Autologous Transplantation May Still Effectively Treat Relapsed Diffuse Large B-Cell Lymphoma in Selected Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Patient Characteristics
Statistical Analysis
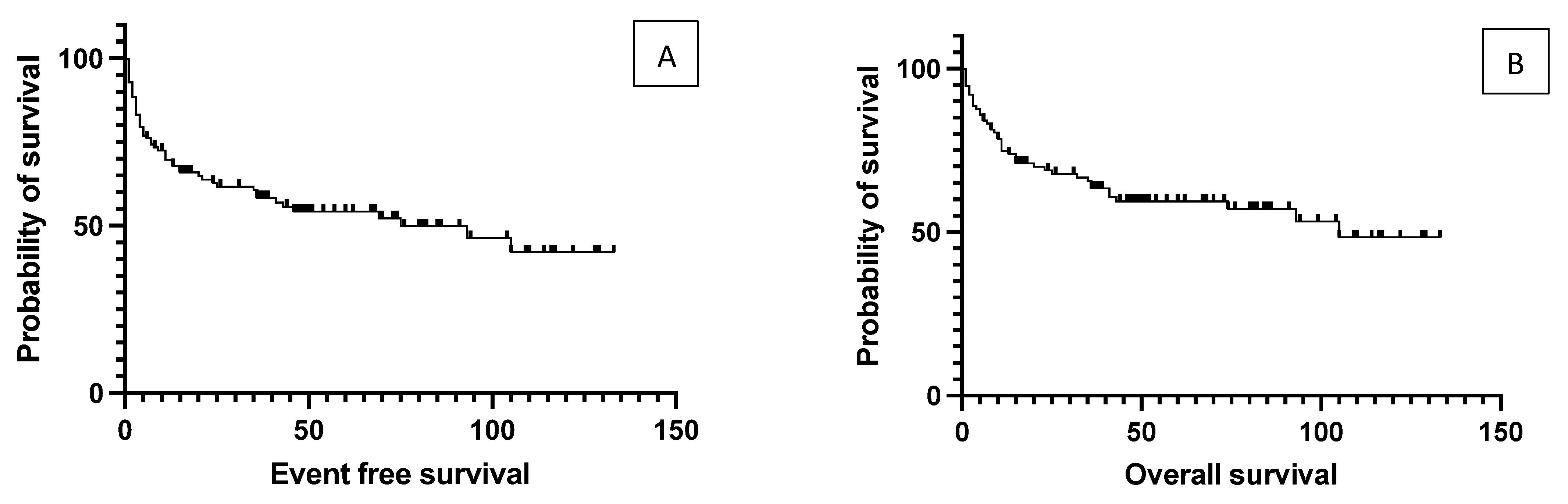
4. Results
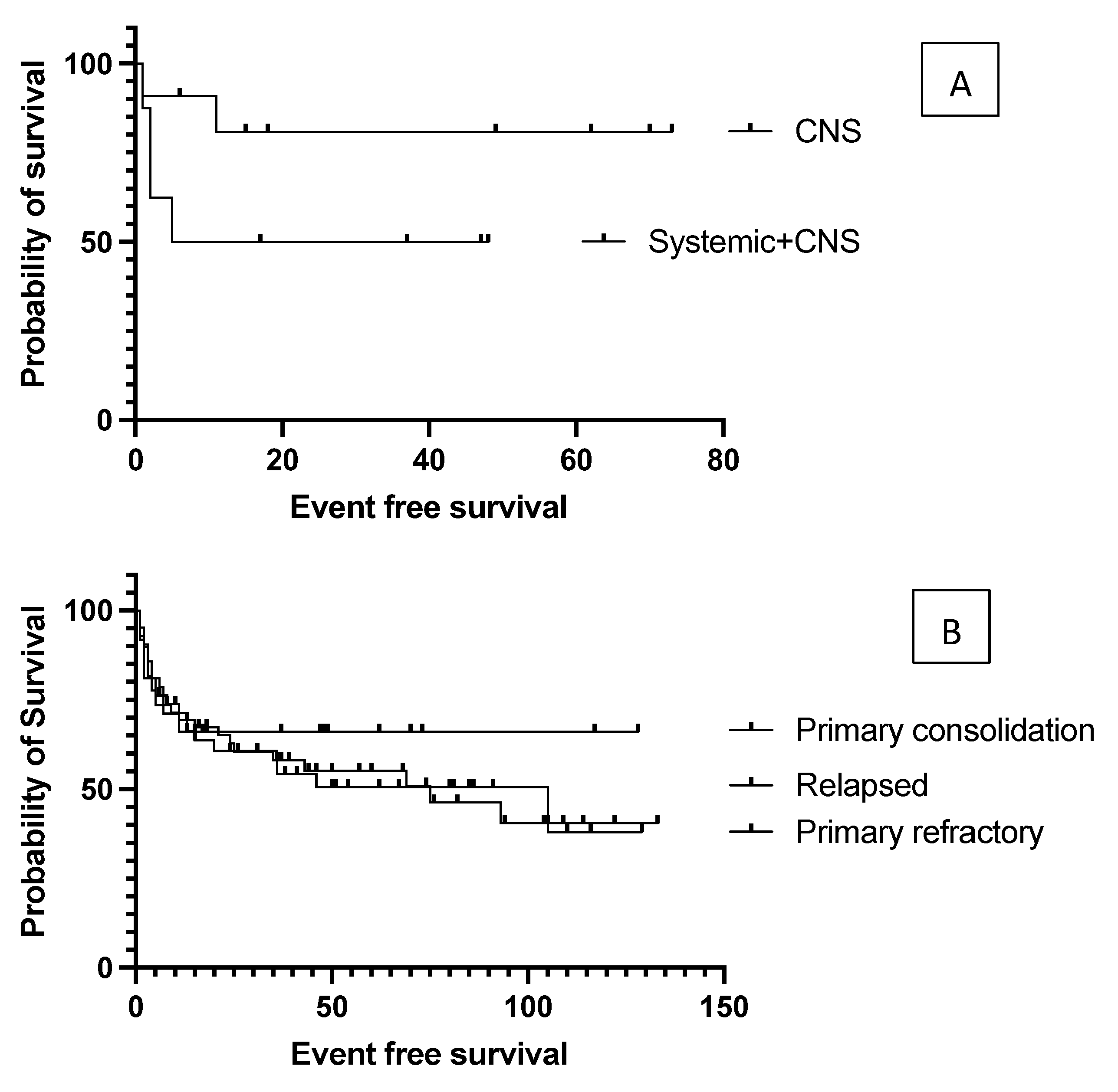
4.1. The Effect of Initial Prognostic Factors Established at Diagnosis on the Outcome of Transplantation
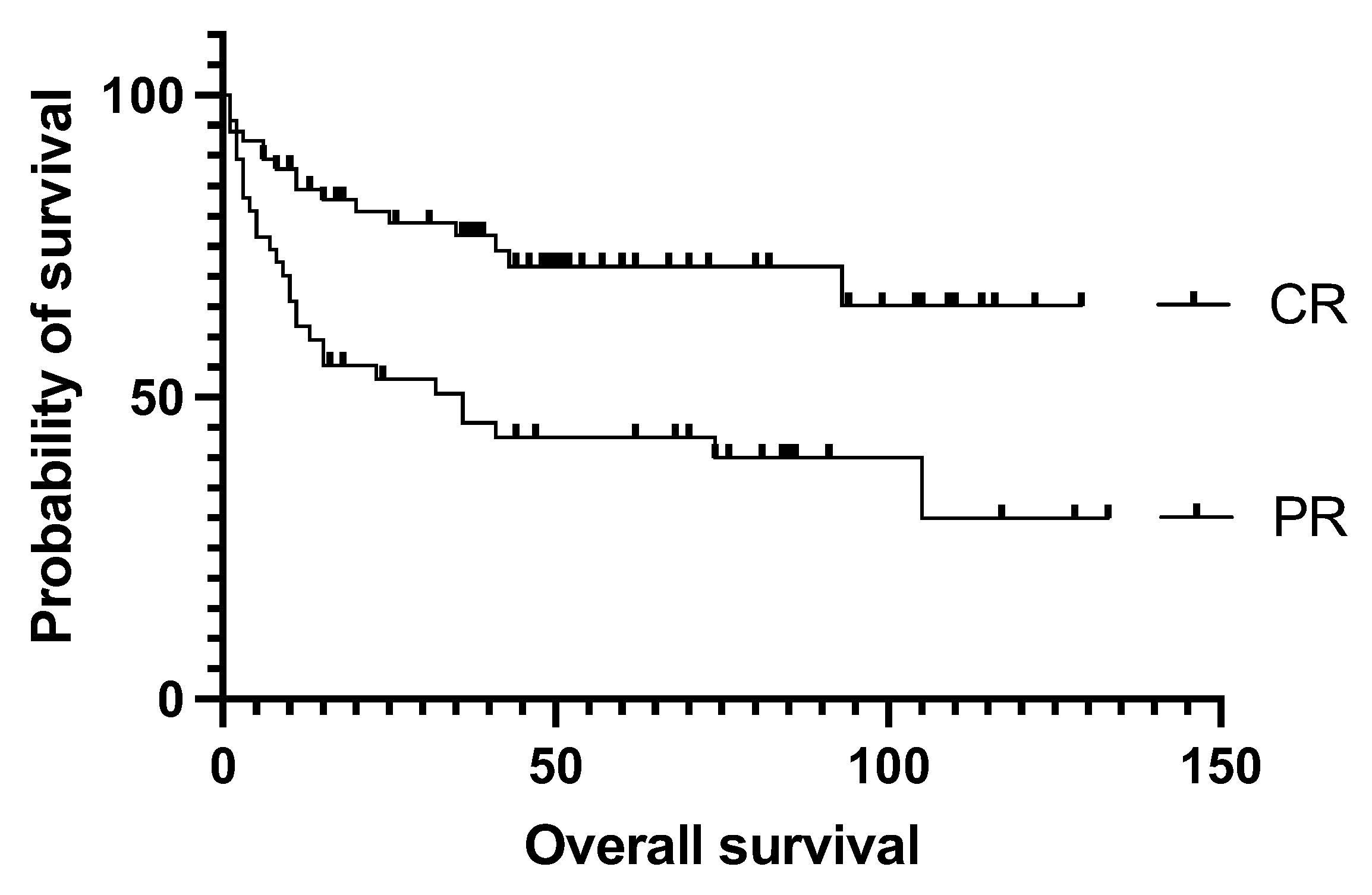
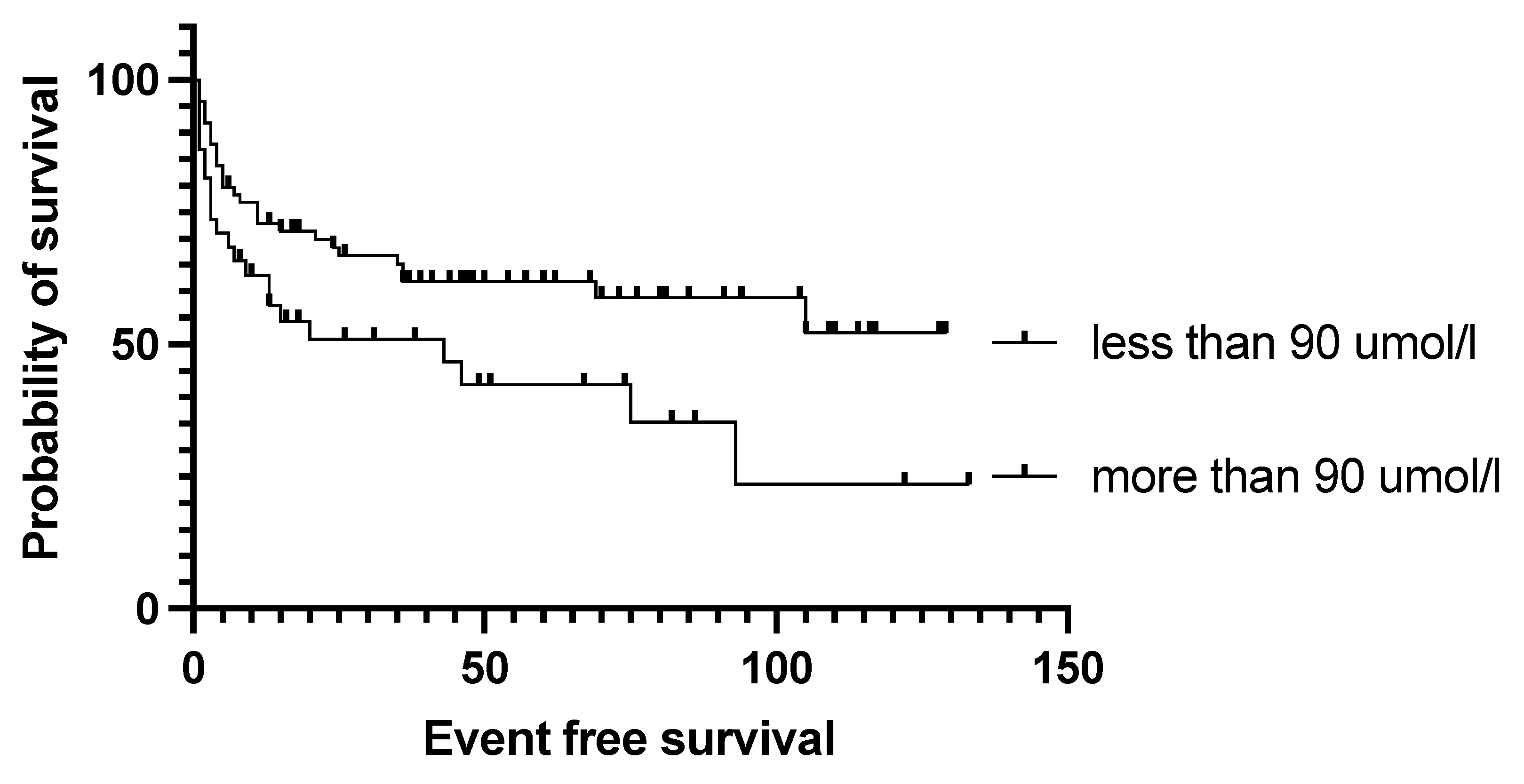
4.2. Effect of Pre-Transplantation Prognostic Factors on Outcome
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coiffier, B.; Lepage, E.; Briere, J.; Herbrecht, R.; Tilly, H.; Bouabdallah, R.; Morel, P.; Van Den Neste, E.; Salles, G.; Gaulard, P.; et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N. Engl. J. Med. 2002, 346, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Tilly, H.; Morschhauser, F.; Sehn, L.H.; Friedberg, J.W.; Trněný, M.; Sharman, J.P.; Herbaux, C.; Burke, J.M.; Matasar, M.; Rai, S.; et al. Polatuzumab Vedotin in Previously Untreated Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2022, 386, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, H.; Gisselbrecht, C.; Coral Study Group. Randomised phase III study of R-ICE versus R-DHAP in relapsed patients with CD20 diffuse large B-cell lymphoma (DLBCL) followed by high-dose therapy and a second randomisation to maintenance treatment with rituximab or not: An update of the CORAL study. Ann. Oncol. 2006, 17 (Suppl. S4), iv31–iv32. [Google Scholar] [CrossRef]
- Locke, F.L.; Miklos, D.B.; Jacobson, C.A.; Perales, M.A.; Kersten, M.J.; Oluwole, O.O.; Ghobadi, A.; Rapoport, A.P.; McGuirk, J.; Pagel, J.M.; et al. Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. N. Engl. J. Med. 2022, 386, 640–654. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Wang, M.; Arnason, J.; Mehta, A.; Purev, E.; Maloney, D.G.; Andreadis, C.; et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): A multicentre seamless design study. Lancet 2020, 396, 839–852. [Google Scholar] [CrossRef]
