New Insights in the Era of Clinical Biomarkers as Potential Predictors of Systemic Therapy-Induced Cardiotoxicity in Women with Breast Cancer: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategies
2.2. Study Population
2.3. Selection Criteria
2.4. Data Extraction
2.5. Data Synthesis
3. Results
3.1. Cancer Therapy and Cardiotoxicity
3.1.1. Chemotherapy
3.1.2. Targeted Therapy
3.2. Traditional Biomarkers
3.2.1. Troponins
3.2.2. Natriuretic Peptides (NPs)
3.2.3. Left Ventricular Ejection Fraction (LVEF) and Strain Changes
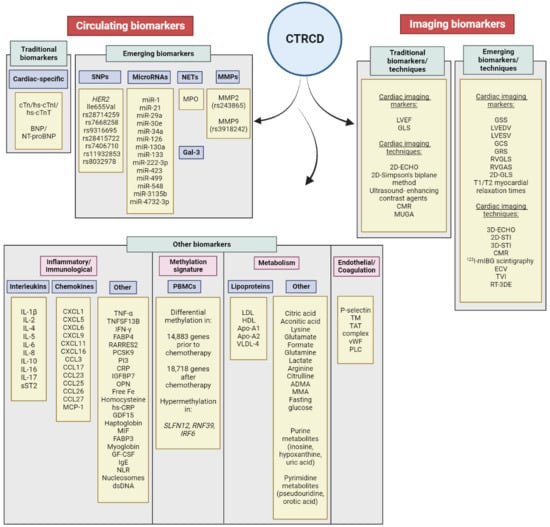
3.3. Emerging Biomarkers
3.3.1. Genetic Susceptibility to CTRCD
SNPs in HER2/neu
Other SNPs
3.3.2. MicroRNAs
3.3.3. Myeloperoxidase (MPO)
3.3.4. Galectin-3 (Gal-3)
3.3.5. Matrix Metalloproteinases (MMPs)
3.4. Other Biomarkers
3.4.1. Inflammatory Biomarkers
3.4.2. Neutrophil-to-lymphocyte Ratio (NLR)
3.4.3. Metabolism
| Biomarker | Subjects | Treatment | Time Points | Clinical Outcome | Definition of Cardiotoxicity | References |
|---|---|---|---|---|---|---|
| IL-10, IL-1β, IL-6, TNF | n = 22 cases n = 42 control | Doxorubicin |
|
| LVEF < 50% or declined LVEF > 10% resulting in LVEF < 50% compared to the baseline and/or troponin and NT-proBNP increased by 20% at T1 or T2 compared to T0 | Alves et al., 2022 [125] |
| TNF-α, Homocysteine levels, Free iron | n = 33 doxorubicin group; n = 35 paclitaxel group; n = 52 trastuzumab group; n = 50 healthy individuals | Doxorubicin, Paclitaxel and trastuzumab |
|
PTX group: 38.27 ± 9.12 pg/mL (p = 0.023); TZ group: 89.6 ± 12.11 pg/mL (p = 0.032); compared to the control group: 9.47 ± 1.56 pg/mL
PTX group: 10.95 ± 0.86 μmol/L (p = 0.005) TZ group: 9.95 ± 1.15 μmol/L (p = 0.0396) compared to the control group: 7.80 ± 0.397μmol/L
PTX group: 113 ± 18.6 ug/dL (p = 0.045) TZ group: 120.5 ± 4.64 ug/dL (p = 0.0058) | Use total CK, CK-MB, and hs-CRP for evaluating cardiac toxicity
DOX group: 4.80 ± 1.23 mg/dL (p = 0.0005); PTX group: 7.12 ± 1.87 mg/dL (p = 0.0006); TZ group: 3.12 ± 0.68 mg/dL (p = 0.095) compared to the control group: 0.66 ± 0.18 mg/dL
PTX group: 63.24 ± 8.6 U/L; TZ group: 81.65 ± 6.4 U/L compared to the control group: 68.33 ± 6.5 U/L | Micheletti et al., 2021 [286] |
| 92 CV-related proteins in plasma samples | n = 342 survivors (≥5 years since diagnosis and cancer-free since treatment) n = 346 controls (no cancer diagnosis) | Anthracyclines, trastuzumab. Anti-hormonal therapy | BC survivors at ≥5 years since diagnosis | Significant upregulation (p < 0.05) of the genes: TNFSD13B, GAL4, MCP1, KLK6, FABP4, GDF15, SCGB3A2, RARRES2, CXCL16, PI3, IGFBP7, CNTN1, TIMP4, OPN, PCSK9, PLC, CTSZ, GAL3, TFPI in the BC survivors compared to the healthy individuals. Correlation of elevated levels of TNFSF13B (p = 0.02), FABP4 (p = 0.033), MCP1 (p = 0.011), RARRES2 (p = 0.017), GDF15 (p = 0.002), CXCL16 (p = 0.019), PI3 (p = 0.041), IGFBP7 (p = 0.026), PCSK9 (p = 0.041), OPN (p = 0.04), PLC (p = 0.011) with lower LVEF in the BC survivors. | ECHO assessment of LVEF reduction | Tromp et al., 2020 [153] |
| 40 chemokines 9 matrix MMPs 33 cardiac markers using multiplex immunoassays in plasma samples and cTnT using the fourth-generation assay | n = 17 normal group n = 5 intermediate group (average LVEF reduction of 6.4%) n = 5 cardiotoxicity (average LVEF reduction 13.2%) | Doxorubicin and cyclophosphamide |
| Baseline Reduced protein levels in intermediate vs. normal: CXCL1 (196 ± 55 vs. 340 ± 203 pg/mL, p = 0.022); CCL3 (10 ± 1.5 versus 17 ± 19 pg/mL. p = 0.027); GDF15 (0.66 ± 0.13 versus 1.01 ± 0.48 ng/mL, p = 0.027); Haptoglobin (669 ± 237 versus 1181 ± 741 μg/mL, p = 0.031) Differential protein abundance in abnormal vs. normal: CCL23 (357 ± 89 versus 201 ± 168 pg/mL, p = 0.003); CCL27 (985 ± 239 versus 643 ± 240 pg/mL, p = 0.008); CXCL6 (28 ± 11 versus 74 ± 48 pg/mL, p = 0.013); sICAM-1 (120 ± 29 vs. 222 ± 98 ng/mL, p = 0.003) After Dox (T1) Reduced protein levels in intermediate vs. normal: IL-I6 (315 ± 62 pg/mL, p = 0.019) FABP3 (977 ± 137 pg/mL, p = 0.011) Myoglobin (26 ± 5 ng/mL, p = 0.049) Differential protein abundance in abnormal vs. normal: Increased levels of: CCL23 (422 ± 123 pg/mL, p = 0.010) Decreased levels of: CXCL5 (485 ± 54 pg/mL, p = 0.002) CCL26 (24 ± 3 pg/mL, p = 0.011) CXCL6 (25 ± 9 pg/mL, p = 0.004) GM-CSF (15 ± 4 pg/mL, p = 0.019) CXCL1 (206 ± 38 pg/mL, p = 0.003) IFN-γ (37 ± 5 pg/mL, p = 0.012) IL-2 (15 ± 3 pg/mL, p = 0.036) IL-8 (10 ± 1 pg/mL, p = 0.001) CXCL11 (39 ± 24 pg/mL, p = 0.047) CXCL9 (136 ± 21 pg/mL, p = 0.006) >CCL17 (60 ± 35 pg/mL, p = 0.033) CCL25 (416 ± 61 pg/mL, p = 0.023) After Dox (T2) Reduced protein levels in intermediate vs. normal: Myoglobin (24 ± 5 ng/mL, p = 0.010) CCL23 (98 ± 65 pg/mL, p = 0.020) Elevated protein levels in abnormal vs. normal: MIF (4.0 ± 2.4 ng/mL, p = 0.031) CCL23 (442 ± 83 pg/mL, p = 0.041). | Abnormal (i.e., cardiotoxicity): asymptomatic reduction in LVEF > 10% or LVEF < 50% or reduced LVEF > 5% to LVEF < 55% with HF Intermediate stage (sub-cardiotoxicity): Reduced LVEF of 5–10% Subclinical cardiotoxicity: reduced LVEF > 4% or >5% Normal: Reduced LVEF of <5% | Yu et al., 2018 [287] |
| NLR and LV GLS | n = 74 received treatment n = 44 with NLR ≥ 2.58 at T2 | All patients received Dox, cyclophosphamide, paclitaxel (in most cases) HER2-positive patients were treated with trastuzumab and then pertuzumab | Blood samples were collected at baseline (T1), during dox (T2) ECHO assessment at baseline (T1) and at the end of dox treatment (T3) | Patients with NLR ≥ 2.58 (T2) showed significant reduction in LV GLS at T3 from baseline compared to patients with NLR < 2.58 (10.6 ± 9.6 vs. 6.1 ± 6.9, p = 0.02). Patients with NLR ≥ 2.58 (T2) had twice as higher risk for LV GLS reduction ≥ 10% (50%, p = 0.009) as compared to patients with NLR < 2.58 (20%). Baseline NLR (T1) could not significantly predict future LV GLS deterioration after the end of treatment (ROC curve analysis and binary logistic regression) (AUC: 0.59; 95% CI (0.44, 0.75); p = 0.24 and OR: 1.07; 95% CI (0.81, 1.4); p = 0.65, respectively). No statistically significant change was documented in the absolute neutrophil counts at T2 (4.0 (2.2, 5.6); p = 0.90) compared to T1 (4.1 (2.8, 5.2)). The majority of HER2-positive breast cancer patients treated with trastuzumab (11 out of 44, 25%) developed NLR ≥ 2.58. | Significant reduction in LV GLS by ≥10% from baseline | Baruch et al., 2023 [288] |
| CRP TM, TAT, MPO, vWF, p-selectin, nucleosomes, dsRNA | n = 21 with asymptomatic decreased LVEF > 10% n = 30 with LVEF ≤ 10% | DOX and cyclophosphamide | Baseline (T0) and after 1 cycle of DOX chemotherapy (T1) | Elevation in the levels of nucleosomes (mean ± SD 144.3 ± 78.9 vs. 113.4 ± 73.3 AU), TM (4.0 ± 1.1 vs. 3.6 ± 0.9 pg/mL) , vWF (5.4 ± 2.4 vs. 4.9 ± 1.7 mIU/mL), TAT complex (14.3 ± 5.7 vs. 12.4 ± 4.6 ng/mL), dsDNA (135.1 ± 39.2 vs. 131.2 ± 26.9 ng/mL), p-selectin (0.104 ± 0.07 vs. 0.099 ± 0.08 ug/mL) and CRP (6.8 ± 2.0 vs. 5.5 ± 2.5 mg/L) in the cardiotoxicity versus non-cardiotoxicity group. | Decline in LVEF > 10% or LVEF < 50% compared to baseline | Todorova et al., 2020 [163] |
| Metabolic profile (BMI, TC, fasting glucose) | n = 64 NCT n = 59 NET | Goserelin acetate with tamoxifen or adriamycin and cyclophosphamide followed by docetaxel | Baseline and after 24 weeks of treatment and 3 years after the initial clinical visit | Patients with hypertension were higher in the NET group compared to the NCT group. BMI significantly changed over the 3 years of follow-up in patients treated with NCT (22.84 kg/m2 at baseline (95% CI 21.94–23.74) and 23.87 kg/m2 (95% CI 23.06-24.68) after NCT). BMI then reached baseline BMI (22.82 kg/m2, 95% CI (22.04–23.61)) after 3 years. No changes were noted in patients treated with NET. Significantly increased TC in patients after NCT (from 181.44 mg/dl, 95% CI (172.96–189.91 at baseline to 215.23 mg/dl, 95% CI (206.75–223.70); p < 0.05) followed by decrease to 176.15 mg/dL (95% CI (167.58–184.72)) after 3 years. No significant changes induced by NET. Fasting glucose increased from 95.36 mg/dL (95% CI (92.55–98.26)) at baseline to 111.36 mg/dL (92% CI (106.02–116.98) after NCT followed by decreased to 99.02 mg/dL (95% CI (95.71–102.45) after 3 years. No significant changes noted in the NET group. Significant increase in the NLR was noted in in NCT group (1.83, 95% CI (1.65–2.02) to 3.18, 95% CI (2.68–3.78); p < 0.01) followed by a decrease (1.42, 95% CI (1.04–1.93)) after three years and significant decrease in the NET group (1.98, 95% CI (1.78–2.21) to 1.43, 95% CI (1.20–1.72); p < 0.05) followed by an increase to 1.61 (95% CU (1.16–2.22)) after 3 years. | No association with cancer therapy–induced cardiotoxicity was investigated | Ryu et al., 2021 [289] |
| Arginine–nitric oxide metabolites | ECHO in n = 139 n = 32 experienced cardiotoxicity (23%) | DOX, cyclophosphamide followed by paclitaxel or DOX and cyclophosphamide followed by paclitaxel and trastuzumab | Blood samples at baseline, after cycle 2 of DOX (month 1), and after DOX completion (month 2) | Decreased levels of arginine and citrulline levels (p < 0.001) and increased ADMA levels (p < 0.001) at month 1 and persistent at month. Decrease in arginine and increase in ADMA and MMA were associated with cardiotoxicity at month 1 and a month 2, respectively. Increased levels of ADMA and MMA by 1.5-fold was associated with cardiotoxicity in response to DOX at month 2 (n = 117) with HR of 3.33 (95% CI (1.12, 9.96); p = 0.03)) and 2.70 (95% CI (1.35, 5.41); p = 0.005, respectively). No statistically significant association was observed between the levels of symmetric dimethylarginine (SDMA) (p = 0.90 month 1; p = 0.88 month 2), citrulline (p = 0.53 month 1; p = 0.28 month 2) and ornithine (p = 10 month 1; p = 0.06 month 2) at either time point. | Reduction in LVEF by ≥10% from baseline to LVEF < 50% | Finkelman et al., 2017 [291] |
| Lipoprotein subfractions and circulating metabolites | n = 250 |
| Serum samples were collected prior to RT (T1, n = 229), after RT completion (T2, n = 211), at 3 months (T3, n = 198), 6 months (T4, n = 195) and 12 months (T5, n = 146) Long-term survival and disease recurrence were assessed 10 years after enrolment to the study | Changes in lipoprotein composition (increased levels of LDL-cholesterol, lower levels of HDL-cholesterol, apo-A1 and apo-A2) was noted after treatment. VLDL-4 triglyceride levels were significantly decreased in patients treated with chemotherapy with or without endocrine treatment. All treated patients showed significantly lower levels in VLDL-5 triglyceride levels. Lysine (group 1: 0.018; group 2: 0.062; group 3: 0.036; group 4: 0.041), glutamate (group 1:0.081; group 2: 0.199; group 3: 0.251; group 4: −0.258) and formate (group 1: −0.322; group 2: −0.091; group 3: 0.167; group 4: 0.217) levels were upregulated during treatment whilst lower levels of glutamine (group 1: -0.091; group 2; −0.167; group 3; −0.126; group 4: 0.090) and lactate (group 1: −0.040; group 2: 0.124; group 3: −0.147; group 4: 0.174) were observed (linear mixed model analysis of longitudinal changes). | / | Giskeødegård et al., 2022 [290] |
| 71 metabolites, previously associated with CV diseases | n = 19 cardiotoxicity n = 19 no cardiotoxicity | DOX, cyclophosphamide, paclitaxel and trastuzumab | ECHO at baseline and every 3 months Blood samples collected at baseline, 3 months (after completion of DOX) and 6 months (after completion of paclitaxel and trastuzumab) | Metabolites related to citric acid cycle, purine and pyrimidine were significantly altered (p < 0.05) in patients with cardiotoxicity. Patients without cardiotoxicity:
No statistically significant differences noted in the levels of glucose/fructose/galactose (monosaccharides) or pyruvic acid in patients with or without cardiotoxicity. | LVEF decline by >10% resulting to LVEF < 55% compared to baseline | Asnani et al., 2020 [292] |
| Methylation signature of PBMCs using infinium HumanMethylation450 BeadChip | n = 9 abnormal LVEF n = 10 normal LVEF | DOX and cyclophosphamide | Blood samples were collected at baseline and after the first cycle of chemotherapy | A total of 14,883 and 18,718 differentially methylated CpGs were identified prior to and after the first cycle of chemotherapy, respectively, and correlated with changes in LVEF (p ≤ 0.05). Significant differential methylation was noted in SLFN12, IRF6 and RNF39. SLFN12 and IRF6 were hypermethylated and transcriptionally downregulated after the first cycle of chemotherapy compared to the baseline in all treatment groups (IRF6 p = 0.8837 normal LVEF; IRF6 p = 0.8119 abnormal LVEF; SLNF12 p = 0.5718 normal LVEF; SLNF12 p = 0.8575 abnormal LVEF). | Decrease in LVEF by >10% or LVEF < 50% compared to baseline | Bauer et al., 2021 [293] |
| High-throughput proteomic profiling using plasma samples | Case/control pairs 1 and 2 Case/control pair 3 Validation study of 35 participants | DOX plus cyclophosphamide followed by trastuzumab and paclitaxel. | ECHO at baseline, after DOX treatment completion and every 3 months of trastuzumab treatment. Blood samples collected at baseline, during treatment, upon diagnosis of CTRCD and, after CTRCD diagnosis. | 862 proteins were identified using liquid chromatography-mass spectrometry (LC-MS) from case/control pairs 1 and 2 and 1360 proteins from case/control pair 3. IgE was significantly upregulated (5- to 58-fold; p = 0.018) in the control groups (non-cardiotoxicity patients) compared to the patients with cardiotoxicity at baseline and at all time points. This was verified (n = 35) using Luminex assay. Baseline IgE was higher in the non-cardiotoxicity group (mean 498.8 ng/mL ± 401.0; median 389.3 ng/mL with range 60.5–1392.1) compared to the cardiotoxicity group (mean 234.9 ng/mL ± 285.9; median 167 ng/mL with range 23.2–1059.2). In addition to IgE, baseline IgG4 levels and IgE related cytokines such as IL4, IL5 and IL17 were all lower in the case group compared to the control group. | Cardiotoxicity: LVEF reduction by ≥10% resulting in LVEF < 50% from baseline plus symptoms of HF Normal: LVEF change by <10% and LVEF > 50% | Beer et al., 2016 [294] |
