Bibliometric Analysis of Global Research on Tumor Dormancy
Abstract
Simple Summary
Abstract
1. Introduction
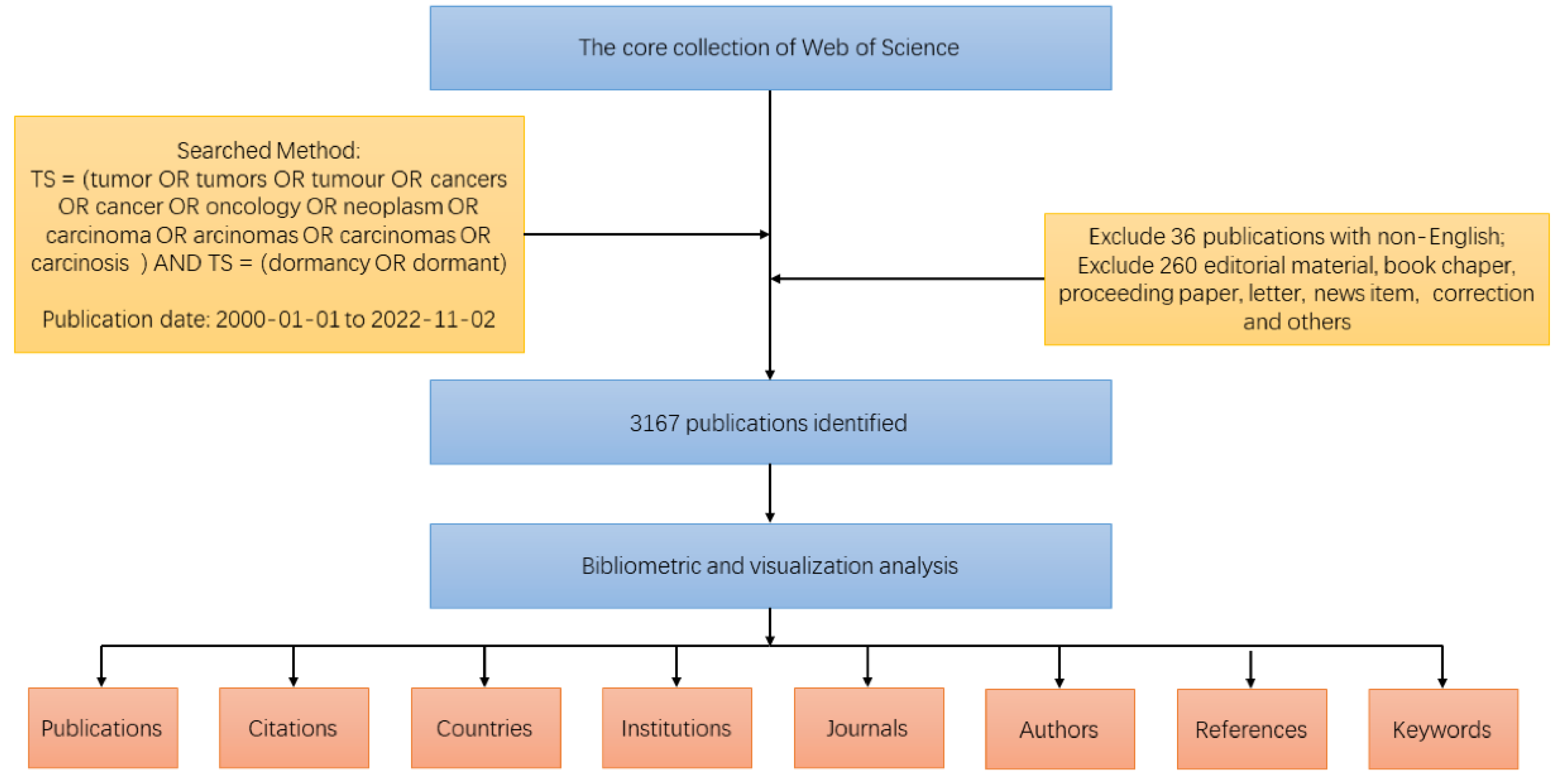
2. Materials and Methods
3. Results
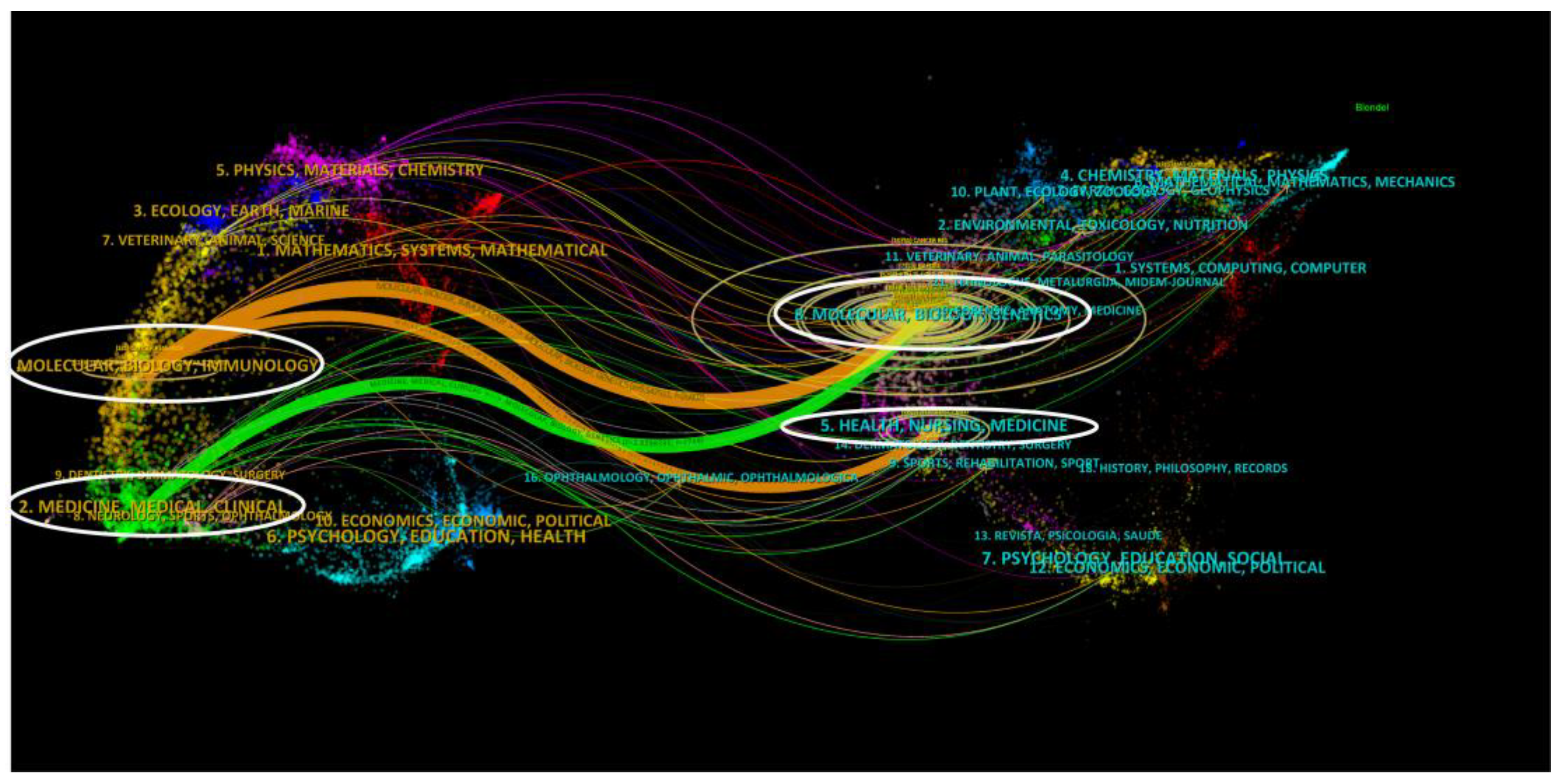
3.1. Posting Volume Worldwide and Country Cooperation
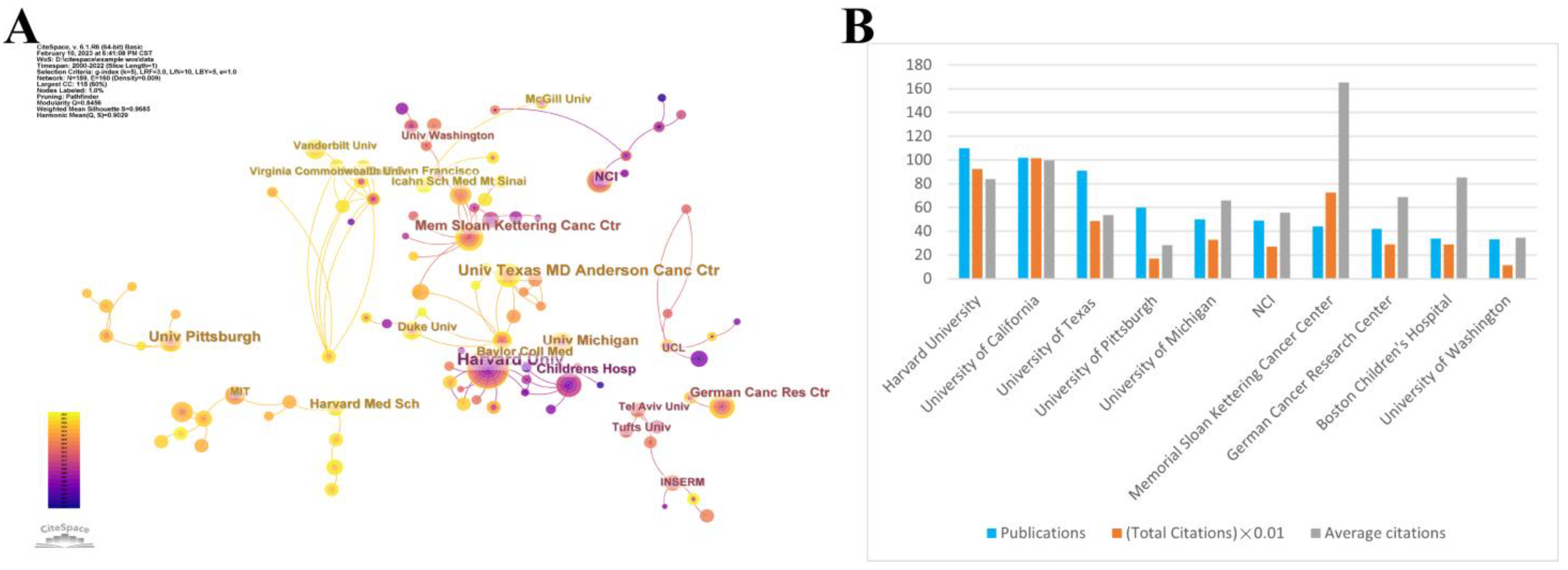
3.2. Analysis of Institutions
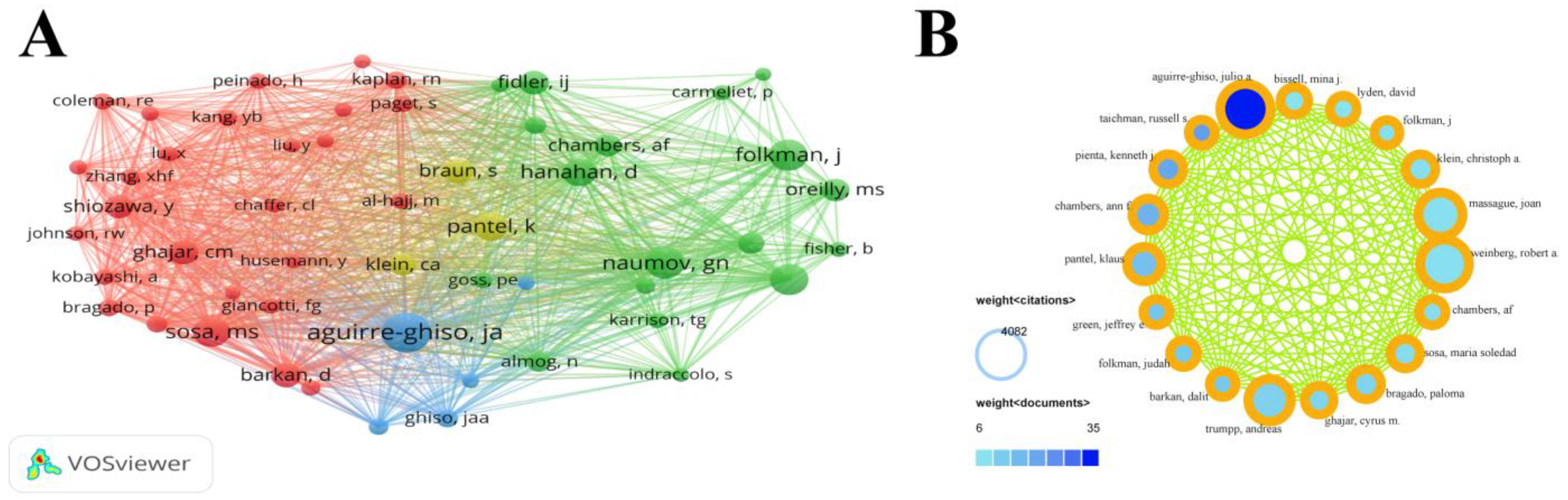
3.3. Author Collaborations
3.4. Examination of Publications and Journals
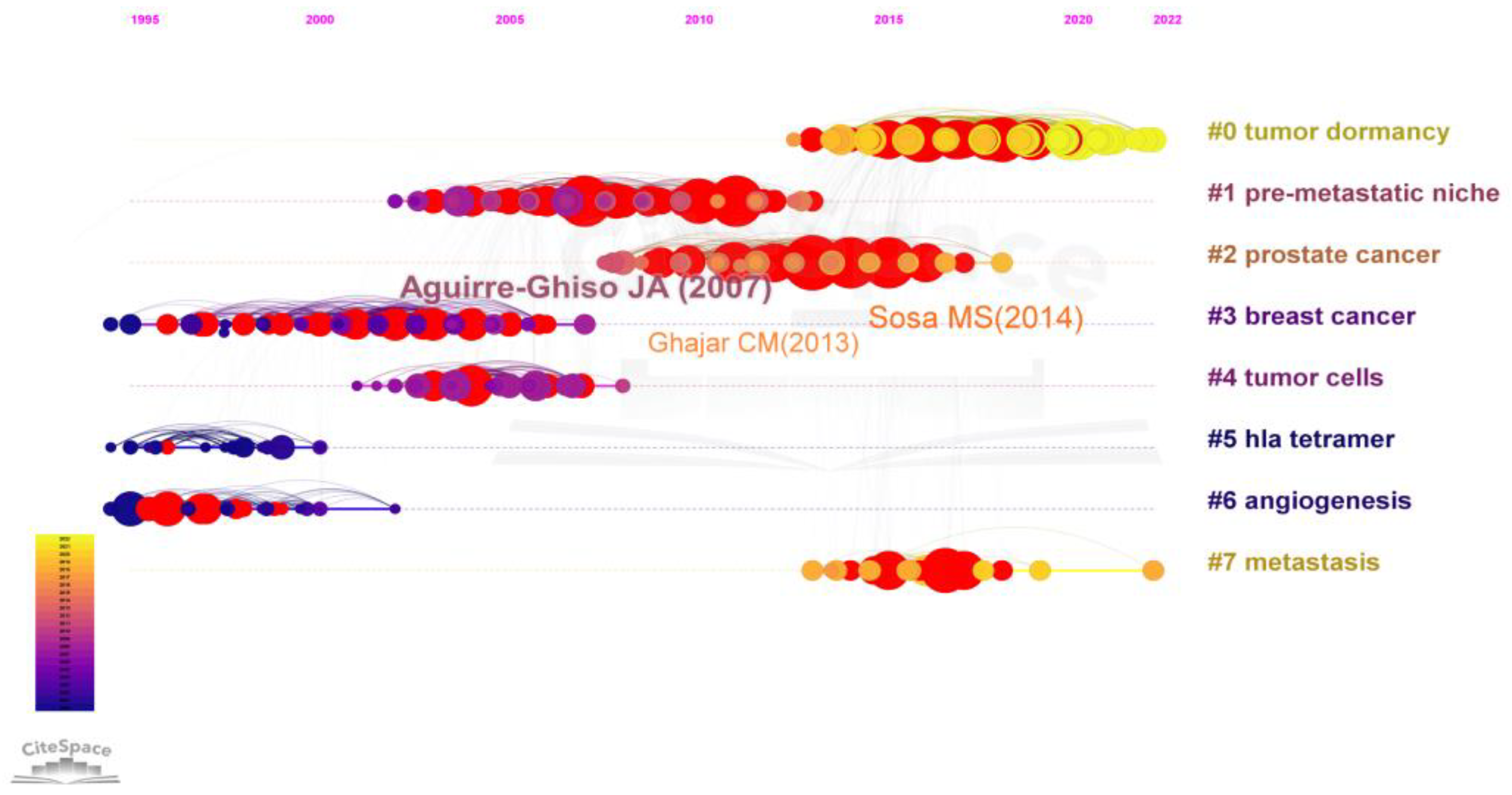
3.5. Analysis of Co-Cited References
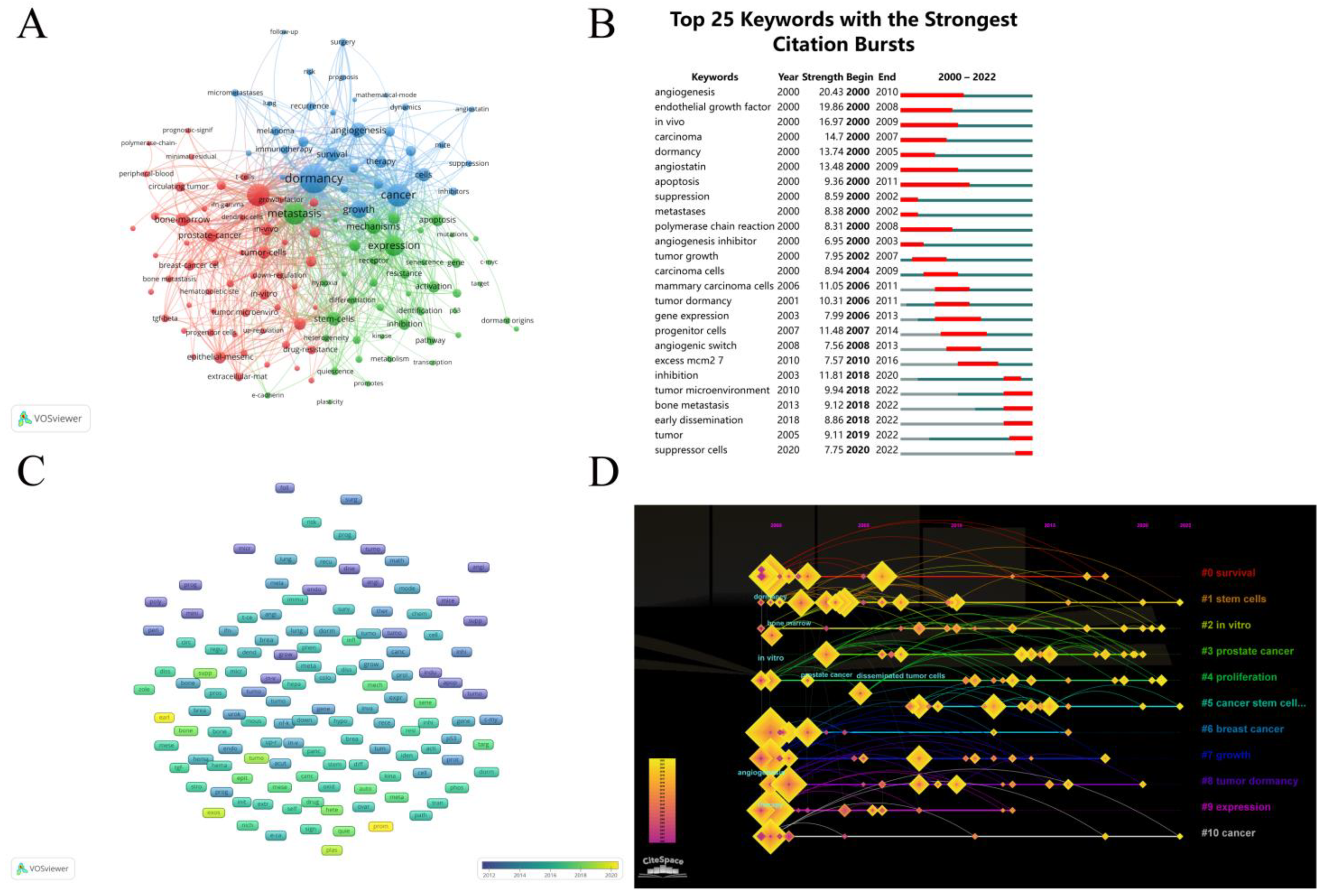
3.6. Analysis of Keywords
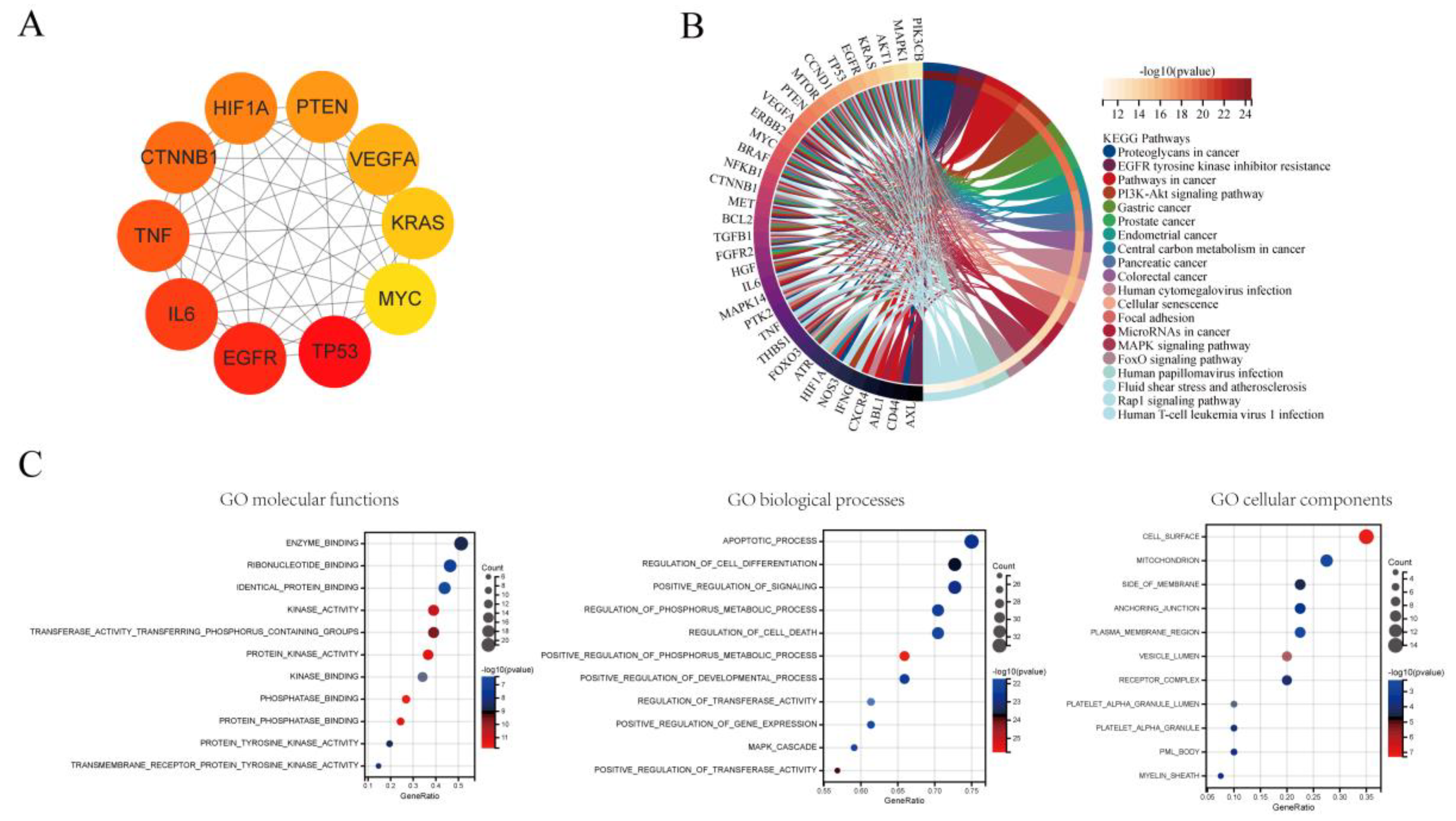
3.7. Hub Genes and Pathways Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Butturini, E.; Carcereri de Prati, A.; Boriero, D.; Mariotto, S. Tumor Dormancy and Interplay with Hypoxic Tumor Microenvironment. IJMS 2019, 20, 4305. [Google Scholar] [CrossRef] [PubMed]
- Elkholi, I.E.; Lalonde, A.; Park, M.; Côté, J.-F. Breast Cancer Metastatic Dormancy and Relapse: An Enigma of Microenvironment(s). Cancer Res. 2022, 82, 4479–4510. [Google Scholar] [CrossRef]
- Goss, P.E.; Chambers, A.F. Does Tumour Dormancy Offer a Therapeutic Target? Nat. Rev. Cancer 2010, 10, 871–877. [Google Scholar] [CrossRef]
- Santos-de-Frutos, K.; Djouder, N. When Dormancy Fuels Tumour Relapse. Commun. Biol. 2021, 4, 747. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.F.; Walker, C.K. A Descriptive and Historical Review of Bibliometrics with Applications to Medical Sciences. Pharmacotherapy 2015, 35, 551–559. [Google Scholar] [CrossRef]
- Zielińska, A.; Karczewski, J.; Eder, P.; Kolanowski, T.; Szalata, M.; Wielgus, K.; Szalata, M.; Kim, D.; Shin, S.R.; Słomski, R.; et al. Scaffolds for Drug Delivery and Tissue Engineering: The Role of Genetics. J. Control. Release 2023, 359, 207–223. [Google Scholar] [CrossRef]
- Ding, X.; Yang, Z. Knowledge Mapping of Platform Research: A Visual Analysis Using VOSviewer and CiteSpace. Electron. Commer. Res. 2022, 22, 787–809. [Google Scholar] [CrossRef]
- da Silva, P.B.V.; Brenelli, L.B.; Mariutti, L.R.B. Waste and By-Products as Sources of Lycopene, Phytoene, and Phytofluene—Integrative Review with Bibliometric Analysis. Food Res. Int. 2023, 169, 112838. [Google Scholar] [CrossRef]
