Tumorigenesis in Inflammatory Bowel Disease: Microbiota-Environment Interconnections
Abstract
Simple Summary
Abstract
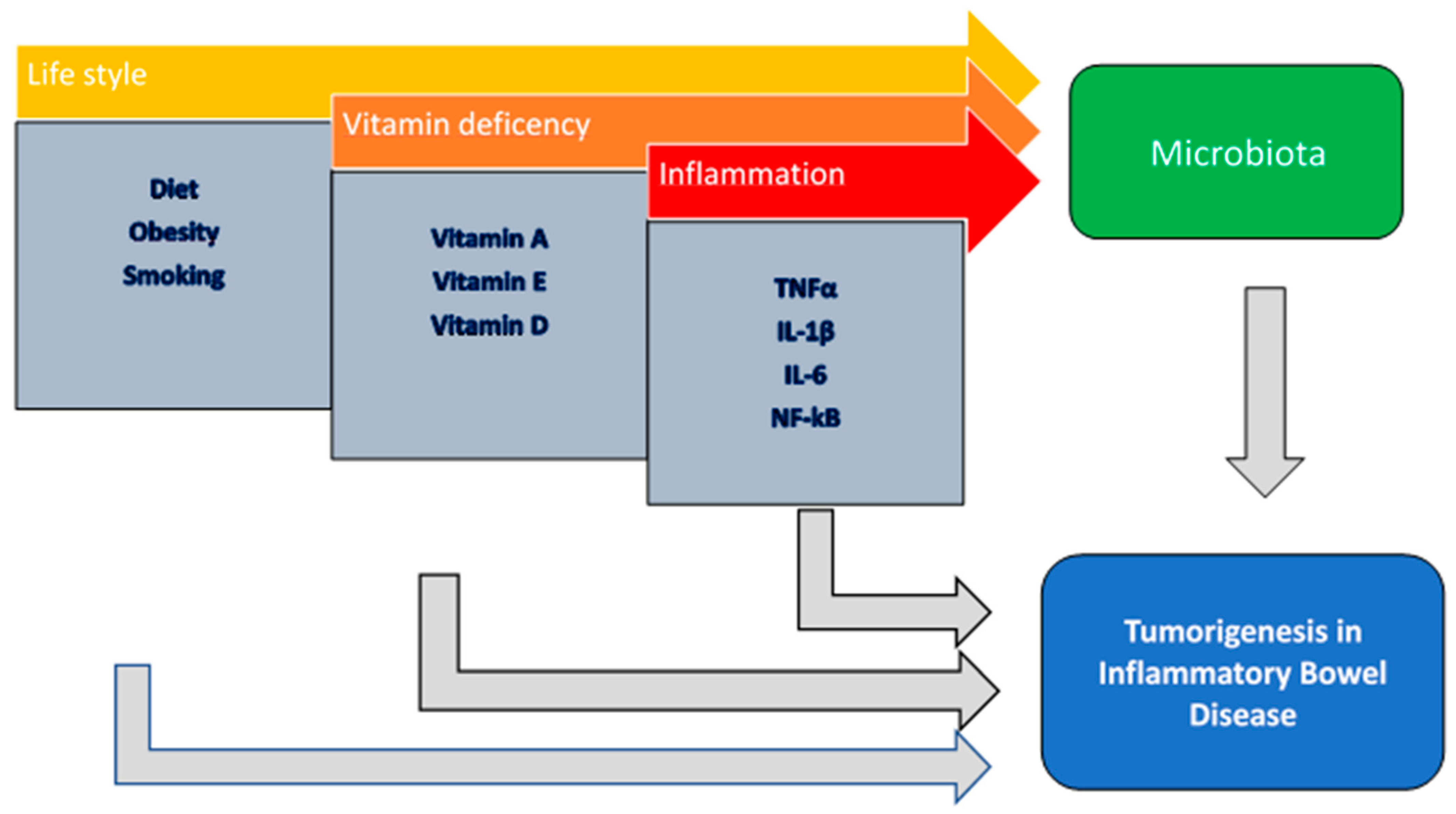
1. Introduction
2. Microbiota
2.1. Lactobacillus spp.
2.2. Bifidobacterium
2.3. Clostridiaceae
2.4. Bacteroides fragilis
2.5. Fusobacterium
2.6. Akkermansia
2.7. Microbiota and Host Interactions
3. Diet and Obesity
3.1. Obesity and Diabetes
3.2. Red Meat
3.3. Vitamins
3.3.1. Vitamin A
3.3.2. Vitamin D
3.3.3. Vitamin E
4. Smoking
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Laine, L.; Kaltenbach, T.; Barkun, A.; McQuaid, K.R.; Subramanian, V.; Soetikno, R. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastroenterology 2015, 148, 639–651.e28. [Google Scholar] [CrossRef] [PubMed]
- Annese, V.; Beaugerie, L.; Egan, L.; Biancone, L.; Bolling, C.; Brandts, C.; Dierickx, D.; Dummer, R.; Fiorino, G.; Gornet, J.M.; et al. European Evidence-based Consensus: Inflammatory Bowel Disease and Malignancies. J. Crohn’s Colitis 2015, 9, 945–965. [Google Scholar] [CrossRef] [PubMed]
- Pouw, R.E.; Bisschops, R.; Gecse, K.B.; de Hertogh, G.; Iacucci, M.; Rutter, M.; Barret, M.; Biermann, K.; Czakó, L.; Hucl, T.; et al. Endoscopic tissue sampling—Part 2: Lower gastrointestinal tract. European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2021, 53, 1261–1273. [Google Scholar] [CrossRef]
- Lee, E.; Lee, G.H.; Park, B.; Ahn, S.S.; Noh, C.K. Positive faecal immunochemical test predicts the onset of inflammatory bowel disease: A nationwide, propensity score-matched study. Front. Immunol. 2023, 14, 1128736. [Google Scholar] [CrossRef] [PubMed]
- Kato, J.; Hiraoka, S.; Nakarai, A.; Takashima, S.; Inokuchi, T.; Ichinose, M. Fecal immunochemical test as a biomarker for inflammatory bowel diseases: Can it rival fecal calprotectin? Intest. Res. 2016, 14, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Duran-Sanchon, S.; Herrera-Pariente, C.; Moreira, L. New non-invasive biomarkers for colorectal cancer screening. Rev. Esp. Enferm. Dig. 2020, 112, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Chiu, J.; Chen, Y.; Huang, Y.; Higashimori, A.; Fang, J.; Brim, H.; Ashktorab, H.; Ng, S.C.; Ng, S.S.M.; et al. Fecal Bacteria Act as Novel Biomarkers for Noninvasive Diagnosis of Colorectal Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 2061–2070. [Google Scholar] [CrossRef]
- Coker, O.O.; Liu, C.; Wu, W.K.K.; Wong, S.H.; Jia, W.; Sung, J.J.Y.; Yu, J. Altered gut metabolites and microbiota interactions are implicated in colorectal carcinogenesis and can be non-invasive diagnostic biomarkers. Microbiome 2022, 10, 35. [Google Scholar] [CrossRef]
- Itzkowitz, S.H.; Yio, X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: The role of inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G7–G17. [Google Scholar] [CrossRef]
- Song, M.; Chan, A.T.; Sun, J. Influence of the Gut Microbiome, Diet, and Environment on Risk of Colorectal Cancer. Gastroenterology 2020, 158, 322–340. [Google Scholar] [CrossRef]
- Scott, A.J.; Alexander, J.L.; Merrifield, C.A.; Cunningham, D.; Jobin, C.; Brown, R.; Alverdy, J.; O’Keefe, S.J.; Gaskins, H.R.; Teare, J.; et al. International Cancer Microbiome Consortium consensus statement on the role of the human microbiome in carcinogenesis. Gut 2019, 68, 1624–1632. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Rhee, K.J.; Zhang, M.; Franco, A.; Sears, C.L. Bacteroides fragilis toxin stimulates intestinal epithelial cell shedding and gamma-secretase-dependent E-cadherin cleavage. J. Cell Sci. 2007, 120, 1944–1952. [Google Scholar] [CrossRef]
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions between the microbiota and the immune system. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de Los Reyes-Gavilán, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Vich Vila, A.; Imhann, F.; Collij, V.; Jankipersadsing, S.A.; Gurry, T.; Mujagic, Z.; Kurilshikov, A.; Bonder, M.J.; Jiang, X.; Tigchelaar, E.F.; et al. Gut microbiota composition and functional changes in inflammatory bowel disease and irritable bowel syndrome. Sci. Transl. Med. 2018, 10, eaap8914. [Google Scholar] [CrossRef]
- Lopetuso, L.R.; Petito, V.; Graziani, C.; Schiavoni, E.; Paroni Sterbini, F.; Poscia, A.; Gaetani, E.; Franceschi, F.; Cammarota, G.; Sanguinetti, M.; et al. Gut Microbiota in Health, Diverticular Disease, Irritable Bowel Syndrome, and Inflammatory Bowel Diseases: Time for Microbial Marker of Gastrointestinal Disorders. Dig. Dis. 2018, 36, 56–65. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- De Sire, R.; Talocco, C.; Petito, V.; Lopetuso, L.R.; Graziani, C.; Gasbarrini, A.; Scaldaferri, F. Microbiota and inflammatory bowel disease: An update. Recent. Progress. Med. 2018, 109, 570–573. [Google Scholar] [CrossRef]
- Weiss, G.A.; Hennet, T. Mechanisms and consequences of intestinal dysbiosis. Cell. Mol. Life Sci. CMLS 2017, 74, 2959–2977. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, W.; Lan, P.; Mou, X. The microbiome in inflammatory bowel diseases: From pathogenesis to therapy. Protein Cell 2021, 12, 331–345. [Google Scholar] [CrossRef]
- Di Tommaso, N.; Gasbarrini, A.; Ponziani, F.R. Intestinal Barrier in Human Health and Disease. Int. J. Environ. Res. Public Health 2021, 18, 12836. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.M. Immunomodulatory mechanisms of lactobacilli. Microb. Cell Factories 2011, 10 (Suppl. 1), S17. [Google Scholar] [CrossRef]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Yitbarek, A.; Taha-Abdelaziz, K.; Hodgins, D.C.; Read, L.; Nagy, É.; Weese, J.S.; Caswell, J.L.; Parkinson, J.; Sharif, S. Gut microbiota-mediated protection against influenza virus subtype H9N2 in chickens is associated with modulation of the innate responses. Sci. Rep. 2018, 8, 13189. [Google Scholar] [CrossRef] [PubMed]
- Keir, M.; Yi, T.; Lu, T.; Ghilardi, N. The role of IL-22 in intestinal health and disease. J. Exp. Med. 2020, 217, e20192195. [Google Scholar] [CrossRef] [PubMed]
- Glal, D.; Sudhakar, J.N.; Lu, H.-H.; Liu, M.-C.; Chiang, H.-Y.; Liu, Y.-C.; Cheng, C.-F.; Shui, J.-W. ATF3 Sustains IL-22-Induced STAT3 Phosphorylation to Maintain Mucosal Immunity Through Inhibiting Phosphatases. Front. Immunol. 2018, 9, 2522. [Google Scholar] [CrossRef]
- Bernink, J.H.; Peters, C.P.; Munneke, M.; te Velde, A.A.; Meijer, S.L.; Weijer, K.; Hreggvidsdottir, H.S.; Heinsbroek, S.E.; Legrand, N.; Buskens, C.J.; et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat. Immunol. 2013, 14, 221–229. [Google Scholar] [CrossRef]