- Locke, F.L.; Ghobadi, A.; Jacobson, C.A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019, 20, 31–42. [Google Scholar] [CrossRef]
- Morschhauser, F.; Flinn, I.W.; Advani, R.; Sehn, L.H.; Diefenbach, C.; Kolibaba, K.; Press, O.W.; Salles, G.; Tilly, H.; Chen, A.I.; et al. Polatuzumab vedotin or pinatuzumab vedotin plus rituximab in patients with relapsed or refractory non-Hodgkin lymphoma: Final results from a phase 2 randomised study (ROMULUS). Lancet Haematol. 2019, 6, e254–e265. [Google Scholar] [CrossRef]
- Wang, Y.W.; Tsai, X.C.; Hou, H.A.; Tien, F.M.; Liu, J.H.; Chou, W.C.; Ko, B.S.; Chen, Y.W.; Lin, C.C.; Cheng, C.L.; et al. Polatuzumab vedotin-based salvage immunochemotherapy as third-line or beyond treatment for patients with diffuse large B-cell lymphoma: A real-world experience. Ann. Hematol. 2022, 101, 349–358. [Google Scholar] [CrossRef]
- Shargian, L.; Amit, O.; Bernstine, H.; Gurion, R.; Gafter-Gvili, A.; Rozovski, U.; Pasvolsky, O.; Perets, G.; Horowitz, N.A.; Halloun, J.; et al. The role of additional chemotherapy prior to autologous HCT in patients with relapse/refractory DLBCL in partial remission-A retrospective multicenter study. Eur. J. Haematol. 2023, 110, 149–156. [Google Scholar] [CrossRef]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A.; Alliance, Australasian Leukaemia and Lymphoma Group; Eastern Cooperative Oncology Group; European Mantle Cell Lymphoma Consortium; et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J. Clin. Oncol. 2014, 32, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