- Hasan, M.; Abedin, M.Z.; Amin, M.B.; Nekmahmud, M.; Oláh, J. Sustainable Biofuel Economy: A Mapping through Bibliometric Research. J. Env. Manag. 2023, 336, 117644. [Google Scholar] [CrossRef] [PubMed]
- van Eck, N.J.; Waltman, L. Software Survey: VOSviewer, a Computer Program for Bibliometric Mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Wang, M.; Xie, X.; Zhao, S.; Han, W.; Zhang, Y. Global Research Trends and Hotspots of Fecal Microbiota Transplantation: A Bibliometric and Visualization Study. Front. Microbiol. 2022, 13, 990800. [Google Scholar] [CrossRef]
- Settles, B. ABNER: An Open Source Tool for Automatically Tagging Genes, Proteins and Other Entity Names in Text. Bioinformatics 2005, 21, 3191–3192. [Google Scholar] [CrossRef] [PubMed]
- Maglott, D.; Ostell, J.; Pruitt, K.D.; Tatusova, T. Entrez Gene: Gene-Centered Information at NCBI. Nucleic Acids Res. 2011, 39, D52–D57. [Google Scholar] [CrossRef]
- von Mering, C.; Huynen, M.; Jaeggi, D.; Schmidt, S.; Bork, P.; Snel, B. STRING: A Database of Predicted Functional Associations between Proteins. Nucleic Acids Res. 2003, 31, 258–261. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Aguirre-Ghiso, J.A. Translating the Science of Cancer Dormancy to the Clinic. Cancer Res. 2021, 81, 4673–4675. [Google Scholar] [CrossRef] [PubMed]
- Hensel, J.A.; Flaig, T.W.; Theodorescu, D. Clinical Opportunities and Challenges in Targeting Tumour Dormancy. Nat. Rev. Clin. Oncol. 2013, 10, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Gomatou, G.; Syrigos, N.; Vathiotis, I.A.; Kotteas, E.A. Tumor Dormancy: Implications for Invasion and Metastasis. Int. J. Mol. Sci. 2021, 22, 4862. [Google Scholar] [CrossRef]
- Recasens, A.; Munoz, L. Targeting Cancer Cell Dormancy. Trends Pharmacol. Sci. 2019, 40, 128–141. [Google Scholar] [CrossRef]
- Aguirre-Ghiso, J.A. Models, Mechanisms and Clinical Evidence for Cancer Dormancy. Nat. Rev. Cancer 2007, 7, 834–846. [Google Scholar] [CrossRef]
- Cheung, T.H.; Rando, T.A. Molecular Regulation of Stem Cell Quiescence. Nat. Rev. Mol. Cell Biol. 2013, 14, 329–340. [Google Scholar] [CrossRef]
- Triana-Martínez, F.; Loza, M.I.; Domínguez, E. Beyond Tumor Suppression: Senescence in Cancer Stemness and Tumor Dormancy. Cells 2020, 9, 346. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Heidrich, I.; Pantel, K. Clinical Management and Biology of Tumor Dormancy in Breast Cancer. Semin. Cancer Biol. 2022, 78, 49–62. [Google Scholar] [CrossRef]