| MMP2, MMP9 | Month 12 n = 114 had anthracycline induced cardiotoxicity (AIC) of NYHA class I-III n = 70 no AIC After BC remission: Group 1: n = 54 (with AIC) Group 2: n = 60 (without AIC) | DOX and cyclophosphamide or DOX, and cyclophosphamide and docetaxel | Baseline, 12 months, and 24 months of treatment | Group 1: Elevated levels of MMP2: by 8% (p = 0.017) from 376.8 (329.5; 426.7) to 481.4 (389.8; 518.7) pg/mL and MMP9: by 18.4% (p < 0.001) from 23.6 (21.4; 24.6) to 26.0 (23.3; 27.0) pg/mL at 24 months. Group 2: MMP2 and MMP9 levels were lower at month 24. Increased levels of MMP2 and MMP9 could predict anthracycline induced CHF (AUC = 0.64; p = 0.013 and AUC = 0.9; p < 0.001, respectively). The presence of gene polymorphisms of MMP2 (rs243865) and MMP9 (rs3918242) were associated with anthracycline induced adverse events (OR = 4.76; p = 0.029; OR = 15.23; p < 0.000, respectively). | Decreased LVEF > 10% or LVEF < 55% with HF and NT-proBNP > 125pg/mL at 12 months after chemotherapy completion | Grakova et al., 2022 [284] |
| GDF-15 | n = 30 placebo n = 33 perindopril n = 31 bisoprolol | DOX, carboplatin, trastuzumab (TCH) or 5-FU, epirubicin, cyclophosphamide (FEC) followed by docetaxel and trastuzumab | Baseline, post-cycle 4, post-cycle 17 | Increase in GDF-15 at post-cycle 4 (+130 ± 150%, p ≤ 0.001) compared to baseline. | LVEF and GLS changes | Kirkham et al., 2022 [285] |
| CRP | n = 121 | Epirubicin (with or without metoprolol succinate or candesartan) in combination with 5-FU and cyclophosphamide | Baseline and after completion of treatment | Increased levels of CRP after epirubicin treatment (p = 0.002) compared to baseline. | Cardiotoxicity was defined as previously described [67] | Gulati et al., 2017 [92] |
| hs-CRP | n = 56 (HER2-negative BC patients) | Adjuvant adriamycin, cyclophosphamide, or adriamycin, cyclophosphamide, taxane | Before each cycle, 12, 24 and 48 weeks after treatment completion | Elevated levels of hsCRP in response to chemotherapy (median levels 8.4 (4.95–16.5). | Changes in LVEF (not specified) | Hasan et al., 2021 [295] |
| hs-CRP GDF-15 | n = 78 (HER2-positive BC patients) | DOX, cyclophosphamide followed by paclitaxel and trastuzumab | Baseline, every 3 months until month 15 | Increased levels of hs-CRP and GDF-15 in response to therapy from baseline to visit 2 (mean changes between baseline and visit 2 (3.5 ± 5.0 mg/l, p < 0.001 and 575.4 ± 291.5 ng/l p < 0.001, respectively). | Cardiotoxicity was defined as previously described [67] | Ky et al., 2014 [105] |
3.4.4. Peripheral Blood Mononuclear Cells (PMBCs)
3.4.5. Immunoglobulin E (IgE)
3.4.6. C-Reactive Protein (CRP)
3.4.7. Growth Differentiation Factor 15 (GDF-15)
3.4.8. Other Biomarkers under Investigation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zamorano, J.L.; Lancellotti, P.; Muñoz, D.R.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart. J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernánde, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klei, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.; Eiermann, W.; Robert, N.; Pienkowski, T.; Martin, M.; Press, M.; Mackey, J.; Glaspy, J.; Chan, A.; Pawlicki, M.; et al. Adjuvant trastuzumab in HER2-positive breast cancer. N. Engl. J. Med. 2011, 365, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Suter, T.M.; Procter, M.; van Veldhuisen, D.J.; Muscholl, M.; Bergh, J.; Carlomagno, C.; Perren, T.; Passalacqua, R.; Bighin, C.; Klijn, J.G.; et al. Trastuzumab-associated cardiac adverse effects in the herceptin adjuvant trial. J. Clin. Oncol. 2007, 25, 3859–3865. [Google Scholar] [CrossRef] [PubMed]
- Calvillo-Argüelles, O.; Abdel-Qadir, H.; Suntheralingam, S.; Michalowska, M.; Amir, E.; Thavendiranathan, P. Trastuzumab-Related Cardiotoxicity and Cardiac Care in Patients with HER2 Positive Metastatic Breast Cancer. Am. J. Cardiol. 2020, 125, 1270–1275. [Google Scholar] [CrossRef]
- Cardinale, D.; Ciceri, F.; Latini, R.; Franzosi, M.G.; Sandri, M.T.; Civelli, M.; Cucchi, G.; Menatti, E.; Mangiavacchi, M.; Cavina, R.; et al. Anthracycline-induced cardiotoxicity: A multicenter randomised trial comparing two strategies for guiding prevention with enalapril: The International CardioOncology Society-one trial. Eur. J. Cancer 2018, 94, 126–137. [Google Scholar] [CrossRef]
- Cardinale, D.; Colombo, A.; Bacchiani, G.; Tedeschi, I.; Meroni, C.A.; Veglia, F.; Civelli, M.; Lamantia, G.; Colombo, N.; Curigliano, G.; et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015, 131, 1981–1988. [Google Scholar] [CrossRef]
- Cardinale, D.; Colombo, A.; Lamantia, G.; Colombo, N.; Civelli, M.; De Giacomi, G.; Rubino, M.; Veglia, F.; Fiorentini, C.; Cipolla, C.M. Anthracycline-induced cardiomyopathy: Clinical relevance and response to pharmacologic therapy. J. Am. Coll. Cardiol. 2010, 55, 213–220. [Google Scholar] [CrossRef]
- Sawaya, H.; Sebag, I.A.; Plana, J.C.; Januzzi, J.L.; Ky, B.; Tan, T.C.; Cohen, V.; Banchs, J.; Carver, J.R.; Wiegers, S.E.; et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ. Cardiovasc. Imaging 2012, 5, 596–603. [Google Scholar] [CrossRef]
- Zardavas, D.; Suter, T.M.; Van Veldhuisen, D.J.; Steinseifer, J.; Noe, J.; Lauer, S.; Al-Sakaff, N.; Piccart-Gebhart, M.J.; de Azambuja, E. Role of Troponins I and T and N-Terminal Prohormone of Brain Natriuretic Peptide in Monitoring Cardiac Safety of Patients with Early-Stage Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer Receiving Trastuzumab: A Herceptin Adjuvant Study Cardiac Marker Substudy. J. Clin. Oncol. 2017, 35, 878–884. [Google Scholar] [CrossRef]
- Martel, S.; Maurer, C.; Lambertini, M.; Pondé, N.; De Azambuja, E. Breast cancer treatment-induced cardiotoxicity. Expert Opin. Drug Saf. 2017, 16, 1021–1038. [Google Scholar] [CrossRef]
- Henry, M.L.; Niu, J.; Zhang, N.; Giordano, S.H.; Chavez-MacGregor, M. Cardiotoxicity and Cardiac Monitoring among Chemotherapy-Treated Breast Cancer Patients. JACC Cardiovasc. Imaging 2018, 11, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Ewer, M.S.; Lippman, S.M. Type II chemotherapy-related cardiac dysfunction: Time to recognize a new entity. J. Clin. Oncol. 2005, 23, 2900–2902. [Google Scholar] [CrossRef]
- Herrmann, J.; Lerman, A.; Sandhu, N.P.; Villarraga, H.R.; Mulvagh, S.L.; Kohli, M. Evaluation and management of patients with heart disease and cancer: Cardio-oncology. Mayo Clin. Proc. 2014, 89, 1287–1306. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, K.; Fujino, T.; Ide, T.; Funakoshi, K.; Sakamoto, I.; Hiasa, K.I.; Higo, T.; Kamezaki, K.; Akashi, K.; Tsutsui, H. Recovery from left ventricular dysfunction was associated with the early introduction of heart failure medical treatment in cancer patients with anthracycline-induced cardiotoxicity. Clin. Res. Cardiol. 2019, 108, 600–611. [Google Scholar] [CrossRef]
- Florescu, D.R.; Nistor, D.E. Therapy-induced cardiotoxicity in breast cancer patients: A well-known yet unresolved problem. Discoveries 2019, 7, e89. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Zhu, W.; Wagar, E.A.; Meng, Q.H. Biomarkers for monitoring chemotherapy-induced cardiotoxicity. Crit. Rev. Clin. Lab. Sci. 2017, 54, 87–101. [Google Scholar] [CrossRef]
- Curigliano, G.; Cardinale, D.; Suter, T.; Plataniotis, G.; de Azambuja, E.; Sandri, M.T.; Criscitiello, C.; Goldhirsch, A.; Cipolla, C.; Roila, F. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann. Oncol. 2012, 23 (Suppl. S7), vii155–viii166. [Google Scholar] [CrossRef]
- Lyon, A.R.; Dent, S.; Stanway, S.; Earl, H.; Brezden-Masley, C.; Cohen-Solal, A.; Tocchetti, C.G.; Moslehi, J.J.; Groarke, J.D.; Bergler-Klein, J.; et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: A position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur. J. Heart Fail. 2020, 22, 1945–1960. [Google Scholar] [CrossRef]
- Vittorini, S.; Clerico, A. Cardiovascular biomarkers: Increasing impact of laboratory medicine in cardiology practice. Clin. Chem. Lab. Med. 2008, 46, 748–763. [Google Scholar] [CrossRef]
- Curigliano, G.; de Azambuja, E.; Lenihan, D.; Calabrò, M.G.; Cardinale, D.; Cipolla, C.M. Prevention, Monitoring, and Management of Cardiac Dysfunction in Patients with Metastatic Breast Cancer. Oncologist 2019, 24, e1034–e1043. [Google Scholar] [CrossRef] [PubMed]
- Bergamini, C.; Torelli, F.; Ghiselli, L.; Rossi, A.; Trevisani, L.; Vinco, G.; Truong, S.; Benfari, G.; La Russa, F.; Golia, G.; et al. Left ventricular end-diastolic volume as early indicator of trastuzumab-related cardiotoxicity in HER2+ breast cancer patients: Results from a single-center retrospective study. Minerva Cardioangiol. 2017, 65, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Gottdiener, J.S.; McClelland, R.L.; Marshall, R.; Shemanski, L.; Furberg, C.D.; Kitzman, D.W.; Cushman, M.; Polak, J.; Gardin, J.M.; Gersh, B.J.; et al. Outcome of congestive heart failure in elderly persons: Influence of left ventricular systolic function. The Cardiovascular Health Study. Ann. Intern. Med. 2002, 137, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Curigliano, G.; Cardinale, D.; Dent, S.; Criscitiello, C.; Aseyev, O.; Lenihan, D.; Cipolla, C.M. Cardiotoxicity of anticancer treatments: Epidemiology, detection, and management. CA Cancer J. Clin. 2016, 66, 309–325. [Google Scholar] [CrossRef]
- Touyz, R.M.; Herrmann, J. Cardiotoxicity with vascular endothelial growth factor inhibitor therapy. NPJ Precis. Oncol. 2018, 2, 13. [Google Scholar] [CrossRef]
- Cardinale, D.; Stivala, F.; Cipolla, C.M. Oncologic therapies associated with cardiac toxicities: How to minimize the risks. Expert Rev. Anticancer Ther. 2019, 19, 359–374. [Google Scholar] [CrossRef]
- Ananthan, K.; Lyon, A.R. The Role of Biomarkers in Cardio-Oncology. J. Cardiovasc. Transl. Res. 2020, 13, 431–450. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B. PRISMA-S: An extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2020, 12, 55–61. [Google Scholar] [CrossRef]
- Coombes, R.C.; Kilburn, L.S.; Snowdon, C.F.; Paridaens, R.; Coleman, R.E.; Jones, S.E.; Jassem, J.; Van de Velde, C.J.; Delozier, T.; Alvarez, I.; et al. Survival and safety of exemestane versus tamoxifen after 2-3 years’ tamoxifen treatment (Intergroup Exemestane Study): A randomised controlled trial. Lancet 2007, 369, 559–570. [Google Scholar] [CrossRef]
- Buzdar, A.; Howell, A.; Cuzick, J.; Wale, C.; Distler, W.; Hoctin-Boes, G.; Houghton, J.; Locker, G.Y.; Nabholtz, J.M. Comprehensive side-effect profile of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: Long-term safety analysis of the ATAC trial. Lancet Oncol. 2006, 7, 633–643. [Google Scholar] [CrossRef]
- Speers, C.; Murthy, V.L.; Walker, E.M.; Glide-Hurst, C.K.; Marsh, R.; Tang, M.; Morris, E.L.; Schipper, M.J.; Weinberg, R.L.; Gits, H.C.; et al. Cardiac Magnetic Resonance Imaging and Blood Biomarkers for Evaluation of Radiation-Induced Cardiotoxicity in Patients with Breast Cancer: Results of a Phase 2 Clinical Trial. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 417–425. [Google Scholar] [CrossRef]
- Cardinale, D.; Iacopo, F.; Cipolla, C.M. Cardiotoxicity of Anthracyclines. Front. Cardiovasc. Med. 2020, 7, 26. [Google Scholar] [CrossRef]
- Zhu, W.; Soonpaa, M.H.; Chen, H.; Shen, W.; Payne, R.M.; Liechty, E.A.; Caldwell, R.L.; Shou, W.; Field, L.J. Acute doxorubicin cardiotoxicity is associated with p53-induced inhibition of the mammalian target of rapamycin pathway. Circulation 2009, 119, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, Y.; Ghanefar, M.; Bayeva, M.; Wu, R.; Khechaduri, A.; Naga Prasad, S.V.; Mutharasan, R.K.; Naik, T.J.; Ardehali, H. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J. Clin. Investig. 2014, 124, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Christidi, E.; Huang, H.; Shafaattalab, S.; Maillet, A.; Lin, E.; Huang, K.; Laksman, Z.; Davis, M.K.; Tibbits, G.F.; Brunham, L.R. Variation in RARG increases susceptibility to doxorubicin-induced cardiotoxicity in patient specific induced pluripotent stem cell-derived cardiomyocytes. Sci. Rep. 2020, 10, 10363. [Google Scholar] [CrossRef]
- Christidi, E.; Brunham, L.R. Regulated cell death pathways in doxorubicin-induced cardiotoxicity. Cell Death Dis. 2021, 12, 339. [Google Scholar] [CrossRef] [PubMed]
- Minotti, G.; Ronchi, R.; Salvatorelli, E.; Menna, P.; Cairo, G. Doxorubicin irreversibly inactivates iron regulatory proteins 1 and 2 in cardiomyocytes: Evidence for distinct metabolic pathways and implications for iron-mediated cardiotoxicity of antitumor therapy. Cancer Res. 2001, 61, 8422–8428. [Google Scholar] [PubMed]
- Liu, Y.; Zeng, L.; Yang, Y.; Chen, C.; Wang, D.; Wang, H. Acyl-CoA thioesterase 1 prevents cardiomyocytes from Doxorubicin-induced ferroptosis via shaping the lipid composition. Cell Death Dis. 2020, 11, 756. [Google Scholar] [CrossRef]
- Meng, L.; Lin, H.; Zhang, J.; Lin, N.; Sun, Z.; Gao, F.; Luo, H.; Ni, T.; Luo, W.; Chi, J.; et al. Doxorubicin induces cardiomyocyte pyroptosis via the TINCR-mediated posttranscriptional stabilization of NLR family pyrin domain containing 3. J. Mol. Cell. Cardiol. 2019, 136, 15–26. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, B. Doxorubicin induces cardiotoxicity through upregulation of death receptors mediated apoptosis in cardiomyocytes. Sci. Rep. 2017, 7, 44735. [Google Scholar] [CrossRef]
- Appelbaum, F.; Strauchen, J.A.; Graw, R.G., Jr.; Savage, D.D.; Kent, K.M.; Ferrans, V.J.; Herzig, G.P. Acute lethal carditis caused by high-dose combination chemotherapy. A unique clinical and pathological entity. Lancet 1976, 1, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Dhesi, S.; Chu, M.P.; Blevins, G.; Paterson, I.; Larratt, L.; Oudit, G.Y.; Kim, D.H. Cyclophosphamide-Induced Cardiomyopathy: A Case Report, Review, and Recommendations for Management. J. Investig. Med. High Impact Case Rep. 2013, 1, 2324709613480346. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Song, M.; Li, L. Grading Evaluation of Cardiotoxicity in Patients with Breast Cancer Treated with Adjuvant Paclitaxel Anthracycline/Cyclophosphamide Chemotherapy: A Meta-Analysis. Comput. Math. Methods Med. 2022, 2022, 7963146. [Google Scholar] [CrossRef]
- Mackey, J.R.; Martin, M.; Pienkowski, T.; Rolski, J.; Guastalla, J.P.; Sami, A.; Glaspy, J.; Juhos, E.; Wardley, A.; Fornander, T.; et al. Adjuvant docetaxel, doxorubicin, and cyclophosphamide in node-positive breast cancer: 10-year follow-up of the phase 3 randomised BCIRG 001 trial. Lancet Oncol. 2013, 14, 72–80. [Google Scholar] [CrossRef]
- Cho, H.S.; Mason, K.; Ramyar, K.X.; Stanley, A.M.; Gabelli, S.B.; Denney, D.W., Jr.; Leahy, D.J. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 2003, 421, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Christianson, T.A.; Doherty, J.K.; Lin, Y.J.; Ramsey, E.E.; Holmes, R.; Keenan, E.J.; Clinton, G.M. NH2-terminally truncated HER-2/neu protein: Relationship with shedding of the extracellular domain and with prognostic factors in breast cancer. Cancer Res. 1998, 58, 5123–5129. [Google Scholar]
