Characteristics and Outcomes of COVID-19 Cancer Patients Admitted to a Portuguese Intensive Care Unit: A Case-Control Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
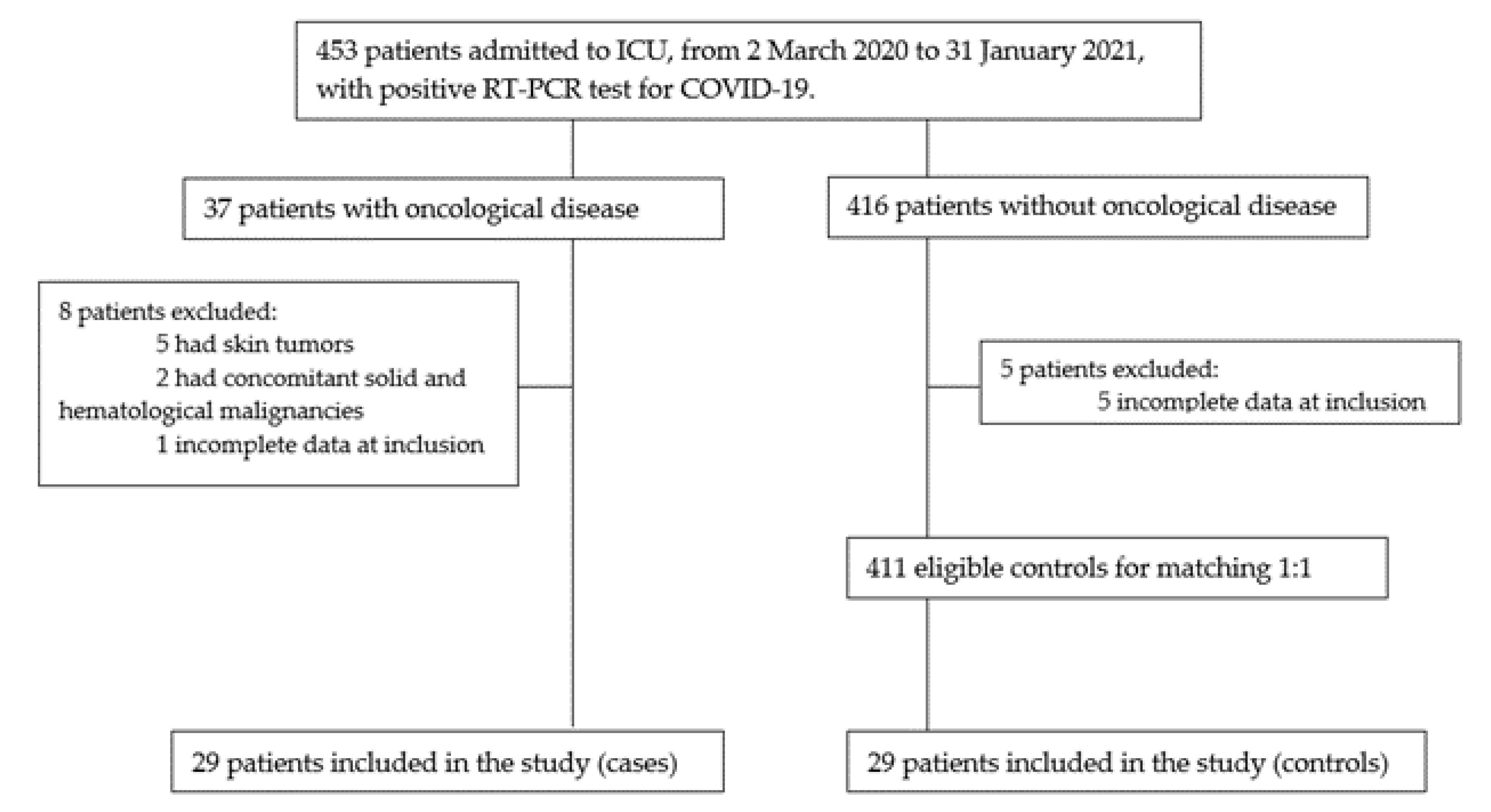
2.1. Study Design and Patient Selection
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Clinical Presentation and Laboratorial and Radiological Findings
3.3. Treatment and Complications
3.4. Effect of Oncological Disease on Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Univariate Logistic Regression | OR | 95% CI | p-Value a |
|---|---|---|---|
Fever Dyspnea Cough Asthenia Dysgeusia Diarrhea Desaturation Abdominal pain Odynophagia Headache Myalgias Leukopenia Lymphopenia Anemia Thrombocytopenia Elevated CRP Elevated PCT Elevated LDH Elevated D-dimers Consolidation Ground-glass opacities Nodular pattern Respiratory dysfunction Cardiovascular dysfunction Liver dysfunction Renal dysfunction Hematological dysfunction CNS dysfunction Secondary infection Septic shock PTE | |||
| 1 0.574 0.322 0.799 1.558 1.558 2.074 1 0.482 0.482 0.171 2.812 0.768 3.868 1.302 1 1.544 1.302 0.871 2.750 0.286 1.071 1 1.414 0.756 1.544 0.724 0.866 1.569 1.518 0.310 | 0.355–2.817 0.203–1.623 0.064–9.081 0.214–2.982 0.240–10.091 0.240–10.091 0.178–24.228 0.060–16.791 0.041–5.632 0.041–5.632 0.919–1.571 0.499–15.857 0.184–3.206 1.260–11.860 0.312–5.436 0.339–2.953 0.535–4.452 0.312–5.436 0.311–2.440 0.610–12.407 0.046–1.780 0.061–18.820 0.389–2.974 0.449–4.507 0.268–2.135 0.535–4.452 0.237–2.213 0.303–2.481 0.533–4.161 0.538–4.284 0.030–3.166 | 1 0.295 0.650 0.738 0.642 0.642 0.561 1 0.561 0.561 0.119 0.241 0.717 0.018 0.717 1 0.422 0.717 0.793 0.188 0.180 0.962 1 0.558 0.598 0.422 0.571 0.788 0.414 0.431 0.325 |
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Singhal, T. A Review of Coronavirus Disease-2019 (COVID-19). Indian J. Pediatr. 2020, 87, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72,314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Aiyegbusi, O.L.; Hughes, S.E.; Turner, G.; Rivera, S.C.; McMullan, C.; Chandan, J.S.; Haroon, S.; Price, G.; Davies, E.H.; Nirantharakumar, K.; et al. Symptoms, complications and management of long COVID: A review. J. R. Soc. Med. 2021, 114, 428–442. [Google Scholar] [CrossRef]
- Hajjar, L.A.; Costa, I.B.S.D.S.; Rizk, S.I.; Biselli, B.; Gomes, B.R.; Bittar, C.S.; Oliveira, G.Q.; Almeida, J.P.; Oliveira Bello, M.V.; Garzillo, C.; et al. Intensive care management of patients with COVID-19: A practical approach. Ann. Intensive Care. 2021, 11, 36. [Google Scholar] [CrossRef]