- Humtsoe, J.O.; Kramer, R.H. Differential Epidermal Growth Factor Receptor Signaling Regulates Anchorage-Independent Growth by Modulation of the PI3K/AKT Pathway. Oncogene 2010, 29, 1214–1226. [Google Scholar] [CrossRef]
- Schewe, D.M.; Aguirre-Ghiso, J.A. ATF6alpha-Rheb-MTOR Signaling Promotes Survival of Dormant Tumor Cells in Vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 10519–10524. [Google Scholar] [CrossRef]
- Bragado, P.; Estrada, Y.; Parikh, F.; Krause, S.; Capobianco, C.; Farina, H.G.; Schewe, D.M.; Aguirre-Ghiso, J.A. TGF-Β2 Dictates Disseminated Tumour Cell Fate in Target Organs through TGF-β-RIII and P38α/β Signalling. Nat. Cell Biol. 2013, 15, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Luo, R.Z.; Lu, Y.; Zhang, X.; Yu, Q.; Khare, S.; Kondo, S.; Kondo, Y.; Yu, Y.; Mills, G.B.; et al. The Tumor Suppressor Gene ARHI Regulates Autophagy and Tumor Dormancy in Human Ovarian Cancer Cells. J. Clin. Investig. 2008, 118, 3917. [Google Scholar] [CrossRef]
- Balz, L.M.; Bartkowiak, K.; Andreas, A.; Pantel, K.; Niggemann, B.; Zänker, K.S.; Brandt, B.H.; Dittmar, T. The Interplay of HER2/HER3/PI3K and EGFR/HER2/PLC-Γ1 Signalling in Breast Cancer Cell Migration and Dissemination. J. Pathol. 2012, 227, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Sosa, M.S.; Bragado, P.; Aguirre-Ghiso, J.A. Mechanisms of Disseminated Cancer Cell Dormancy: An Awakening Field. Nat. Rev. Cancer 2014, 14, 611–622. [Google Scholar] [CrossRef]
- Neophytou, C.M.; Kyriakou, T.-C.; Papageorgis, P. Mechanisms of Metastatic Tumor Dormancy and Implications for Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 6158. [Google Scholar] [CrossRef]
- Manjili, S.H.; Isbell, M.; Ghochaghi, N.; Perkinson, T.; Manjili, M.H. Multifaceted Functions of Chronic Inflammation in Regulating Tumor Dormancy and Relapse. Semin. Cancer Biol. 2022, 78, 17–22. [Google Scholar] [CrossRef]
- Cackowski, F.C.; Heath, E.I. Prostate Cancer Dormancy and Recurrence. Cancer Lett. 2022, 524, 103–108. [Google Scholar] [CrossRef]
- Ghajar, C.M.; Peinado, H.; Mori, H.; Matei, I.R.; Evason, K.J.; Brazier, H.; Almeida, D.; Koller, A.; Hajjar, K.A.; Stainier, D.Y.R.; et al. The Perivascular Niche Regulates Breast Tumour Dormancy. Nat. Cell Biol. 2013, 15, 807–817. [Google Scholar] [CrossRef]