- Haeno, T.; Rai, S.; Miyake, Y.; Inoue, M.; Fujimoto, K.; Fujii, A.; Iwata, Y.; Minamoto, S.; Taniguchi, T.; Kakutani, H.; et al. Long-term effectiveness and safety of high dose chemotherapy followed by autologous stem cell transplantation in daily practice in patients with diffuse large B-cell lymphoma. J. Clin. Exp. Hematop. 2023. [Google Scholar] [CrossRef] [PubMed]
- Mounier, N.; Canals, C.; Gisselbrecht, C.; Cornelissen, J.; Foa, R.; Conde, E.; Maertens, J.; Attal, M.; Rambaldi, A.; Crawley, C.; et al. High-dose therapy and autologous stem cell transplantation in first relapse for diffuse large B cell lymphoma in the rituximab era: An analysis based on data from the European Blood and Marrow Transplantation Registry. Biol. Blood Marrow. Transplant. 2012, 18, 788–793. [Google Scholar] [CrossRef]
- Iqbal, M.; Castano, Y.G.; Paludo, J.; Rosenthal, A.; Li, Z.; Beltran, M.; Moustafa, M.A.; Inwards, D.; Porrata, L.; Micallef, I.; et al. Impact of Cell of Origin on Outcomes After Autologous Hematopoietic Cell Transplant in Diffuse Large B-Cell Lymphoma. Clin. Lymphoma Myeloma Leuk. 2022, 22, e89–e95. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.J.; Feldman, A.L.; Micallef, I.N.; Inwards, D.J.; Johnston, P.B.; Porrata, L.F.; Ansell, S.M. Germinal center B (GCB) and non-GCB cell-like diffuse large B cell lymphomas have similar outcomes following autologous haematopoietic stem cell transplantation. Br. J. Haematol. 2008, 142, 404–412. [Google Scholar] [CrossRef]
- Moskowitz, C.H.; Zelenetz, A.D.; Kewalramani, T.; Hamlin, P.; Lessac-Chenen, S.; Houldsworth, J.; Olshen, A.; Chaganti, R.; Nimer, S.; Teruya-Feldstein, J. Cell of origin, germinal center versus nongerminal center, determined by immunohistochemistry on tissue microarray, does not correlate with outcome in patients with relapsed and refractory DLBCL. Blood 2005, 106, 3383–3385. [Google Scholar] [CrossRef] [PubMed]
- Gisselbrecht, C.; Glass, B.; Mounier, N.; Singh Gill, D.; Linch, D.C.; Trneny, M.; Bosly, A.; Ketterer, N.; Shpilberg, O.; Hagberg, H.; et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J. Clin. Oncol. 2010, 28, 4184–4190. [Google Scholar] [CrossRef]
