Personalized [177Lu]Lutetium-PSMA Therapy for Patients with Pre-Treated Castration-Resistant Prostate Cancer: A Single Institution Experience from a Comprehensive Cancer Centre
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- Documented progressive mCRPC after at least one line of systemic therapy for mCRPC after ADT.
- Evidence of PSMA positive metastases on PSMA PET/CT without any PSMA negative suspicious lesions on CT.
- In case of discrepancy between PSMA, PET, and CT, the lesions, if amenable for biopsy, were confirmed either by histopathology or with best supporting evidence.
- An Eastern Cooperative Oncology Group (ECOG) performance status up to 3.
- Hb > 6g/dL, Thrombocytes > 50 × 109/L. In patients with values lower than the aforementioned cut-offs, either no Lu-PSMA therapy was offered or, depending on the ECOG status of the patients, they went for pre Lu-PSMA therapy blood transfusion (erythrocytes or thrombocytes without any G-CSF or erythropoietin)
- In patients with brain metastases and/or intraspinal metastases and/or painful bone lesions and/or the presence of acute pathological fracture or a suspicion of a high probability of bone-related events from the lesions, Lu-PSMA therapy was preceded by external beam radiation therapy (EBRT). After EBRT, patients were treated with Lu-PSMA within 3–4 weeks. In patients with mild pain without any need urgent need for opiates or pain palliation with EBRT, Lu-PSMA therapy was started first, and it was then followed by EBRT within 1–2 weeks.
- In patients with evidence of bone marrow or renal function deterioration or diffuse liver metastases with elevated GGT and/or Bilirubin, the dose of Lu-PSMA therapy was reduced by 25–75%.
- In patients with high tumor burden and/or evidence of rapid tumor kinetic on pre-therapy PSMA, PET/CT, or PSA value or clinical symptoms, the time interval between two treatment cycles was reduced from 6 weeks to 4–5 weeks.