- Slamon, D.J.; Godolphin, W.; Jones, L.A.; Holt, J.A.; Wong, S.G.; Keith, D.E.; Levin, W.J.; Stuart, S.G.; Udove, J.; Ullrich, A.; et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989, 244, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.K.; Borgen, P.I.; Wong, G.Y.; Cordon-Cardo, C.; Osborne, M.P. HER-2/neu amplification and overexpression in primary human breast cancer is associated with early metastasis. Anticancer Res. 1992, 12, 419–425. [Google Scholar]
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Lane, H.A.; Beuvink, I.; Motoyama, A.B.; Daly, J.M.; Neve, R.M.; Hynes, N.E. ErbB2 potentiates breast tumor proliferation through modulation of p27(Kip1)-Cdk2 complex formation: Receptor overexpression does not determine growth dependency. Mol. Cell. Biol. 2000, 20, 3210–3223. [Google Scholar] [CrossRef]
- Beerli, R.R.; Graus-Porta, D.; Woods-Cook, K.; Chen, X.; Yarden, Y.; Hynes, N.E. Neu differentiation factor activation of ErbB-3 and ErbB-4 is cell specific and displays a differential requirement for ErbB-2. Mol. Cell. Biol. 1995, 15, 6496–6505. [Google Scholar] [CrossRef]
- Spencer, K.S.; Graus-Porta, D.; Leng, J.; Hynes, N.E.; Klemke, R.L. ErbB2 is necessary for induction of carcinoma cell invasion by ErbB family receptor tyrosine kinases. J. Cell Biol. 2000, 148, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001, 2, 127–137. [Google Scholar] [CrossRef]
- Kokai, Y.; Myers, J.N.; Wada, T.; Brown, V.I.; LeVea, C.M.; Davis, J.G.; Dobashi, K.; Greene, M.I. Synergistic interaction of p185c-neu and the EGF receptor leads to transformation of rodent fibroblasts. Cell 1989, 58, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Shastry, M.; Hamilton, E. Targeting HER2-positive breast cancer: Advances and future directions. Nat. Rev. Drug Discov. 2023, 22, 101–126. [Google Scholar] [CrossRef]
- Giordano, S.H.; Temin, S.; Chandarlapaty, S.; Crews, J.R.; Esteva, F.J.; Kirshner, J.J.; Krop, I.E.; Levinson, J.; Lin, N.U.; Modi, S.; et al. Systemic Therapy for Patients with Advanced Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 2736–2740. [Google Scholar] [CrossRef]
- Piccart-Gebhart, M.J.; Procter, M.; Leyland-Jones, B.; Goldhirsch, A.; Untch, M.; Smith, I.; Gianni, L.; Baselga, J.; Bell, R.; Jackisch, C.; et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N. Engl. J. Med. 2005, 353, 1659–1672. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Procter, M.; de Azambuja, E.; Zardavas, D.; Benyunes, M.; Viale, G.; Suter, T.; Arahmani, A.; Rouchet, N.; Clark, E.; et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N. Engl. J. Med. 2017, 377, 122–131. [Google Scholar] [CrossRef]
- Simmons, C.; Rayson, D.; Joy, A.A.; Henning, J.W.; Lemieux, J.; McArthur, H.; Card, P.B.; Dent, R.; Brezden-Masley, C. Current and future landscape of targeted therapy in HER2-positive advanced breast cancer: Redrawing the lines. Ther. Adv. Med. Oncol. 2022, 14, 17588359211066677. [Google Scholar] [CrossRef]
- von Arx, C.; De Placido, P.; Caltavituro, A.; Di Rienzo, R.; Buonaiuto, R.; De Laurentiis, M.; Arpino, G.; Puglisi, F.; Giuliano, M.; Del Mastro, L. The evolving therapeutic landscape of trastuzumab-drug conjugates: Future perspectives beyond HER2-positive breast cancer. Cancer Treat. Rev. 2023, 113, 102500. [Google Scholar] [CrossRef]
- Yu, A.F.; Yadav, N.U.; Lung, B.Y.; Eaton, A.A.; Thaler, H.T.; Hudis, C.A.; Dang, C.T.; Steingart, R.M. Trastuzumab interruption and treatment-induced cardiotoxicity in early HER2-positive breast cancer. Breast Cancer Res. Treat. 2015, 149, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Meng, W.; Zhao, W.; Tong, Z. Cardiac safety analysis of anti-HER2-targeted therapy in early breast cancer. Sci. Rep. 2022, 12, 14312. [Google Scholar] [CrossRef] [PubMed]
- Pondé, N.F.; Lambertini, M.; de Azambuja, E. Twenty years of anti-HER2 therapy-associated cardiotoxicity. ESMO Open 2016, 1, e000073. [Google Scholar] [CrossRef]
- Eiger, D.; Pondé, N.F.; Agbor-Tarh, D.; Moreno-Aspitia, A.; Piccart, M.; Hilbers, F.S.; Werner, O.; Chumsri, S.; Dueck, A.; Kroep, J.R.; et al. Long-term cardiac outcomes of patients with HER2-positive breast cancer treated in the adjuvant lapatinib and/or trastuzumab Treatment Optimization Trial. Br. J. Cancer 2020, 122, 1453–1460. [Google Scholar] [CrossRef]
- Seidman, A.; Hudis, C.; Pierri, M.K.; Shak, S.; Paton, V.; Ashby, M.; Murphy, M.; Stewart, S.J.; Keefe, D. Cardiac dysfunction in the trastuzumab clinical trials experience. J. Clin. Oncol. 2002, 20, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Sardesai, S.; Sukumar, J.; Kassem, M.; Palettas, M.; Stephens, J.; Morgan, E.; Addison, D.; Baliga, R.; Stover, D.G.; VanDeusen, J.; et al. Clinical impact of interruption in adjuvant Trastuzumab therapy in patients with operable HER-2 positive breast cancer. Cardiooncology 2020, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Gong, I.Y.; Verma, S.; Yan, A.T.; Ko, D.T.; Earle, C.C.; Tomlinson, G.A.; Trudeau, M.E.; Krahn, M.D.; Krzyzanowska, M.K.; Brezden-Masley, C.B.; et al. Long-term cardiovascular outcomes and overall survival of early-stage breast cancer patients with early discontinuation of trastuzumab: A population-based study. Breast Cancer Res. Treat. 2016, 157, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Jacquinot, Q.; Paget-Bailly, S.; Fumoleau, P.; Romieu, G.; Pierga, J.Y.; Espié, M.; Lortholary, A.; Nabholtz, J.M.; Mercier, C.F.; Pauporté, I.; et al. Fluctuation of the left ventricular ejection fraction in patients with HER2-positive early breast cancer treated by 12 months of adjuvant trastuzumab. Breast 2018, 41, 1–7. [Google Scholar] [CrossRef]
- Crone, S.A.; Zhao, Y.Y.; Fan, L.; Gu, Y.; Minamisawa, S.; Liu, Y.; Peterson, K.L.; Chen, J.; Kahn, R.; Condorelli, G.; et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat. Med. 2002, 8, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.A.; Koehler, M.; Byrne, J.; Preston, A.J.; Rappold, E.; Ewer, M.S. Cardiac safety of lapatinib: Pooled analysis of 3689 patients enrolled in clinical trials. Mayo Clin. Proc. 2008, 83, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Alhussein, M.M.; Mokbel, A.; Cosman, T.; Aghel, N.; Yang, E.H.; Mukherjee, S.D.; Dent, S.; Ellis, P.M.; Dhesy-Thind, S.; Leong, D.P. Pertuzumab Cardiotoxicity in Patients with HER2-Positive Cancer: A Systematic Review and Meta-analysis. CJC Open 2021, 3, 1372–1382. [Google Scholar] [CrossRef]
- Marjot, J.; Kaier, T.E.; Martin, E.D.; Reji, S.S.; Copeland, O.; Iqbal, M.; Goodson, B.; Hamren, S.; Harding, S.E.; Marber, M.S. Quantifying the Release of Biomarkers of Myocardial Necrosis from Cardiac Myocytes and Intact Myocardium. Clin. Chem. 2017, 63, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; White, H.D. Universal Definition of Myocardial Infarction. J. Am. Coll. Cardiol. 2007, 50, 2173–2195. [Google Scholar] [CrossRef]
- Cardinale, D.; Sandri, M.T.; Colombo, A.; Colombo, N.; Boeri, M.; Lamantia, G.; Civelli, M.; Peccatori, F.; Martinelli, G.; Fiorentini, C.; et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation 2004, 109, 2749–2754. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Sandri, M.T.; Martinoni, A.; Borghini, E.; Civelli, M.; Lamantia, G.; Cinieri, S.; Martinelli, G.; Fiorentini, C.; Cipolla, C.M. Myocardial injury revealed by plasma troponin I in breast cancer treated with high-dose chemotherapy. Ann. Oncol. 2002, 13, 710–715. [Google Scholar] [CrossRef]
- Sandri, M.T.; Cardinale, D.; Zorzino, L.; Passerini, R.; Lentati, P.; Martinoni, A.; Martinelli, G.; Cipolla, C.M. Minor Increases in Plasma Troponin I Predict Decreased Left Ventricular Ejection Fraction after High-Dose Chemotherapy. Clin. Chem. 2003, 49, 248–252. [Google Scholar] [CrossRef]
- Cardinale, D.; Colombo, A.; Torrisi, R.; Sandri, M.T.; Civelli, M.; Salvatici, M.; Lamantia, G.; Colombo, N.; Cortinovis, S.; Dessanai, M.A.; et al. Trastuzumab-induced cardiotoxicity: Clinical and prognostic implications of troponin I evaluation. J. Clin. Oncol. 2010, 28, 3910–3916. [Google Scholar] [CrossRef]
- Apple, F.S.; Sandoval, Y.; Jaffe, A.S.; Ordonez-Llanos, J. Cardiac Troponin Assays: Guide to Understanding Analytical Characteristics and Their Impact on Clinical Care. Clin. Chem. 2017, 63, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Clerico, A.; Zaninotto, M.; Aimo, A.; Cardinale, D.M.; Dittadi, R.; Sandri, M.T.; Perrone, M.A.; Belloni, L.; Fortunato, A.; Trenti, T.; et al. Variability of cardiac troponin levels in normal subjects and in patients with cardiovascular diseases: Analytical considerations and clinical relevance. Clin. Chem. Lab. Med. 2023, 61, 1209–1229. [Google Scholar] [CrossRef] [PubMed]
- Tzolos, E.; Adamson, P.D.; Hall, P.S.; Macpherson, I.R.; Oikonomidou, O.; MacLean, M.; Lewis, S.C.; McVicars, H.; Newby, D.E.; Mills, N.L.; et al. Dynamic Changes in High-Sensitivity Cardiac Troponin I in Response to Anthracycline-Based Chemotherapy. Clin. Oncol. 2020, 32, 292–297. [Google Scholar] [CrossRef]
- Shafi, A.; Siddiqui, N.; Imtiaz, S.; Din Sajid, M.U. Left Ventricular Systolic Dysfunction Predicted By Early Troponin I Release after Anthracycline Based Chemotherapy In Breast Cancer Patients. J. Ayub Med. Coll. Abbottabad 2017, 29, 266–269. [Google Scholar] [PubMed]
- Blaes, A.H.; Rehman, A.; Vock, D.M.; Luo, X.; Menge, M.; Yee, D.; Missov, E.; Duprez, D. Utility of high-sensitivity cardiac troponin T in patients receiving anthracycline chemotherapy. Vasc. Health Risk Manag. 2015, 11, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Antón, B.; Madurga, R.; Zorita, B.; Wasniewski, S.; Moreno-Arciniegas, A.; López-Melgar, B.; Merino, N.R.; Martín-Asenjo, R.; Barrio, P.; Escañuela, M.G.A.; et al. Early detection of anthracycline- and trastuzumab-induced cardiotoxicity: Value and optimal timing of serum biomarkers and echocardiographic parameters. ESC Heart Fail. 2022, 9, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Howden, E.J.; Foulkes, S.; Dillon, H.T.; Bigaran, A.; Wright, L.; Janssens, K.; Comie, P.; Costello, B.; La Gerche, A. Traditional markers of cardiac toxicity fail to detect marked reductions in cardiorespiratory fitness among cancer patients undergoing anti-cancer treatment. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 451–458. [Google Scholar] [CrossRef]
- Curigliano, G.; Lenihan, D.; Fradley, M.; Ganatra, S.; Barac, A.; Blaes, A.; Herrmann, J.; Porter, C.; Lyon, A.R.; Lancellotti, P.; et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann. Oncol. 2020, 31, 171–190. [Google Scholar] [CrossRef]
- Pillai, S.S.; Pereira, D.G.; Bonsu, G.; Chaudhry, H.; Puri, N.; Lakhani, H.V.; Tirona, M.T.; Sodhi, K.; Thompson, E. Biomarker panel for early screening of trastuzumab-induced cardiotoxicity among breast cancer patients in west virginia. Front. Pharmacol. 2022, 13, 953178. [Google Scholar] [CrossRef]
- Bisoc, A.; Ciurescu, D.; Rădoi, M.; Tântu, M.M.; Rogozea, L.; Sweidan, A.J.; Bota, D.A. Elevations in High-Sensitive Cardiac Troponin T and N-Terminal Prohormone Brain Natriuretic Peptide Levels in the Serum Can Predict the Development of Anthracycline-Induced Cardiomyopathy. Am. J. Ther. 2020, 27, e142–e150. [Google Scholar] [CrossRef]
- Goel, S.; Liu, J.; Guo, H.; Barry, W.; Bell, R.; Murray, B.; Lynch, J.; Bastick, P.; Chantrill, L.; Kiely, B.E.; et al. Decline in Left Ventricular Ejection Fraction Following Anthracyclines Predicts Trastuzumab Cardiotoxicity. JACC Heart Fail. 2019, 7, 795–804. [Google Scholar] [CrossRef]
- Gulati, G.; Heck, S.L.; Røsjø, H.; Ree, A.H.; Hoffmann, P.; Hagve, T.A.; Norseth, J.; Gravdehaug, B.; Steine, K.; Geisler, J.; et al. Neurohormonal Blockade and Circulating Cardiovascular Biomarkers during Anthracycline Therapy in Breast Cancer Patients: Results From the PRADA (Prevention of Cardiac Dysfunction during Adjuvant Breast Cancer Therapy) Study. J. Am. Heart Assoc. 2017, 6, e006513. [Google Scholar] [CrossRef] [PubMed]
- Gullo, G.; Eustace, A.J.; Canonici, A.; Collins, D.M.; Kennedy, M.J.; Grogan, L.; Breathhnach, O.; McCaffrey, J.; Keane, M.; Martin, M.J.; et al. Pilot study of bevacizumab in combination with docetaxel and cyclophosphamide as adjuvant treatment for patients with early stage HER-2 negative breast cancer, including analysis of candidate circulating markers of cardiac toxicity: ICORG 08-10 trial. Ther. Adv. Med. Oncol. 2019, 11, 1758835919864236. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, A.A.; Haykowsky, M.J.; Beaudry, R.I.; Grenier, J.G.; Mackey, J.R.; Pituskin, E.; Paterson, D.I.; Thompson, R.B. Cardiac and skeletal muscle predictors of impaired cardiorespiratory fitness post-anthracycline chemotherapy for breast cancer. Sci. Rep. 2021, 11, 14005. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, H.V.; Pillai, S.S.; Zehra, M.; Dao, B.; Tirona, M.T.; Thompson, E.; Sodhi, K. Detecting early onset of anthracyclines-induced cardiotoxicity using a novel panel of biomarkers in West-Virginian population with breast cancer. Sci. Rep. 2021, 11, 7954. [Google Scholar] [CrossRef]
- Malik, A.; Jeyaraj, P.A.; Calton, R.; Uppal, B.; Negi, P.; Shankar, A.; Patil, J.; Mahajan, M.K. Are Biomarkers Predictive of Anthracycline-Induced Cardiac Dysfunction? Asian Pac. J. Cancer Prev. 2016, 17, 2301–2305. [Google Scholar] [CrossRef]
- Ponde, N.; Bradbury, I.; Lambertini, M.; Ewer, M.; Campbell, C.; Ameels, H.; Zardavas, D.; Di Cosimo, S.; Baselga, J.; Huober, J.; et al. Cardiac biomarkers for early detection and prediction of trastuzumab and/or lapatinib-induced cardiotoxicity in patients with HER2-positive early-stage breast cancer: A NeoALTTO sub-study (BIG 1-06). Breast Cancer Res. Treat. 2018, 168, 631–638. [Google Scholar] [CrossRef]
- Putt, M.; Hahn, V.S.; Januzzi, J.L.; Sawaya, H.; Sebag, I.A.; Plana, J.C.; Picard, M.H.; Carver, J.R.; Halpern, E.F.; Kuter, I.; et al. Longitudinal Changes in Multiple Biomarkers Are Associated with Cardiotoxicity in Breast Cancer Patients Treated with Doxorubicin, Taxanes, and Trastuzumab. Clin. Chem. 2015, 61, 1164–1172. [Google Scholar] [CrossRef]
- Rüger, A.M.; Schneeweiss, A.; Seiler, S.; Tesch, H.; van Mackelenbergh, M.; Marmé, F.; Lübbe, K.; Sinn, B.; Karn, T.; Stickeler, E.; et al. Cardiotoxicity and Cardiovascular Biomarkers in Patients with Breast Cancer: Data From the GeparOcto-GBG 84 Trial. J. Am. Heart Assoc. 2020, 9, e018143. [Google Scholar] [CrossRef]