- Mukherjee, A.; Bravo-Cordero, J.J. Regulation of Dormancy during Tumor Dissemination: The Role of the ECM. Cancer Metastasis Rev. 2023, 42, 99–112. [Google Scholar] [CrossRef]
- Tamamouna, V.; Pavlou, E.; Neophytou, C.M.; Papageorgis, P.; Costeas, P. Regulation of Metastatic Tumor Dormancy and Emerging Opportunities for Therapeutic Intervention. Int. J. Mol. Sci. 2022, 23, 13931. [Google Scholar] [CrossRef]
- Qiu, Y.; Qiu, S.; Deng, L.; Nie, L.; Gong, L.; Liao, X.; Zheng, X.; Jin, K.; Li, J.; Tu, X.; et al. Biomaterial 3D Collagen I Gel Culture Model: A Novel Approach to Investigate Tumorigenesis and Dormancy of Bladder Cancer Cells Induced by Tumor Microenvironment. Biomaterials 2020, 256, 120217. [Google Scholar] [CrossRef]
- Hedley, B.D.; Chambers, A.F. Tumor Dormancy and Metastasis. Adv. Cancer Res. 2009, 102, 67–101. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lv, J.; Liang, X.; Yin, X.; Zhang, L.; Chen, D.; Jin, X.; Fiskesund, R.; Tang, K.; Ma, J.; et al. Fibrin Stiffness Mediates Dormancy of Tumor-Repopulating Cells via a Cdc42-Driven Tet2 Epigenetic Program. Cancer Res. 2018, 78, 3926–3937. [Google Scholar] [CrossRef] [PubMed]
- Manjili, M.H. Tumor Dormancy and Relapse: From a Natural Byproduct of Evolution to a Disease State. Cancer Res. 2017, 77, 2564–2569. [Google Scholar] [CrossRef]
- Nanou, A.; Lorenzo-Moldero, I.; Gazouleas, K.D.; Cortese, B.; Moroni, L. 3D Culture Modeling of Metastatic Breast Cancer Cells in Additive Manufactured Scaffolds. ACS Appl. Mater. Interfaces 2022, 14, 28389–28402. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Wirasaputra, A.; Mohammadi, F.; Kundu, A.N.; Esteves, J.A.E.; Heiser, L.M.; Meyer, A.S.; Peyton, S.R. Live Cell Lineage Tracing of Dormant Cancer Cells. Adv. Healthc. Mater. 2023, 12, e2202275. [Google Scholar] [CrossRef] [PubMed]
- Adam, A.P.; George, A.; Schewe, D.; Bragado, P.; Iglesias, B.V.; Ranganathan, A.C.; Kourtidis, A.; Conklin, D.S.; Aguirre-Ghiso, J.A. Computational Identification of a P38SAPK-Regulated Transcription Factor Network Required for Tumor Cell Quiescence. Cancer Res. 2009, 69, 5664–5672. [Google Scholar] [CrossRef]
