFR and MicroRNAs Hands. Int. J. Mol. Sci. 2021, 22, 12496. [Google Scholar] [CrossRef]
- Wu, L.; Chen, X.; Zhao, J.; Martin, B.; Zepp, J.A.; Ko, J.S.; Gu, C.; Cai, G.; Ouyang, W.; Sen, G.; et al. A novel IL-17 signaling pathway controlling keratinocyte proliferation and tumorigenesis via the TRAF4-ERK5 axis. J. Exp. Med. 2015, 212, 1571–1587. [Google Scholar] [CrossRef]
- Wu, W.; Graves, L.M.; Gill, G.N.; Parsons, S.J.; Samet, J.M. Src-dependent phosphorylation of the epidermal growth factor receptor on tyrosine 845 is required for zinc-induced Ras activation. J. Biol. Chem. 2002, 277, 24252–24257. [Google Scholar] [CrossRef] [PubMed]
- Ohara, S.; Suda, K.; Fujino, T.; Hamada, A.; Koga, T.; Nishino, M.; Chiba, M.; Shimoji, M.; Takemoto, T.; Soh, J.; et al. Dose-dependence in acquisition of drug tolerant phenotype and high RYK expression as a mechanism of osimertinib tolerance in lung cancer. Lung Cancer 2021, 154, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Tomas, A.; Futter, C.E.; Eden, E.R. EGF receptor trafficking: Consequences for signaling and cancer. Trends Cell Biol. 2014, 24, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Brand, T.M.; Iida, M.; Li, C.; Wheeler, D.L. The nuclear epidermal growth factor receptor signaling network and its role in cancer. Discov. Med. 2011, 12, 419–432. [Google Scholar] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.-L.; Lai, T.-C.; Lee, W.-J.; Chen, Y.-C.; Ho, K.-H.; Hung, W.-Y.; Yang, Y.-C.; Chan, M.-H.; Hsieh, F.-K.; Chung, C.-L.; et al. Sustaining the Activation of EGFR Signal by Inflammatory Cytokine IL17A Prompts Cell Proliferation and EGFR-TKI Resistance in Lung Cancer. Cancers 2023, 15, 3288. https://doi.org/10.3390/cancers15133288
Lee K-L, Lai T-C, Lee W-J, Chen Y-C, Ho K-H, Hung W-Y, Yang Y-C, Chan M-H, Hsieh F-K, Chung C-L, et al. Sustaining the Activation of EGFR Signal by Inflammatory Cytokine IL17A Prompts Cell Proliferation and EGFR-TKI Resistance in Lung Cancer. Cancers. 2023; 15(13):3288. https://doi.org/10.3390/cancers15133288
Chicago/Turabian StyleLee, Kai-Ling, Tsung-Ching Lai, Wei-Jiunn Lee, Yu-Chieh Chen, Kuo-Hao Ho, Wen-Yueh Hung, Yi-Chieh Yang, Ming-Hsien Chan, Feng-Koo Hsieh, Chi-Li Chung, and et al. 2023. "Sustaining the Activation of EGFR Signal by Inflammatory Cytokine IL17A Prompts Cell Proliferation and EGFR-TKI Resistance in Lung Cancer" Cancers 15, no. 13: 3288. https://doi.org/10.3390/cancers15133288
APA StyleLee, K.-L., Lai, T.-C., Lee, W.-J., Chen, Y.-C., Ho, K.-H., Hung, W.-Y., Yang, Y.-C., Chan, M.-H., Hsieh, F.-K., Chung, C.-L., Chang, J.-H., & Chien, M.-H. (2023). Sustaining the Activation of EGFR Signal by Inflammatory Cytokine IL17A Prompts Cell Proliferation and EGFR-TKI Resistance in Lung Cancer. Cancers, 15(13), 3288. https://doi.org/10.3390/cancers15133288