- Hamlin, P.A.; Zelenetz, A.D.; Kewalramani, T.; Qin, J.; Satagopan, J.M.; Verbel, D.; Noy, A.; Portlock, C.S.; Straus, D.J.; Yahalom, J.; et al. Age-adjusted International Prognostic Index predicts autologous stem cell transplantation outcome for patients with relapsed or primary refractory diffuse large B-cell lymphoma. Blood 2003, 102, 1989–1996. [Google Scholar] [CrossRef]
- Wullenkord, R.; Berning, P.; Niemann, A.L.; Wethmar, K.; Bergmann, S.; Lutz, M.; Schliemann, C.; Mesters, R.; Keßler, T.; Schmitz, N.; et al. The role of autologous stem cell transplantation (ASCT) in aggressive B-cell lymphomas: Real-world data from a retrospective single-center analysis. Ann. Hematol. 2021, 100, 2733–2744. [Google Scholar] [CrossRef]
- Armand, P.; Welch, S.; Kim, H.T.; LaCasce, A.S.; Jacobsen, E.D.; Davids, M.S.; Jacobson, C.; Fisher, D.C.; Brown, J.R.; Coughlin, E.; et al. Prognostic factors for patients with diffuse large B cell lymphoma and transformed indolent lymphoma undergoing autologous stem cell transplantation in the positron emission tomography era. Br. J. Haematol. 2013, 160, 608–617. [Google Scholar] [CrossRef]
- Sauter, C.S.; Matasar, M.J.; Meikle, J.; Schoder, H.; Ulaner, G.A.; Migliacci, J.C.; Hilden, P.; Devlin, S.M.; Zelenetz, A.D.; Moskowitz, C.H. Prognostic value of FDG-PET prior to autologous stem cell transplantation for relapsed and refractory diffuse large B-cell lymphoma. Blood 2015, 125, 2579–2581. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, M.; Hoyt, R.; Roberts, A.W.; Grigg, A.; Seymour, J.F.; Prince, H.M.; Szer, J.; Ritchie, D. Improved survival for relapsed diffuse large B cell lymphoma is predicted by a negative pre-transplant FDG-PET scan following salvage chemotherapy. Br. J. Haematol. 