- Yu-Lee, L.-Y.; Yu, G.; Lee, Y.-C.; Lin, S.-C.; Pan, J.; Pan, T.; Yu, K.-J.; Liu, B.; Creighton, C.J.; Rodriguez-Canales, J.; et al. Osteoblast-Secreted Factors Mediate Dormancy of Metastatic Prostate Cancer in the Bone via Activation of the TGFβRIII–P38MAPK–PS249/T252RB Pathway. Cancer Res. 2018, 78, 2911–2924. [Google Scholar] [CrossRef]
- Ren, D.; Dai, Y.; Yang, Q.; Zhang, X.; Guo, W.; Ye, L.; Huang, S.; Chen, X.; Lai, Y.; Du, H.; et al. Wnt5a Induces and Maintains Prostate Cancer Cells Dormancy in Bone. J. Exp. Med. 2019, 216, 428–449. [Google Scholar] [CrossRef] [PubMed]
- Capulli, M.; Hristova, D.; Valbret, Z.; Carys, K.; Arjan, R.; Maurizi, A.; Masedu, F.; Cappariello, A.; Rucci, N.; Teti, A. Notch2 Pathway Mediates Breast Cancer Cellular Dormancy and Mobilisation in Bone and Contributes to Haematopoietic Stem Cell Mimicry. Br. J. Cancer 2019, 121, 157–171. [Google Scholar] [CrossRef]
- Attaran, S.; Bissell, M.J. The Role of Tumor Microenvironment and Exosomes in Dormancy and Relapse. Semin. Cancer Biol. 2022, 78, 35–44. [Google Scholar] [CrossRef]
- Globig, P.; Willumeit-Römer, R.; Martini, F.; Mazzoni, E.; Luthringer-Feyerabend, B.J.C. Slow Degrading Mg-Based Materials Induce Tumor Cell Dormancy on an Osteosarcoma-Fibroblast Coculture Model. Bioact. Mater. 2022, 16, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Rossari, F.; Zucchinetti, C.; Buda, G.; Orciuolo, E. Tumor Dormancy as an Alternative Step in the Development of Chemoresistance and Metastasis—Clinical Implications. Cell. Oncol. 2020, 43, 155–176. [Google Scholar] [CrossRef]
- Fornetti, J.; Welm, A.L.; Stewart, S.A. Understanding the Bone in Cancer Metastasis. J. Bone Min. Res. 2018, 33, 2099–2113. [Google Scholar] [CrossRef]
- Ring, A.; Spataro, M.; Wicki, A.; Aceto, N. Clinical and Biological Aspects of Disseminated Tumor Cells and Dormancy in Breast Cancer. Front. Cell Dev. Biol. 2022, 10, 929893. [Google Scholar] [CrossRef]
- Pranzini, E.; Raugei, G.; Taddei, M.L. Metabolic Features of Tumor Dormancy: Possible Therapeutic Strategies. Cancers 2022, 14, 547. [Google Scholar] [CrossRef]
- Showalter, L.; Czerniecki, B.J.; Koski, G.K. Th1 Cytokines in Conjunction with Pharmacological Akt Inhibition Potentiate Apoptosis of Breast Cancer Cells in vitro and Suppress Tumor Growth in vivo. Oncotarget 2020, 11, 2873–2888. [Google Scholar] [CrossRef]
- Bianchini, G.; Gianni, L. The Immune System and Response to HER2-Targeted Treatment in Breast Cancer. Lancet Oncol. 2014, 15, e58–e68. [Google Scholar] [CrossRef] [PubMed]