- De Sanctis, V.; Alfò, M.; Vitiello, C.; Vullo, G.; Facondo, G.; Marinelli, L.; Burocchi, S.; Gallo, G.; Valeriani, M.; Campanella, B.; et al. Markers of Cardiotoxicity in Early Breast Cancer Patients Treated with a Hypofractionated Schedule: A Prospective Study. Clin. Breast Cancer 2021, 21, e141–e149. [Google Scholar] [CrossRef]
- de Vries Schultink, A.H.M.; Boekhout, A.H.; Gietema, J.A.; Burylo, A.M.; Dorlo, T.P.C.; van Hasselt, J.G.C.; Schellens, J.H.M.; Huitema, A.D.R. Pharmacodynamic modeling of cardiac biomarkers in breast cancer patients treated with anthracycline and trastuzumab regimens. J. Pharmacokinet. Pharmacodyn. 2018, 45, 431–442. [Google Scholar] [CrossRef]
- Silva, F.B.; Romero, W.G.; Carvalho, A.; Souza, G.A.A.; Claudio, E.R.G.; Abreu, G.R. Effects of treatment with chemotherapy and/or tamoxifen on the biomarkers of cardiac injury and oxidative stress in women with breast cancer. Medicine 2017, 96, e8723. [Google Scholar] [CrossRef] [PubMed]
- Simões, R.; Silva, L.M.; de Oliveira, A.N.; Alves, M.T.; Pestana, R.M.C.; de Souza, I.D.P.; Oliveira, H.H.M.; Soares, C.E.; Sabino, A.P.; Gomes, K.B. Identification of Clinical and Laboratory Variables Associated with Cardiotoxicity Events Due to Doxorubicin in Breast Cancer Patients: A 1-Year Follow-Up Study. Cardiovasc. Toxicol. 2021, 21, 106–114. [Google Scholar] [CrossRef]
- Ürun, Y.; Utkan, G.; Yalcin, B.; Akbulut, H.; Onur, H.; Oztuna, D.G.; Şenler, F.C.; Demirkazık, A.; İçli, F. The role of cardiac biomarkers as predictors of trastuzumab cardiotoxicity in patients with breast cancer. Exp. Oncol. 2015, 37, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Ky, B.; Putt, M.; Sawaya, H.; French, B.; Januzzi, J.L., Jr.; Sebag, I.A.; Plana, J.C.; Cohen, V.; Banchs, J.; Carver, J.R.; et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J. Am. Coll. Cardiol. 2014, 63, 809–816. [Google Scholar] [CrossRef]
- Anber, Z.N.H.; Saleh, B.O.M.; Al-Rawi, S.A. The cardiotoxicity effect of different chemotherapeutic regimens in Iraqi patients with breast cancer: A follow up study. Heliyon 2019, 5, e02194. [Google Scholar] [CrossRef]
- Dhesy-Thind, S.; Ellis, P.M.; Mukherjee, S.D.; Mackett, K.; Bordeleau, L.; Kavsak, P.A. Longitudinal High-Sensitivity Cardiac Troponin I Measurements in Patients with Breast Cancer Receiving Trastuzumab. Can. J. Cardiol. 2019, 35, e541–e545. [Google Scholar] [CrossRef]
- Dovganych, N.V.; Kozhukhov, S.M.; Smolanka, I.I.; Lygyrda, O.F.; Bazyka, O.Y.; Lyalkin, S.A.; Ivankova, O.M.; Yarynkina, O.A.; Tkhor, N.V. Cardiotoxicity in breast cancer patients: Relationship of HS-troponin T changes and heart function in cancer treatment. Probl. Radiatsiinoi Medytsyny Radiobiolohii 2022, 27, 440–454. [Google Scholar] [CrossRef] [PubMed]
- Finke, D.; Romann, S.W.; Heckmann, M.B.; Hund, H.; Bougatf, N.; Kantharajah, A.; Katus, H.A.; Müller, O.J.; Frey, N.; Giannitsis, E.; et al. High-sensitivity cardiac troponin T determines all-cause mortality in cancer patients: A single-centre cohort study. ESC Heart Fail. 2021, 8, 3709–3719. [Google Scholar] [CrossRef]
- Florido, R.; Lee, A.K.; McEvoy, J.W.; Hoogeveen, R.C.; Koton, S.; Vitolins, M.Z.; Shenoy, C.; Russell, S.D.; Blumenthal, R.S.; Ndumele, C.E.; et al. Cancer survivorship and subclinical myocardial damage. Am. J. Epidemiol. 2019, 188, 2188–2195. [Google Scholar] [CrossRef]
- Henriksen, P.A.; Hall, P.; Oikonomidou, O.; MacPherson, I.R.; Maclean, M.; Lewis, S.; McVicars, H.; Broom, A.; Scott, F.; McKay, P.; et al. Rationale and Design of the Cardiac CARE Trial: A Randomized Trial of Troponin-Guided Neurohormonal Blockade for the Prevention of Anthracycline Cardiotoxicity. Circ. Heart Fail. 2022, 15, e009445. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, Y.H.; Ishardyanto, H.; Anniwati, L. High Sensitive Troponin I and Extended Range C-Reactive Protein as Markers to Predict Cardiotoxicity in Locally Advanced Breast Cancer with Neoadjuvant CAF (Cyclophoshpamide, Adriamycin/Doxorubicin, 5Fluorouracil) Therapy. Folia Med. Indones. 2020, 56, 91–98. [Google Scholar] [CrossRef]
- Hinrichs, L.; Mrotzek, S.M.; Mincu, R.I.; Pohl, J.; Röll, A.; Michel, L.; Mahabadi, A.A.; Al-Rashid, F.; Totzeck, M.; Rassaf, T. Troponins and Natriuretic Peptides in Cardio-Oncology Patients-Data From the ECoR Registry. Front. Pharmacol. 2020, 11, 740. [Google Scholar] [CrossRef] [PubMed]
- Isemede, D.A.; Sharma, A.; Bailey, J. Assessing the cardiotoxicity of Epirubicin-based chemotherapy in patients with breast cancer using high-sensitivity cardiac troponin T, N-terminal pro b-type natriuretic peptide and soluble suppression of tumorigenicity-2. Ann. Clin. Biochem. 2022, 59, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; O’Gorman, P.; Kelly, C.; Mahon, N.; Fitzgibbon, M.C. High-sensitive cardiac troponin-I facilitates timely detection of subclinical anthracycline-mediated cardiac injury. Ann. Clin. Biochem. 2017, 54, 149–157. [Google Scholar] [CrossRef]
- Mokuyasu, S.; Suzuki, Y.; Kawahara, E.; Seto, T.; Tokuda, Y. High-sensitivity cardiac troponin I detection for 2 types of drug-induced cardiotoxicity in patients with breast cancer. Breast Cancer 2015, 22, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Rosenkaimer, S.L.; Winter, L.; Sieburg, T.; Maier, S.; Mavratzas, A.; Hofmann, W.K.; Akin, I.; Duerschmied, D.; Hofheinz, R.D.; Hohneck, A. Diagnostic Value of sST2, VCAM-1, and Adiponectin in Patients with Breast Cancer to Predict Anti-Tumour Treatment-Related Cardiac Events: A Pilot Study. Oncol. Res. Treat. 2022, 45, 598–607. [Google Scholar] [CrossRef]
- Posch, F.; Niedrist, T.; Glantschnig, T.; Firla, S.; Moik, F.; Kolesnik, E.; Wallner, M.; Verheyen, N.; Jost, P.J.; Zirlik, A.; et al. Left ventricular ejection fraction and cardiac biomarkers for dynamic prediction of cardiotoxicity in early breast cancer. Front. Cardiovasc. Med. 2022, 9, 933428. [Google Scholar] [CrossRef]
- Ben Kridis, W.; Sghaier, S.; Charfeddine, S.; Toumi, N.; Daoud, J.; Kammoun, S.; Khanfir, A. A Prospective Study About Trastuzumab-induced Cardiotoxicity in HER2-positive Breast Cancer. Am. J. Clin. Oncol. 2020, 43, 510–516. [Google Scholar] [CrossRef]
- Clerico, A.; Fontana, M.; Zyw, L.; Passino, C.; Emdin, M. Comparison of the diagnostic accuracy of brain natriuretic peptide (BNP) and the N-terminal part of the propeptide of BNP immunoassays in chronic and acute heart failure: A systematic review. Clin. Chem. 2007, 53, 813–822. [Google Scholar] [CrossRef]
- Rudolf, H.; Mügge, A.; Trampisch, H.J.; Scharnagl, H.; März, W.; Kara, K. NT-proBNP for risk prediction of cardiovascular events and all-cause mortality: The getABI-study. IJC Heart Vasc. 2020, 29, 100553. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.E.; Linderholm, B.; Giglio, D. Delta NT-proBNP predicts cardiotoxicity in HER2-positive breast cancer patients treated with trastuzumab. Acta Oncol. 2021, 60, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Blancas, I.; Martín-Pérez, F.J.; Garrido, J.M.; Rodríguez-Serrano, F. NT-proBNP as predictor factor of cardiotoxicity during trastuzumab treatment in breast cancer patients. Breast 2020, 54, 106–113. [Google Scholar] [CrossRef]
- Bouwer, N.I.; Liesting, C.; Kofflard, M.J.M.; Sprangers-Van Campen, S.M.; Brugts, J.J.; Kitzen, J.J.E.M.; Fouraux, M.A.; Levin, M.D.; Boersma, E. NT-proBNP correlates with LVEF decline in HER2-positive breast cancer patients treated with trastuzumab. Cardio-Oncol. 2019, 5, 4. [Google Scholar] [CrossRef]
- Alves, M.T.; Simões, R.; Pestana, R.M.C.; de Oliveira, A.N.; Oliveira, H.H.M.; Soares, C.E.; Sabino, A.D.P.; Silva, L.M.; Gomes, K.B. Interleukin-10 Levels are Associated with Doxorubicin-Related Cardiotoxicity in Breast Cancer Patients in a One-Year Follow-Up Study. Immunol. Investig. 2022, 51, 883–898. [Google Scholar] [CrossRef] [PubMed]
- Matos, E.; Jug, B.; Blagus, R.; Zakotnik, B. A Prospective Cohort Study on Cardiotoxicity of Adjuvant Trastuzumab Therapy in Breast Cancer Patients. Arq. Bras. Cardiol. 2016, 107, 40–47. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, Y.; Chen, C.; Han, C.; Xue, L.; Xing, D.; Huang, O.; Tao, M. BNP as a marker for early prediction of anthracycline-induced cardiotoxicity in patients with breast cancer. Oncol. Lett. 2019, 18, 4992–5001. [Google Scholar] [CrossRef]
- Kouloubinis, A.; Sofroniadou, S.; Panoulas, V.F.; Makaritsis, K.; Revela, I.; Karavolias, G.; Voudris, V.; Adamopoulos, S. The role of TNF-α, Fas/Fas ligand system and NT-proBNP in the early detection of asymptomatic left ventricular dysfunction in cancer patients treated with anthracyclines. Int. J. Cardiol. Heart Vasc. 2015, 6, 85–90. [Google Scholar] [CrossRef]
- De Iuliis, F.; Salerno, G.; Taglieri, L.; De Biase, L.; Lanza, R.; Cardelli, P.; Scarpa, S. Serum biomarkers evaluation to predict chemotherapy-induced cardiotoxicity in breast cancer patients. Tumour Biol. 2016, 37, 3379–3387. [Google Scholar] [CrossRef]
- Demissei, B.G.; Hubbard, R.A.; Zhang, L.; Smith, A.M.; Sheline, K.; McDonald, C.; Narayan, V.; Domchek, S.M.; DeMichele, A.; Shah, P.; et al. Changes in Cardiovascular Biomarkers with Breast Cancer Therapy and Associations with Cardiac Dysfunction. J. Am. Heart Assoc. 2020, 9, e014708. [Google Scholar] [CrossRef]
- Demissei, B.G.; Finkelman, B.S.; Hubbard, R.A.; Zhang, L.; Smith, A.M.; Sheline, K.; McDonald, C.; Narayan, H.K.; Narayan, V.; Waxman, A.J.; et al. Detailed phenotyping reveals distinct trajectories of cardiovascular function and symptoms with exposure to modern breast cancer therapy. Cancer 2019, 125, 2762–2771. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, Q.; Hu, C. Early Predictive Value of NT-proBNP Combined with Echocardiography in Anthracyclines Induced Cardiotoxicity. Front. Surg. 2022, 9, 898172. [Google Scholar] [CrossRef]
- El-Sherbeny, W.S.; Sabry, N.M.; Sharbay, R.M. Prediction of trastuzumab-induced cardiotoxicity in breast cancer patients receiving anthracycline-based chemotherapy. J. Echocardiogr. 2019, 17, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Grela-Wojewoda, A.; Püsküllüoğlu, M.; Sas-Korczyńska, B.; Zemełka, T.; Pacholczak-Madej, R.; Wysocki, W.M.; Wojewoda, T.; Adamczyk, A.; Lompart, J.; Korman, M.; et al. Biomarkers of Trastuzumab-Induced Cardiac Toxicity in HER2-Positive Breast Cancer Patient Population. Cancers 2022, 14, 3353. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Brundage, R.C.; Jacobson, P.A.; Blaes, A.; Kirstein, M.N. Pharmacokinetic-pharmacodynamic modelling of acute N-terminal pro B-type natriuretic peptide after doxorubicin infusion in breast cancer. Br. J. Clin. Pharmacol. 2016, 82, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Herrmann, J.; Vierkant, R.A.; Olson, J.E.; Couch, F.J.; Hazim, A.; Sloan, J.A.; Loprinzi, C.L.; Ruddy, K.J. N-Terminal Pro Brain Natriuretic Peptide, sST2, and Galectin-3 Levels in Breast Cancer Survivors. J. Clin. Med. 2021, 10, 3313. [Google Scholar] [CrossRef] [PubMed]
- Skovgaard, D.; Hasbak, P.; Kjaer, A. BNP predicts chemotherapy-related cardiotoxicity and death: Comparison with gated equilibrium radionuclide ventriculography. PLoS ONE 2014, 9, e96736. [Google Scholar] [CrossRef] [PubMed]
- Caram, M.E.V.; Guo, C.; Leja, M.; Smerage, J.; Henry, N.L.; Giacherio, D.; Rubenfire, M.; Schott, A.; Davis, M.; Hayes, D.F.; et al. Doxorubicin-induced cardiac dysfunction in unselected patients with a history of early-stage breast cancer. Breast Cancer Res. Treat. 2015, 152, 163–172. [Google Scholar] [CrossRef]
- Dores, H.; Abecasis, J.; Correia, M.J.; Gândara, F.; Fonseca, C.; Azevedo, J.; Arroja, I.; Martins, A.; Mendes, M. Detection of early sub-clinical trastuzumab-induced cardiotoxicity in breast cancer patients. Arq. Bras. Cardiol. 2013, 100, 328–332. [Google Scholar] [CrossRef]
- Kittiwarawut, A.; Vorasettakarnkij, Y.; Tanasanvimon, S.; Manasnayakorn, S.; Sriuranpong, V. Serum NT-proBNP in the early detection of doxorubicin-induced cardiac dysfunction. Asia Pac. J. Clin. Oncol. 2013, 9, 155–161. [Google Scholar] [CrossRef]
- Özbay, B.; Şimşek, E.; Kemal, H.; Çakar, B.; Yavuzgil, O. Anthracycline Chemotherapy-Induced Electro-Mechanical Changes: Strain Echocardiography Combined with Repolarization Parameters on Electrocardiography to Predict Early Cardiotoxicity. Turk. Kardiyol. Dern. Ars. 2022, 50, 478–484. [Google Scholar] [CrossRef]
- Dhir, V.; Yan, A.T.; Nisenbaum, R.; Sloninko, J.; Connelly, K.A.; Barfett, J.; Haq, R.; Kirpalani, A.; Chan, K.K.W.; Petrella, T.M.; et al. Assessment of left ventricular function by CMR versus MUGA scans in breast cancer patients receiving trastuzumab: A prospective observational study. Int. J. Cardiovasc. Imaging 2019, 35, 2085–2093. [Google Scholar] [CrossRef]
- Esmaeilzadeh, M.; Fresno, C.M.U.; Somerset, E.; Shalmon, T.; Amir, E.; Fan, C.S.; Brezden-Masley, C.; Thampinathan, B.; Thevakumaran, Y.; Yared, K.; et al. A Combined Echocardiography Approach for the Diagnosis of Cancer Therapy-Related Cardiac Dysfunction in Women with Early-Stage Breast Cancer. JAMA Cardiol. 2022, 7, 330–340. [Google Scholar] [CrossRef]
- Feng, Q.; Ren, Y.; Hou, A.; Guo, J.; Mao, Z.; Liu, S.; Wang, B.; Bai, Z.; Hou, X. MicroRNA-130a Increases and Predicts Cardiotoxicity during Adjuvant Chemotherapy in Human Epidermal Growth Factor Receptor-2-Positive Breast Cancer. J. Breast Cancer 2021, 24, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Gherghe, M.; Lazar, A.M.; Mutuleanu, M.D.; Bordea, C.I.; Ionescu, S.; Mihaila, R.I.; Petroiu, C.; Stanciu, A.E. Evaluating Cardiotoxicity in Breast Cancer Patients Treated with HER2 Inhibitors: Could a Combination of Radionuclide Ventriculography and Cardiac Biomarkers Predict the Cardiac Impact? Cancers 2022, 15, 207. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, P.W.; Richards, D.A.; Boyd, A.; Hui, R.; Harnett, P.R.; Meikle, S.R.; Clarke, J.L.; Thomas, L. Altered left ventricular longitudinal diastolic function correlates with reduced systolic function immediately after anthracycline chemotherapy. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 228–234. [Google Scholar] [CrossRef]
- Stoodley, P.W.; Richards, D.A.; Boyd, A.; Hui, R.; Harnett, P.R.; Meikle, S.R.; Byth, K.; Stuart, K.; Clarke, J.L.; Thomas, L. Left ventricular systolic function in HER2/neu negative breast cancer patients treated with anthracycline chemotherapy: A comparative analysis of left ventricular ejection fraction and myocardial strain imaging over 12 months. Eur. J. Cancer 2013, 49, 3396–3403. [Google Scholar] [CrossRef] [PubMed]
- Suerken, C.K.; D’Agostino, R.B., Jr.; Jordan, J.H.; Meléndez, G.C.; Vasu, S.; Lamar, Z.S.; Hundley, W.G. Simultaneous Left Ventricular Volume and Strain Changes during Chemotherapy Associate with 2-Year Postchemotherapy Measures of Left Ventricular Ejection Fraction. J. Am. Heart Assoc. 2020, 9, e015400. [Google Scholar] [CrossRef]
