Predicting Chemotherapy Benefit across Different Races in Early-Stage Breast Cancer Patients Using the Oncotype DX Score
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. SEER-Oncotype DX Database
2.2. Survival Analysis
2.3. Statistical Analysis
3. Results
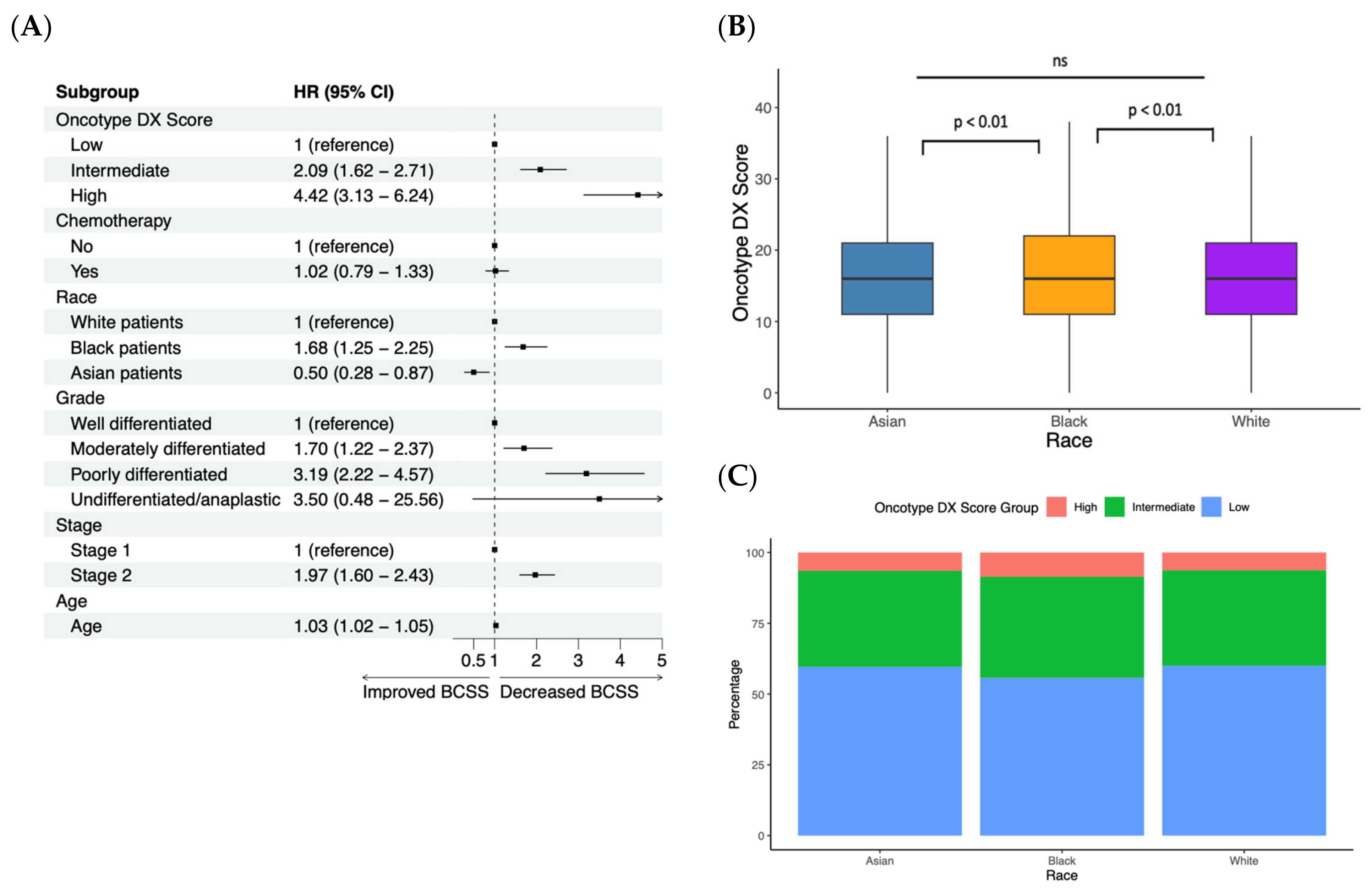
3.1. Characterizing Important Clinical Parameters with a Linear Cox Proportional Hazards Regression
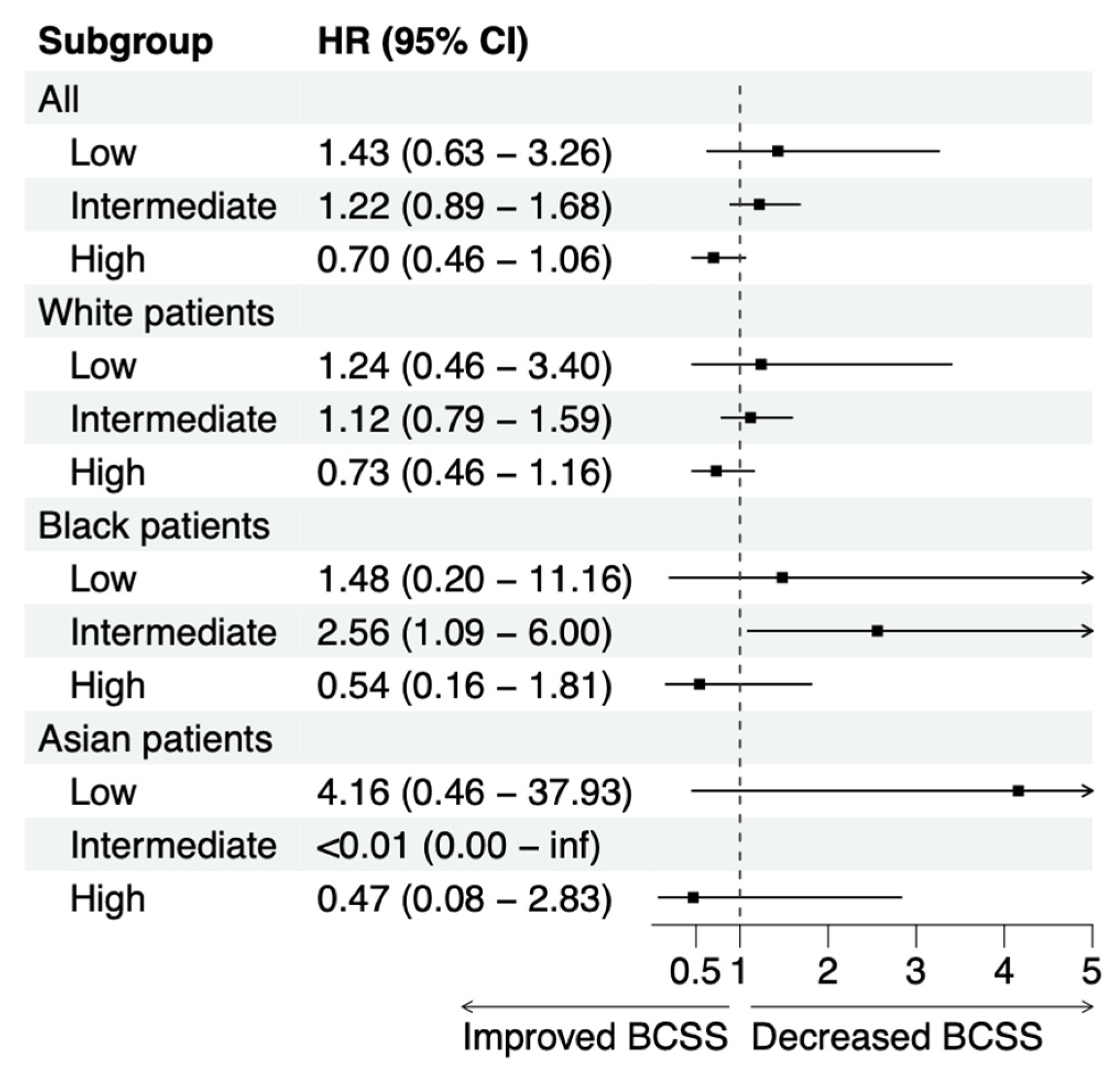
3.2. Patients with High Oncotype DX Scores May Benefit from Chemotherapy
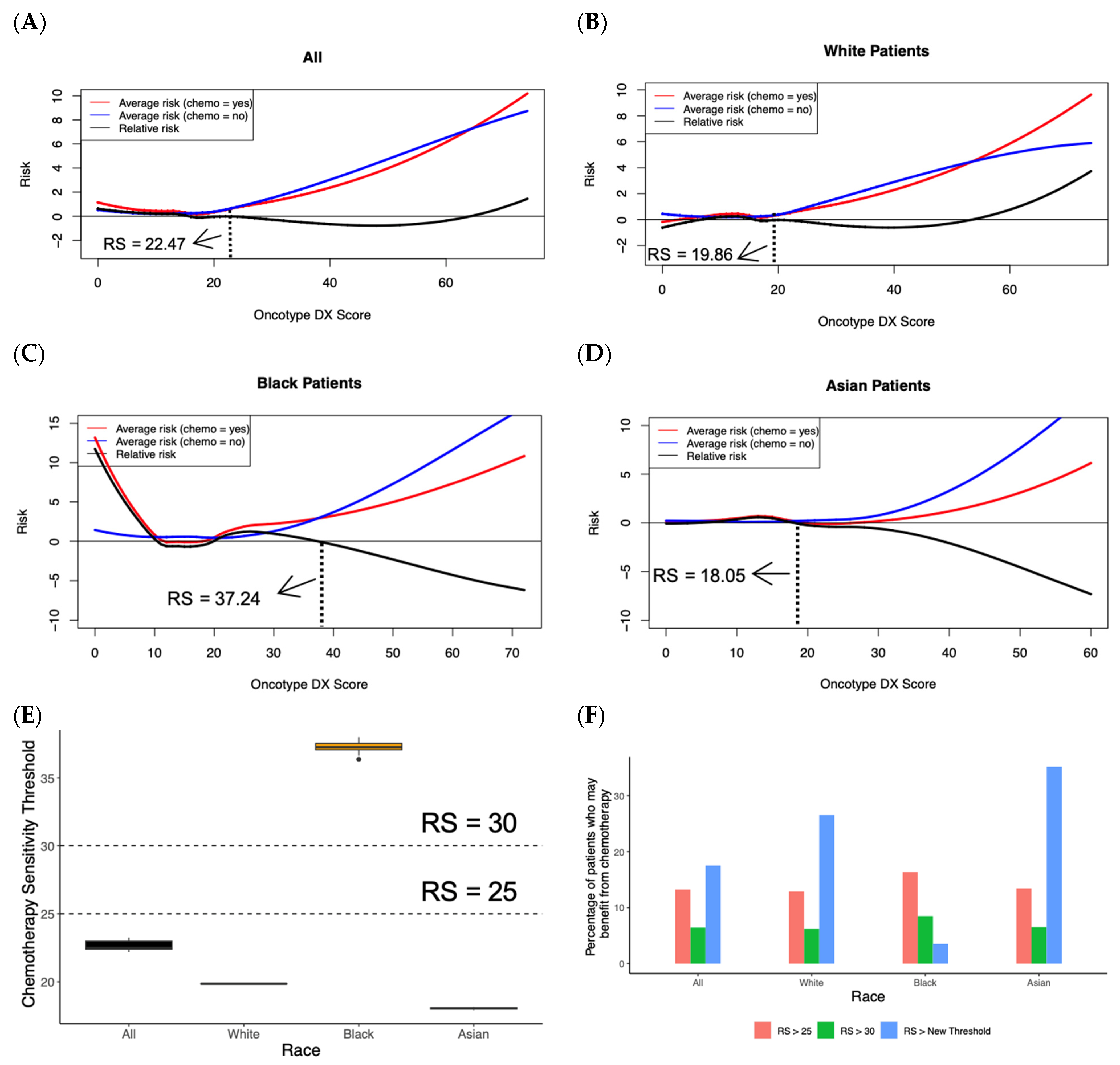
3.3. Random Forest Survival Model Identifies the Optimal Oncotype DX Score Threshold for Adopting Chemotherapy
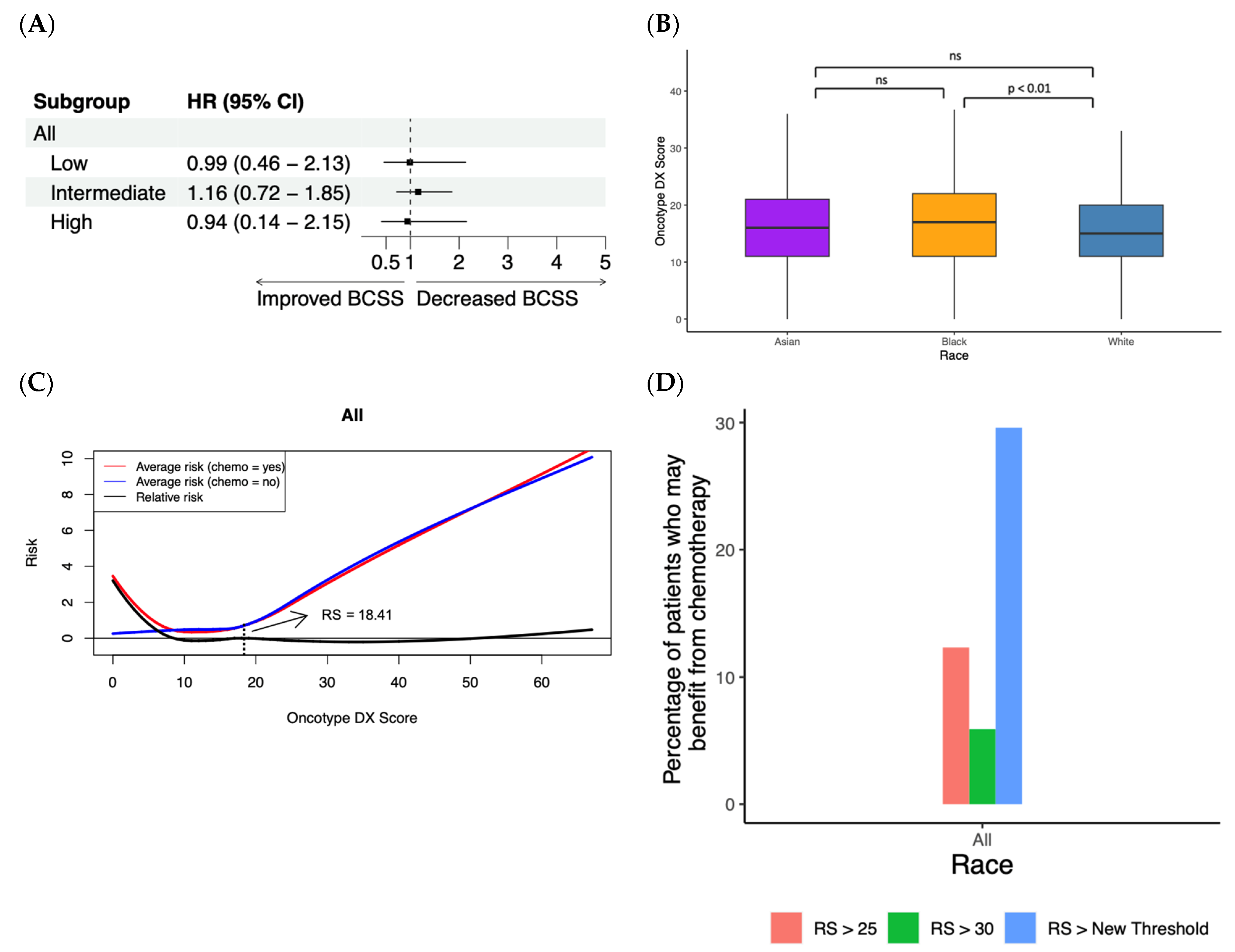
3.4. Random Forest Survival Model Demonstrates Chemotherapy Benefit in LN+ Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harbeck, N.; Gnant, M. Breast cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef] [PubMed]
- Ghoncheh, M.; Pournamdar, Z.; Salehiniya, H. Incidence and Mortality and Epidemiology of Breast Cancer in the World. Asian Pac. J. Cancer Prev. 2016, 17, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Pease, A.M.; Riba, L.A.; Gruner, R.A.; Tung, N.M.; James, T.A. Oncotype DX® Recurrence Score as a Predictor of Response to Neoadjuvant Chemotherapy. Ann. Surg. Oncol. 2019, 26, 366–371. [Google Scholar] [CrossRef]