- Azoulay, E.; Waele, J.; Ferrer, R.; Staudinger, T.; Borkowska, M.; Povoa, P.; Iliopoulou, K.; Artigas, A.; Schaller, S.J.; Shankar-Hari, M.; et al. International variation in the management of severe COVID-19 patients. Crit. Care. 2020, 24, 486. [Google Scholar] [CrossRef]
- Heo, J.; Han, D.; Kim, H.J.; Kim, D.; Lee, Y.K.; Lim, D.; Hong, S.O.; Park, M.J.; Ha, B.; Seog, W. Prediction of patients requiring intensive care for COVID-19: Development and validation of an integer-based score using data from Centers for Disease Control and Prevention of South Korea. J. Intensive Care. 2021, 9, 16. [Google Scholar] [CrossRef]
- Yang, L.; Chai, P.; Yu, J.; Fan, X. Effects of cancer on patients with COVID-19: A systematic review and meta-analysis of 63,019 participants. Cancer Biol. Med. 2021, 18, 298–307. [Google Scholar] [CrossRef]
- Russell, C.D.; Lone, N.I.; Baillie, J.K. Comorbidities, multimorbidity and COVID-19. Nat. Med. 2023, 29, 334–343. [Google Scholar] [CrossRef]
- Dai, M.; Liu, D.; Liu, M.; Zhou, F.; Li, G.; Chen, Z.; Zhang, Z.; You, H.; Wu, M.; Zheng, Q.; et al. Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multicenter Study during the COVID-19 Outbreak. Cancer Discov. 2020, 10, 783–791. [Google Scholar] [CrossRef]
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H.; et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020, 21, 335–337. [Google Scholar] [CrossRef]
- Tian, J.; Yuan, X.; Xiao, J.; Zhong, Q.; Yang, C.; Liu, B.; Cai, Y.; Lu, Z.; Wang, J.; Wang, Y.; et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: A multicentre, retrospective, cohort study. Lancet Oncol. 2020, 21, 893–903. [Google Scholar] [CrossRef]
- Yang, K.; Sheng, Y.; Huang, C.; Jin, Y.; Xiong, N.; Jiang, K.; Lu, H.; Liu, J.; Yang, J.; Dong, Y.; et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: A multicentre, retrospective, cohort study. Lancet Oncol. 2020, 21, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Kuderer, N.; Choueiri, T.; Shah, D.; Shyr, Y.; Rubinstein, S.M.; Rivera, D.R.; Shete, S.; Hsu, C.Y.; Desai, A.; Lima Lopes, G.; et al. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 2020, 395, 1907–1918. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Lu, W.; Guo, E.; Liu, J.; Yang, B.; Wu, P.; Lin, S.; Peng, T.; Fu, Y.; Li, F.; et al. Cancer history is an independent risk factor for mortality in hospitalized COVID-19 patients: A propensity score-matched analysis. J. Hematol. Oncol. 