- Correia, A.L.; Guimaraes, J.C.; Auf der Maur, P.; De Silva, D.; Trefny, M.P.; Okamoto, R.; Bruno, S.; Schmidt, A.; Mertz, K.; Volkmann, K.; et al. Hepatic Stellate Cells Suppress NK Cell-Sustained Breast Cancer Dormancy. Nature 2021, 594, 566–571. [Google Scholar] [CrossRef]
- Jiang, X.; Liang, L.; Chen, G.; Liu, C. Modulation of Immune Components on Stem Cell and Dormancy in Cancer. Cells 2021, 10, 2826. [Google Scholar] [CrossRef]
- Chen, Y.; Gibson, S.B. Three Dimensions of Autophagy in Regulating Tumor Growth: Cell Survival/Death, Cell Proliferation, and Tumor Dormancy. Biochim. Et Biophys. Acta (BBA) Mol. Basis Dis. 2021, 1867, 166265. [Google Scholar] [CrossRef]
- Borriello, L.; Coste, A.; Traub, B.; Sharma, V.P.; Karagiannis, G.S.; Lin, Y.; Wang, Y.; Ye, X.; Duran, C.L.; Chen, X.; et al. Primary Tumor Associated Macrophages Activate Programs of Invasion and Dormancy in Disseminating Tumor Cells. Nat. Commun. 2022, 13, 626. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, K.; He, H.; Yang, Y.; Li, W.; Liu, T.; Li, J. The Relationship Between Mesenchymal Stem Cells and Tumor Dormancy. Front. Cell Dev. Biol. 2021, 9, 731393. [Google Scholar] [CrossRef] [PubMed]
- Stanford-Moore, G.B.; Canick, J.; Kaplan, S.; Lee, W.T. International Collaboration Trends in Facial Plastic and Reconstructive Surgery: A Systematic Bibliometric Scoping Review. JAMA Otolaryngol. Head Neck Surg. 2023, 149, 540–545. [Google Scholar] [CrossRef] [PubMed]
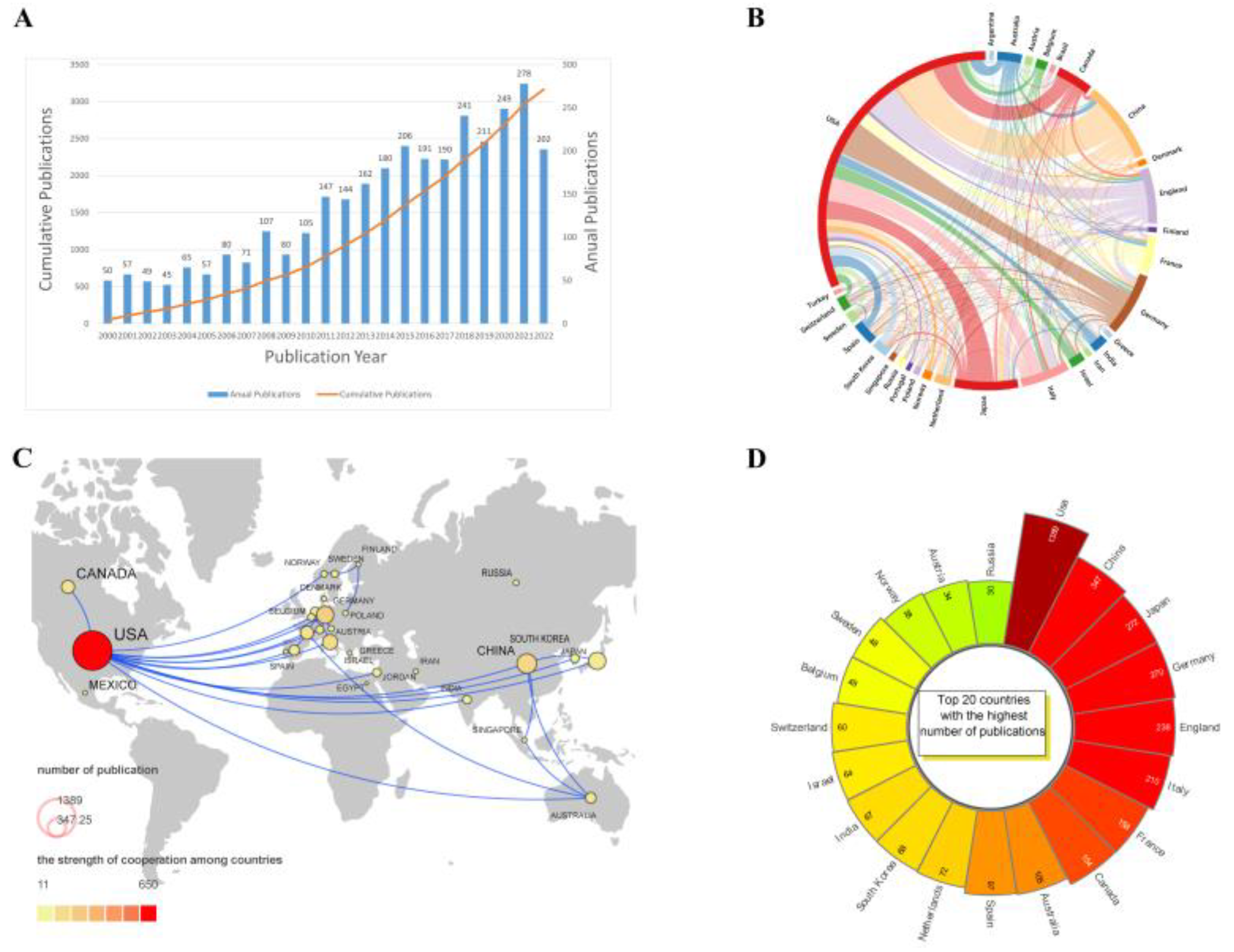
| Rank | Country | Documents | Citations | Average Citations |
|---|---|---|---|---|
| 1 | USA | 1389 | 82,193 | 59.17 |
| 2 | China | 347 | 8814 | 25.40 |
| 3 | Japan | 272 | 8954 | 32.92 |
| 4 | Germany | 270 | 19,499 | 72.22 |
| 5 | England | 238 | 13,470 | 56.60 |
| 6 | Italy | 210 | 9530 | 45.38 |
| 7 | France | 158 | 6907 | 43.72 |
| 8 | Canada | 154 | 8238 | 53.49 |
| 9 | Australia | 105 | 7586 | 72.25 |
| 10 | Spain | 97 | 5427 | 55.95 |
| Rank | Author | Affiliation | Total Link Strength | Documents | Citations |
|---|---|---|---|---|---|
| 1 | Aguirre-Ghiso, Julio A. | Albert Einstein College of Medicine | 2165 | 35 | 4082 |
| 2 | Weinberg, Robert A. | Whitehead Institute | 187 | 7 | 3862 |
| 3 | Massague, Joan | Memorial Sloan Kettering Cancer Center | 233 | 7 | 3153 |