- Schaafsma, E.; Zhang, B.; Schaafsma, M.; Tong, C.Y.; Zhang, L.; Cheng, C. Impact of Oncotype DX testing on ER+ breast cancer treatment and survival in the first decade of use. Breast Cancer Res. 2021, 23, 74. [Google Scholar] [CrossRef] [PubMed]
- Baker, J. Genomic Health, Inc. Pharmacogenomics 2007, 8, 397–399. [Google Scholar] [CrossRef]
- Dowsett, M.; Cuzick, J.; Wale, C.; Forbes, J.; Mallon, E.A.; Salter, J.; Quinn, E.; Dunbier, A.; Baum, M.; Buzdar, A.; et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: A TransATAC study. J. Clin. Oncol. 2010, 28, 1829–1834. [Google Scholar] [CrossRef] [PubMed]
- Paik, S.; Tang, G.; Shak, S.; Kim, C.; Baker, J.; Kim, W.; Cronin, M.; Baehner, F.L.; Watson, D.; Bryant, J.; et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J. Clin. Oncol. 2006, 24, 3726–3734. [Google Scholar] [CrossRef]
- Paik, S.; Shak, S.; Tang, G.; Kim, C.; Baker, J.; Cronin, M.; Baehner, F.L.; Walker, M.G.; Watson, D.; Park, T.; et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N. Engl. J. Med. 2004, 351, 2817–2826. [Google Scholar] [CrossRef]
- Bello, D.M.; Russell, C.; McCullough, D.; Tierno, M.; Morrow, M. Lymph Node Status in Breast Cancer Does Not Predict Tumor Biology. Ann. Surg. Oncol. 2018, 25, 2884–2889. [Google Scholar] [CrossRef]