- Patients were intended to be treated with a minimum of 4 Lu-PSMA cycles. However, the decision to carry on the 3rd and the higher therapy cycles was assessed in MDT after every 2 therapy cycles. Apart from PSA response, improvement/deterioration in clinical symptoms, clinical need, ECOG status, posttherapy scans including SPECT/CT, and PSMA PET/CT response were also all considered when making the decision of whether to continue or discontinue treatment.
3. Results
3.1. Characteristics of the Study Cohort
3.2. Treatment Exposure and Tolerability
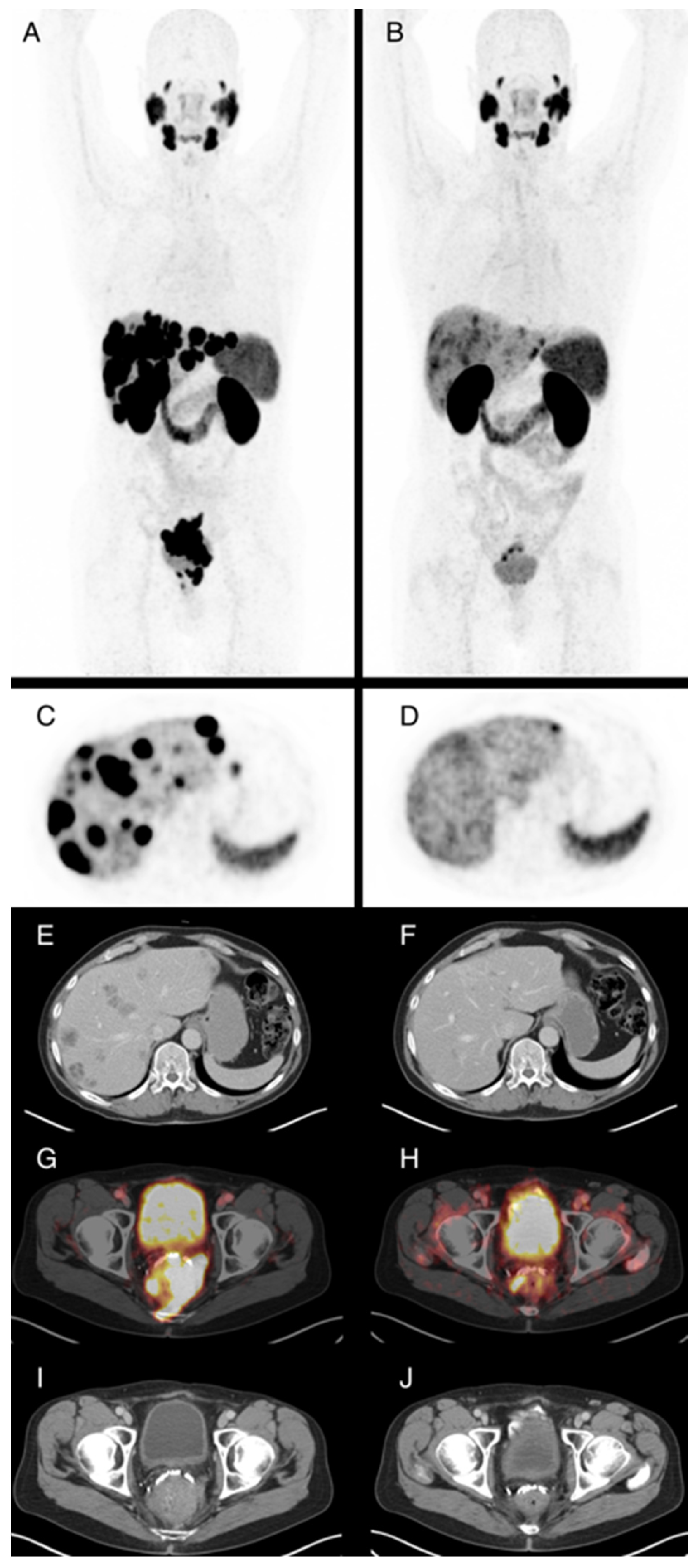
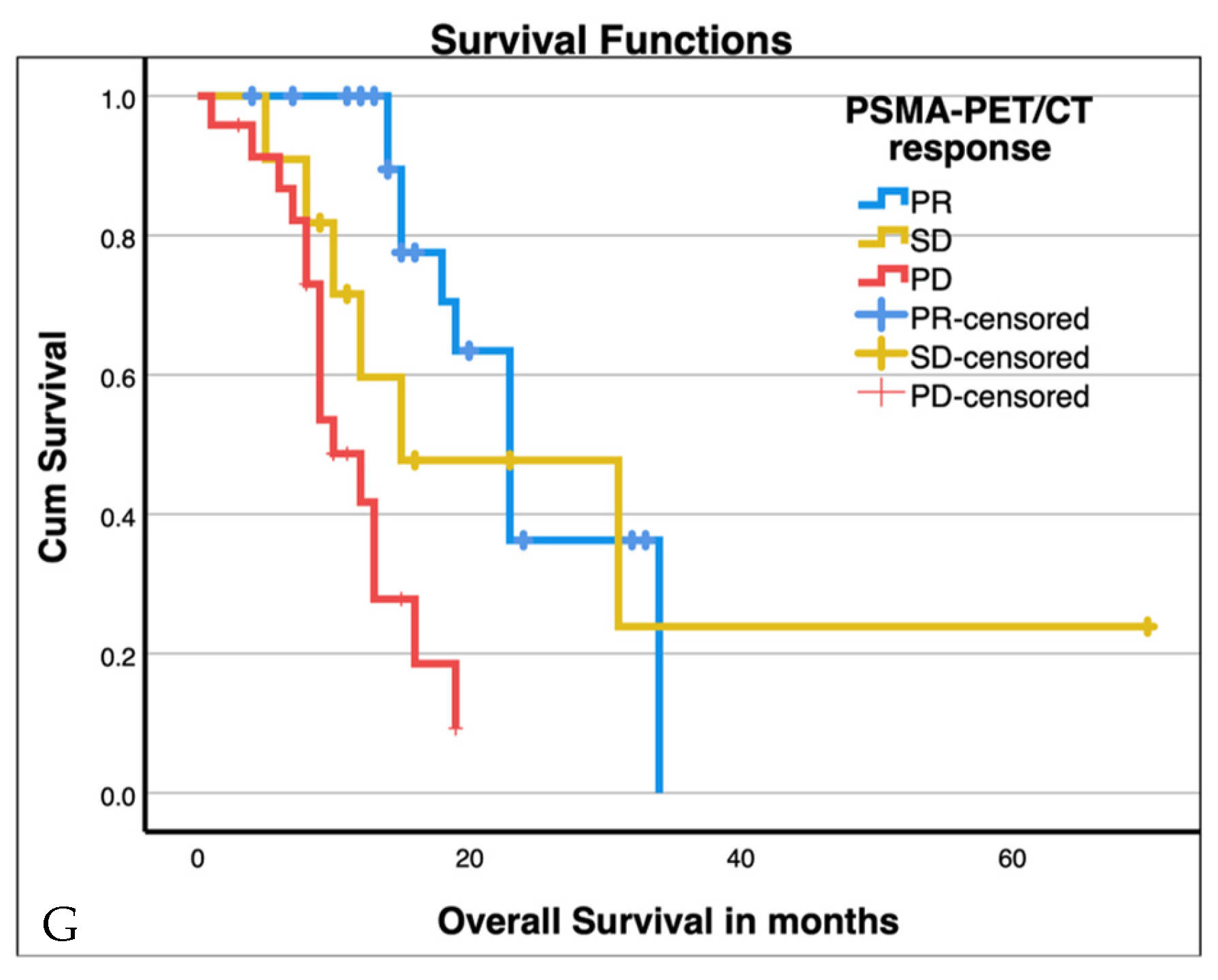
3.3. PSMA-PET/CT Response
3.4. PSA Response
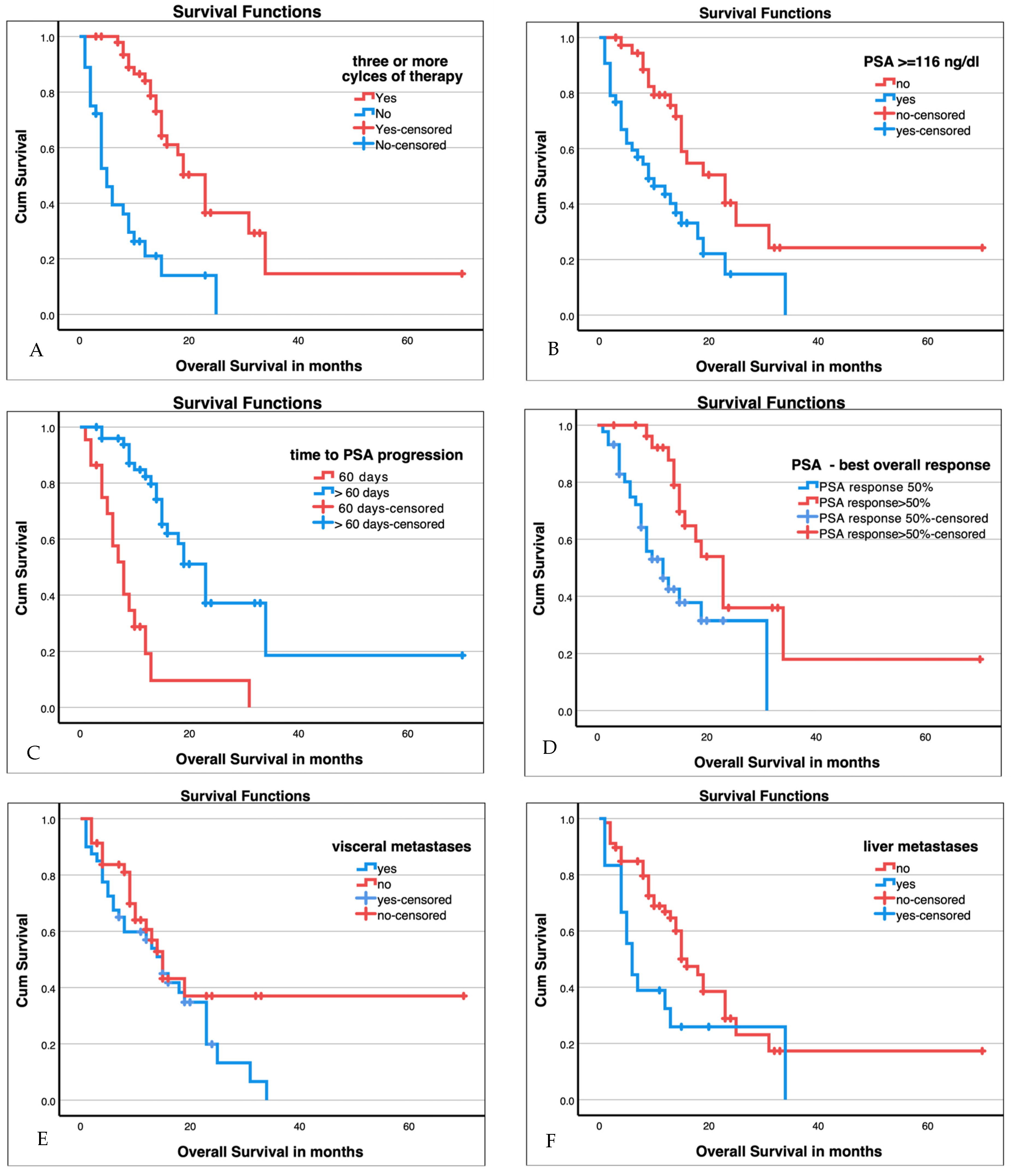
3.5. Survival Analysis
3.6. Baseline Predictors for Improved Overall Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morris, M.J.; Rumble, R.B.; Basch, E.; Hotte, S.J.; Loblaw, A.; Rathkopf, D.; Celano, P.; Bangs, R.; Milowsky, M.I. Optimizing Anticancer Therapy in Metastatic Non-Castrate Prostate Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1521–1539. [Google Scholar] [CrossRef] [PubMed]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II-2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2021, 79, 263–282. [Google Scholar] [CrossRef] [PubMed]
- Farolfi, A.; Calderoni, L.; Mattana, F.; Mei, R.; Telo, S.; Fanti, S.; Castellucci, P. Current and Emerging Clinical Applications of PSMA PET Diagnostic Imaging for Prostate Cancer. J. Nucl. Med. 2021, 62, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Fendler, W.P.; Rahbar, K.; Herrmann, K.; Kratochwil, C.; Eiber, M. 177Lu-PSMA Radioligand Therapy for Prostate Cancer. J. Nucl. Med. 2017, 58, 1196–1200. [Google Scholar] [CrossRef]