- Hammer, A.M.; Morris, N.L.; Cannon, A.R.; Khan, O.M.; Gagnon, R.C.; Movtchan, N.V.; van Langeveld, I.; Li, X.; Gao, B.; Choudhry, M.A. Interleukin-22 Prevents Microbial Dysbiosis and Promotes Intestinal Barrier Regeneration Following Acute Injury. Shock 2017, 48, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Okamura, Y.; Kinoshita, M.; Kono, T.; Sakai, M.; Hikima, J.I. Deficiency of interleukin-17 receptor A1 induces microbiota disruption in the intestine of Japanese medaka, Oryzias latipes. Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 40, 100885. [Google Scholar] [CrossRef] [PubMed]
- Natividad, J.M.; Agus, A.; Planchais, J.; Lamas, B.; Jarry, A.C.; Martin, R.; Michel, M.-L.; Chong-Nguyen, C.; Roussel, R.; Straube, M.; et al. Impaired Aryl Hydrocarbon Receptor Ligand Production by the Gut Microbiota Is a Key Factor in Metabolic Syndrome. Cell Metab. 2018, 28, 737–749.e4. [Google Scholar] [CrossRef] [PubMed]
- Etienne-Mesmin, L.; Chassaing, B.; Gewirtz, A.T. Tryptophan: A gut microbiota-derived metabolites regulating inflammation. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 7–9. [Google Scholar] [CrossRef]
- Macho Fernandez, E.; Valenti, V.; Rockel, C.; Hermann, C.; Pot, B.; Boneca, I.G.; Grangette, C. Anti-inflammatory capacity of selected lactobacilli in experimental colitis is driven by NOD2-mediated recognition of a specific peptidoglycan-derived muropeptide. Gut 2011, 60, 1050–1059. [Google Scholar] [CrossRef]
- Kramer, M.; Netea, M.G.; de Jong, D.J.; Kullberg, B.J.; Adema, G.J. Impaired dendritic cell function in Crohn’s disease patients with NOD2 3020insC mutation. J. Leukoc. Biol. 2006, 79, 860–866. [Google Scholar] [CrossRef]
- Saez-Lara, M.J.; Gomez-Llorente, C.; Plaza-Diaz, J.; Gil, A. The role of probiotic lactic acid bacteria and bifidobacteria in the prevention and treatment of inflammatory bowel disease and other related diseases: A systematic review of randomized human clinical trials. BioMed Res. Int. 2015, 2015, 505878. [Google Scholar] [CrossRef]
- Ghouri, Y.A.; Richards, D.M.; Rahimi, E.F.; Krill, J.T.; Jelinek, K.A.; DuPont, A.W. Systematic review of randomized controlled trials of probiotics, prebiotics, and synbiotics in inflammatory bowel disease. Clin. Exp. Gastroenterol. 2014, 7, 473–487. [Google Scholar] [CrossRef]
- Anjum, N.; Maqsood, S.; Masud, T.; Ahmad, A.; Sohail, A.; Momin, A. Lactobacillus acidophilus: Characterization of the species and application in food production. Crit. Rev. Food Sci. Nutr. 2014, 54, 1241–1251. [Google Scholar] [CrossRef]
- Hrdý, J.; Couturier-Maillard, A.; Boutillier, D.; Lapadatescu, C.; Blanc, P.; Procházka, J.; Pot, B.; Ryffel, B.; Grangette, C.; Chamaillard, M. Oral supplementation with selected Lactobacillus acidophilus triggers IL-17-dependent innate defense response, activation of innate lymphoid cells type 3 and improves colitis. Sci. Rep. 2022, 12, 17591. [Google Scholar] [CrossRef]
- Ann, S.; Choi, Y.; Yoon, Y. Comparative Genomic Analysis and Physiological Properties of Limosilactobacillus fermentum SMFM2017-NK2 with Ability to Inflammatory Bowel Disease. Microorganisms 2023, 11, 547. [Google Scholar] [CrossRef] [PubMed]
- Rattanaprasert, M.; van Pijkeren, J.-P.; Ramer-Tait, A.E.; Quintero, M.; Kok, C.R.; Walter, J.; Hutkins, R.W. Genes Involved in Galactooligosaccharide Metabolism in Lactobacillus reuteri and Their Ecological Role in the Gastrointestinal Tract. Appl. Environ. Microbiol. 2019, 85, e01788-19. [Google Scholar] [CrossRef] [PubMed]
- Oh, P.L.; Benson, A.K.; Peterson, D.A.; Patil, P.B.; Moriyama, E.N.; Roos, S.; Walter, J. Diversification of the gut symbiont Lactobacillus reuteri as a result of host-driven evolution. ISME J. 2010, 4, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.; Britton, R.A.; Roos, S. Host-microbial symbiosis in the vertebrate gastrointestinal tract and the Lactobacillus reuteri paradigm. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4645–4652. [Google Scholar] [CrossRef] [PubMed]
- Spinler, J.K.; Sontakke, A.; Hollister, E.B.; Venable, S.F.; Oh, P.L.; Balderas, M.A.; Saulnier, D.M.A.; Mistretta, T.-A.; Devaraj, S.; Walter, J.; et al. From prediction to function using evolutionary genomics: Human-specific ecotypes of Lactobacillus reuteri have diverse probiotic functions. Genome Biol. Evol. 2014, 6, 1772–1789. [Google Scholar] [CrossRef]
- Schreiber, O.; Petersson, J.; Phillipson, M.; Perry, M.; Roos, S.; Holm, L. Lactobacillus reuteri prevents colitis by reducing P-selectin-associated leukocyte- and platelet-endothelial cell interactions. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G534–G542. [Google Scholar] [CrossRef]
- Preidis, G.A.; Saulnier, D.M.; Blutt, S.E.; Mistretta, T.-A.; Riehle, K.P.; Major, A.M.; Venable, S.F.; Barrish, J.P.; Finegold, M.J.; Petrosino, J.F.; et al. Host response to probiotics determined by nutritional status of rotavirus-infected neonatal mice. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 299–307. [Google Scholar] [CrossRef]
- Gao, C.; Ganesh, B.P.; Shi, Z.; Shah, R.R.; Fultz, R.; Major, A.; Venable, S.; Lugo, M.; Hoch, K.; Chen, X.; et al. Gut Microbe-Mediated Suppression of Inflammation-Associated Colon Carcinogenesis by Luminal Histamine Production. Am. J. Pathol. 2017, 187, 2323–2336. [Google Scholar] [CrossRef]
- Dias, A.M.M.; Douhard, R.; Hermetet, F.; Regimbeau, M.; Lopez, T.E.; Gonzalez, D.; Masson, S.; Marcion, G.; Chaumonnot, K.; Uyanik, B.; et al. Lactobacillus stress protein GroEL prevents colonic inflammation. J. Gastroenterol. 2021, 56, 442–455. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Gu, F.; Zhu, C.; Yuan, L.; Zhu, C.; Zhu, M.; Yao, J.; Hu, P.; Zhang, Y.; Dicksved, J.; et al. Epithelial Heat Shock Proteins Mediate the Protective Effects of Limosilactobacillus reuteri in Dextran Sulfate Sodium-Induced Colitis. Front. Immunol. 2022, 13, 865982. [Google Scholar] [CrossRef]
- Wang, G.; Huang, S.; Cai, S.; Yu, H.; Wang, Y.; Zeng, X.; Qiao, S. Lactobacillus reuteri Ameliorates Intestinal Inflammation and Modulates Gut Microbiota and Metabolic Disorders in Dextran Sulfate Sodium-Induced Colitis in Mice. Nutrients 2020, 12, 2298. [Google Scholar] [CrossRef] [PubMed]
- Bell, H.N.; Rebernick, R.J.; Goyert, J.; Singhal, R.; Kuljanin, M.; Kerk, S.A.; Huang, W.; Das, N.K.; Andren, A.; Solanki, S.; et al. Reuterin in the healthy gut microbiome suppresses colorectal cancer growth through altering redox balance. Cancer Cell 2022, 40, 185–200.e6. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Ke, C.; Guo, J.; Zhang, X.; Li, B. Lactobacillus plantarum L15 Alleviates Colitis by Inhibiting LPS-Mediated NF-κB Activation and Ameliorates DSS-Induced Gut Microbiota Dysbiosis. Front. Immunol. 2020, 11, 575173. [Google Scholar] [CrossRef]
- Prantera, C.; Scribano, M.L.; Falasco, G.; Andreoli, A.; Luzi, C. Ineffectiveness of probiotics in preventing recurrence after curative resection for Crohn’s disease: A randomised controlled trial with Lactobacillus GG. Gut 2002, 51, 405–409. [Google Scholar] [CrossRef]