- Iles, K.; Roberson, M.L.; Spanheimer, P.; Gallagher, K.; Ollila, D.W.; Strassle, P.D.; Downs-Canner, S. The impact of age and nodal status on variations in oncotype DX testing and adjuvant treatment. NPJ Breast Cancer 2022, 8, 27. [Google Scholar] [CrossRef]
- Sparano, J.A.; Gray, R.J.; Makower, D.F.; Pritchard, K.I.; Albain, K.S.; Hayes, D.F.; Geyer, C.E., Jr.; Dees, E.C.; Goetz, M.P.; Olson, J.A., Jr.; et al. Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer. N. Engl. J. Med. 2018, 379, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, K.F.; Danciu, O.C.; Ko, N.Y.; Calip, G.S. Association of Race/Ethnicity and the 21-Gene Recurrence Score with Breast Cancer–Specific Mortality Among US Women. JAMA Oncol. 2021, 7, 370. [Google Scholar] [CrossRef] [PubMed]
- Van’t Veer, L.J.; Dai, H.; Van De Vijver, M.J.; He, Y.D.; Hart, A.A.; Mao, M.; Peterse, H.L.; Van Der Kooy, K.; Marton, M.J.; Witteveen, A.T.; et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature 2002, 415, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wu, K.; Zhang, P.; Zhang, M.; Ding, A.; Chen, H. The Prognostic Significance of the Oncotype DX Recurrence Score in T1-2N1M0 Estrogen Receptor-Positive HER2-Negative Breast Cancer Based on the Prognostic Stage in the Updated AJCC 8th Edition. Ann. Surg. Oncol. 2019, 26, 1227–1235. [Google Scholar] [CrossRef]