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Baum, R.; Bozkurt, M.F.; Czernin, J.; Delgado Bolton, R.C.; Ezziddin, S.; Forrer, F.; Hicks, R.J.; et al. EANM Procedure Guidelines for Radionuclide Therapy with 177Lu-Labelled PSMA-Ligands (177Lu-PSMA-RLT). Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2536–2544. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; et al. [177Lu]Lu-PSMA-617 versus Cabazitaxel in Patients with Metastatic Castration-Resistant Prostate Cancer (TheraP): A Randomised, Open-Label, Phase 2 Trial. Lancet 2021, 397, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Ravi Kumar, A.; Murphy, D.G.; et al. [177Lu]-PSMA-617 Radionuclide Treatment in Patients with Metastatic Castration-Resistant Prostate Cancer (LuPSMA Trial): A Single-Centre, Single-Arm, Phase 2 Study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Privé, B.M.; Janssen, M.J.R.; van Oort, I.M.; Muselaers, C.H.J.; Jonker, M.A.; van Gemert, W.A.; de Groot, M.; Westdorp, H.; Mehra, N.; Verzijlbergen, J.F.; et al. Update to a Randomized Controlled Trial of Lutetium-177-PSMA in Oligo-Metastatic Hormone-Sensitive Prostate Cancer: The BULLSEYE Trial. Trials 2021, 22, 768. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, A.; Rosellini, M.; Nuvola, G.; Tassinari, E.; Mollica, V.; Rizzo, A.; Santoni, M.; Cimadamore, A.; Farolfi, A.; Montironi, R.; et al. PARP Inhibitors and Radiometabolic Approaches in Metastatic Castration-Resistant Prostate Cancer: What’s Now, What’s New, and What’s Coming? Cancers 2022, 14, 907. [Google Scholar] [CrossRef] [PubMed]
- Emmett, L.; Subramaniam, S.; Joshua, A.M.; Crumbaker, M.; Martin, A.; Zhang, A.Y.; Rana, N.; Langford, A.; Mitchell, J.; Yip, S.; et al. ENZA-p Trial Protocol: A Randomized Phase II Trial Using Prostate-Specific Membrane Antigen as a Therapeutic Target and Prognostic Indicator in Men with Metastatic Castration-Resistant Prostate Cancer Treated with Enzalutamide (ANZUP 1901). BJU Int. 2021, 128, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Fendler, W.P.; Kratochwil, C.; Ahmadzadehfar, H.; Rahbar, K.; Baum, R.P.; Schmidt, M.; Pfestroff, A.; Lützen, U.; Prasad, V.; Heinzel, A.; et