- Rogler, G. Chronic ulcerative colitis and colorectal cancer. Cancer Lett. 2014, 345, 235–241. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Corrigendum: Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 1486. [Google Scholar] [CrossRef]
- Vetuschi, A.; Battista, N.; Pompili, S.; Cappariello, A.; Prete, R.; Taticchi, A.; Selvaggini, R.; Latella, G.G.; Corsetti, A.; Sferra, R. The antiinflammatory and antifibrotic effect of olive phenols and Lactiplantibacillus plantarum IMC513 in dextran sodium sulfate-induced chronic colitis. Nutrition 2022, 94, 111511. [Google Scholar] [CrossRef] [PubMed]
- Paolillo, R.; Romano Carratelli, C.; Sorrentino, S.; Mazzola, N.; Rizzo, A. Immunomodulatory effects of Lactobacillus plantarum on human colon cancer cells. Int. Immunopharmacol. 2009, 9, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Chuah, L.-O.; Foo, H.L.; Loh, T.C.; Mohammed Alitheen, N.B.; Yeap, S.K.; Abdul Mutalib, N.E.; Abdul Rahim, R.; Yusoff, K. Postbiotic metabolites produced by Lactobacillus plantarum strains exert selective cytotoxicity effects on cancer cells. BMC Complement. Altern. Med. 2019, 19, 114. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Kim, Y.; Park, S.; Lee, D.; Lee, J.; Hlaing, S.P.; Yoo, J.-W.; Rhee, S.H.; Im, E. Lactobacillus plantarum Metabolites Elicit Anticancer Effects by Inhibiting Autophagy-Related Responses. Molecules 2023, 28, 1890. [Google Scholar] [CrossRef]
- Kim, H.J.; An, J.; Ha, E.M. Lactobacillus plantarum-derived metabolites sensitize the tumor-suppressive effects of butyrate by regulating the functional expression of SMCT1 in 5-FU-resistant colorectal cancer cells. Korean J. Microbiol. 2022, 60, 100–117. [Google Scholar] [CrossRef] [PubMed]
- Ze, X.; Le Mougen, F.; Duncan, S.H.; Louis, P.; Flint, H.J. Some are more equal than others: The role of “keystone” species in the degradation of recalcitrant substrates. Gut Microbes 2013, 4, 236–240. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.G.; Laiño, J.E.; del Valle, M.J.; Vannini, V.; van Sinderen, D.; Taranto, M.P.; de Valdez, G.F.; de Giori, G.S.; Sesma, F. B-group vitamin production by lactic acid bacteria—Current knowledge and potential applications. J. Appl. Microbiol. 2011, 111, 1297–1309. [Google Scholar] [CrossRef] [PubMed]
- de Kivit, S.; Tobin, M.C.; Forsyth, C.B.; Keshavarzian, A.; Landay, A.L. Regulation of Intestinal Immune Responses through TLR Activation: Implications for Pro- and Prebiotics. Front. Immunol. 2014, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Zeuthen, L.H.; Fink, L.N.; Frøkiaer, H. Toll-like receptor 2 and nucleotide-binding oligomerization domain-2 play divergent roles in the recognition of gut-derived lactobacilli and bifidobacteria in dendritic cells. Immunology 2008, 124, 489–502. [Google Scholar] [CrossRef]
- Thompson-Chagoyán, O.C.; Maldonado, J.; Gil, A. Aetiology of inflammatory bowel disease (IBD): Role of intestinal microbiota and gut-associated lymphoid tissue immune response. Clin. Nutr. 2005, 24, 339–352. [Google Scholar] [CrossRef]
- Sood, A.; Midha, V.; Makharia, G.K.; Ahuja, V.; Singal, D.; Goswami, P.; Tandon, R.K. The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin. Gastroenterol. Hepatol. 2009, 7, 1202–1209.e1. [Google Scholar] [CrossRef]
- Tursi, A.; Brandimarte, G.; Giorgetti, G.M.; Forti, G.; Modeo, M.E.; Gigliobianco, A. Low-dose balsalazide plus a high-potency probiotic preparation is more effective than balsalazide alone or mesalazine in the treatment of acute mild-to-moderate ulcerative colitis. Med. Sci. Monit. 2004, 10, PI126–PI131. [Google Scholar]
- Dong, Y.; Liao, W.; Tang, J.; Fei, T.; Gai, Z.; Han, M. Bifidobacterium BLa80 mitigates colitis by altering gut microbiota and alleviating inflammation. AMB Express 2022, 12, 67. [Google Scholar] [CrossRef]
- Fujimori, S.; Tatsuguchi, A.; Gudis, K.; Kishida, T.; Mitsui, K.; Ehara, A.; Kobayashi, T.; Sekita, Y.; Seo, T.; Sakamoto, C. High dose probiotic and prebiotic cotherapy for remission induction of active Crohn’s disease. J. Gastroenterol. Hepatol. 2007, 22, 1199–1204. [Google Scholar] [CrossRef]
- Steed, H.; Macfarlane, G.T.; Blackett, K.L.; Bahrami, B.; Reynolds, N.; Walsh, S.V.; Cummings, J.H.; Macfarlane, S. Clinical trial: The microbiological and immunological effects of synbiotic consumption—A randomized double-blind placebo-controlled study in active Crohn’s disease. Aliment. Pharmacol. Ther. 2010, 32, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-S.-E.; Li, W.-B.; Wang, H.-Y.; Ma, Y.-M.; Zhao, X.-H.; Yang, H.; Qian, J.-M.; Li, J.-N. VSL#3 can prevent ulcerative colitis-associated carcinogenesis in mice. World J. Gastroenterol. 2018, 24, 4254–4262. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Y.; Wang, C.; Wang, H.; Ma, Y.; Yang, H.; Zhao, X.; Hu, X.; Kao, J.Y.; Qian, J.; et al. Probiotic mixture VSL#3 prevents ulcerative colitis-associated carcinogenesis in mice and cells by regulating the inflammatory and Wnt/β-catenin pathway. Chin. Med. J. 2022, 135, 2357–2359. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xie, W.; Wan, X.; Deng, T. Clostridium butyricum modulates gut microbiota and reduces colitis associated colon cancer in mice. Int. Immunopharmacol. 2020, 88, 106862. [Google Scholar] [CrossRef]
- Li, Y.; Liu, M.; Liu, H.; Sui, X.; Liu, Y.; Wei, X.; Liu, C.; Cheng, Y.; Ye, W.; Gao, B.; et al. The Anti-Inflammatory Effect and Mucosal Barrier Protection of Clostridium butyricum RH2 in Ceftriaxone-Induced Intestinal Dysbacteriosis. Front. Cell. Infect. Microbiol. 2021, 11, 647048. [Google Scholar] [CrossRef]
- Choi, Y.; Choi, S.I.; Kim, N.; Nam, R.H.; Jang, J.Y.; Na, H.Y.; Shin, C.M.; Lee, D.H.; Min, H.; Kim, Y.-R.; et al. Effect of Clostridium butyricum on High-Fat Diet-Induced Intestinal Inflammation and Production of Short-Chain Fatty Acids. Dig. Dis. Sci. 2023, 68, 2427–2440. [Google Scholar] [CrossRef]
- Shao, X.; Sun, S.; Zhou, Y.; Wang, H.; Yu, Y.; Hu, T.; Yao, Y.; Zhou, C. Bacteroides fragilis restricts colitis-associated cancer via negative regulation of the NLRP3 axis. Cancer Lett. 2021, 523, 170–181. [Google Scholar] [CrossRef]
- Zamani, S.; Hesam Shariati, S.; Zali, M.R.; Asadzadeh Aghdaei, H.; Sarabi Asiabar, A.; Bokaie, S.; Nomanpour, B.; Sechi, L.A.; Feizabadi, M.M. Detection of enterotoxigenic Bacteroides fragilis in patients with ulcerative colitis. Gut Pathog. 2017, 9, 53. [Google Scholar] [CrossRef]
- Zamani, S.; Taslimi, R.; Sarabi, A.; Jasemi, S.; Sechi, L.A.; Feizabadi, M.M. Multi-cohort analysis of colorectal cancer metagenome identified altered bacteria across populations and universal bacterial markers. Microbiome 2018, 6, 70. [Google Scholar] [CrossRef]