- Bulten, B.F.; Verberne, H.J.; Bellersen, L.; Oyen, W.J.G.; Sabaté-Llobera, A.; Mavinkurve-Groothuis, A.M.C.; Kapusta, L.; Van Laarhoven, H.W.M.; De Geus-Oei, L.F. Relationship of promising methods in the detection of anthracycline-induced cardiotoxicity in breast cancer patients. Cancer Chemother. Pharmacol. 2015, 76, 957–967. [Google Scholar] [CrossRef]
- Tahir, E.; Azar, M.; Shihada, S.; Seiffert, K.; Goy, Y.; Beitzen-Heineke, A.; Molwitz, I.; Muellerleile, K.; Stehning, C.; Schön, G.; et al. Myocardial injury detected by T1 and T2 mapping on CMR predicts subsequent cancer therapy-related cardiac dysfunction in patients with breast cancer treated by epirubicin-based chemotherapy or left-sided RT. Eur. Radiol. 2022, 32, 1853–1865. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, C.; Li, H.; Liu, K.; Yu, M.; Zhang, P. 3D-STI evaluation of the effect of dexrazoxane on the mechanical properties of right ventricular myocardium in breast cancer patients treated with pirarubicin. Ann. Palliat. Med. 2020, 9, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Ganatra, S.; Nohria, A.; Shah, S.; Groarke, J.D.; Sharma, A.; Venesy, D.; Patten, R.; Gunturu, K.; Zarwan, C.; Neilan, T.G.; et al. Upfront dexrazoxane for the reduction of anthracycline-induced cardiotoxicity in adults with preexisting cardiomyopathy and cancer: A consecutive case series. Cardio-Oncol. 2019, 5, 1. [Google Scholar] [CrossRef]
- Tromp, J.; Boerman, L.M.; Sama, I.E.; Maass, S.; Maduro, J.H.; Hummel, Y.M.; Berger, M.Y.; de Bock, G.H.; Gietema, J.A.; Berendsen, A.J.; et al. Long-term survivors of early breast cancer treated with chemotherapy are characterized by a pro-inflammatory biomarker profile compared to matched controls. Eur. J. Heart Fail. 2020, 22, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.; Lamacie, M.; Thampinathan, B.; Altaha, M.A.; Esmaeilzadeh, M.; Nolan, M.; Fresno, C.U.; Somerset, E.; Amir, E.; Marwick, T.H.; et al. Variability in echocardiography and MRI for detection of cancer therapy cardiotoxicity. Heart 2020, 106, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Plana, J.C.; Thavendiranathan, P.; Bucciarelli-Ducci, C.; Lancellotti, P. Multi-Modality Imaging in the Assessment of Cardiovascular Toxicity in the Cancer Patient. JACC Cardiovasc. Imaging 2018, 11, 1173–1186. [Google Scholar] [CrossRef]
- Houbois, C.P.; Nolan, M.; Somerset, E.; Shalmon, T.; Esmaeilzadeh, M.; Lamacie, M.M.; Amir, E.; Brezden-Masley, C.; Koch, C.A.; Thevakumaran, Y.; et al. Serial Cardiovascular Magnetic Resonance Strain Measurements to Identify Cardiotoxicity in Breast Cancer: Comparison with Echocardiography. JACC Cardiovasc. Imaging 2021, 14, 962–974. [Google Scholar] [CrossRef]
- Liu, J.E.; Barac, A.; Thavendiranathan, P.; Scherrer-Crosbie, M. Strain Imaging in Cardio-Oncology. JACC CardioOncol. 2020, 2, 677–689. [Google Scholar] [CrossRef]
- Florescu, M.; Magda, L.S.; Enescu, O.A.; Jinga, D.; Vinereanu, D. Early detection of epirubicin-induced cardiotoxicity in patients with breast cancer. J. Am. Soc. Echocardiogr. 2014, 27, 83–92. [Google Scholar] [CrossRef]
- Bulten, B.F.; Mavinkurve-Groothuis, A.M.C.; De Geus-Oei, L.F.; De Haan, A.F.J.; De Korte, C.L.; Bellersen, L.; Van Laarhoven, H.W.M.; Kapusta, L. Early myocardial deformation abnormalities in breast cancer survivors. Breast Cancer Res. Treat. 2014, 146, 127–135. [Google Scholar] [CrossRef]
- Huang, F.; Brezden-Masley, C.; Chan, K.K.W.; Barfett, J.J.; Kirpalani, A.; Deva, D.P.; Jimenez-Juan, L.; Barthur, A.; Song, L.; Chacko, B.; et al. Evaluation of left atrial remodeling using cardiovascular magnetic resonance imaging in breast cancer patients treated with adjuvant trastuzumab. Eur. Radiol. 2022, 32, 4234–4242. [Google Scholar] [CrossRef]
- Huang, P.; Dai, S.; Ye, Z.; Liu, Y.; Chen, Z.; Zheng, Y.; Shao, X.; Lei, L.; Wang, X. Long-term tolerance and cardiac function in breast cancer patients receiving trastuzumab therapy. Oncotarget 2017, 8, 2069–2075. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Shen, H.; Liu, R.; Wang, X.; Li, X.; Yuan, X.; Chen, Q.; Wang, Y.; Ran, Z.; Lan, X.; et al. Myocardial extracellular volume derived from contrast-enhanced chest computed tomography for longitudinal evaluation of cardiotoxicity in patients with breast cancer treated with anthracyclines. Insights Imaging 2022, 13, 85. [Google Scholar] [CrossRef] [PubMed]
- Todorova, V.K.; Hsu, P.C.; Wei, J.Y.; Lopez-Candales, A.; Chen, J.Z.; Su, L.J.; Makhoul, I. Biomarkers of inflammation, hypercoagulability and endothelial injury predict early asymptomatic doxorubicin-induced cardiotoxicity in breast cancer patients. Am. J. Cancer Res. 2020, 10, 2933–2945. [Google Scholar]
- Cruz, M.C.; Branco, L.M.; Portugal, G.; Galrinho, A.; Timóteo, A.T.; Rio, P.; Moreira, R.I.; Mendonça, T.; Leal, A.; Gameiro, F.; et al. Three-dimensional speckle-tracking echocardiography for the global and regional assessments of left ventricle myocardial deformation in breast cancer patients treated with anthracyclines. Clin. Res. Cardiol. 2020, 109, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.; Guo, H.; Najita, J.; Yardley, D.; Marcom, K.; Albain, K.; Rugo, H.; Miller, K.; Ellis, M.; Shapira, I.; et al. Cardiac Outcomes of Patients Receiving Adjuvant Weekly Paclitaxel and Trastuzumab for Node-Negative, ERBB2-Positive Breast Cancer. JAMA Oncol. 2016, 2, 29–36. [Google Scholar] [CrossRef]
- Demissei, B.G.; Fan, Y.; Qian, Y.; Cheng, H.G.; Smith, A.M.; Shimamoto, K.; Vedage, N.; Narayan, H.K.; Scherrer-Crosbie, M.; Davatzikos, C.; et al. Left ventricular segmental strain and the prediction of cancer therapy-related cardiac dysfunction. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 418–426. [Google Scholar] [CrossRef]
- de Souza, T.F.; Silva, T.Q.A.C.; Costa, F.O.; Shah, R.; Neilan, T.G.; Velloso, L.; Nadruz, W.; Brenelli, F.; Sposito, A.C.; Matos-Souza, J.R.; et al. Anthracycline Therapy Is Associated with Cardiomyocyte Atrophy and Preclinical Manifestations of Heart Disease. JACC Cardiovasc. Imaging 2018, 11, 1045–1055. [Google Scholar] [CrossRef]
- Ferreira, V.V.; Mano, T.B.; Cardoso, I.; Cruz, M.C.; Branco, L.M.; Almeida-Morais, L.; Timóteo, A.; Galrinho, A.; Castelo, A.; Brás, P.G.; et al. Myocardial Work Brings New Insights into Left Ventricular Remodelling in Cardio-Oncology Patients. Int. J. Environ. Res. Public Health 2022, 19, 2826. [Google Scholar] [CrossRef]
- Gavila, J.; Seguí, M.; Calvo, L.; López, T.; Alonso, J.J.; Farto, M.; Sánchez-de la Rosa, R. Evaluation and management of chemotherapy-induced cardiotoxicity in breast cancer: A Delphi study. Clin. Transl. Oncol. 2017, 19, 91–104. [Google Scholar] [CrossRef]
- Honda, K.; Takeshita, K.; Murotani, K.; Mitsuma, A.; Hayashi, H.; Tsunoda, N.; Kikumori, T.; Murohara, T.; Ando, Y. Assessment of left ventricular diastolic function during trastuzumab treatment in patients with HER2-positive breast cancer. Breast Cancer 2017, 24, 312–318. [Google Scholar] [CrossRef]
- Huang, G.; Zhai, J.; Huang, X.; Zheng, D. Predictive value of soluble ST-2 for changes of cardiac function and structure in breast cancer patients receiving chemotherapy. Medicine 2018, 97, e12447. [Google Scholar] [CrossRef] [PubMed]
- Jordan, J.H.; D’Agostino, R.B., Jr.; Hamilton, C.A.; Vasu, S.; Hall, M.E.; Kitzman, D.W.; Thohan, V.; Lawrence, J.A.; Ellis, L.R.; Lash, T.L.; et al. Longitudinal assessment of concurrent changes in left ventricular ejection fraction and left ventricular myocardial tissue characteristics after administration of cardiotoxic chemotherapies using T1-weighted and T2-weighted cardiovascular magnetic resonance. Circ. Cardiovasc. Imaging 2014, 7, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Kibudde, S.; Mondo, C.K.; Kibirige, D.; Walusansa, V.; Orem, J. Anthracycline induced cardiotoxicity in adult cancer patients: A prospective cohort study from a specialized oncology treatment centre in Uganda. Afr. Health Sci. 2019, 19, 1647–1656. [Google Scholar] [CrossRef]
- Kim, E.K.; Cho, J.; Kim, J.Y.; Chang, S.A.; Park, S.J.; Choi, J.O.; Lee, S.C.; Ahn, J.S.; Park, S.W.; Im, Y.H.; et al. Early Decline in Left Ventricular Ejection Fraction Can Predict Trastuzumab-Related Cardiotoxicity in Patients with Breast Cancer: A Study Using 13 Years of Registry Data. Cancer Res. Treat. 2019, 51, 727–736. [Google Scholar] [CrossRef]
- Liu, X.; Tao, L.; Wang, M.; Li, H.; Xu, W. ABSDELL Model: Development and Internal Validation of a Risk Prediction Model of LVEF Decline in Breast Cancer Patients Treated with Trastuzumab. Clin. Breast Cancer 2023, 23, 23–31. [Google Scholar] [CrossRef]
- Lorenzini, C.; Lamberti, C.; Aquilina, M.; Rocca, A.; Cortesi, P.; Corsi, C. Reliability of Left Ventricular Ejection Fraction from Three-Dimensional Echocardiography for Cardiotoxicity Onset Detection in Patients with Breast Cancer. J. Am. Soc. Echocardiogr. 2017, 30, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Mescher, C.; Ding, C.; Defor, T.; Konety, S.; Blaes, A. Left Ventricular Ejection Fraction Screening and Clinical Decision-making in Metastatic HER2-positive Breast Cancer. Anticancer Res. 2017, 37, 3751–3755. [Google Scholar] [CrossRef]
- Sun, Y.; Li, T.; Zhang, Y.; Zhang, Q. Evaluation of Left Ventricular Ejection Fractions in Breast Cancer Patients Undergoing Long-Term Trastuzumab Treatment. Med. Sci. Monit. 2016, 22, 5035–5040. [Google Scholar] [CrossRef]
- Yamashita, K.; Tanaka, H.; Hatazawa, K.; Tanaka, Y.; Sumimoto, K.; Shono, A.; Suzuki, M.; Yokota, S.; Suto, M.; Mukai, J.; et al. Association between clinical risk factors and left ventricular function in patients with breast cancer following chemotherapy. Int. J. Cardiovasc. Imaging 2021, 37, 197–205. [Google Scholar] [CrossRef]
- Abdallah, I.B.; Nasr, S.B.; Chourabi, C.; Boukhris, M.; Zribi, A.; Fendri, S.; Balti, M.; Fehri, W.; Chraiet, N.; Haddaoui, A. The predictive value of 2D myocardial strain for epirubicin-induced cardiotoxicity. J. Oncol. 2020, 2020, 5706561. [Google Scholar] [CrossRef]
- Altaha, M.A.; Nolan, M.; Marwick, T.H.; Somerset, E.; Houbois, C.; Amir, E.; Yip, P.; Connelly, K.A.; Michalowska, M.; Sussman, M.S.; et al. Can Quantitative CMR Tissue Characterization Adequately Identify Cardiotoxicity during Chemotherapy?: Impact of Temporal and Observer Variability. JACC Cardiovasc. Imaging 2020, 13, 951–962. [Google Scholar] [CrossRef]
- Calle, M.C.A.; Sandhu, N.P.; Xia, H.; Cha, S.S.; Pellikka, P.A.; Ye, Z.; Herrmann, J.; Villarraga, H.R. Two-dimensional speckle tracking echocardiography predicts early subclinical cardiotoxicity associated with anthracycline-trastuzumab chemotherapy in patients with breast cancer. BMC Cancer 2018, 18, 1037. [Google Scholar] [CrossRef]
- Calvillo-Argüelles, O.; Thampinathan, B.; Somerset, E.; Shalmon, T.; Amir, E.; Steve Fan, C.P.; Moon, S.; Abdel-Qadir, H.; Thevakumaran, Y.; Day, J.; et al. Diagnostic and Prognostic Value of Myocardial Work Indices for Identification of Cancer Therapy-Related Cardiotoxicity. JACC Cardiovasc. Imaging 2022, 15, 1361–1376. [Google Scholar] [CrossRef]
- Chang, W.T.; Shih, J.Y.; Feng, Y.H.; Chiang, C.Y.; Kuo, Y.H.; Chen, W.Y.; Wu, H.C.; Cheng, J.T.; Wang, J.J.; Chen, Z.C. The Early Predictive Value of Right Ventricular Strain in Epirubicin-Induced Cardiotoxicity in Patients with Breast Cancer. Acta Cardiol. Sin. 2016, 32, 550–559. [Google Scholar] [CrossRef]
- Chang, W.T.; Feng, Y.H.; Kuo, Y.H.; Chen, W.Y.; Wu, H.C.; Huang, C.T.; Huang, T.L.; Chen, Z.C. Layer-specific distribution of myocardial deformation from anthracycline-induced cardiotoxicity in patients with breast cancer—From bedside to bench. Int. J. Cardiol. 2020, 311, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Choe, J.C.; Choi, J.H.; Choi, J.H.; Ahn, J.; Park, J.S.; Lee, H.W.; Oh, J.H.; Lee, H.C.; Cha, K.S.; Hong, T.J. Prolonged electromechanical delay as an early predictor of trastuzumab-induced cardiotoxicity in patients undergoing treatment for breast cancer. Clin. Cardiol. 2018, 41, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- de Barros, M.V.L.; Macedo, A.V.S.; Sarvari, S.I.; Faleiros, M.H.; Felipe, P.T.; Silva, J.L.P.; Edvardsen, T. Left ventricular regional wall motion abnormality is a strong predictor of cardiotoxicity in breast cancer patients undergoing chemotherapy. Arq. Bras. Cardiol. 2019, 112, 50–56. [Google Scholar] [CrossRef]
- de Souza, T.F.; Silva, T.Q.; Antunes-Correa, L.; Drobni, Z.D.; Costa, F.O.; Dertkigil, S.S.J.; Nadruz, W.; Brenelli, F.; Sposito, A.C.; Matos-Souza, J.R., Jr.; et al. Cardiac magnetic resonance assessment of right ventricular remodeling after anthracycline therapy. Sci. Rep. 2021, 11, 17132. [Google Scholar] [CrossRef] [PubMed]
- Gong, I.Y.; Ong, G.; Brezden-Masley, C.; Dhir, V.; Deva, D.P.; Chan, K.K.W.; Graham, J.J.; Chow, C.M.; Thavendiranathan, P.; Dai, D.; et al. Early diastolic strain rate measurements by cardiac MRI in breast cancer patients treated with trastuzumab: A longitudinal study. Int. J. Cardiovasc. Imaging 2019, 35, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Gripp, E.A.; Oliveira, G.E.; Feijó, L.A.; Garcia, M.I.; Xavier, S.S.; Sousa, A.S. Global Longitudinal Strain Accuracy for Cardiotoxicity Prediction in a Cohort of Breast Cancer Patients during Anthracycline and/or Trastuzumab Treatment. Arq. Bras. Cardiol. 2018, 110, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Bao, W.; Xu, Y.; Yang, W.; Li, M.; Xu, M.; Zhang, Y.; Zhang, M. Assessment of Myocardial Work in Cancer Therapy-Related Cardiac Dysfunction and Analysis of CTRCD Prediction by Echocardiography. Front. Pharmacol. 2021, 12, 770580. [Google Scholar] [CrossRef]
- Guerra, F.; Marchesini, M.; Contadini, D.; Menditto, A.; Morelli, M.; Piccolo, E.; Battelli, N.; Pistelli, M.; Berardi, R.; Cascinu, S.; et al. Speckle-tracking global longitudinal strain as an early predictor of cardiotoxicity in breast carcinoma. Support Care Cancer 2016, 24, 3139–3145. [Google Scholar] [CrossRef] [PubMed]
- Hochstadt, A.; Arnold, J.; Rosen, R.; Sherez, C.; Sherez, J.; Mor, L.; Derakhshesh, M.; Moshkovits, Y.; Merdler, I.; Arbel, Y.; et al. Diastolic strain time as predictor for systolic dysfunction among patients with active breast cancer. Echocardiography 2020, 37, 1890–1896. [Google Scholar] [CrossRef] [PubMed]