al. 177Lu-PSMA-617 therapy, dosimetry and follow-up in patients with metastatic castration-resistant prostate cancer. Nuklearmedizin 2016, 55, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.; Huang, K.; Prasad, S.; Makowski, M.R.; Czech, N.; Brenner, W. In Comparison to PSA, Interim Ga-68-PSMA PET/CT Response Evaluation Based on Modified RECIST 1.1 After 2nd Cycle Is Better Predictor of Overall Survival of Prostate Cancer Patients Treated With 177Lu-PSMA. Front. Oncol. 2021, 11, 578093. [Google Scholar] [CrossRef] [PubMed]
- Gafita, A.; Calais, J.; Grogan, T.R.; Hadaschik, B.; Wang, H.; Weber, M.; Sandhu, S.; Kratochwil, C.; Esfandiari, R.; Tauber, R.; et al. Nomograms to Predict Outcomes after 177Lu-PSMA Therapy in Men with Metastatic Castration-Resistant Prostate Cancer: An International, Multicentre, Retrospective Study. Lancet Oncol. 2021, 22, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Current, K.; Meyer, C.; Magyar, C.E.; Mona, C.E.; Almajano, J.; Slavik, R.; Stuparu, A.D.; Cheng, C.; Dawson, D.W.; Radu, C.G.; et al. Investigating PSMA-Targeted Radioligand Therapy Efficacy as a Function of Cellular PSMA Levels and Intratumoral PSMA Heterogeneity. Clin. Cancer Res. 2020, 26, 2946–2955. [Google Scholar] [CrossRef] [PubMed]
- Paschalis, A.; Sheehan, B.; Riisnaes, R.; Rodrigues, D.N.; Gurel, B.; Bertan, C.; Ferreira, A.; Lambros, M.B.K.; Seed, G.; Yuan, W.; et al. Prostate-Specific Membrane Antigen Heterogeneity and DNA Repair Defects in Prostate Cancer. Eur. Urol. 2019, 76, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Emmett, L. Tumour Heterogeneity and Resistance to Therapy in Prostate Cancer: A Fundamental Limitation of Prostate-Specific Membrane Antigen Theranostics or a Key Strength? Eur. Urol. 2019, 76, 479–481. [Google Scholar] [CrossRef] [PubMed]