- Zamani, S.; Taslimi, R.; Sarabi, A.; Jasemi, S.; Sechi, L.A.; Feizabadi, M.M. Enterotoxigenic Bacteroides fragilis: A Possible Etiological Candidate for Bacterially-Induced Colorectal Precancerous and Cancerous Lesions. Front. Cell. Infect. Microbiol. 2019, 9, 449. [Google Scholar] [CrossRef]
- Dai, Z.; Coker, O.O.; Nakatsu, G.; Wu, W.K.K.; Zhao, L.; Chen, Z.; Chan, F.K.L.; Kristiansen, K.; Sung, J.J.Y.; Wong, S.H.; et al. The Bacteroides fragilis toxin gene is prevalent in the colon mucosa of colorectal cancer patients. Clin. Infect. Dis. 2015, 60, 208–215. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Z.; Yan, Y.; Ji, L.; He, J.; Xuan, B.; Shen, C.; Ma, Y.; Jiang, S.; Ma, D.; et al. Enterotoxigenic Bacteroides fragilis Promotes Intestinal Inflammation and Malignancy by Inhibiting Exosome-Packaged miR-149-3p. Gastroenterology 2021, 161, 1552–1566.e12. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.T.; Kantilal, H.K.; Davamani, F. The Mechanism of Bacteroides fragilis Toxin Contributes to Colon Cancer Formation. Malays. J. Med. Sci. MJMS 2020, 27, 9–21. [Google Scholar] [CrossRef]
- Lee, C.-G.; Hwang, S.; Gwon, S.-Y.; Park, C.; Jo, M.; Hong, J.-E.; Rhee, K.-J. Bacteroides fragilis Toxin Induces Intestinal Epithelial Cell Secretion of Interleukin-8 by the E-Cadherin/β-Catenin/NF-κB Dependent Pathway. Biomedicines 2022, 10, 827. [Google Scholar] [CrossRef] [PubMed]
- Castellarin, M.; Warren, R.L.; Freeman, J.D.; Dreolini, L.; Krzywinski, M.; Strauss, J.; Barnes, R.; Watson, P.; Allen-Vercoe, E.; Moore, R.A.; et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012, 22, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.D.; Gevers, D.; Pedamallu, C.S.; Michaud, M.; Duke, F.; Earl, A.M.; Ojesina, A.I.; Jung, J.; Bass, A.J.; Tabernero, J.; et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012, 22, 292–298. [Google Scholar] [CrossRef]
- Hashemi Goradel, N.; Heidarzadeh, S.; Jahangiri, S.; Farhood, B.; Mortezaee, K.; Khanlarkhani, N.; Negahdari, B. Fusobacterium nucleatum and colorectal cancer: A mechanistic overview. J. Cell. Physiol. 2019, 234, 2337–2344. [Google Scholar] [CrossRef]
- Li, R.; Shen, J.; Xu, Y. Fusobacterium nucleatum and Colorectal Cancer. Infect. Drug Resist. 2022, 15, 1115–1120. [Google Scholar] [CrossRef]
- Rubinstein, M.R.; Baik, J.E.; Lagana, S.M.; Han, R.P.; Raab, W.J.; Sahoo, D.; Dalerba, P.; Wang, T.C.; Han, Y.W. Fusobacterium nucleatum promotes colorectal cancer by inducing Wnt/β-catenin modulator Annexin A1. EMBO Rep. 2019, 20, e47638. [Google Scholar] [CrossRef]
- Li, D.H.; Li, Z.P.; Yan Zhang Zhou, G.Z.; Ren, R.R.; Zhao, H.J.; Zhang, N.N.; Li, J.F.; Peng, L.H.; Yang, Y.S. Fecal Fusobacterium nucleatum harbored virulence gene fadA are associated with ulcerative colitis and clinical outcomes. Microb. Pathog. 2021, 157, 104964. [Google Scholar] [CrossRef]
- Yu, M.R.; Kim, H.J.; Park, H.R. Fusobacterium nucleatum Accelerates the Progression of Colitis-Associated Colorectal Cancer by Promoting EMT. Cancers 2020, 12, 2728. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, L.; Li, M.; Zhang, Y.; Sun, M.; Wang, L.; Lin, J.; Cui, Y.; Chen, Q.; Jin, C.; et al. Fusobacterium nucleatum reduces METTL3-mediated m6A modification and contributes to colorectal cancer metastasis. Nat. Commun. 2022, 13, 1248. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-D.; Zhang, X.; Zhang, Y.-P.; Yue, C.-B.; Wang, Y.-L.; Pan, H.-W.; Zhang, Y.-L.; Liu, H.; Zhang, Y. Fusobacterium Nucleatum Is a Risk Factor for Metastatic Colorectal Cancer. Curr. Med. Sci. 2022, 42, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Fang, J.Y. Fusobacterium nucleatum, a key pathogenic factor and microbial biomarker for colorectal cancer. Trends Microbiol. 2023, 31, 159–172. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, C.G.; Kim, W.K.; Kim, K.-A.; Yoo, J.; Min, B.S.; Paik, S.; Shin, S.J.; Lee, H.; Lee, K.; et al. Fusobacterium nucleatum induces a tumor microenvironment with diminished adaptive immunity against colorectal cancers. Front. Cell. Infect. Microbiol. 2023, 13, 1101291. [Google Scholar] [CrossRef]
- Gao, Y.; Bi, D.; Xie, R.; Li, M.; Guo, J.; Liu, H.; Guo, X.; Fang, J.; Ding, T.; Zhu, H.; et al. Fusobacterium nucleatum enhances the efficacy of PD-L1 blockade in colorectal cancer. Signal Transduct. Target. Ther. 2021, 6, 398. [Google Scholar] [CrossRef]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’Byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. Tor. Ont. 2022, 29, 3044–3060. [Google Scholar] [CrossRef]
- Gao, Y.; Zou, T.; Xu, P.; Wang, Y.; Jiang, Y.; Chen, Y.-X.; Chen, H.; Hong, J.; Fang, J.-Y. Fusobacterium nucleatum stimulates cell proliferation and promotes PD-L1 expression via IFIT1-related signal in colorectal cancer. Neoplasia 2023, 35, 100850. [Google Scholar] [CrossRef]
- Chang, C.-C.; Liu, C.-Y.; Su, I.-C.; Lee, Y.-J.; Yeh, H.-J.; Chen, W.-C.; Yu, C.-J.; Kao, W.-Y.; Liu, Y.-C.; Huang, C.-J. Functional Plasmon-Activated Water Increases Akkermansia muciniphila Abundance in Gut Microbiota to Ameliorate Inflammatory Bowel Disease. Int. J. Mol. Sci. 2022, 23, 11422. [Google Scholar] [CrossRef]
- López-Cauce, B.; Puerto, M.; García, J.J.; Ponce-Alonso, M.; Becerra-Aparicio, F.; Del Campo, R.; Peligros, I.; Fernández-Aceñero, M.J.; Gómez-Navarro, Y.; Lara, J.M.; et al. Akkermansia deficiency and mucin depletion are implicated in intestinal barrier dysfunction as earlier event in the development of inflammation in interleukin-10-deficient mice. Front. Microbiol. 2022, 13, 1083884. [Google Scholar] [CrossRef]
- Macchione, I.G.; Lopetuso, L.R.; Ianiro, G.; Napoli, M.; Gibiino, G.; Rizzatti, G.; Petito, V.; Gasbarrini, A.; Scaldaferri, F. Akkermansia muciniphila: Key player in metabolic and gastrointestinal disorders. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8075–8083. [Google Scholar] [CrossRef] [PubMed]
- Pittayanon, R.; Lau, J.T.; Leontiadis, G.I.; Tse, F.; Yuan, Y.; Surette, M.; Moayyedi, P. Differences in Gut Microbiota in Patients With vs Without Inflammatory Bowel Diseases: A Systematic Review. Gastroenterology 2020, 158, 930–946.e1. [Google Scholar] [CrossRef]
- Ahn, I.S.; Lang, J.M.; Olson, C.A.; Diamante, G.; Zhang, G.; Ying, Z.; Byun, H.R.; Cely, I.; Ding, J.; Cohn, P.; et al. Host Genetic Background and Gut Microbiota Contribute to Differential Metabolic Responses to Fructose Consumption in Mice. J. Nutr. 2020, 150, 2716–2728. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wu, W.; Wang, Q.; Yang, L.; Bian, X.; Jiang, X.; Lv, L.; Yan, R.; Xia, J.; Han, S.; et al. The negative effect of Akkermansia muciniphila-mediated post-antibiotic reconstitution of the gut microbiota on the development of colitis-associated colorectal cancer in mice. Front. Microbiol. 2022, 13, 932047. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Cai, K.; Xiao, Q.; He, L.; Xie, L.; Liu, Z. Akkermansia muciniphila administration exacerbated the development of colitis-associated colorectal cancer in mice. J. Cancer 2022, 13, 124–133. [Google Scholar] [CrossRef]