2020, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- Garassino, M.C.; Whisenant, J.G.; Huang, L.C.; Trama, A.; Torri, V.; Agustoni, F.; Baena, J.; Banna, G.; Berardi, R.; Bettini, A.C.; et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): First results of an international, registry-based, cohort study. Lancet Oncol. 2020, 21, 914–922. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, F.; Xie, L.; Wang, C.; Wang, J.; Chen, R.; Jia, P.; Guan, H.Q.; Peng, L.; Chen, Y.; et al. Clinical characteristics of COVID-19-infected cancer patients: A retrospective case study in three hospitals within Wuhan, China. Ann. Oncol. 2020, 31, 894–901. [Google Scholar] [CrossRef]
- Richards, M.; Anderson, M.; Carter, P.; Ebert, B.L.; Mossialos, E. The impact of the COVID-19 pandemic on cancer care. Nat. Cancer 2020, 1, 565–567. [Google Scholar] [CrossRef]
- Arayici, M.E.; Kipcak, N.; Kayacik, U.; Kelbat, C.; Keskin, D.; Kilicarslan, M.E.; Kilinc, A.V.; Kirgoz, S.; Kirilmaz, A.; Kizilkaya, M.A.; et al. Effects of SARS-CoV-2 infections in patients with cancer on mortality, ICU admission and incidence: A systematic review with meta-analysis involving 709,908 participants and 31,732 cancer patients. J. Cancer Res. Clin. Oncol. 2022, 13, 1–14. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Y.; Okwan-Duodu, D.; Basho, R.; Cui, X. COVID-19 in cancer patients: Risk, clinical features, and management. Cancer Biol. Med. 2020, 17, 519–527. [Google Scholar] [CrossRef]
- Lenti, M.V.; Klersy, C.; Brera, A.S.; Ballesio, A.; Croce, G.; Padovini, L.; Ciccocioppo, R.; Bertolino, G.; Di Sabatino, A.; Corazza, G.R. Aging underlies heterogeneity between comorbidity and multimorbidity frameworks. Intern. Emerg. Med. 2022, 17, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Jin, R.; Zhao, J.; Li, W.; Shen, H. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020, 21, e180. [Google Scholar] [CrossRef] [PubMed]
- Malard, F.; Genthon, A.; Brissot, E.; van de Wyngaert, Z.; Marjanovic, Z.; Ikhlef, S.; Banet, A.; Lapusan, S.; Sestilli, S.; Corre, E.; et al. COVID-19 outcomes in patients with hematologic disease. Bone Marrow Transplant. 2020, 55, 2180–2184. [Google Scholar] [CrossRef]
- Höllein, A.; Bojko, P.; Schulz, S.; Neitz, J.; Stötzer, O.; Pihusch, R.; Abedinpour, F.; Schmidt, B.; Hentrich, M. Characteristics and outcomes of patients with cancer and COVID-19: Results from a cohort study. Acta Oncol. 2021, 60, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.Y.W.; Cazier, J.B.; Starkey, T.; Briggs, S.E.W.; Arnold, R.; Bisht, V.; Booth, S.; Campton, N.A.; Cheng, V.W.T.; Collins, G.; et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: A prospective cohort study. Lancet Oncol. 2020, 21, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, L.; Chen, Y.; Shen, X.; Wang, Q.; Yan, Y.; Yu, Y.; Wu, Q.; Zhong, Y.; Chua, M.L.K.; et al. A multicentre study of 2019 novel coronavirus disease outcomes of cancer patients in Wuhan, China. MedRxiv 2020, 41, 145. [Google Scholar]