2010, 150, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.N.; Ahn, K.W.; Litovich, C.; He, Y.; Sauter, C.; Fenske, T.S.; Hamadani, M. Is autologous transplant in relapsed DLBCL patients achieving only a PET+ PR appropriate in the CAR T-cell era. Blood 2021, 137, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Minson, A.; Hofman, M.; Dickinson, M. A PET in a time of need: Toward early PET-adapted therapy in DLBCL in first relapse. Leuk. Lymphoma. 2022, 63, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Vajavaara, H.; Leivonen, S.K.; Jørgensen, J.; Holte, H.; Leppä, S. Low lymphocyte-to-monocyte ratio predicts poor outcome in high-risk aggressive large B-cell lymphoma. EJHaem 2022, 3, 681–687. [Google Scholar] [CrossRef]
- Schuster, S.J.; Tam, C.S.; Borchmann, P.; Worel, N.; McGuirk, J.P.; Holte, H.; Waller, E.K.; Jaglowski, S.; Bishop, M.R.; Damon, L.E.; et al. Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B-cell lymphomas (JULIET): A multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021, 22, 1403–1415. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Reagan, P.M.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; et al. Comparison of 2-year outcomes with CAR T cells (ZUMA-1) vs salvage chemotherapy in refractory large B-cell lymphoma. Blood Adv. 2021, 5, 4149–4155. [Google Scholar] [CrossRef]
- Tian, L.; Li, C.; Sun, J.; Zhai, Y.; Wang, J.; Liu, S.; Jiang, Y.; Wu, W.; Xing, D.; Lv, Y.; et al. Efficacy of chimeric antigen receptor T cell therapy and autologous stem cell transplant in relapsed or refractory diffuse large B-cell lymphoma: A systematic review. Front. Immunol. 2022, 13, 1041177. [Google Scholar] [CrossRef]
- Harrysson, S.; Eloranta, S.; Ekberg, S.; Enblad, G.; El-Galaly, T.C.; Sander, B.; Sonnevi, K.; Andersson, P.O.; Jerkeman, M.; Smedby, K.E. Outcomes of relapsed/refractory diffuse large B-cell lymphoma and influence of chimaeric antigen receptor T trial eligibility criteria in second line—A population-based study of 736 patients. Br. J. Haematol. 2022, 198, 267–277. [Google Scholar] [CrossRef]