- Wang, L.; Tang, L.; Feng, Y.; Zhao, S.; Han, M.; Zhang, C.; Yuan, G.; Zhu, J.; Cao, S.; Wu, Q.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of CD8+ T cells in mice. Gut 2020, 69, 1988–1997. [Google Scholar] [CrossRef]
- Steele, L.; Mayer, L.; Berin, M.C. Mucosal immunology of tolerance and allergy in the gastrointestinal tract. Immunol. Res. 2012, 54, 75–82. [Google Scholar] [CrossRef]
- Weström, B.; Arévalo Sureda, E.; Pierzynowska, K.; Pierzynowski, S.G.; Pérez-Cano, F.J. The Immature Gut Barrier and Its Importance in Establishing Immunity in Newborn Mammals. Front. Immunol. 2020, 11, 1153. [Google Scholar] [CrossRef]
- Saez, A.; Gomez-Bris, R.; Herrero-Fernandez, B.; Mingorance, C.; Rius, C.; Gonzalez-Granado, J.M. Innate Lymphoid Cells in Intestinal Homeostasis and Inflammatory Bowel Disease. Int. J. Mol. Sci. 2021, 22, 7618. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Macfarlane, S.; Macfarlane, G.T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2003, 62, 67–72. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Li, G.; Lin, J.; Zhang, C.; Gao, H.; Lu, H.; Gao, X.; Zhu, R.; Li, Z.; Li, M.; Liu, Z. Microbiota metabolite butyrate constrains neutrophil functions and ameliorates mucosal inflammation in inflammatory bowel disease. Gut Microbes 2021, 13, 1968257. [Google Scholar] [CrossRef]
- Segain, J.P.; Raingeard de la Blétière, D.; Bourreille, A.; Leray, V.; Gervois, N.; Rosales, C.; Ferrier, L.; Bonnet, C.; Blottière, H.M.; Galmiche, J.P. Butyrate inhibits inflammatory responses through NFkappaB inhibition: Implications for Crohn’s disease. Gut 2000, 47, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, S.; Huang, S.; Wu, Z.; Pang, J.; Wu, Y.; Wang, J.; Han, D. Resistant Maltodextrin Alleviates Dextran Sulfate Sodium-Induced Intestinal Inflammatory Injury by Increasing Butyric Acid to Inhibit Proinflammatory Cytokine Levels. BioMed Res. Int. 2020, 2020, 7694734. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, C.; Zhu, J.; Lin, Q.; Yu, M.; Wen, J.; Feng, J.; Hu, C. Sodium Butyrate Ameliorates Oxidative Stress-Induced Intestinal Epithelium Barrier Injury and Mitochondrial Damage through AMPK-Mitophagy Pathway. Oxidative Med. Cell. Longev. 2022, 2022, 3745135. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.W.; Yin, F.Y.; Zhang, Z.F.; Gong, X.; Yang, Y. Butyrate Suppresses Glucose Metabolism of Colorectal Cancer Cells via GPR109a-AKT Signaling Pathway and Enhances Chemotherapy. Front. Mol. Biosci. 2021, 8, 634874. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cao, L.; Tian, Y.; Zhang, P.; Ding, C.; Lu, W.; Jia, C.; Shao, C.; Liu, W.; Wang, D.; et al. Butyrate Suppresses the Proliferation of Colorectal Cancer Cells via Targeting Pyruvate Kinase M2 and Metabolic Reprogramming. Mol. Cell. Proteom. MCP 2018, 17, 1531–1545. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A Double-Edged Sword for Health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Ji, X.; Song, Z.; Wu, F.; Qu, Y.; Jin, X.; Xue, X.; Wang, F.; Huang, Y. Butyrate Inhibits Gastric Cancer Cells by Inducing Mitochondriamediated Apoptosis. Comb. Chem. High Throughput Screen. 2023, 26, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Yang, M.; Wang, Y.; Yang, C.; Shafiq, M.; Wang, G.; Li, L. Sodium propionate protect the blood-milk barrier integrity, relieve lipopolysaccharide-induced inflammatory injury and cells apoptosis. Life Sci. 2021, 270, 119138. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, J.; Garces, L.; Quintero, M.A.; Pignac-Kobinger, J.; Santander, A.M.; Fernández, I.; Ban, Y.J.; Kwon, D.; Phillips, M.C.; Knight, K.; et al. Low-Fat, High-Fiber Diet Reduces Markers of Inflammation and Dysbiosis and Improves Quality of Life in Patients With Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2021, 19, 1189–1199.e30. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.W.; Kwon, Y.H. Regulation of gene expression in the development of colitis-associated colon cancer in mice fed a high-fat diet. Biochem. Biophys. Res. Commun. 2022, 592, 81–86. [Google Scholar] [CrossRef]
- Chen, J.; Wellens, J.; Kalla, R.; Fu, T.; Deng, M.; Zhang, H.; Yuan, S.; Wang, X.; Theodoratou, E.; Li, X.; et al. Intake of ultra-processed foods is associated with an increased risk of Crohn’s disease: A cross-sectional and prospective analysis of 187,154 participants in the UK Biobank. J. Crohns Colitis. 2023, 17, 535–552. [Google Scholar] [CrossRef] [PubMed]
- Cassotta, M.; Cianciosi, D.; De Giuseppe, R.; Navarro-Hortal, M.D.; Armas Diaz, Y.; Forbes-Hernández, T.Y.; Pifarre, K.T.; Pascual Barrera, A.E.; Grosso, G.; Xiao, J.; et al. Possible role of nutrition in the prevention of inflammatory bowel disease-related colorectal cancer: A focus on human studies. Nutrition 2023, 110, 111980. [Google Scholar] [CrossRef]
- Overgaard, S.H.; Sørensen, S.B.; Munk, H.L.; Nexøe, A.B.; Glerup, H.; Henriksen, R.H.; Guldmann, T.; Pedersen, N.; Saboori, S.; Hvid, L.; et al. Impact of fibre and red/processed meat intake on treatment outcomes among patients with chronic inflammatory diseases initiating biological therapy: A prospective cohort study. Front. Nutr. 2022, 9, 985732. [Google Scholar] [CrossRef]
- Dong, C.; Chan, S.S.M.; Jantchou, P.; Racine, A.; Oldenburg, B.; Weiderpass, E.; Heath, A.K.; Tong, T.Y.N.; Tjønneland, A.; Kyrø, C.; et al. Meat Intake Is Associated with a Higher Risk of Ulcerative Colitis in a Large European Prospective Cohort Studyø. J. Crohn’s Colitis 2022, 16, 1187–1196. [Google Scholar] [CrossRef]
- Miquel, S.; Martín, R.; Rossi, O.; Bermúdez-Humarán, L.G.; Chatel, J.M.; Sokol, H.; Thomas, M.; Wells, J.M.; Langella, P. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 2013, 16, 255–261. [Google Scholar] [CrossRef]
- Leylabadlo, H.E.; Ghotaslou, R.; Feizabadi, M.M.; Farajnia, S.; Moaddab, S.Y.; Ganbarov, K.; Khodadadi, E.; Tanomand, A.; Sheykhsaran, E.; Yousefi, B.; et al. The critical role of Faecalibacterium prausnitzii in human health: An overview. Microb. Pathog. 2020, 149, 104344. [Google Scholar] [CrossRef]
- Liu, B.N.; Liu, X.T.; Liang, Z.H.; Wang, J.H. Gut microbiota in obesity. World J. Gastroenterol. 2021, 27, 3837–3850. [Google Scholar] [CrossRef]
- Breton, J.; Galmiche, M.; Déchelotte, P. Dysbiotic Gut Bacteria in Obesity: An Overview of the Metabolic Mechanisms and Therapeutic Perspectives of Next-Generation Probiotics. Microorganisms 2022, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Ni, Q.; Sun, W.; Li, L.; Feng, X. The links between gut microbiota and obesity and obesity related diseases. Biomed. Pharmacother. 2022, 147, 112678. [Google Scholar] [CrossRef] [PubMed]