- Stroppa, E.M.; Toscani, I.; Citterio, C.; Anselmi, E.; Zaffignani, E.; Codeluppi, M.; Cavanna, L. Coronavirus disease-2019 in cancer patients. A report of the first 25 cancer patients in a western country (Italy). Future Oncol. 2020, 16, 1425–1432. [Google Scholar] [CrossRef]
- Rogado, J.; Obispo, B.; Pangua, C.; Serrano-Montero, G.; Martín Marino, A.; Pérez-Pérez, M.; López-Alfonso, A.; Gullón, P.; Lara, M.Á. COVID-19 transmission, outcome and associated risk factors in cancer patients at the first month of the pandemic in a Spanish hospital in Madrid. Clin. Transl. Oncol. 2020, 22, 2364–2368. [Google Scholar] [CrossRef]
- Rogado, J.; Pangua, C.; Serrano-Montero, G.; Obispo, B.; Marino, A.M.; Pérez-Pérez, M.; López-Alfonso, A.; Gullón, P.; Lara, M.Á. COVID-19 and lung cancer: A greater fatality rate? Lung Cancer 2020, 146, 19–22. [Google Scholar] [CrossRef]
- Yarza, R.; Bover, M.; Paredes, D.; López-López, F.; Jara-Casas, D.; Castelo-Loureiro, A.; Baena, J.; Mazarico, J.M.; Folgueira, M.D.; Meléndez-Carmona, M.Á.; et al. SARS-CoV-2 infection in cancer patients undergoing active treatment: Analysis of clinical features and predictive factors for severe respiratory failure and death. Eur. J. Cancer 2020, 135, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Lemos, A.E.G.; Silva, G.R.; Gimba, E.R.P.; Matos, A.D.R. Susceptibility of lung cancer patients to COVID-19: A review of the pandemic data from multiple nationalities. Thorac. Cancer. 2021, 12, 2637–2647. [Google Scholar] [CrossRef] [PubMed]
- Passaro, A.; Bestvina, C.; Velez Velez, M.; Garassino, M.C.; Garon, E.; Peters, S. Severity of COVID-19 in patients with lung cancer: Evidence and challenges. J. Immunother. Cancer. 2021, 9, e002266. [Google Scholar] [CrossRef]
- Zhao, Q.; Meng, M.; Kumar, R.; Wu, Y.; Huang, J.; Deng, Y.; Weng, Z.; Yang, L. Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A systemic review and meta-analysis. Int. J. Infect. Dis. 2020, 96, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, A.; Jafarzadeh, S.; Nozari, P.; Mokhtari, P.; Nemati, M. Lymphopenia an important immunological abnormality in patients with COVID-19: Possible mechanisms. Scand. J. Immunol. 2021, 93, e12967. [Google Scholar] [CrossRef]