- Hochstadt, A.; Arnold, J.; Rosen, R.; Sherez, C.; Sherez, J.; Mor, L.; Moshkovits, Y.; Merdler, I.; Szekely, Y.; Arbel, Y.; et al. Longitudinal diastolic strain slope as an early sign for systolic dysfunction among patients with active cancer. Clin. Res. Cardiol. 2021, 110, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.A.; Small, A.D.; Ray, S.; Hamilton, D.J.; Martin, W.; Robinson, J.; Goodfield, N.E.R.; Paterson, C.A. Radionuclide ventriculography phase analysis for risk stratification of patients undergoing cardiotoxic cancer therapy. J. Nucl. Cardiol. 2022, 29, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Monti, C.B.; Zanardo, M.; Bosetti, T.; Alì, M.; De Benedictis, E.; Luporini, A.; Secchi, F.; Sardanelli, F. Assessment of myocardial extracellular volume on body computed tomography in breast cancer patients treated with anthracyclines. Quant. Imaging Med. Surg. 2020, 10, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Nakano, S.; Takahashi, M.; Kimura, F.; Senoo, T.; Saeki, T.; Ueda, S.; Tanno, J.; Senbonmatsu, T.; Kasai, T.; Nishimura, S. Cardiac magnetic resonance imaging-based myocardial strain study for evaluation of cardiotoxicity in breast cancer patients treated with trastuzumab: A pilot study to evaluate the feasibility of the method. Cardiol. J. 2016, 23, 270–280. [Google Scholar] [CrossRef]
- Narayan, H.K.; French, B.; Khan, A.M.; Plappert, T.; Hyman, D.; Bajulaiye, A.; Domchek, S.; DeMichele, A.; Clark, A.; Matro, J.; et al. Noninvasive Measures of Ventricular-Arterial Coupling and Circumferential Strain Predict Cancer Therapeutics-Related Cardiac Dysfunction. JACC Cardiovasc. Imaging 2016, 9, 1131–1141. [Google Scholar] [CrossRef]
- Narayan, H.K.; Finkelman, B.; French, B.; Plappert, T.; Hyman, D.; Smith, A.M.; Margulies, K.B.; Ky, B. Detailed Echocardiographic Phenotyping in Breast Cancer Patients: Associations with Ejection Fraction Decline, Recovery, and Heart Failure Symptoms Over 3 Years of Follow-Up. Circulation 2017, 135, 1397–1412. [Google Scholar] [CrossRef]
- Negishi, K.; Negishi, T.; Hare, J.L.; Haluska, B.A.; Plana, J.C.; Marwick, T.H. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J. Am. Soc. Echocardiogr. 2013, 26, 493–498. [Google Scholar] [CrossRef]
- Negishi, T.; Thavendiranathan, P.; Negishi, K.; Marwick, T.H.; Aakhus, S.; Murbræch, K.; Massey, R.; Bansal, M.; Fukuda, N.; Hristova, K.; et al. Rationale and Design of the Strain Surveillance of Chemotherapy for Improving Cardiovascular Outcomes: The SUCCOUR Trial. JACC Cardiovasc. Imaging 2018, 11, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Ong, G.; Brezden-Masley, C.; Dhir, V.; Deva, D.P.; Chan, K.K.W.; Chow, C.M.; Thavendiranathan, D.; Haq, R.; Barfett, J.J.; Petrella, T.M.; et al. Myocardial strain imaging by cardiac magnetic resonance for detection of subclinical myocardial dysfunction in breast cancer patients receiving trastuzumab and chemotherapy. Int. J. Cardiol. 2018, 261, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kim, K.H.; Kim, H.Y.; Cho, J.Y.; Yoon, H.J.; Hong, Y.J.; Park, H.W.; Kim, J.H.; Ahn, Y.; Jeong, M.H.; et al. Left atrial longitudinal strain as a predictor of Cancer therapeutics-related cardiac dysfunction in patients with breast Cancer. Cardiovasc. Ultrasound 2020, 18, 28. [Google Scholar] [CrossRef] [PubMed]
- Perone, F.; Zamora Auñon, P.; Rodríguez, L.; Vinal, D.; Caro-Codon, J.; Pertejo, A.; Martínez Marín, V.; Espinosa, E.; López-Fernández, T. Cardiac monitoring during trastuzumab therapy in metastatic breast cancer: Early incidence of cardiac dysfunction. Monaldi Arch. Chest Dis. 2022, 92. [Google Scholar] [CrossRef]
- Piotrowski, G.; Gawor, R.; Bourge, R.C.; Stasiak, A.; Potemski, P.; Gawor, Z.; Nanda, N.C.; Banach, M. Heart remodeling induced by adjuvant trastuzumab-containing chemotherapy for breast cancer overexpressing human epidermal growth factor receptor type 2: A prospective study. Pharmacol. Res. 2013, 78, 41–48. [Google Scholar] [CrossRef]
- Reuvekamp, E.J.; Bulten, B.F.; Nieuwenhuis, A.A.; Meekes, M.R.; de Haan, A.F.; Tol, J.; Maas, A.H.; Elias-Smale, S.E.; de Geus-Oei, L.F. Does diastolic dysfunction precede systolic dysfunction in trastuzumab-induced cardiotoxicity? Assessment with multigated radionuclide angiography (MUGA). J. Nucl. Cardiol. 2016, 23, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Rushton, M.; Johnson, C.; Dent, S. Trastuzumab-induced cardiotoxicity: Testing a clinical risk score in a real-world cardio-oncology population. Curr. Oncol. 2017, 24, 176–180. [Google Scholar] [CrossRef]
- Sato, A.; Yoshihisa, A.; Miyata-Tatsumi, M.; Oikawa, M.; Kobayashi, A.; Ishida, T.; Ohtake, T.; Takeishi, Y. Valvular heart disease as a possible predictor of trastuzumab-induced cardiotoxicity in patients with breast cancer. Mol. Clin. Oncol. 2019, 10, 37–42. [Google Scholar] [CrossRef]
- Seferina, S.C.; de Boer, M.; Derksen, M.W.; van den Berkmortel, F.; van Kampen, R.J.; van de Wouw, A.J.; Joore, M.; Peer, P.G.; Voogd, A.C.; Tjan-Heijnen, V.C. Cardiotoxicity and Cardiac Monitoring during Adjuvant Trastuzumab in Daily Dutch Practice: A Study of the Southeast Netherlands Breast Cancer Consortium. Oncologist 2016, 21, 555–562. [Google Scholar] [CrossRef]
- Tan, R.; Cong, T.; Xu, G.; Hao, Z.; Liao, J.; Xie, Y.; Lin, Y.; Yang, X.; Li, Q.; Liu, Y.; et al. Anthracycline-Induced Atrial Structural and Electrical Remodeling Characterizes Early Cardiotoxicity and Contributes to Atrial Conductive Instability and Dysfunction. Antioxid. Redox Signal. 2022, 37, 19–39. [Google Scholar] [CrossRef]
- Terui, Y.; Sugimura, K.; Ota, H.; Tada, H.; Nochioka, K.; Sato, H.; Katsuta, Y.; Fujiwara, J.; Harada-Shoji, N.; Sato-Tadano, A.; et al. Usefulness of cardiac magnetic resonance for early detection of cancer therapeutics-related cardiac dysfunction in breast cancer patients. Int. J. Cardiol. 2023, 371, 472–479. [Google Scholar] [CrossRef] [PubMed]
- van der Linde, D.; van Hagen, I.; Veen, K.; Zuetenhorst, H.; van Dalen, B. Global longitudinal strain: An early marker for cardiotoxicity in patients treated for breast cancer. Neth. Heart J. 2022, 31, 103–108. [Google Scholar] [CrossRef]
- Xu, H.; Mao, L.; Liu, H.; Zhang, Y.; Yang, J. Assessment of Subclinical Deterioration of Right Ventricular Function by Three-Dimensional Speckle Tracking Echocardiography in Breast Cancer Patients Undergoing Anthracycline-Based Chemotherapy. Int. J. Gen. Med. 2021, 14, 885–893. [Google Scholar] [CrossRef]
- Yaylali, Y.T.; Saricopur, A.; Yurtdas, M.; Senol, H.; Gokoz-Dogu, G. Atrial function in patients with breast cancer after treatment with anthracyclines. Arq. Bras. Cardiol. 2016, 107, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Yersal, Ö.; Eryilmaz, U.; Akdam, H.; Meydan, N.; Barutca, S. Arterial Stiffness in Breast Cancer Patients Treated with Anthracycline and Trastuzumab-Based Regimens. Cardiol. Res. Pract. 2018, 2018, 5352914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Azibani, F.; Okello, E.; Kayima, J.; Sinabulya, I.; Leeta, J.; Walusansa, V.; Orem, J.; Sliwa, K. Clinical characterization, cardiovascular risk factor profile and cardiac strain analysis in a Uganda cancer population: The SATRACD study. PLoS ONE 2021, 16, e0249717. [Google Scholar] [CrossRef]
- Zhang, K.W.; Finkelman, B.S.; Gulati, G.; Narayan, H.K.; Upshaw, J.; Narayan, V.; Plappert, T.; Englefield, V.; Smith, A.M.; Zhang, C.; et al. Abnormalities in 3-Dimensional Left Ventricular Mechanics with Anthracycline Chemotherapy Are Associated with Systolic and Diastolic Dysfunction. JACC Cardiovasc. Imaging 2018, 11, 1059–1068. [Google Scholar] [CrossRef]
- Feng, Y.; Qin, Z.; Yang, Z. Deceleration capacity of heart rate predicts trastuzumab-related cardiotoxicity in patients with HER2-positive breast cancer: A prospective observational study. J. Clin. Pharm. Ther. 2021, 46, 93–98. [Google Scholar] [CrossRef]
- Zhou, F.; Niu, L.; Zhao, M.; Ni, W.X.; Liu, J. Real-time three-dimensional echocardiography predicts cardiotoxicity induced by postoperative chemotherapy in breast cancer patients. World J. Clin. Cases 2020, 8, 2542–2553. [Google Scholar] [CrossRef]
- Chen, J.; Wang, L.; Wu, F.F.; Sun, G. Early detection of cardiotoxicity by 3D speckle tracking imaging of area strain in breast cancer patients receiving chemotherapy. Echocardiography 2019, 36, 1682–1688. [Google Scholar] [CrossRef]
- Tan, L.; Su, X.; Li, X.; Li, H.; Hu, B. Correlation of HER2 codon 655 polymorphism with cardiotoxicity risk in Chinese HER2-positive breast cancer patients undergoing epirubicin/cyclophosphamide followed by docetaxel plus trastuzumab adjuvant chemotherapy. Int. J. Clin. Exp. Pathol. 2020, 13, 286–294. [Google Scholar]
- Stanton, S.E.; Ward, M.M.; Christos, P.; Sanford, R.; Lam, C.; Cobham, M.V.; Donovan, D.; Scheff, R.J.; Cigler, T.; Moore, A.; et al. Pro1170 Ala polymorphism in HER2-neu is associated with risk of trastuzumab cardiotoxicity. BMC Cancer 2015, 15, 267. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.H.; Udagawa, C.; Shimo, A.; Kojima, Y.; Yoshie, R.; Zaha, H.; Abe, N.; Motonari, T.; Unesoko, M.; Tamura, K.; et al. A Genome-Wide Association Study Identifies Five Novel Genetic Markers for Trastuzumab-Induced Cardiotoxicity in Japanese Population. Biol. Pharm. Bull. 2019, 42, 2045–2053. [Google Scholar] [CrossRef] [PubMed]
- Reinbolt, R.E.; Patel, R.; Pan, X.; Timmers, C.D.; Pilarski, R.; Shapiro, C.L.; Lustberg, M.B. Risk factors for anthracycline-associated cardiotoxicity. Support Care Cancer 2016, 24, 2173–2180. [Google Scholar] [CrossRef] [PubMed]
- Vivenza, D.; Feola, M.; Garrone, O.; Monteverde, M.; Merlano, M.; Lo Nigro, C. Role of the renin-angiotensin-aldosterone system and the glutathione S-transferase Mu, Pi and Theta gene polymorphisms in cardiotoxicity after anthracycline chemotherapy for breast carcinoma. Int. J. Biol. Markers 2013, 28, e336–e347. [Google Scholar] [CrossRef] [PubMed]
- Serie, D.J.; Crook, J.E.; Necela, B.M.; Dockter, T.J.; Wang, X.; Asmann, Y.W.; Fairweather, D.; Bruno, K.A.; Colon-Otero, G.; Perez, E.A.; et al. Genome-wide association study of cardiotoxicity in the NCCTG N9831 (Alliance) adjuvant trastuzumab trial. Pharm. Genom. 2017, 27, 378–385. [Google Scholar] [CrossRef]
- Roca, L.; Diéras, V.; Roché, H.; Lappartient, E.; Kerbrat, P.; Cany, L.; Chieze, S.; Canon, J.-L.; Spielmann, M.; Penault-Llorca, F.; et al. Correlation of HER2, FCGR2A, and FCGR3A gene polymorphisms with trastuzumab related cardiac toxicity and efficacy in a subgroup of patients from UNICANCER-PACS04 trial. Breast Cancer Res. Treat. 2013, 139, 789–800. [Google Scholar] [CrossRef]
- Gómez Peña, C.; Dávila-Fajardo, C.L.; Martínez-González, L.J.; Carmona-Sáez, P.; Soto Pino, M.J.; Sánchez Ramos, J.; Moreno Escobar, E.; Blancas, I.; Fernández, J.J.; Fernández, D.; et al. Influence of the HER2 Ile655Val polymorphism on trastuzumab-induced cardiotoxicity in HER2-positive breast cancer patients: A meta-analysis. Pharm. Genom. 2015, 25, 388–393. [Google Scholar] [CrossRef]
- Lemieux, J.; Diorio, C.; Côté, M.A.; Provencher, L.; Barabé, F.; Jacob, S.; St-Pierre, C.; Demers, E.; Tremblay-Lemay, R.; Nadeau-Larochelle, C.; et al. Alcohol and HER2 polymorphisms as risk factor for cardiotoxicity in breast cancer treated with trastuzumab. Anticancer Res. 2013, 33, 2569–2576. [Google Scholar]
- Beauclair, S.; Formento, P.; Fischel, J.L.; Lescaut, W.; Largillier, R.; Chamorey, E.; Hofman, P.; Ferrero, J.M.; Pagès, G.; Milano, G. Role of the HER2 [Ile655Val] genetic polymorphism in tumorogenesis and in the risk of trastuzumab-related cardiotoxicity. Ann. Oncol. 2007, 18, 1335–1341. [Google Scholar] [CrossRef]
- Schneider, B.P.; Shen, F.; Gardner, L.; Radovich, M.; Li, L.; Miller, K.D.; Jiang, G.; Lai, D.; O’Neill, A.; Sparano, J.A.; et al. Genome-Wide Association Study for Anthracycline-Induced Congestive Heart Failure. Clin. Cancer Res. 2017, 23, 43–51. [Google Scholar] [CrossRef]
- Wu, X.; Shen, F.; Jiang, G.; Xue, G.; Philips, S.; Gardner, L.; Cunningham, G.; Bales, C.; Cantor, E.; Schneider, B.P. A non-coding GWAS variant impacts anthracycline-induced cardiotoxic phenotypes in human iPSC-derived cardiomyocytes. Nat. Commun. 2022, 13, 7171. [Google Scholar] [CrossRef] [PubMed]
- Gvaldin, D.Y.; Timoshkina, N.N.; Vladimirova, L.Y.; Svetitskaya, Y.V.; Vaschenko, L.S. Polymorphisms rs4673 and rs28714259 in predicting anthracycline-mediated cardiotoxicity in patients with breast cancer. Klin. Onkol. 2021, 34, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hu, B.; Guo, Z.; Jiang, X.; Su, X.; Zhang, X. Correlation of UGT2B7 Polymorphism with Cardiotoxicity in Breast Cancer Patients Undergoing Epirubicin/Cyclophosphamide-Docetaxel Adjuvant Chemotherapy. Yonsei Med. J. 2019, 60, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Luo, H.; Liu, Y.Y.; Chen, L.X.; Zhu, M.Q.; Deng, Q.T.; Zhu, D.M.; Wang, Z.M.; Xu, J.F. Value of UGT2B7-161 Single Nucleotide Polymorphism in Predicting the Risk of Cardiotoxicity in HER-2 Positive Breast Cancer Patients Who Underwent Pertuzumab Combined with Trastuzumab Therapy by PSL. Pharmgenom. Pers. Med. 2022, 15, 215–225. [Google Scholar] [CrossRef]
- Sawyer, M.B.; Pituskin, E.; Damaraju, S.; Bies, R.R.; Vos, L.J.; Prado, C.M.M.; Kuzma, M.; Scarfe, A.G.; Clemons, M.; Tonkin, K.; et al. A Uridine Glucuronosyltransferase 2B7 Polymorphism Predicts Epirubicin Clearance and Outcomes in Early-Stage Breast Cancer. Clin. Breast Cancer 2016, 16, 139–144.e133. [Google Scholar] [CrossRef]
- Udagawa, C.; Kuah, S.; Shimoi, T.; Kato, K.; Yoshida, T.; Nakano, M.H.; Shimo, A.; Kojima, Y.; Yoshie, R.; Tsugawa, K.; et al. Replication Study for the Association of Five SNPs Identified by GWAS and Trastuzumab-Induced Cardiotoxicity in Japanese and Singaporean Cohorts. Biol. Pharm. Bull. 2022, 45, 1198–1202. [Google Scholar] [CrossRef]
- Ovchinnikova, E.S.; Schmitter, D.; Vegter, E.L.; Ter Maaten, J.M.; Valente, M.A.; Liu, L.C.; van der Harst, P.; Pinto, Y.M.; de Boer, R.A.; Meyer, S.; et al. Signature of circulating microRNAs in patients with acute heart failure. Eur. J. Heart Fail. 2016, 18, 414–423. [Google Scholar] [CrossRef]
- Zile, M.R.; Mehurg, S.M.; Arroyo, J.E.; Stroud, R.E.; DeSantis, S.M.; Spinale, F.G. Relationship between the temporal profile of plasma microRNA and left ventricular remodeling in patients after myocardial infarction. Circ. Cardiovasc. Genet. 2011, 4, 614–619. [Google Scholar] [CrossRef]
- Cheng, M.; Yang, J.; Zhao, X.; Zhang, E.; Zeng, Q.; Yu, Y.; Yang, L.; Wu, B.; Yi, G.; Mao, X.; et al. Circulating myocardial microRNAs from infarcted hearts are carried in exosomes and mobilise bone marrow progenitor cells. Nat. Commun. 2019, 10, 959. [Google Scholar] [CrossRef]