- Flowers, C.R.; Odejide, O.O. Sequencing therapy in relapsed DLBCL. Hematol. Am. Soc. Hematol. Educ. Program 2022, 2022, 146–154. [Google Scholar] [CrossRef]

| Characteristics | Variable | Value |
|---|---|---|
| Sex | Male | 63 (54.3%) |
| Female | 53 (45.7%) | |
| Age at diagnosis (years) | Median | 55 |
| Range | 17–73 | |
| Subtype DLBCL | Germinal center | 19 (16.3%) |
| Non-germinal center | 36 (31%) | |
| Not otherwise specified | 61 (52.5%) | |
| PCNSL | 11 (9%) | |
| B symptoms | Present | 43 (37%) |
| Absent | 73 (63%) | |
| Ann Arbor stage at diagnosis | I–II | 23 (19.8%) |
| III–IV | 93 (80.2%) | |
| IPI at diagnosis | 0–1 | 30 (25.8%) |
| 2 | 39 (33.6%) | |
| 3–5 | 47 (40.5%) | |
| ECOG PS | 0–1 | 102 (88%) |
| 2 | 14 (12%) | |
| Age at auto-SCT (years) | Median | 58 |
| Range | 18–74 | |
| Lines of therapy before auto-SCT | 1 | 16 (13.8%) |
| 2 | 58 (50%) | |
| 3 | 40 (34.5%) | |
| 4 | 2 (1.7%) | |
| Response before auto-SCT | Partial response | 47 (40.5%) |
| Complete response | 69 (59.5%) | |
| Conditioning regimen | R-BEAM | 92 (79.4%) |
| TBC | 24 (20.6%) | |
| Number of stem cells administered | Range (×106/bwkg) | 2.68–9.69 |
| Time to engraftment (days) | Range | 7–18 |
| Average | 9.4 |
| Univariate Analysis | ||||
|---|---|---|---|---|
| EFS | OS | |||
| Hazard Ratio | p-Value | Hazard Ratio | p-Value | |
| Factors at diagnosis | ||||
| Ann Arbor stage III–IV | 2.254 | 0.1053 | 1.728 | 0.2435 |
| IPI 3–5 | 1.425 | 0.1934 | 1.411 | 0.2412 |
| Bulky disease | 1.385 | 0.4724 | 1.042 | 0.9140 |
| B symptoms | 1.922 | 0.0760 | 1.606 | 0.1006 |
| CG histology | 0.765 | 0.5259 | 0.887 | 0.7456 |
| Normal LDH | 0.810 | 0.5468 | 0.828 | 0.6033 |