| 4 | Trumpp, Andreas | German Cancer Research Center | 48 | 12 | 2940 |
| 5 | Pantel, Klaus | University Medical Center Hamburg-Eppendorf | 403 | 16 | 1922 |
| 6 | Chambers, Ann F. | University of Western Ontario | 722 | 19 | 1653 |
| 7 | Bragado, Paloma | Hospital Clinico San Carlos | 795 | 11 | 1476 |
| 8 | Pienta, Kenneth J. | Johns Hopkins Medicine | 657 | 20 | 1450 |
| 9 | Klein, Christoph | University of Regensburg | 306 | 7 | 1422 |
| 10 | Sosa, Maria Soledad | Icahn School of Medicine at Mount Sinai | 685 | 8 | 1400 |
| Rank | Journal | JCR (2021) | Documents | Citations | Average Citations | Average Publication Year |
|---|---|---|---|---|---|---|
| 1 | Cancer Research | Q1 | 221 | 8191 | 37.06 | 2013.33 |
| 2 | Cancers | Q1 | 70 | 779 | 11.13 | 2020.66 |
| 3 | Plos one | Q2 | 62 | 2361 | 38.08 | 2013.79 |
| 4 | International Journal of Molecular Sciences | Q1 | 52 | 771 | 14.83 | 2019.25 |
| 5 | Clinical Cancer Research | Q1 | 47 | 3614 | 76.89 | 2010.94 |
| 6 | Scientific Reports | Q1 | 44 | 791 | 17.98 | 2018.25 |
| 7 | Seminars in Cancer Biology | Q1 | 44 | 2689 | 61.11 | 2014.68 |
| 8 | Clinical & Experimental Metastasis | Q2 | 41 | 1009 | 24.61 | 2011.71 |
| 9 | Oncogene | Q1 | 41 | 2230 | 54.39 | 2013.17 |
| 10 | Oncotarget | - | 41 | 1868 | 45.56 | 2015.95 |
| Rank | Year | First Author | Title | Journals | Citations |
|---|---|---|---|---|---|
| 1 | 2007 | Aguirre-Ghiso, JA | Models, mechanisms and clinical evidence for cancer dormancy | Nature Reviews Cancer | 428 |
| 2 | 2014 | Sosa, MS | Mechanisms of disseminated cancer cell dormancy: an awakening field | Nature Reviews Cancer | 266 |
| 3 | 1995 | HOLMGREN, L | Dormancy of micrometastases-balanced proliferation and apoptosis in the presence of angiogenesis suppression | Nature Medicine | 245 |
| 4 | 2013 | Ghajar, CM | The perivascular niche regulates breast tumor dormancy | Nature Cell Biology | 236 |
| 5 | 2011 | Hanahan, D | Hallmarks of Cancer: The Next Generation | Cell | 185 |
| 6 | 1998 | Luzzi, KJ | Multistep nature of metastatic inefficiency - Dormancy of solitary cells after successful extravasation and limited survival of early micrometastases | American Journal of Pathology | 183 |
| 7 | 2013 | Bragado, P | TGF-beta 2 dictates disseminated tumor cell fate in target organs through TGF-beta-RIII and p38 alpha/beta signaling | Nature Cell Biology | 166 |