- Chen, W.M.; Sheu, W.H.; Tseng, P.C.; Lee, T.S.; Lee, W.J.; Chang, P.J.; Chiang, A.N. Modulation of microRNA Expression in Subjects with Metabolic Syndrome and Decrease of Cholesterol Efflux from Macrophages via microRNA-33-Mediated Attenuation of ATP-Binding Cassette Transporter A1 Expression by Statins. PLoS ONE 2016, 11, e0154672. [Google Scholar] [CrossRef] [PubMed]
- Pordzik, J.; Jakubik, D.; Jarosz-Popek, J.; Wicik, Z.; Eyileten, C.; De Rosa, S.; Indolfi, C.; Siller-Matula, J.M.; Czajka, P.; Postula, M. Significance of circulating microRNAs in diabetes mellitus type 2 and platelet reactivity: Bioinformatic analysis and review. Cardiovasc. Diabetol. 2019, 18, 113. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Lucena, R.; Rangel-Zúñiga, O.A.; Alcalá-Díaz, J.F.; López-Moreno, J.; Roncero-Ramos, I.; Molina-Abril, H.; Yubero-Serrano, E.M.; Caballero-Villarraso, J.; Delgado-Lista, J.; Castaño, J.P.; et al. Circulating miRNAs as Predictive Biomarkers of Type 2 Diabetes Mellitus Development in Coronary Heart Disease Patients from the CORDIOPREV Study. Mol. Ther. Nucleic Acids 2018, 12, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Zampetaki, A.; Kiechl, S.; Drozdov, I.; Willeit, P.; Mayr, U.; Prokopi, M.; Mayr, A.; Weger, S.; Oberhollenzer, F.; Bonora, E.; et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ. Res. 2010, 107, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Friedman, R.C.; Farh, K.K.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef]
- Zhou, S.S.; Jin, J.P.; Wang, J.Q.; Zhang, Z.G.; Freedman, J.H.; Zheng, Y.; Cai, L. miRNAS in cardiovascular diseases: Potential biomarkers, therapeutic targets and challenges. Acta Pharmacol. Sin. 2018, 39, 1073–1084. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Y.; Lu, R.; Liu, Q.; Hong, S.; Zhang, Z.; Hu, G. MicroRNA-381-3p signatures as a diagnostic marker in patients with sepsis and modulates sepsis-steered cardiac damage and inflammation by binding HMGB1. Bioengineered 2021, 12, 11936–11946. [Google Scholar] [CrossRef]
- Momen-Heravi, F.; Saha, B.; Kodys, K.; Catalano, D.; Satishchandran, A.; Szabo, G. Increased number of circulating exosomes and their microRNA cargos are potential novel biomarkers in alcoholic hepatitis. J. Transl. Med. 2015, 13, 261. [Google Scholar] [CrossRef]
- Glinge, C.; Clauss, S.; Boddum, K.; Jabbari, R.; Jabbari, J.; Risgaard, B.; Tomsits, P.; Hildebrand, B.; Kääb, S.; Wakili, R.; et al. Stability of Circulating Blood-Based MicroRNAs—Pre-Analytic Methodological Considerations. PLoS ONE 2017, 12, e0167969. [Google Scholar] [CrossRef]
- Brown, C.; Mantzaris, M.; Nicolaou, E.; Karanasiou, G.; Papageorgiou, E.; Curigliano, G.; Cardinale, D.; Filippatos, G.; Memos, N.; Naka, K.K.; et al. A systematic review of miRNAs as biomarkers for chemotherapy-induced cardiotoxicity in breast cancer patients reveals potentially clinically informative panels as well as key challenges in miRNA research. Cardiooncology 2022, 8, 16. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, R.; Reinal, I.; Peiró-Molina, E.; Buigues, M.; Tejedor, S.; Hernándiz, A.; Selva, M.; Hervás, D.; Cañada, A.J.; Dorronsoro, A.; et al. MicroRNA-4732-3p Is Dysregulated in Breast Cancer Patients with Cardiotoxicity, and Its Therapeutic Delivery Protects the Heart from Doxorubicin-Induced Oxidative Stress in Rats. Antioxidants 2022, 11, 1955. [Google Scholar] [CrossRef] [PubMed]
- Zare, N.; Dana, N.; Mosayebi, A.; Vaseghi, G.; Javanmard, S.H. Evaluation of Expression Level of miR-3135b-5p in Blood Samples of Breast Cancer Patients Experiencing Chemotherapy-Induced Cardiotoxicity. Indian J. Clin. Biochem. 2022. [CrossRef]
- Zhang, S.; Wang, Y.; Wang, Y.; Peng, J.; Yuan, C.; Zhou, L.; Xu, S.; Lin, Y.; Du, Y.; Yang, F.; et al. Serum miR-222-3p as a Double-Edged Sword in Predicting Efficacy and Trastuzumab-Induced Cardiotoxicity for HER2-Positive Breast Cancer Patients Receiving Neoadjuvant Target Therapy. Front. Oncol. 2020, 10, 631. [Google Scholar] [CrossRef] [PubMed]
- Gioffré, S.; Chiesa, M.; Cardinale, D.M.; Ricci, V.; Vavassori, C.; Cipolla, C.M.; Masson, S.; Sandri, M.T.; Salvatici, M.; Ciceri, F.; et al. Circulating MicroRNAs as Potential Predictors of Anthracycline-Induced Troponin Elevation in Breast Cancer Patients: Diverging Effects of Doxorubicin and Epirubicin. J. Clin. Med. 2020, 9, 1418. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.K.; Halley, C.; Duan, Z.H.; Lappe, J.; Viterna, J.; Jana, S.; Augoff, K.; Mohan, M.L.; Vasudevan, N.T.; Na, J.; et al. miRNA-548c: A specific signature in circulating PBMCs from dilated cardiomyopathy patients. J. Mol. Cell. Cardiol. 2013, 62, 131–141. [Google Scholar] [CrossRef]
- Holmgren, G.; Synnergren, J.; Andersson, C.X.; Lindahl, A.; Sartipy, P. MicroRNAs as potential biomarkers for doxorubicin-induced cardiotoxicity. Toxicol. Vitr. 2016, 34, 26–34. [Google Scholar] [CrossRef]
- Liu, H.; Hu, X.; Wang, L.; Du, T.; Feng, J.; Li, M.; Liu, L.; Liu, X. Longitude Variation of the microRNA-497/FGF-23 Axis during Treatment and Its Linkage with Neoadjuvant/Adjuvant Trastuzumab-Induced Cardiotoxicity in HER2-Positive Breast Cancer Patients. Front. Surg. 2022, 9, 862617. [Google Scholar] [CrossRef]
- Qin, X.; Chang, F.; Wang, Z.; Jiang, W. Correlation of circulating pro-angiogenic miRNAs with cardiotoxicity induced by epirubicin/cyclophosphamide followed by docetaxel in patients with breast cancer. Cancer Biomark 2018, 23, 473–484. [Google Scholar] [CrossRef]
- Rigaud, V.O.; Ferreira, L.R.; Ayub-Ferreira, S.M.; Ávila, M.S.; Brandão, S.M.; Cruz, F.D.; Santos, M.H.; Cruz, C.B.; Alves, M.S.; Issa, V.S.; et al. Circulating miR-1 as a potential biomarker of doxorubicin-induced cardiotoxicity in breast cancer patients. Oncotarget 2017, 8, 6994–7002. [Google Scholar] [CrossRef]
- Todorova, V.K.; Makhoul, I.; Wei, J.; Klimberg, V.S. Circulating miRNA Profiles of Doxorubicin-induced Cardiotoxicity in Breast Cancer Patients. Ann. Clin. Lab. Sci. 2017, 47, 115–119. [Google Scholar]
- Yadi, W.; Shurui, C.; Tong, Z.; Suxian, C.; Qing, T.; Dongning, H. Bioinformatic analysis of peripheral blood miRNA of breast cancer patients in relation with anthracycline cardiotoxicity. BMC Cardiovasc. Disord. 2020, 20, 43. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, X.; Dong, H.; Ke, S.; Zheng, W.H. Let-7f and miRNA-126 correlate with reduced cardiotoxicity risk in triple-negative breast cancer patients who underwent neoadjuvant chemotherapy. Int. J. Clin. Exp. Pathol. 2018, 11, 4987–4995. [Google Scholar]
- Oren, A.; Taylor, J.M. The subcellular localization of defensins and myeloperoxidase in human neutrophils: Immunocytochemical evidence for azurophil granule heterogeneity. J. Lab. Clin. Med. 1995, 125, 340–347. [Google Scholar] [PubMed]
- Schultz, J.; Kaminker, K. Myeloperoxidase of the leucocyte of normal human blood. I. Content and localization. Arch. Biochem. Biophys. 1962, 96, 465–467. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Strzepa, A.; Pritchard, K.A.; Dittel, B.N. Myeloperoxidase: A new player in autoimmunity. Cell. Immunol. 2017, 317, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Rizo, V.; Martínez-Guzmán, M.A.; Iñiguez-Gutierrez, L.; García-Orozco, A.; Alvarado-Navarro, A.; Fafutis-Morris, M. Neutrophil Extracellular Traps and Its Implications in Inflammation: An Overview. Front. Immunol. 2017, 8, 81. [Google Scholar] [CrossRef]
- Liu, J.; Yang, D.; Wang, X.; Zhu, Z.; Wang, T.; Ma, A.; Liu, P. Neutrophil extracellular traps and dsDNA predict outcomes among patients with ST-elevation myocardial infarction. Sci. Rep. 2019, 9, 11599. [Google Scholar] [CrossRef]
- Anatoliotakis, N.; Deftereos, S.; Bouras, G.; Giannopoulos, G.; Tsounis, D.; Angelidis, C.; Kaoukis, A.; Stefanadis, C. Myeloperoxidase: Expressing inflammation and oxidative stress in cardiovascular disease. Curr. Top. Med. Chem. 2013, 13, 115–138. [Google Scholar] [CrossRef]
- Scandolara, T.B.; Panis, C. Neutrophil traps, anti-myeloperoxidase antibodies and cancer: Are they linked? Immunol. Lett. 2020, 221, 33–38. [Google Scholar] [CrossRef]
- Wanderley, M.R.B., Jr.; Ávila, M.S.; Fernandes-Silva, M.M.; Cruz, F.D.D.; Brandão, S.M.G.; Rigaud, V.O.C.; Hajjar, L.A.; Filho, R.K.; Cunha-Neto, E.; Bocchi, E.A.; et al. Plasma biomarkers reflecting high oxidative stress in the prediction of myocardial injury due to anthracycline chemotherapy and the effect of carvedilol: Insights from the CECCY Trial. Oncotarget 2022, 13, 214–223. [Google Scholar] [CrossRef]
- Putt, M.; Ky, B.; Sawaya, H.; French, B.; Januzzi, J.L.; Sebag, I.; Planas, J.; Cohen, V.; Blanchs, J.; Carver, J. Early Increases in Biomarkers Predict Subsequent Cardiotoxicity in Breast Cancer Patients Treated with Doxorubicin, Taxanes, and Trastuzumab. Am. Heart Assoc. 2013, 128, A15849. [Google Scholar]
- Baldus, S.; Heeschen, C.; Meinertz, T.; Zeiher, A.M.; Eiserich, J.P.; Münzel, T.; Simoons, M.L.; Hamm, C.W. Myeloperoxidase Serum Levels Predict Risk in Patients with Acute Coronary Syndromes. Circulation 2003, 108, 1440–1445. [Google Scholar] [CrossRef] [PubMed]
- Madrigal-Matute, J.; Lindholt, J.S.; Fernandez-Garcia, C.E.; Benito-Martin, A.; Burillo, E.; Zalba, G.; Beloqui, O.; Llamas-Granda, P.; Ortiz, A.; Egido, J.; et al. Galectin-3, a biomarker linking oxidative stress and inflammation with the clinical outcomes of patients with atherothrombosis. J. Am. Heart Assoc. 2014, 3, e000785. [Google Scholar] [CrossRef]
- Sharma, U.C.; Pokharel, S.; van Brakel, T.J.; van Berlo, J.H.; Cleutjens, J.P.; Schroen, B.; André, S.; Crijns, H.J.; Gabius, H.J.; Maessen, J.; et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation 2004, 110, 3121–3128. [Google Scholar] [CrossRef]
- Zaborska, B.; Sygitowicz, G.; Smarż, K.; Pilichowska-Paszkiet, E.; Budaj, A. Galectin-3 is related to right ventricular dysfunction in heart failure patients with reduced ejection fraction and may affect exercise capacity. Sci. Rep. 2020, 10, 16682. [Google Scholar] [CrossRef]
- Ho, J.E.; Liu, C.; Lyass, A.; Courchesne, P.; Pencina, M.J.; Vasan, R.S.; Larson, M.G.; Levy, D. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J. Am. Coll. Cardiol. 2012, 60, 1249–1256. [Google Scholar] [CrossRef]
- Magalhaes, A.; Menezes, M.; Cortez-Dias, N.; Saraiva, M.; Silva, D.; Santos, L.; Calisto, C.; Costa, L.; Pinto, F.; Fiuza, M. Galectin-3 and longitudinal global strain predict drug-related cardiotoxicity in patients with breast cancer. Eur. Heart J. 2015, 36, 704. [Google Scholar]
- van Boxtel, W.; Bulten, B.F.; Mavinkurve-Groothuis, A.M.; Bellersen, L.; Mandigers, C.M.; Joosten, L.A.; Kapusta, L.; de Geus-Oei, L.F.; van Laarhoven, H.W. New biomarkers for early detection of cardiotoxicity after treatment with docetaxel, doxorubicin and cyclophosphamide. Biomarkers 2015, 20, 143–148. [Google Scholar] [CrossRef]
- Camacho, A.X.A.; Ramírez, A.R.G.; Alonso, A.J.P.; García, J.D.R.; Urbano, M.A.O.; Poyatos, P.T.; Arrabal, S.R.; Núñez, M.I. Metalloproteinases 1 and 3 as Potential Biomarkers in Breast Cancer Development. Int. J. Mol. Sci. 2021, 22, 9012. [Google Scholar] [CrossRef] [PubMed]
- Belkin, A.M.; Akimov, S.S.; Zaritskaya, L.S.; Ratnikov, B.I.; Deryugina, E.I.; Strongin, A.Y. Matrix-dependent proteolysis of surface transglutaminase by membrane-type metalloproteinase regulates cancer cell adhesion and locomotion. J. Biol. Chem. 2001, 276, 18415–18422. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, N.I.; Gomes, J.A.S.; Fiuza, J.A.; Sousa, G.R.; Almeida, E.F.; Novaes, R.O.; Rocha, V.L.S.; Chaves, A.T.; Dutra, W.O.; Rocha, M.O.C.; et al. MMP-2 and MMP-9 plasma levels are potential biomarkers for indeterminate and cardiac clinical forms progression in chronic Chagas disease. Sci. Rep. 2019, 9, 14170. [Google Scholar] [CrossRef] [PubMed]
- Van Linthout, S.; Seeland, U.; Riad, A.; Eckhardt, O.; Hohl, M.; Dhayat, N.; Richter, U.; Fischer, J.W.; Böhm, M.; Pauschinger, M.; et al. Reduced MMP-2 activity contributes to cardiac fibrosis in experimental diabetic cardiomyopathy. Basic Res. Cardiol. 2008, 103, 319–327. [Google Scholar] [CrossRef]
- Grakova, E.V.; Shilov, S.N.; Kopeva, K.V.; Berezikova, E.N.; Popova, A.A.; Neupokoeva, M.N.; Ratushnyak, E.T.; Teplyakov, A.T. Extracellular matrix remodeling in anthracycline-induced cardiotoxicity: What place on the pedestal? Int. J. Cardiol. 2022, 350, 55–61. [Google Scholar] [CrossRef]
- Kirkham, A.A.; Pituskin, E.; Thompson, R.B.; Mackey, J.R.; Koshman, S.L.; Jassal, D.; Pitz, M.; Haykowsky, M.J.; Pagano, J.J.; Chow, K.; et al. Cardiac and cardiometabolic phenotyping of trastuzumab-mediated cardiotoxicity: A secondary analysis of the MANTICORE trial. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Micheletti, P.L.; Carla-da-Silva, J.; Scandolara, T.B.; Kern, R.; Alves, V.D.; Malanowski, J.; Victorino, V.J.; Herrera, A.C.S.A.; Rech, D.; Souza, J.A.O.; et al. Proinflammatory circulating markers: New players for evaluating asymptomatic acute cardiovascular toxicity in breast cancer treatment. J. Chemother. 2021, 33, 106–115. [Google Scholar] [CrossRef]
- Yu, L.R.; Cao, Z.; Makhoul, I.; Daniels, J.R.; Klimberg, S.; Wei, J.Y.; Bai, J.P.; Li, J.; Lathrop, J.T.; Beger, R.D.; et al. Immune response proteins as predictive biomarkers of doxorubicin-induced cardiotoxicity in breast cancer patients. Exp. Biol. Med. 2018, 243, 248–255. [Google Scholar] [CrossRef]
- Baruch, R.; Zahler, D.; Zornitzki, L.; Arbel, Y.; Rozenbaum, Z.; Arnold, J.H.; Raphael, A.; Khoury, S.; Banai, S.; Topilsky, Y.; et al. High neutrophil-to-lymphocyte ratio as an early sign of cardiotoxicity in breast cancer patients treated with anthracycline. Clin. Cardiol. 2023, 46, 328–335. [Google Scholar] [CrossRef]
- Ryu, H.H.; Ahn, S.H.; Kim, S.O.; Kim, J.E.; Kim, J.S.; Ahn, J.-H.; Jung, K.H.; Kim, S.-B.; Ko, B.S.; Lee, J.W.; et al. Comparison of metabolic changes after neoadjuvant endocrine and chemotherapy in ER-positive, HER2-negative breast cancer. Sci. Rep. 2021, 11, 10510. [Google Scholar] [CrossRef]
- Giskeødegård, G.F.; Madssen, T.S.; Sangermani, M.; Lundgren, S.; Wethal, T.; Andreassen, T.; Reidunsdatter, R.J.; Bathen, T.F. Longitudinal Changes in Circulating Metabolites and Lipoproteins after Breast Cancer Treatment. Front. Oncol. 2022, 12, 919522. [Google Scholar] [CrossRef]
- Finkelman, B.S.; Putt, M.; Wang, T.; Wang, L.; Narayan, H.; Domchek, S.; DeMichele, A.; Fox, K.; Matro, J.; Shah, P.; et al. Arginine-Nitric Oxide Metabolites and Cardiac Dysfunction in Patients with Breast Cancer. J. Am. Coll. Cardiol. 2017, 70, 152–162. [Google Scholar] [CrossRef]