| Factors at transplantation | ||||
| PET-negative | 0.422 | 0.0015 | 0.382 | 0.0010 |
| More than one salvage therapy | 1.527 | 0.133 | 1.599 | 0.1158 |
| Primary refractory disease | 1.014 | 0.9624 | 1.024 | 0.9566 |
| Engraftment before 9 days | 0.531 | 0.0253 | 0.452 | 0.0101 |
| More than 4 × 106/kg CD34+ graft | 0.684 | 0.2195 | 1.031 | 0.9402 |
| Hgb over 100 g/L | 0.89 | 0.7276 | 0.779 | 0.4666 |
| Abs. lymphocyte over 1 G/L | 1.078 | 0.8176 | 1.212 | 0.5321 |
| Thrombocyte over 100 G/L | 0.704 | 0.2103 | 0.491 | 0.0241 |
| CRP less than 6 mg/L | 0.558 | 0.0453 | 0.530 | 0.0382 |
| Creatinine below 90 umol/L | 0.504 | 0.0288 | 0.550 | 0.0408 |
| BUN less than 4.5 mmol/L | 0.437 | 0.0184 | 0.375 | 0.0121 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bicsko, R.R.; Antal, L.; Magyari, F.; Szász, R.; Udvardy, M.; Illes, A.; Gergely, L. Autologous Transplantation May Still Effectively Treat Relapsed Diffuse Large B-Cell Lymphoma in Selected Patients. Cancers 2023, 15, 3223. https://doi.org/10.3390/cancers15123223
Bicsko RR, Antal L, Magyari F, Szász R, Udvardy M, Illes A, Gergely L. Autologous Transplantation May Still Effectively Treat Relapsed Diffuse Large B-Cell Lymphoma in Selected Patients. Cancers. 2023; 15(12):3223. https://doi.org/10.3390/cancers15123223
Chicago/Turabian StyleBicsko, Reka Rahel, Lili Antal, Ferenc Magyari, Róbert Szász, Miklós Udvardy, Arpad Illes, and Lajos Gergely. 2023. "Autologous Transplantation May Still Effectively Treat Relapsed Diffuse Large B-Cell Lymphoma in Selected Patients" Cancers 15, no. 12: 3223. https://doi.org/10.3390/cancers15123223
APA StyleBicsko, R. R., Antal, L., Magyari, F., Szász, R., Udvardy, M., Illes, A., & Gergely, L. (2023). Autologous Transplantation May Still Effectively Treat Relapsed Diffuse Large B-Cell Lymphoma in Selected Patients. Cancers, 15(12), 3223. https://doi.org/10.3390/cancers15123223