- Cheng, R.; Wang, Z.; Kong, X.; Wang, J.; Fang, Y.; Qi, L. Factors associated with chemotherapy benefit in breast cancer patients with midrange Oncotype DX breast recurrence scores. Cancer Lett. 2021, 503, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Rigatti, S.J. Random Forest. J. Insur. Med. 2017, 47, 31–39. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program; Published Online. 2019. Available online: https://seer.cancer.gov/ (accessed on 1 January 2021).
- Holm, K.; Hegardt, C.; Staaf, J.; Vallon-Christersson, J.; Jönsson, G.; Olsson, H.; Borg, Å.; Ringnér, M. Molecular subtypes of breast cancer are associated with characteristic DNA methylation patterns. Breast Cancer Res. 2010, 12, R36. [Google Scholar] [CrossRef]
- Qiu, X.; Gao, J.; Yang, J.; Hu, J.; Hu, W.; Kong, L.; Lu, J.J. A Comparison Study of Machine Learning (Random Survival Forest) and Classic Statistic (Cox Proportional Hazards) for Predicting Progression in High-Grade Glioma after Proton and Carbon Ion Radiotherapy. Front. Oncol. 2020, 10, 551420. [Google Scholar] [CrossRef]
- Mogensen, U.B.; Ishwaran, H.; Gerds, T.A. Evaluating Random Forests for Survival Analysis using Prediction Error Curves. J. Stat. Softw. 2012, 50, 1. [Google Scholar] [CrossRef]