- Khreish, F.; Ghazal, Z.; Marlowe, R.J.; Rosar, F.; Sabet, A.; Maus, S.; Linxweiler, J.; Bartholomä, M.; Ezziddin, S. 177 Lu-PSMA-617 Radioligand Therapy of Metastatic Castration-Resistant Prostate Cancer: Initial 254-Patient Results from a Prospective Registry (REALITY Study). Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Sadaghiani, M.S.; Sheikhbahaei, S.; Werner, R.A.; Pienta, K.J.; Pomper, M.G.; Solnes, L.B.; Gorin, M.A.; Wang, N.-Y.; Rowe, S.P. A Systematic Review and Meta-Analysis of the Effectiveness and Toxicities of Lutetium-177-Labeled Prostate-Specific Membrane Antigen-Targeted Radioligand Therapy in Metastatic Castration-Resistant Prostate Cancer. Eur. Urol. 2021, 80, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Morris, M.J.; Basch, E.; Heller, G. End Points and Outcomes in Castration-Resistant Prostate Cancer: From Clinical Trials to Clinical Practice. J. Clin. Oncol. 2011, 29, 3695–3704. [Google Scholar] [CrossRef] [PubMed]

| Age (n = 86)—Years | 71 (52–95) |
|---|---|
| ECOG (n = 86) | |
| • ECOG 0 | 36 (41.9%) |
| • ECOG 1 | 34 (39.5%) |
| • ECOG 2 | 14 (16.3%) |
| • ECOG 3 | 2 (2.3%) |
| PSA at Treatment Start (n = 83)—ng/mL | 116 (0.19–3160) |
| GFR (n = 86)—mL/min | 85 (14–123) |
| • GFR < 60 mL/min | 13 (15.1%) |
| • GFR ≥ 60 mL/min | 73 (84.9%) |
| Alkaline Phosphatase (n = 84)—U/L | 117 (31–1764) |
| Lactate Dehydrogenase (n = 82)—U/L | 244 (103–2500) |
| Gleason-Score (n = 86): | |
| • Score ≤ 6 | 4 (4.7%) |
| • Score 7 | 21 (24.4%) |
| • Score 8 | 22 (25.6%) |
| • Score 9 | 30 (34.9%) |
| • Score 10 | 2 (2.3%) |
| • Unknown | 7 (8.1%) |
| Previous local therapy (n = 86) | |
| • Prostatectomy | 46 (53.5%) |
| • Radiation | 6 (7.0%) |
| • TURP | 8 (9.3%) |
| • No local therapy | 26 (30.2%) |
| Previous new hormonal agent (n = 86) | Yes: 81 (94.2%) No: 5 (5.8%) |
| Previous taxane therapy (n = 86) | Yes: 65 (75.6%) No: 21 1 (24.4%) |
| Site of disease (baseline PET/CT) (n = 86) | |
| • Lymph Node Metastases | Yes: 60 (70.0%) No: 26 (30.0%) |