- Szarpak, L.; Ruetzler, K.; Safiejko, K.; Hampel, M.; Pruc, M.; Kanczuga-Koda, L.; Filipiak, K.J.; Jaguszewski, M.J. Lactate dehydrogenase level as a COVID-19 severity marker. Am. J. Emerg. Med. 2021, 45, 638–639. [Google Scholar] [CrossRef]
- Zuin, M.; Rigatelli, G.; Quadretti, L.; Fogato, L.; Zuliani, G.; Roncon, L. Prognostic Role of Anemia in COVID-19 Patients: A Meta-Analysis. Infect. Dis. Rep. 2021, 13, 930–937. [Google Scholar] [CrossRef]
- Armstrong, R.A.; Kane, A.D.; Cook, T.M. Outcomes from intensive care in patients with COVID-19: A systematic review and meta-analysis of observational studies. Anaesthesia 2020, 75, 1340–1349. [Google Scholar] [CrossRef]
- Nadkarni, A.R.; Vijayakumaran, S.C.; Gupta, S.; Divatia, J.V. Mortality in Cancer Patients With COVID-19 Who Are Admitted to an ICU or Who Have Severe COVID-19: A Systematic Review and Meta-Analysis. JCO Glob. Oncol. 2021, 7, 1286–1305. [Google Scholar] [CrossRef]
- Plais, H.; Labruyère, M.; Creutin, T.; Nay, P.; Plantefeve, G.; Tapponnier, R.; Jonas, M.; Ngapmen, N.T.; Le Guennec, L.; De Roquetaillade, C.; et al. Outcomes of Patients with Active Cancer and COVID-19 in the Intensive-Care Unit: A Multicenter Ambispective Study. Front. Oncol. 2022, 12, 858276. [Google Scholar] [CrossRef]
- Zavras, P.D.; Mehta, V.; Goel, S.; Pradhan, K.; Billett, H.H. Increased Incidence of Thrombosis in a Cohort of Cancer Patients with COVID-19. Acta Haematol. 2022, 145, 152–159. [Google Scholar] [CrossRef] [PubMed]
| Characteristics of Matching | With Cancer—Case (n = 29) n (%) | Without Cancer—Control (n = 29) n (%) | p-Value |
|---|---|---|---|
| Age (years) Median (range) | 77 (63.5–80) | 74 (64.5–80.5) | 0.988 |
| Male Sex | 17 (59%) | 17 (59%) | 1 |
| Comorbidities Current or past smoker Overweight/obesity Arterial hypertension Diabetes mellitus Dyslipidemia Cardiovascular disease Respiratory disease Kidney disease | |||
| 11 (38%) 13 (45%) 19 (66%) 12 (41%) 11 (38%) 9 (31%) 9 (31%) 6 (21%) | 10 (34%) 14 (48%) 19 (66%) 14 (48%) 10 (34%) 11 (38%) 10 (34%) 6 (21%) | 0.785 0.792 1 0.792 0.785 0.783 0.780 1 |
| Patient Characteristics | With Cancer—Case (n = 29) n (%) |
|---|---|
| Type of cancer Solid Hematological | |
| 23 (79.3%) | |
| 6 (20.7%) | |
| Stage of solid malignancy I–III IV | 20 (87%) |
| 3 (13%) | |
| Cancer classification according to ICD-10 Malignant cancer of digestive tract (C15–C26) Malignant cancer of respiratory and intrathoracic organs (C30–C39) Malignant cancer of bone and articular cartilage (C40–C41) Malignant cancer of soft tissue (C45–C49) Malignant cancer of breast (C50–C50) Malignant cancer of male genital tract (C60–C63) Malignant cancer of the urinary tract (C64–C68) Malignant cancer of central nervous system (C69–C72) Malignant cancer of thyroid and other endocrine glands (C73–C75) Leukemia (C81–C96) Lymphoma (C81–C96) Multiple myeloma (C81–C96) | |