- Di Nardo, P.; Lisanti, C.; Garutti, M.; Buriolla, S.; Alberti, M.; Mazzeo, R.; Puglisi, F. Chemotherapy in patients with early breast cancer: Clinical overview and management of long-term side effects. Expert. Opin. Drug. Saf. 2022, 21, 1341–1355. [Google Scholar] [CrossRef]
- Albain, K.S.; Gray, R.J.; Makower, D.F.; Faghih, A.; Hayes, D.F.; Geyer, C.E., Jr.; Dees, E.C.; Goetz, M.P.; Olson, J.A., Jr.; Lively, T.; et al. Race, Ethnicity, and Clinical Outcomes in Hormone Receptor-Positive, HER2-Negative, Node-Negative Breast Cancer in the Randomized TAILORx Trial. J. Natl. Cancer Inst. 2021, 113, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Collin, L.J.; Yan, M.; Jiang, R.; Ward, K.C.; Crawford, B.; Torres, M.A.; Gogineni, K.; Subhedar, P.D.; Puvanesarajah, S.; Gaudet, M.M.; et al. Oncotype DX recurrence score implications for disparities in chemotherapy and breast cancer mortality in Georgia. NPJ Breast Cancer 2019, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Dignam, J.; Emir, B.; Bryant, J.; DeCillis, A.; Wolmark, N.; Wickerham, D.L.; Dimitrov, N.V.; Abramson, N.; Atkins, J.N.; et al. Tamoxifen and chemotherapy for lymph node-negative, estrogen receptor-positive breast cancer. J. Natl. Cancer Inst. 1997, 89, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Petkov, V.I.; Miller, D.P.; Howlader, N.; Gliner, N.; Howe, W.; Schussler, N.; Cronin, K.; Baehner, F.L.; Cress, R.; Deapen, D.; et al. Breast-cancer-specific mortality in patients treated based on the 21-gene assay: A SEER population-based study. NPJ Breast Cancer 2016, 2, 16017. [Google Scholar] [CrossRef] [PubMed]