- Sanz, Y.; Santacruz, A.; Gauffin, P. Gut microbiota in obesity and metabolic disorders. Proc. Nutr. Soc. 2010, 69, 434–441. [Google Scholar] [CrossRef]
- de La Serre, C.B.; Ellis, C.L.; Lee, J.; Hartman, A.L.; Rutledge, J.C.; Raybould, H.E. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G440–G448. [Google Scholar] [CrossRef]
- Hamilton, M.K.; Boudry, G.; Lemay, D.G.; Raybould, H.E. Changes in intestinal barrier function and gut microbiota in high-fat diet-fed rats are dynamic and region dependent. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G840–G851. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Kong, L.-C.; Tap, J.; Aron-Wisnewsky, J.; Pelloux, V.; Basdevant, A.; Bouillot, J.-L.; Zucker, J.-D.; Doré, J.; Clément, K. Gut microbiota after gastric bypass in human obesity: Increased richness and associations of bacterial genera with adipose tissue genes. Am. J. Clin. Nutr. 2013, 98, 16–24. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef]
- Feng, J.; Tang, H.; Li, M.; Pang, X.; Wang, L.; Zhang, M.; Zhao, Y.; Zhang, X.; Shen, J. The abundance of fecal Faecalibacterium prausnitzii in relation to obesity and gender in Chinese adults. Arch. Microbiol. 2014, 196, 73–77. [Google Scholar] [CrossRef]
- Clarke, S.F.; Murphy, E.F.; Nilaweera, K.; Ross, P.R.; Shanahan, F.; O’Toole, P.W.; Cotter, P.D. The gut microbiota and its relationship to diet and obesity: New insights. Gut Microbes 2012, 3, 186–202. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Tremaroli, V.; Karlsson, F.; Werling, M.; Ståhlman, M.; Kovatcheva-Datchary, P.; Olbers, T.; Fändriks, L.; le Roux, C.W.; Nielsen, J.; Bäckhed, F. Roux-en-Y Gastric Bypass and Vertical Banded Gastroplasty Induce Long-Term Changes on the Human Gut Microbiome Contributing to Fat Mass Regulation. Cell Metab. 2015, 22, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.W.M.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Xu, Y.C.; Niu, L. Obesity and colorectal cancer risk: A meta-analysis of cohort studies. World J. Gastroenterol. 2007, 13, 4199–4206. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Painter, J.E.; Atkin, W.S.; Potten, C.S.; Shalet, S.M.; O’Dwyer, S.T. High-risk colorectal adenomas and serum insulin-like growth factors. Br. J. Surg. 2001, 88, 107–113. [Google Scholar] [CrossRef]
- Renehan, A.G.; Zwahlen, M.; Minder, C.; O’Dwyer, S.T.; Shalet, S.M.; Egger, M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: Systematic review and meta-regression analysis. Lancet 2004, 363, 1346–1353. [Google Scholar] [CrossRef]
- Renehan, A.G.; Frystyk, J.; Flyvbjerg, A. Obesity and cancer risk: The role of the insulin-IGF axis. Trends Endocrinol. Metab. TEM 2006, 17, 328–336. [Google Scholar] [CrossRef]
- Larsson, S.C.; Orsini, N.; Wolk, A. Diabetes mellitus and risk of colorectal cancer: A meta-analysis. J. Natl. Cancer Inst. 2005, 97, 1679–1687. [Google Scholar] [CrossRef]
- Yang, G.; Fan, W.; Luo, B.; Xu, Z.; Wang, P.; Tang, S.; Xu, P.; Yu, M. Circulating Resistin Levels and Risk of Colorectal Cancer: A Meta-Analysis. BioMed Res. Int. 2016, 2016, 7367485. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, Y.; Wang, F.; Zhang, P.; Shi, C.; Zou, Y.; Qin, H. Obesity and risk of colorectal cancer: A systematic review of prospective studies. PLoS ONE 2013, 8, e53916. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, P.; Sarma, D.K.; Kumawat, M.; Tiwari, R.; Verma, V.; Nagpal, R.; Kumar, M. Implication of Obesity and Gut Microbiome Dysbiosis in the Etiology of Colorectal Cancer. Cancers 2023, 15, 1913. [Google Scholar] [CrossRef] [PubMed]
- Vigneri, P.G.; Tirrò, E.; Pennisi, M.S.; Massimino, M.; Stella, S.; Romano, C.; Manzella, L. The Insulin/IGF System in Colorectal Cancer Development and Resistance to Therapy. Front. Oncol. 2015, 5, 230. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Alcoholado, L.; Ordóñez, R.; Otero, A.; Plaza-Andrade, I.; Laborda-Illanes, A.; Medina, J.A.; Ramos-Molina, B.; Gómez-Millán, J.; Queipo-Ortuño, M.I. Gut Microbiota-Mediated Inflammation and Gut Permeability in Patients with Obesity and Colorectal Cancer. Int. J. Mol. Sci. 2020, 21, 6782. [Google Scholar] [CrossRef] [PubMed]
- Noureldein, M.; Nawfal, R.; Bitar, S.; Maxwell, S.S.; Khurana, I.; Kassouf, H.K.; Khuri, F.R.; El-Osta, A.; Eid, A.A. Intestinal microbiota regulates diabetes and cancer progression by IL-1β and NOX4 dependent signaling cascades. Cell. Mol. Life Sci. CMLS 2022, 79, 502. [Google Scholar] [CrossRef]
- Campisciano, G.; de Manzini, N.; Delbue, S.; Cason, C.; Cosola, D.; Basile, G.; Ferrante, P.; Comar, M.; Palmisano, S. The Obesity-Related Gut Bacterial and Viral Dysbiosis Can Impact the Risk of Colon Cancer Development. Microorganisms 2020, 8, 431. [Google Scholar] [CrossRef]
- O’Mahony, C.; Clooney, A.; Clarke, S.F.; Aguilera, M.; Gavin, A.; Simnica, D.; Ahern, M.; Fanning, A.; Stanley, M.; Rubio, R.C.; et al. Dietary-Induced Bacterial Metabolites Reduce Inflammation and Inflammation-Associated Cancer via Vitamin D Pathway. Int. J. Mol. Sci. 2023, 24, 1864. [Google Scholar] [CrossRef]
- Peters, V.; Bolte, L.; Schuttert, E.M.; Andreu-Sánchez, S.; Dijkstra, G.; Weersma, R.K.; Campmans-Kuijpers, M.J.E. Western and Carnivorous Dietary Patterns are Associated with Greater Likelihood of IBD Development in a Large Prospective Population-based Cohort. J. Crohn’s Colitis 2022, 16, 931–939. [Google Scholar] [CrossRef]
- Li, T.; Qiu, Y.; Yang, H.S.; Li, M.Y.; Zhuang, X.J.; Zhang, S.H.; Feng, R.; Chen, B.L.; He, Y.; Zeng, Z.R.; et al. Systematic review and meta-analysis: Association of a pre-illness Western dietary pattern with the risk of developing inflammatory bowel disease. J. Dig. Dis. 2020, 21, 362–371. [Google Scholar] [CrossRef]
- Narula, N.; Wong, E.C.L.; Dehghan, M.; Mente, A.; Rangarajan, S.; Lanas, F.; Lopez-Jaramillo, P.; Rohatgi, P.; Lakshmi, P.V.M.; Varma, R.P.; et al. Association of ultra-processed food intake with risk of inflammatory bowel disease: Prospective cohort study. BMJ 2021, 374, n1554. [Google Scholar] [CrossRef]
- Albenberg, L.; Brensinger, C.M.; Wu, Q.; Gilroy, E.; Kappelman, M.D.; Sandler, R.S.; Lewis, J.D. A Diet Low in Red and Processed Meat Does Not Reduce Rate of Crohn’s Disease Flares. Gastroenterology 2019, 157, 128–136.e5. [Google Scholar] [CrossRef]