| 3 (10.3%) 2 (6.9%) 1 (3.4%) 3 (10.3%) 4 (13.8%) 4 (13.8%) 4 (13.8%) 1 (3.4%) 1 (3.4%) 3 (10.3%) 2 (6.9%) 1 (3.4%) | |
| Status of cancer Surveillance Active | 9 (31%) 20 (69%) |
| Prior treatment Surgery Radiotherapy Chemotherapy Endocrine therapy Target therapy | 20 (69%) 8 (27.6%) 8 (27.6%) 6 (20.7%) 1 (3.4%) |
| Patient Characteristics | With Cancer—Case (n = 29) n (%) | Without Cancer—Control (n = 29) n (%) | p-Value |
|---|---|---|---|
| Symptoms or signs at initial presentation Fever Dyspnea Cough Asthenia Dysgeusia Diarrhea Prostration Desaturation Abdominal pain Odynophagia Headache Myalgias | |||
| 13 (44.8%) 12 (41.4%) 10 (34.5%) 5 (17.2%) 3 (10.3%) 3 (10.3%) 2 (6.9%) 2 (6.9%) 1 (3.4%) 1 (3.4%) 1 (3.4%) 1 (3.4%) | 13 (44.8%) 16 (55.2%) 18 (62.1%) 6 (20.7%) 2 (6.9%) 2 (6.9%) - 1 (3.4%) 1 (3.4%) 2 (6.9%) 2 (6.9%) 5 (17.2%) | 1 0.431 0.650 0.738 0.640 0.640 - 0.553 1 0.553 0.553 0.194 | |
| Analytical parameters at ICU admission Leukopenia a Lymphopenia b Anemia c Thrombocytopenia d Elevated CRP e Elevated PCT f Elevated LDH g Elevated D-dimers h | |||
| 5 (17.2%) 24 (82.8%) 22 (75.9%) 5 (17.2%) 19 (65.5%) 13 (44.8%) 25 (86.2%) 14 (48.3%) | 2 (6.9%) 25 (86.2%) 13 (44.8%) 4 (13.8%) 19 (65.5%) 10 (34.5%) 24 (82.8%) 15 (51.7%) | 0.423 0.717 0.031 0.717 1 0.592 0.717 0.793 | |
| Findings on chest X-ray at ICU admission Unilateral Bilateral | 1 (3.4%) 28 (96.6%) | 2 (6.9%) 27 (93.1%) | 0.553 |
| CT pattern at ICU admission Consolidation Ground-glass opacities Nodular pattern | 11/15 (73.3%) 10/15 (66.7%) 1/15 (6.7%) | 8/16 (50%) 16/16 (100%) 1/16 (6.3%) | 0.273 0.018 0.963 |
| SAPS II at ICU admission SOFA at ICU admission | 44.24 ± 18.27 6 (2–8) | 34.31 ± 19.99 4 (2–9) | 0.067 0.396 |
| Characteristics | With Cancer—Case (n = 29) n (%) | Without Cancer—Control (n = 29) n (%) | p-Value |
|---|---|---|---|
| Organs/Systems Dysfunction Respiratory Cardiovascular Liver Renal Hematological CNS | 29 (100%) 22 (75.9%) 12 (41.4%) 19 (65.5%) 8 (27.6%) 11 (38%) | 29 (100%) 20 (69%) 14 (48.3%) 16 (55.2%) 10 (34.5%) 12 (41.4%) | 1 0.770 0.792 0.592 0.777 0.914 |
| Secondary Infection VAP CVC-associated infection | 20 (69%) 12 (60%) 1 (5%) | 17 (58.6%) 9 (52.9%) 1 (5.9%) | 0.414 |
| Septic shock | 17 (58.6%) | 14 (48.3%) | 0.599 |
| PTE | 1 (3.4%) | 3 (10.3%) | 0.300 |