- Cobleigh, M.A.; Tabesh, B.; Bitterman, P.; Baker, J.; Cronin, M.; Liu, M.L.; Borchik, R.; Mosquera, J.M.; Walker, M.G.; Shak, S. Tumor gene expression and prognosis in breast cancer patients with 10 or more positive lymph nodes. Clin. Cancer Res. 2005, 11 Pt 1, 8623–8631. [Google Scholar] [CrossRef]
- Pepe, M.S.; Feng, Z.; Janes, H.; Bossuyt, P.M.; Potter, J.D. Pivotal evaluation of the accuracy of a biomarker used for classification or prediction: Standards for study design. J. Natl. Cancer Inst. 2008, 100, 1432–1438. [Google Scholar] [CrossRef]
- Petrelli, F.; Cortellini, A.; Indini, A.; Tomasello, G.; Ghidini, M.; Nigro, O.; Salati, M.; Dottorini, L.; Iaculli, A.; Varricchio, A.; et al. Association of Obesity with Survival Outcomes in Patients With Cancer: A Systematic Review and Meta-analysis. JAMA Netw. Open. 2021, 4, e213520. [Google Scholar] [CrossRef]
- Macacu, A.; Autier, P.; Boniol, M.; Boyle, P. Active and passive smoking and risk of breast cancer: A meta-analysis. Breast Cancer Res. Treat. 2015, 154, 213–224. [Google Scholar] [CrossRef]
- DeSantis, C.E.; Ma, J.; Goding Sauer, A.; Newman, L.A.; Jemal, A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J. Clin. 2017, 67, 439–448. [Google Scholar] [CrossRef]