- Limketkai, B.N.; Godoy-Brewer, G.; Parian, A.M.; Noorian, S.; Krishna, M.; Shah, N.D.; White, J.; Mullin, G.E. Dietary Interventions for the Treatment of Inflammatory Bowel Diseases: An Updated Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2022, 99, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Limketkai, B.N.; Iheozor-Ejiofor, Z.; Gjuladin-Hellon, T.; Parian, A.; Matarese, L.E.; Bracewell, K.; MacDonald, J.K.; Gordon, M.; Mullin, G.E. Dietary interventions for induction and maintenance of remission in inflammatory bowel disease. Cochrane Database Syst. Rev. 2019, 2, CD012839. [Google Scholar] [CrossRef] [PubMed]
- Peters, V.; Spooren, C.E.G.M.; Pierik, M.J.; Weersma, R.K.; van Dullemen, H.M.; Festen, E.A.M.; Visschedijk, M.C.; Masclee, A.A.M.; Hendrix, E.M.B.; Almeida, R.J.; et al. Dietary Intake Pattern is Associated with Occurrence of Flares in IBD Patients. J. Crohn’s Colitis 2021, 15, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Fu, T.; Dan, L.; Chen, X.; Sun, Y.; Chen, J.; Wang, X.; Hesketh, T. Meat consumption and all-cause mortality in 5763 patients with inflammatory bowel disease: A retrospective cohort study. EClinicalMedicine 2022, 47, 101406. [Google Scholar] [CrossRef]
- Diallo, A.; Deschasaux, M.; Latino-Martel, P.; Hercberg, S.; Galan, P.; Fassier, P.; Allès, B.; Guéraud, F.; Pierre, F.H.; Touvier, M. Red and processed meat intake and cancer risk: Results from the prospective NutriNet-Santé cohort study. Int. J. Cancer 2018, 142, 230–237. [Google Scholar] [CrossRef]
- Huang, Y.; Cao, D.; Chen, Z.; Chen, B.; Li, J.; Guo, J.; Dong, Q.; Liu, L.; Wei, Q. Red and processed meat consumption and cancer outcomes: Umbrella review. Food Chem. 2021, 356, 129697. [Google Scholar] [CrossRef]
- Schepens, M.A.A.; Vink, C.; Schonewille, A.J.; Dijkstra, G.; van der Meer, R.; Bovee-Oudenhoven, I.M.J. Dietary heme adversely affects experimental colitis in rats, despite heat-shock protein induction. Nutrition 2011, 27, 590–597. [Google Scholar] [CrossRef]
- IJssennagger, N.; Derrien, M.; van Doorn, G.M.; Rijnierse, A.; van den Bogert, B.; Müller, M.; Dekker, J.; Kleerebezem, M.; van der Meer, R. Dietary heme alters microbiota and mucosa of mouse colon without functional changes in host-microbe cross-talk. PLoS ONE 2012, 7, e49868. [Google Scholar] [CrossRef]
- Constante, M.; Fragoso, G.; Calvé, A.; Samba-Mondonga, M.; Santos, M.M. Dietary Heme Induces Gut Dysbiosis, Aggravates Colitis, and Potentiates the Development of Adenomas in Mice. Front. Microbiol. 2017, 8, 1809. [Google Scholar] [CrossRef]
- Li, D.P.; Cui, M.; Tan, F.; Liu, X.Y.; Yao, P. High Red Meat Intake Exacerbates Dextran Sulfate-Induced Colitis by Altering Gut Microbiota in Mice. Front. Nutr. 2021, 8, 646819. [Google Scholar] [CrossRef]
- Steinert, R.E.; Lee, Y.K.; Sybesma, W. Vitamins for the Gut Microbiome. Trends Mol. Med. 2020, 26, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Dong, W.; Sun, Y.; Gu, Y.; Ma, J.; Wang, B.; Cao, H. Vitamin-Microbiota Crosstalk in Intestinal Inflammation and Carcinogenesis. Nutrients 2022, 14, 3383. [Google Scholar] [CrossRef] [PubMed]
- Bakdash, G.; Vogelpoel, L.T.C.; van Capel, T.M.M.; Kapsenberg, M.L.; de Jong, E.C. Retinoic acid primes human dendritic cells to induce gut-homing, IL-10-producing regulatory T cells. Mucosal Immunol. 2015, 8, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Mucida, D.; Park, Y.; Kim, G.; Turovskaya, O.; Scott, I.; Kronenberg, M.; Cheroutre, H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 2007, 317, 256–260. [Google Scholar] [CrossRef]
- Bhattacharya, N.; Yuan, R.; Prestwood, T.R.; Penny, H.L.; DiMaio, M.A.; Reticker-Flynn, N.E.; Krois, C.R.; Kenkel, J.A.; Pham, T.D.; Carmi, Y.; et al. Normalizing Microbiota-Induced Retinoic Acid Deficiency Stimulates Protective CD8(+) T Cell-Mediated Immunity in Colorectal Cancer. Immunity 2016, 45, 641–655. [Google Scholar] [CrossRef]
- Okayasu, I.; Hana, K.; Nemoto, N.; Yoshida, T.; Saegusa, M.; Yokota-Nakatsuma, A.; Song, S.-Y.; Iwata, M. Vitamin A Inhibits Development of Dextran Sulfate Sodium-Induced Colitis and Colon Cancer in a Mouse Model. BioMed Res. Int. 2016, 2016, 4874809. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Goulart, R.d.A.; Batista, G.L.D.S.A. Vitamin A and inflammatory bowel diseases: From cellular studies and animal models to human disease. Expert. Rev. Gastroenterol. Hepatol. 2019, 13, 25–35. [Google Scholar] [CrossRef]
- Rampal, R.; Wari, N.; Singh, A.K.; Das, U.; Bopanna, S.; Gupta, V.; Nayak, B.; Velapandian, T.; Kedia, S.; Kumar, D.; et al. Retinoic Acid Is Elevated in the Mucosa of Patients with Active Ulcerative Colitis and Displays a Proinflammatory Role by Augmenting IL-17 and IFNγ Production. Inflamm. Bowel Dis. 2021, 27, 74–83. [Google Scholar] [CrossRef]
- Ramagopalan, S.V.; Heger, A.; Berlanga, A.J.; Maugeri, N.J.; Lincoln, M.R.; Burrell, A.; Handunnetthi, L.; Handel, A.E.; Disanto, G.; Orton, S.-M.; et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: Associations with disease and evolution. Genome Res. 2010, 20, 1352–1360. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Waddell, A. The vitamin D receptor turns off chronically activated T cells. Ann. N. Y. Acad. Sci. 2014, 1317, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Kongsbak, M.; Levring, T.B.; Geisler, C.; von Essen, M.R. The vitamin d receptor and T cell function. Front. Immunol. 2013, 4, 148. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-W.; Wang, P.-Y.; Zhu, J.; Chen, G.-W.; Zhang, J.-L.; Chen, Z.-Y.; Zuo, S.; Liu, Y.-C.; Pan, Y.-S. Protective effect of 1,25-dihydroxyvitamin d3 on lipopolysaccharide-induced intestinal epithelial tight junction injury in caco-2 cell monolayers. Inflammation 2015, 38, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Garrett, S.; Carroll, R.E.; Xia, Y.; Sun, J. Vitamin D receptor upregulates tight junction protein claudin-5 against colitis-associated tumorigenesis. Mucosal Immunol. 2022, 15, 683–697. [Google Scholar] [CrossRef]
- Chatterjee, I.; Zhang, Y.; Zhang, J.; Lu, R.; Xia, Y.; Sun, J. Overexpression of Vitamin D Receptor in Intestinal Epithelia Protects Against Colitis via Upregulating Tight Junction Protein Claudin 15. J. Crohn’s Colitis 2021, 15, 1720–1736. [Google Scholar] [CrossRef]
- Domazetovic, V.; Iantomasi, T.; Bonanomi, A.G.; Stio, M. Vitamin D regulates claudin-2 and claudin-4 expression in active ulcerative colitis by p-Stat-6 and Smad-7 signaling. Int. J. Colorectal Dis. 2020, 35, 1231–1242. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, X.; Li, J.; Wang, H.; Li, Y.; Chen, Y.; Zhang, H. Clinical evaluation of vitamin D status and its relationship with disease activity and changes of intestinal immune function in patients with Crohn’s disease in the Chinese population. Scand. J. Gastroenterol. 2021, 56, 20–29. [Google Scholar] [CrossRef]
- Yeh, C.-L.; Wu, J.-M.; Yang, P.-J.; Lee, P.-C.; Chen, K.-Y.; Huang, C.-C.; Yeh, S.-L.; Lin, M.-T. Intravenous calcitriol administration modulates mesenteric lymph node CD4+ T-cell polarization and attenuates intestinal inflammation in obese mice complicated with polymicrobial sepsis. JPEN J. Parenter. Enteral Nutr. 2022, 46, 1371–1383. [Google Scholar] [CrossRef]