| • Bone Metastases | Yes: 79 (91.9%) No: 7 (8.1%) |
| • Visceral Metastases | Yes: 39 (45.3%) No: 47 (54.7%) |
| of those: | |
| Liver metastases | 18 (46.2%) |
| Adrenal gland | 14 (35.9%) |
| Lung | 10 (25.6%) |
| Peritoneal | 4 (10.3%) |
| Brain | 1 (2.6%) |
| Adverse Event | All Grades | Grade ≥ 3 |
|---|---|---|
| Any adverse event | 71 | 23 |
| Anemia | 71 | 14 |
| Leucopenia | 51 | 13 |
| Thrombocytopenia | 30 | 4 |
| Nausea | 15 | |
| Dry mouth | 13 | 2 |
| Difficulty voiding urine | 11 | 3 |
| Fatigue | 9 | 1 |
| Vomiting | 8 | |
| Bone pain | 7 | |
| Decreased appetite | 1 | |
| Constipation | 1 | |
| Diarrhea | 1 | |
| Peripheral edema | 1 | |
| Headache | 1 |
| Factor | Groups | n | Median Survival in Months | 95% Confidence Interval in Months | Chi-Square Log Rank (Mantle-Cox) | p-Value |
|---|---|---|---|---|---|---|
| VISION criteria met | Yes | 60 | 13 | 8–18 | 2.1 | 0.151 |
| No | 24 | 16 | 12–20 | |||
| ≥3 therapy cycles | Yes | 50 | 23 | 19–27 | 33.3 | <0.001 * |
| No | 36 | 5 | 3–7 | |||
| Visceral metastases | Yes | 38 | 15 | 11–19 | 2.0 | 0.152 |
| No | 44 | 15 | 12–18 | |||
| Liver metastases | Yes | 18 | 6 | 4–8 | 4.8 | 0.029 * |
| No | 68 | 16 | 13–17 | |||
| Haemoglobin | ≥10 g/dL | 58 | 19 | 12–26 | 19.5 | <0.001 * |
| <10 g/dL | 28 | 5 | 1–9 | |||
| AP | Normal | 44 | 18 | 13–23 | 7.9 | 0.005 * |
| Elevated | 40 | 9 | 4–14 | |||
| LDH | Normal | 42 | 18 | 13–23 | 5.6 | 0.018 * |
| Elevated | 40 | 9 | 3–15 | |||
| PSA at start of therapy above median | ≥116 ng/mL | 43 | 9 | 3–15 | 10.5 | 0.001 * |
| <116 ng/mL | 40 | 23 | 14–32 | |||
| PSA best overall response | ≤50% | 44 | 12 | 7–17 | 7.7 | 0.005 * |
| >50% | 29 | 23 | 18–28 | |||
| Time to PSA progression | ≤60 days | 22 | 8 | 5–11 | 29.6 | <0.001 * |
| >60 days | 51 | 23 | 19–27 | |||
| PSMA-PET/CT response | PR | 27 | 23 | 19–27 | 16.8 | <0.001 * |
| SD | 11 | 15 | 0–32 | |||