- Kalinsky, K.; Barlow, W.E.; Gralow, J.R.; Meric-Bernstam, F.; Albain, K.S.; Hayes, D.F.; Lin, N.U.; Perez, E.A.; Goldstein, L.J.; Chia, S.K.; et al. 21-Gene Assay to Inform Chemotherapy Benefit in Node-Positive Breast Cancer. N. Engl. J. Med. 2021, 385, 2336–2347. [Google Scholar] [CrossRef]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaw, V.R.; Amos, C.I.; Cheng, C. Predicting Chemotherapy Benefit across Different Races in Early-Stage Breast Cancer Patients Using the Oncotype DX Score. Cancers 2023, 15, 3217. https://doi.org/10.3390/cancers15123217
Shaw VR, Amos CI, Cheng C. Predicting Chemotherapy Benefit across Different Races in Early-Stage Breast Cancer Patients Using the Oncotype DX Score. Cancers. 2023; 15(12):3217. https://doi.org/10.3390/cancers15123217
Chicago/Turabian StyleShaw, Vikram R., Christopher I. Amos, and Chao Cheng. 2023. "Predicting Chemotherapy Benefit across Different Races in Early-Stage Breast Cancer Patients Using the Oncotype DX Score" Cancers 15, no. 12: 3217. https://doi.org/10.3390/cancers15123217
APA StyleShaw, V. R., Amos, C. I., & Cheng, C. (2023). Predicting Chemotherapy Benefit across Different Races in Early-Stage Breast Cancer Patients Using the Oncotype DX Score. Cancers, 15(12), 3217. https://doi.org/10.3390/cancers15123217