| 8 | 2002 | Chambers | Dissemination and growth of cancer cells in metastatic sites | Nature Reviews Cancer | 164 |
| 9 | 2001 | Aguirre-Ghiso, JA | Urokinase receptors and fibronectin regulate the ERK(MAPK) to p38(MAPK) activity ratios that determine carcinoma cell proliferation or dormancy in vivo. | Molecular Biology of The Cell | 152 |
| 10 | 2003 | Aguirre-Ghiso, JA | ERK(MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38(SAPK). | Cancer Research | 150 |
| Rank | Molecule | Occurrence | State | Occurrence | Disease | Occurrence |
|---|---|---|---|---|---|---|
| 1 | Endothelial growth-factor | 130 | Dormancy | 1012 | Breast cancer | 598 |
| 2 | Transforming growth factor-beta | 79 | Metastasis | 571 | Prostate cancer | 192 |
| 3 | Urokinase receptor | 64 | Angiogenesis | 262 | Colorectal cancer | 131 |
| 4 | Growth-factor | 53 | Epithelial-mesenchymal transition | 170 | Melanoma | 104 |
| 5 | Nuclear factor-kappa-b | 46 | Apoptosis | 159 | Lung cancer | 88 |
| 6 | E-cadherin | 43 | Proliferation | 155 | Ovarian cancer | 58 |
| 7 | P53 | 37 | Invasion | 82 | Hepatocellular carcinoma | 39 |
| 8 | C-myc | 35 | Self-renewal | 76 | Pancreatic cancer | 31 |
| 9 | Mcm2–7 | 32 | Autophagy | 74 | Glioblastoma | 21 |
| 10 | Tumor-necrosis-factor | 32 | Differentiation | 74 | Multiple myeloma | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yan, L.; Wang, Z.; Li, F.; Lv, J.; Liu, J.; Liu, X.; Bao, L.; Zhang, Y. Bibliometric Analysis of Global Research on Tumor Dormancy. Cancers 2023, 15, 3230. https://doi.org/10.3390/cancers15123230
Zhang Y, Yan L, Wang Z, Li F, Lv J, Liu J, Liu X, Bao L, Zhang Y. Bibliometric Analysis of Global Research on Tumor Dormancy. Cancers. 2023; 15(12):3230. https://doi.org/10.3390/cancers15123230
Chicago/Turabian StyleZhang, Yuzhe, Lirong Yan, Zhongqing Wang, Fang Li, Jinqi Lv, Jiaqing Liu, Xuqin Liu, Li Bao, and Ye Zhang. 2023. "Bibliometric Analysis of Global Research on Tumor Dormancy" Cancers 15, no. 12: 3230. https://doi.org/10.3390/cancers15123230
APA StyleZhang, Y., Yan, L., Wang, Z., Li, F., Lv, J., Liu, J., Liu, X., Bao, L., & Zhang, Y. (2023). Bibliometric Analysis of Global Research on Tumor Dormancy. Cancers, 15(12), 3230. https://doi.org/10.3390/cancers15123230