| PD | 24 | 10 | 7–13 |
| p-Value | HR | 95% CI | |
|---|---|---|---|
| VISION Criteria met | 0.98 | 1.01 | 0.5–2.0 |
| Presence of Visceral metastasis | 0.46 | 1.31 | 0.6–2.7 |
| Presence of Liver metastasis | 0.26 | 1.58 | 0.7–3.5 |
| Pre-treatment Haemoglobin ≥ 10 g/dL | <0.01 * | 0.37 | 0.2–0.7 |
| Pre-treatment PSA ≥ 116 ng/mL | 0.03 * | 2.11 | 1.1–4.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thaiss, W.; Zengerling, F.; Friedrich, J.; Hechler, V.; Grunert, M.; Bolenz, C.; Wiegel, T.; Beer, A.J.; Prasad, V. Personalized [177Lu]Lutetium-PSMA Therapy for Patients with Pre-Treated Castration-Resistant Prostate Cancer: A Single Institution Experience from a Comprehensive Cancer Centre. Cancers 2023, 15, 3216. https://doi.org/10.3390/cancers15123216
Thaiss W, Zengerling F, Friedrich J, Hechler V, Grunert M, Bolenz C, Wiegel T, Beer AJ, Prasad V. Personalized [177Lu]Lutetium-PSMA Therapy for Patients with Pre-Treated Castration-Resistant Prostate Cancer: A Single Institution Experience from a Comprehensive Cancer Centre. Cancers. 2023; 15(12):3216. https://doi.org/10.3390/cancers15123216
Chicago/Turabian StyleThaiss, Wolfgang, Friedemann Zengerling, Julia Friedrich, Veronika Hechler, Michael Grunert, Christian Bolenz, Thomas Wiegel, Ambros J. Beer, and Vikas Prasad. 2023. "Personalized [177Lu]Lutetium-PSMA Therapy for Patients with Pre-Treated Castration-Resistant Prostate Cancer: A Single Institution Experience from a Comprehensive Cancer Centre" Cancers 15, no. 12: 3216. https://doi.org/10.3390/cancers15123216
APA StyleThaiss, W., Zengerling, F., Friedrich, J., Hechler, V., Grunert, M., Bolenz, C., Wiegel, T., Beer, A. J., & Prasad, V. (2023). Personalized [177Lu]Lutetium-PSMA Therapy for Patients with Pre-Treated Castration-Resistant Prostate Cancer: A Single Institution Experience from a Comprehensive Cancer Centre. Cancers, 15(12), 3216. https://doi.org/10.3390/cancers15123216