| Length of ICU stay (days) | 8 (3–23.50) | 9 (6–13.50) | 0.944 |
| Variables | With Cancer—Case (n = 29) n (%) | Without Cancer—Control (n = 29) n (%) | p-Value |
|---|---|---|---|
| Oxygen therapy/ventilatory support at ICU admission Oxygen therapy HCM HFOT NIMV IMV | |||
11 (37.9%) 3 (10.3%) 8 (27.6%) 4 (13.8%) 14 (48.3%) | 23 (79.3%) 2 (6.9%) 21 (72.4%) 4 (13.8%) 2 (6.9%) | 0.186 1 0.002 | |
| IMV need during all-length of ICU stay | 22 (75.9%) | 16 (55.2%) | 0.167 |
| Days from ICU admission to IMV Total days on IMV | 2 (1–9) 12 (4.75–28.75) | 2 (1.75–2) 11 (7–14) | 0.685 0.871 |
| Drug Therapy Corticosteroids Remdesivir Antimicrobial treatments Vasopressor support | 22 (75.9%) 5 (17.2%) 20 (69%) 20 (69%) | 29 (100%) 6 (20.7%) 16 (55.2%) 17 (58.6%) | 0.005 0.738 0.279 0.414 |
| Prone positioning | 16 (55.2%) | 19 (65.5%) | 0.421 |
| ECMO | 1 (3.4%) | 1 (3.4%) | 1 |
| RRT | 2 (6.9%) | 1 (3.4%) | 0.998 |
| Days from ICU admission and death | 8 (4–25.5) | 13 (7–18) | 0.299 |
| Univariate Logistic Regression | OR | 95% CI | p-Value a |
|---|---|---|---|
| Death IMV need at ICU admission IMV need during all-length of ICU stay | 2.318 | 0.809–6.644 | 0.118 |
| 12.600 2.554 | 2.517–63.063 0.831–7.842 | 0.002 0.102 | |
| Multivariate Logistic Regression | Adjusted OR b | 95% CI | p-Value a |
| IMV need at ICU admission | 14.036 | 1.337–153.111 | 0.028 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranchor, R.; Pereira, N.; Medeiros, A.R.; Magalhães, M.; Marinho, A.; Araújo, A. Characteristics and Outcomes of COVID-19 Cancer Patients Admitted to a Portuguese Intensive Care Unit: A Case-Control Study. Cancers 2023, 15, 3264. https://doi.org/10.3390/cancers15123264
Ranchor R, Pereira N, Medeiros AR, Magalhães M, Marinho A, Araújo A. Characteristics and Outcomes of COVID-19 Cancer Patients Admitted to a Portuguese Intensive Care Unit: A Case-Control Study. Cancers. 2023; 15(12):3264. https://doi.org/10.3390/cancers15123264
Chicago/Turabian StyleRanchor, Ridhi, Nuno Pereira, Ana R. Medeiros, Manuel Magalhães, Aníbal Marinho, and António Araújo. 2023. "Characteristics and Outcomes of COVID-19 Cancer Patients Admitted to a Portuguese Intensive Care Unit: A Case-Control Study" Cancers 15, no. 12: 3264. https://doi.org/10.3390/cancers15123264
APA StyleRanchor, R., Pereira, N., Medeiros, A. R., Magalhães, M., Marinho, A., & Araújo, A. (2023). Characteristics and Outcomes of COVID-19 Cancer Patients Admitted to a Portuguese Intensive Care Unit: A Case-Control Study. Cancers, 15(12), 3264. https://doi.org/10.3390/cancers15123264