- Asnani, A.; Shi, X.; Farrell, L.; Lall, R.; Sebag, I.A.; Plana, J.C.; Gerszten, R.E.; Scherrer-Crosbie, M. Changes in Citric Acid Cycle and Nucleoside Metabolism Are Associated with Anthracycline Cardiotoxicity in Patients with Breast Cancer. J. Cardiovasc. Transl. Res. 2020, 13, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.A.; Todorova, V.K.; Stone, A.; Carter, W.; Plotkin, M.D.; Hsu, P.C.; Wei, J.Y.; Su, J.L.; Makhoul, I. Genome-Wide DNA Methylation Signatures Predict the Early Asymptomatic Doxorubicin-Induced Cardiotoxicity in Breast Cancer. Cancers 2021, 13, 6291. [Google Scholar] [CrossRef]
- Beer, L.A.; Kossenkov, A.V.; Liu, Q.; Luning Prak, E.; Domchek, S.; Speicher, D.W.; Ky, B. Baseline Immunoglobulin E Levels as a Marker of Doxorubicin- and Trastuzumab-Associated Cardiac Dysfunction. Circ. Res. 2016, 119, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Hasan, D.; Ismail, Y.; Al Tibi, A.; Al-Zeidaneen, S.A.; Ode, M.; Burghel, G.J.; Natsheh, I.; Abdelnour, A. Serum Biomarkers for Chemotherapy Cardiotoxicity Risk Detection of Breast Cancer Patients. Asian Pac. J. Cancer Prev. 2021, 22, 3355–3363. [Google Scholar] [CrossRef]
- Kitani, T.; Ong, S.G.; Lam, C.K.; Rhee, J.W.; Zhang, J.Z.; Oikonomopoulos, A.; Ma, N.; Tian, L.; Lee, J.; Telli, M.L.; et al. Human-Induced Pluripotent Stem Cell Model of Trastuzumab-Induced Cardiac Dysfunction in Patients with Breast Cancer. Circulation 2019, 139, 2451–2465. [Google Scholar] [CrossRef] [PubMed]
- Assadi, A.; Zahabi, A.; Hart, R.A. GDF15, an update of the physiological and pathological roles it plays: A review. Pflügers Arch. Eur. J. Physiol. 2020, 472, 1535–1546. [Google Scholar] [CrossRef]
- Hooks, M.; Sandhu, G.; Maganti, T.; Chen, K.A.; Wang, M.; Cullen, R.; Velangi, P.S.; Gu, C.; Wiederin, J.; Connett, J.; et al. Incidental coronary calcium in cancer patients treated with anthracycline and/or trastuzumab. Eur. J. Prev. Cardiol. 2022, 29, 2200–2210. [Google Scholar] [CrossRef]
- Krishnarao, K.; Bruno, K.A.; Di Florio, D.N.; Edenfield, B.H.; Whelan, E.R.; Macomb, L.P.; McGuire, M.M.; Hill, A.R.; Ray, J.C.; Cornell, L.F.; et al. Upregulation of Endothelin-1 May Predict Chemotherapy-Induced Cardiotoxicity in Women with Breast Cancer. J. Clin. Med. 2022, 11, 3547. [Google Scholar] [CrossRef]
- Geisberg, C.A.; Abdallah, W.M.; da Silva, M.; Silverstein, C.; Smith, H.M.; Abramson, V.; Mayer, I.; Means-Powell, J.; Freehardt, D.; White, B.; et al. Circulating neuregulin during the transition from stage A to stage B/C heart failure in a breast cancer cohort. J. Card. Fail. 2013, 19, 10–15. [Google Scholar] [CrossRef]
- Lammert, J.; Basrai, M.; Struck, J.; Hartmann, O.; Engel, C.; Bischoff, S.C.; Berling-Ernst, A.; Halle, M.; Kiechle, M.; Grill, S. Associations of Plasma Bioactive Adrenomedullin Levels with Cardiovascular Risk Factors in BRCA1/2 Mutation Carriers. Geburtshilfe Frauenheilkd. 2022, 82, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Dural, M.; Demir, L.; Babayiğit, E.; Junushova, B.; Mert, K.U.; Ulus, T.; Çavuşoğlu, Y.; Yıldız, B.; Dinçer, M.; Görenek, B. Fragmented QRS formation and its predictors in patients with breast cancer receiving anthracycline-based chemotherapy. J. Electrocardiol. 2019, 54, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Balmagambetova, S.; Tlegenova, Z.; Zholdin, B.; Kurmanalina, G.; Talipova, I.; Koyshybaev, A.; Nurmanova, D.; Sultanbekova, G.; Baspayeva, M.; Madinova, S.; et al. Early Diagnosis of Chemotherapy-Linked Cardiotoxicity in Breast Cancer Patients Using Conventional Biomarker Panel: A Prospective Study Protocol. Diagnostics 2022, 12, 2714. [Google Scholar] [CrossRef] [PubMed]
- Frères, P.; Bouznad, N.; Servais, L.; Josse, C.; Wenric, S.; Poncin, A.; Thiry, J.; Moonen, M.; Oury, C.; Lancellotti, P.; et al. Variations of circulating cardiac biomarkers during and after anthracycline-containing chemotherapy in breast cancer patients. BMC Cancer 2018, 18, 102. [Google Scholar] [CrossRef] [PubMed]
- Eltohamy, M.I.; Badawy, O.M.; El kinaai, N.; Loay, I.; Nassar, H.R.; Allam, R.M.; Sakr, M.A. Topoisomerase II α Gene alteration in Triple Negative Breast Cancer and Its Predictive Role for Anthracycline-Based Chemotherapy (Egyptian NCI Patients). Asian Pac. J. Cancer Prev. 2018, 19, 3581–3589. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Allen, R.; Jia, L.; Sun, T.; Isakoff, S.J.; Scherrer-Crosbie, M.; Kehlmann, A.M.; Zheng, H.; Ly, A.; Walmsley, C.S.; et al. An Initial Evaluation of Human Plasma cMLC-1: A Potential Protein Biomarker for Trastuzumab-Induced Cardiotoxicity, Breast Cancer Screening and Progression. Front. Oncol. 2022, 12, 809715. [Google Scholar] [CrossRef]
- de la Fuente, A.; Santisteban, M.; Lupón, J.; Aramendía, J.M.; Díaz, A.; Santaballa, A.; Hernándiz, A.; Sepúlveda, P.; Cediel, G.; López, B.; et al. A Fibrosis Biomarker Early Predicts Cardiotoxicity Due to Anthracycline-Based Breast Cancer Chemotherapy. Cancers 2022, 14, 2914. [Google Scholar] [CrossRef]
- Li, W.; Liu, M.; Yu, F.; Jiang, T.; Zhu, W.; Liu, H. Changes in epicardial adipose tissue among women treated with trastuzumab for breast cancer. Int. J. Cardiol. 2022, 348, 163–168. [Google Scholar] [CrossRef]
- Monti, C.B.; Schiaffino, S.; Ortiz, M.D.M.G.; Capra, D.; Zanardo, M.; De Benedictis, E.; Luporini, A.G.; Spagnolo, P.; Secchi, F.; Sardanelli, F. Potential role of epicardial adipose tissue as a biomarker of anthracycline cardiotoxicity. Insights Imaging 2021, 12, 161. [Google Scholar] [CrossRef]
- Vera, T.; D’Agostino, R.B., Jr.; Jordan, J.H.; Whitlock, M.C.; Meléndez, G.C.; Lamar, Z.S.; Porosnicu, M.; Bonkovsky, H.L.; Poole, L.B.; Hundley, W.G. Relation of Pre-anthracycline Serum Bilirubin Levels to Left Ventricular Ejection Fraction after Chemotherapy. Am. J. Cardiol. 2015, 116, 1752–1755. [Google Scholar] [CrossRef]
- Liu, J.; Lane, S.; Lall, R.; Russo, M.; Farrell, L.; Debreli Coskun, M.; Curtin, C.; Araujo-Gutierrez, R.; Scherrer-Crosbie, M.; Trachtenberg, B.H.; et al. Circulating hemopexin modulates anthracycline cardiac toxicity in patients and in mice. Sci. Adv. 2022, 8, eadc9245. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.R.; Briggs, E.R.; Qatanani, M.; Sawaya, H.; Sebag, I.A.; Picard, M.H.; Scherrer-Crosbie, M.; Lazar, M.A. Human resistin in chemotherapy-induced heart failure in humanized male mice and in women treated for breast cancer. Endocrinology 2013, 154, 4206–4214. [Google Scholar] [CrossRef] [PubMed]
- Kopeva, K.V.; Grakova, E.V.; Shilov, S.N.; Berezikova, E.N.; Popova, A.A.; Neupokoeva, M.N.; Ratushnyak, E.T.; Teplyakov, A.T. Anthracycline-induced cardiotoxicity in women without cardiovascular diseases: Molecular and genetic predictors. Acta Cardiol. 2022, 77, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Shilov, S.N.; Teplyakov, A.T.; Popova, A.A.; Berezikova, E.N.; Neupokoeva, M.N.; Grakova, E.V.; Valeeva, A.M.; Tuleutaev, S.M. Prognostic role of p53 gene polymorphism in risk assessment of anthracycline-induced cardiotoxicity. Kardiologiia 2019, 59, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Nyangwara, V.A.; Mazhindu, T.; Chikwambi, Z.; Masimirembwa, C.; Campbell, T.B.; Borok, M.; Ndlovu, N. Cardiotoxicity and pharmacogenetics of doxorubicin in black Zimbabwean breast cancer patients. Br. J. Clin. Pharmacol. 2023. [CrossRef] [PubMed]
- Velasco-Ruiz, A.; Nuñez-Torres, R.; Pita, G.; Wildiers, H.; Lambrechts, D.; Hatse, S.; Delombaerde, D.; Van Brussel, T.; Alonso, M.R.; Alvarez, N.; et al. POLRMT as a Novel Susceptibility Gene for Cardiotoxicity in Epirubicin Treatment of Breast Cancer Patients. Pharmaceutics 2021, 13, 1942. [Google Scholar] [CrossRef]
- Soria-Chacartegui, P.; Villapalos-García, G.; López-Fernández, L.A.; Navares-Gómez, M.; Mejía-Abril, G.; Abad-Santos, F.; Zubiaur, P. Clinical relevance of novel polymorphisms in the dihydropyrimidine dehydrogenase (Dpyd) gene in patients with severe fluoropyrimidine toxicity: A spanish case-control study. Pharmaceutics 2021, 13, 2036. [Google Scholar] [CrossRef]
- Vulsteke, C.; Pfeil, A.M.; Maggen, C.; Schwenkglenks, M.; Pettengell, R.; Szucs, T.D.; Lambrechts, D.; Dieudonné, A.S.; Hatse, S.; Neven, P.; et al. Clinical and genetic risk factors for epirubicin-induced cardiac toxicity in early breast cancer patients. Breast Cancer Res. Treat. 2015, 152, 67–76. [Google Scholar] [CrossRef]
- Varkoly, K.; Tan, S.; Beladi, R.; Fonseca, D.; Zanetti, I.R.; Kraberger, S.; Shah, C.; Yaron, J.R.; Zhang, L.; Juby, M.; et al. RNA Virus Gene Signatures Detected in Patients with Cardiomyopathy after Chemotherapy; A Pilot Study. Front. Cardiovasc. Med. 2022, 9, 821162. [Google Scholar] [CrossRef]
- Herrmann, J. Adverse cardiac effects of cancer therapies: Cardiotoxicity and arrhythmia. Nat. Rev. Cardiol. 2020, 17, 474–502. [Google Scholar] [CrossRef]
- Herrmann, J.; Lenihan, D.; Armenian, S.; Barac, A.; Blaes, A.; Cardinale, D.; Carver, J.; Dent, S.; Ky, B.; Lyon, A.R.; et al. Defining cardiovascular toxicities of cancer therapies: An International Cardio-Oncology Society (IC-OS) consensus statement. Eur. Heart J. 2022, 43, 280–299. [Google Scholar] [CrossRef]
- Clerico, A.; Zaninotto, M.; Plebani, M. High-sensitivity assay for cardiac troponins with POCT methods. The future is soon. Clin. Chem. Lab. Med. 2021, 59, 1477–1478. [Google Scholar] [CrossRef] [PubMed]
- Khoury, E.E.; Kinaneh, S.; Aronson, D.; Amir, O.; Ghanim, D.; Volinsky, N.; Azzam, Z.; Abassi, Z. Natriuretic peptides system in the pulmonary tissue of rats with heart failure: Potential involvement in lung edema and inflammation. Oncotarget 2018, 9, 21715–21730. [Google Scholar] [CrossRef] [PubMed]
- Zhan, W.; Chen, L.; Liu, H.; Long, C.; Liu, J.; Ding, S.; Wu, Q.; Chen, S. Pcsk6 Deficiency Promotes Cardiomyocyte Senescence by Modulating Ddit3-Mediated ER Stress. Genes 2022, 13, 711. [Google Scholar] [CrossRef]
- Wang, H.; Chen, F.; Tong, J.; Li, Y.; Cai, J.; Wang, Y.; Li, P.; Hao, Y.; Tian, W.; Lv, Y.; et al. Circulating microRNAs as novel biomarkers for dilated cardiomyopathy. Cardiol. J. 2017, 24, 65–73. [Google Scholar] [CrossRef]
- Liu, W.; Ling, S.; Sun, W.; Liu, T.; Li, Y.; Zhong, G.; Zhao, D.; Zhang, P.; Song, J.; Jin, X.; et al. Circulating microRNAs correlated with the level of coronary artery calcification in symptomatic patients. Sci. Rep. 2015, 5, 16099. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, D.; Xue, J.; Zuo, Z. Galectin-3 and Myeloperoxidase May Monitor Cancer-Therapy-Related Cardiotoxicity? A Systematic Review and Meta-Analysis. Biomolecules 2022, 12, 1788. [Google Scholar] [CrossRef]
- Lazzarini, E.; Balbi, C.; Altieri, P.; Pfeffer, U.; Gambini, E.; Canepa, M.; Varesio, L.; Bosco, M.C.; Coviello, D.; Pompilio, G.; et al. The human amniotic fluid stem cell secretome effectively counteracts doxorubicin-induced cardiotoxicity. Sci. Rep. 2016, 6, 29994. [Google Scholar] [CrossRef]
- Ethier, J.L.; Desautels, D.; Templeton, A.; Shah, P.S.; Amir, E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: A systematic review and meta-analysis. Breast Cancer Res. 2017, 19, 2. [Google Scholar] [CrossRef]
- Guha, A.; Stabellini, N.; Montero, A.J. Commentary: Longitudinal changes in circulating metabolites and lipoproteins after breast cancer treatment. Front. Cardiovasc. Med. 2022, 9, 962698. [Google Scholar] [CrossRef] [PubMed]
- Lofterød, T.; Mortensen, E.S.; Nalwoga, H.; Wilsgaard, T.; Frydenberg, H.; Risberg, T.; Eggen, A.E.; McTiernan, A.; Aziz, S.; Wist, E.A.; et al. Impact of pre-diagnostic triglycerides and HDL-cholesterol on breast cancer recurrence and survival by breast cancer subtypes. BMC Cancer 2018, 18, 654. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.; Bose, J.C.; Chandrasekar, A.; Tiwary, B.K.; Gajalakshmi, P.; Chatterjee, S. Increased Plasma Nitrite and von Willebrand Factor Indicates Early Diagnosis of Vascular Diseases in Chemotherapy Treated Cancer Patients. Cardiovasc. Toxicol. 2019, 19, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Maier, R.H.; Plummer, C.; Kasim, A.S.; Akhter, N.; Ogundimu, E.; Maddox, J.; Graham, J.; Stewart, M.; Wardley, A.; Haney, S.; et al. Preventing cardiotoxicity in patients with breast cancer and lymphoma: Protocol for a multicentre randomised controlled trial (PROACT). BMJ Open 2022, 12, e066252. [Google Scholar] [CrossRef] [PubMed]
- Borgonovo, G.; Vettus, E.; Greco, A.; Leo, L.A.; Faletra, F.F.; Klersy, C.; Curti, M.; Valli, M. Early Detection of Cardiotoxicity From Systemic and Radiation Therapy in Patients with Breast Cancer: Protocol for a Multi-Institutional Prospective Study. JMIR Res. Protoc. 2022, 11, e31887. [Google Scholar] [CrossRef] [PubMed]
- CardioCare Consortium. An Interdisciplinary Approach for the Management of the Elderly Multimorbid Patient with Breast Cancer Therapy Induced Cardiac Toxicity. Available online: https://cordis.europa.eu/project/id/945175 (accessed on 9 June 2023).
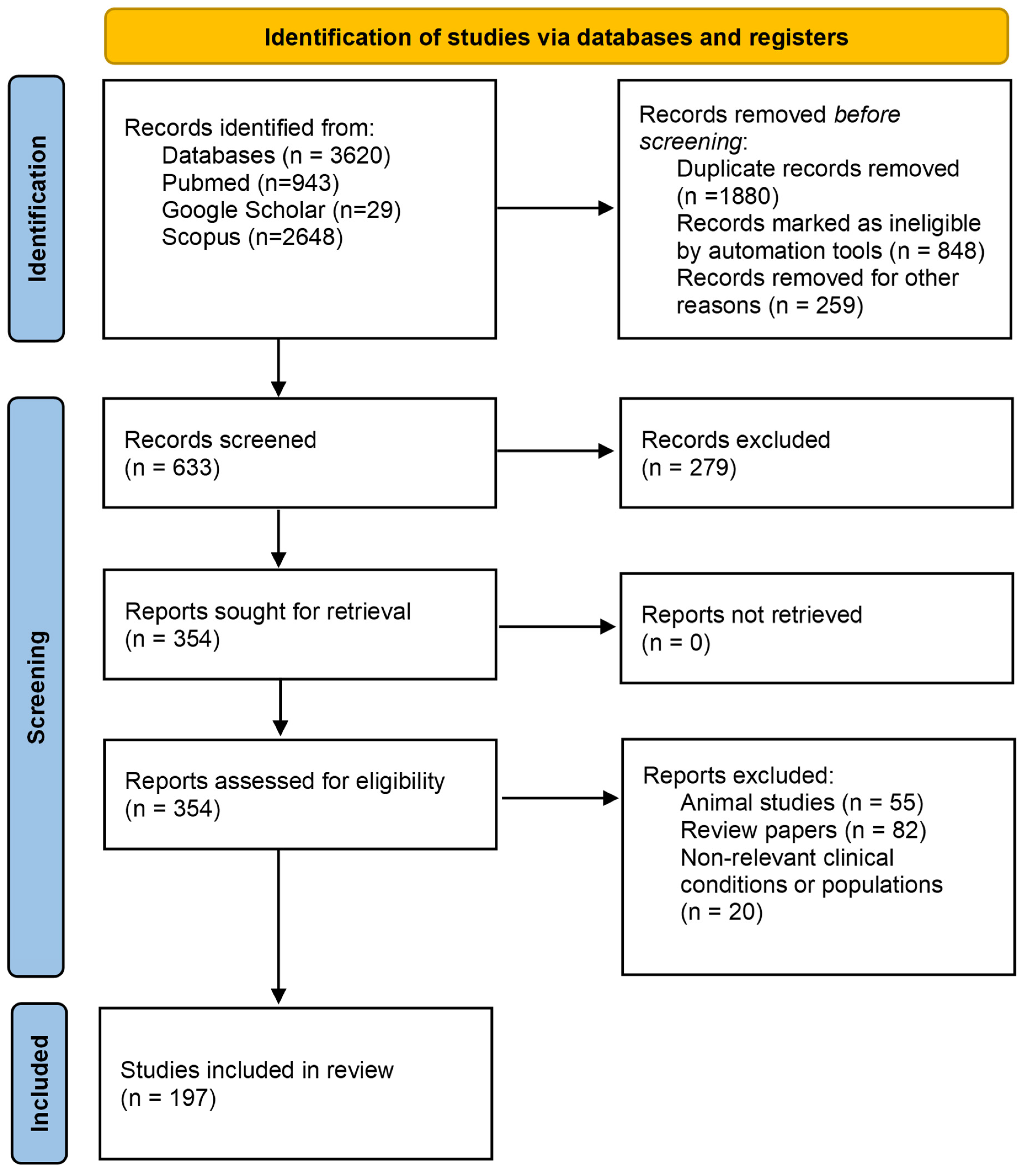
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexandraki, A.; Papageorgiou, E.; Zacharia, M.; Keramida, K.; Papakonstantinou, A.; Cipolla, C.M.; Tsekoura, D.; Naka, K.; Mazzocco, K.; Mauri, D.; et al. New Insights in the Era of Clinical Biomarkers as Potential Predictors of Systemic Therapy-Induced Cardiotoxicity in Women with Breast Cancer: A Systematic Review. Cancers 2023, 15, 3290. https://doi.org/10.3390/cancers15133290
Alexandraki A, Papageorgiou E, Zacharia M, Keramida K, Papakonstantinou A, Cipolla CM, Tsekoura D, Naka K, Mazzocco K, Mauri D, et al. New Insights in the Era of Clinical Biomarkers as Potential Predictors of Systemic Therapy-Induced Cardiotoxicity in Women with Breast Cancer: A Systematic Review. Cancers. 2023; 15(13):3290. https://doi.org/10.3390/cancers15133290
Chicago/Turabian StyleAlexandraki, Alexia, Elisavet Papageorgiou, Marina Zacharia, Kalliopi Keramida, Andri Papakonstantinou, Carlo M. Cipolla, Dorothea Tsekoura, Katerina Naka, Ketti Mazzocco, Davide Mauri, and et al. 2023. "New Insights in the Era of Clinical Biomarkers as Potential Predictors of Systemic Therapy-Induced Cardiotoxicity in Women with Breast Cancer: A Systematic Review" Cancers 15, no. 13: 3290. https://doi.org/10.3390/cancers15133290
APA StyleAlexandraki, A., Papageorgiou, E., Zacharia, M., Keramida, K., Papakonstantinou, A., Cipolla, C. M., Tsekoura, D., Naka, K., Mazzocco, K., Mauri, D., Tsiknakis, M., Manikis, G. C., Marias, K., Marcou, Y., Kakouri, E., Konstantinou, I., Daniel, M., Galazi, M., Kampouroglou, E., ... Constantinidou, A. (2023). New Insights in the Era of Clinical Biomarkers as Potential Predictors of Systemic Therapy-Induced Cardiotoxicity in Women with Breast Cancer: A Systematic Review. Cancers, 15(13), 3290. https://doi.org/10.3390/cancers15133290