- Kong, J.; Zhang, Z.; Musch, M.W.; Ning, G.; Sun, J.; Hart, J.; Bissonnette, M.; Li, Y.C. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G208–G216. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Cheng, S.-C.; Cai, T.; Cagan, A.; Gainer, V.S.; Szolovits, P.; Shaw, S.Y.; Churchill, S.; Karlson, E.W.; Murphy, S.N.; et al. Association between reduced plasma 25-hydroxy vitamin D and increased risk of cancer in patients with inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 2014, 12, 821–827. [Google Scholar] [CrossRef]
- Xin, Y.; He, L.; Luan, Z.; Lv, H.; Yang, H.; Zhou, Y.; Zhao, X.; Zhou, W.; Yu, S.; Tan, B.; et al. E-cadherin Mediates the Preventive Effect of Vitamin D3 in Colitis-associated Carcinogenesis. Inflamm. Bowel Dis. 2017, 23, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Meeker, S.M.; Seamons, A.; Treuting, P.M.; Paik, J.; Brabb, T.; Hsu, C.C.; Grady, W.M.; Maggio-Price, L. Effect of Chronic Vitamin D Deficiency on the Development and Severity of DSS-Induced Colon Cancer in Smad3-/- Mice. Comp. Med. 2020, 70, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.A.; Lee, J.; Oh, J.H.; Chang, H.J.; Sohn, D.K.; Shin, A.; Kim, J. Vitamin D receptor FokI polymorphism and the risks of colorectal cancer, inflammatory bowel disease, and colorectal adenoma. Sci. Rep. 2018, 8, 12899. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, C.S.; Baptistella, M.M.; Siqueira, A.P.; Carvalho, M.O.; Ramos, L.F.; Souto, B.S.; de Almeida, L.A.; Dos Santos, E.G.; Novaes, R.D.; Nogueira, E.S.C.; et al. Combination of vitamin D and probiotics inhibits chemically induced colorectal carcinogenesis in Wistar rats. Life Sci. 2023, 322, 121617. [Google Scholar] [CrossRef]
- Chen, D.; Tang, H.; Li, Y.; Yang, H.; Wang, H.; Tan, B.; Qian, J. Vitamin D3 and Lactobacillus rhamnosus GG/p40 Synergize to Protect Mice From Colitis by Promoting Vitamin D Receptor Expression and Epithelial Proliferation. Inflamm. Bowel Dis. 2023, 29, 620–632. [Google Scholar] [CrossRef]
- Castagliuolo, I.; Scarpa, M.; Brun, P.; Bernabe, G.; Sagheddu, V.; Elli, M.; Fiore, W.; De Vitis, V.; Guglielmetti, S. Co-administration of vitamin D3 and Lacticaseibacillus paracasei DG increase 25-hydroxyvitamin D serum levels in mice. Ann. Microbiol. 2021, 71, 42. [Google Scholar] [CrossRef]
- Costanzo, M.; Cesi, V.; Palone, F.; Pierdomenico, M.; Colantoni, E.; Leter, B.; Vitali, R.; Negroni, A.; Cucchiara, S.; Stronati, L. Krill oil, vitamin D and Lactobacillus reuteri cooperate to reduce gut inflammation. Benef. Microbes 2018, 9, 389–399. [Google Scholar] [CrossRef]
- Fan, X.; Yin, J.; Yin, J.; Weng, X.; Ding, R. Comparison of the anti-inflammatory effects of vitamin E and vitamin D on a rat model of dextran sulfate sodium-induced ulcerative colitis. Exp. Ther. Med. 2023, 25, 98. [Google Scholar] [CrossRef]
- Farhana, L.; Sarkar, S.; Nangia-Makker, P.; Yu, Y.; Khosla, P.; Levi, E.; Azmi, A.; Majumdar, A.P.N. Natural agents inhibit colon cancer cell proliferation and alter microbial diversity in mice. PLoS ONE 2020, 15, e0229823. [Google Scholar] [CrossRef]
- Liu, K.Y.; Wang, Q.; Nakatsu, C.H.; Jones-Hall, Y.; Jiang, Q. Combining gamma-tocopherol and aspirin synergistically suppresses colitis-associated colon tumorigenesis and modulates the gut microbiota in mice, and inhibits the growth of human colon cancer cells. Eur. J. Pharmacol. 2023, 946, 175656. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, Y.; Im, S.; Nakatsu, C.; Jones-Hall, Y.; Jiang, Q. Vitamin E delta-tocotrienol and metabolite 13’-carboxychromanol inhibit colitis-associated colon tumorigenesis and modulate gut microbiota in mice. J. Nutr. Biochem. 2021, 89, 108567. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.Y.; Nakatsu, C.H.; Jones-Hall, Y.; Kozik, A.; Jiang, Q. Vitamin E alpha- and gamma-tocopherol mitigate colitis, protect intestinal barrier function and modulate the gut microbiota in mice. Free Radic. Biol. Med. 2021, 163, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Mahid, S.S.; Minor, K.S.; Soto, R.E.; Hornung, C.A.; Galandiuk, S. Smoking and inflammatory bowel disease: A meta-analysis. Mayo Clin. Proc. 2006, 81, 1462–1471. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef]
- Bastida, G.; Beltrán, B. Ulcerative colitis in smokers, non-smokers and ex-smokers. World J. Gastroenterol. 2011, 17, 2740–2747. [Google Scholar] [CrossRef]
- Dignass, A.; Eliakim, R.; Magro, F.; Maaser, C.; Chowers, Y.; Geboes, K.; Mantzaris, G.; Reinisch, W.; Colombel, J.-F.; Vermeire, S.; et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: Definitions and diagnosis. J. Crohn’s Colitis 2012, 6, 965–990. [Google Scholar] [CrossRef]
- Botteri, E.; Borroni, E.; Sloan, E.K.; Bagnardi, V.; Bosetti, C.; Peveri, G.; Santucci, C.; Specchia, C.; van den Brandt, P.; Gallus, S.; et al. Smoking and Colorectal Cancer Risk, Overall and by Molecular Subtypes: A Meta-Analysis. Am. J. Gastroenterol. 2020, 115, 1940–1949. [Google Scholar] [CrossRef]
- Tomoda, K.; Kubo, K.; Asahara, T.; Andoh, A.; Nomoto, K.; Nishii, Y.; Yamamoto, Y.; Yoshikawa, M.; Kimura, H. Cigarette smoke decreases organic acids levels and population of bifidobacterium in the caecum of rats. J. Toxicol. Sci. 2011, 36, 261–266. [Google Scholar] [CrossRef]
- Hu, J.; Wei, T.; Sun, S.; Zhao, A.; Xu, C. Effects of cigarette smoke condensate on the production and characterization of exopolysaccharides by Bifidobacterium. An. Da Acad. Bras. De Cienc. 2015, 87, 997–1005. [Google Scholar] [CrossRef]
- Benjamin, J.L.; Hedin, C.R.H.; Koutsoumpas, A.; Ng, S.C.; McCarthy, N.E.; Prescott, N.J.; Pessoa-Lopes, P.; Mathew, C.G.; Sanderson, J.; Hart, A.L.; et al. Smokers with active Crohn’s disease have a clinically relevant dysbiosis of the gastrointestinal microbiota. Inflamm. Bowel Dis. 2012, 18, 1092–1100. [Google Scholar] [CrossRef]
- Leite, G.; Barlow, G.M.; Hosseini, A.; Parodi, G.; Pimentel, M.L.; Wang, J.; Fiorentino, A.; Rezaie, A.; Pimentel, M.; Mathur, R. Smoking has disruptive effects on the small bowel luminal microbiome. Sci. Rep. 2022, 12, 6231. [Google Scholar] [CrossRef]
- Opstelten, J.L.; Plassais, J.; van Mil, S.W.C.; Achouri, E.; Pichaud, M.; Siersema, P.D.; Oldenburg, B.; Cervino, A.C.L. Gut Microbial Diversity Is Reduced in Smokers with Crohn’s Disease. Inflamm. Bowel Dis. 2016, 22, 2070–2077. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Wei, H.; Liu, W.; Coker, O.O.; Gou, H.; Liu, C.; Zhao, L.; Li, C.; Zhou, Y.; Wang, G.; et al. Cigarette smoke promotes colorectal cancer through modulation of gut microbiota and related metabolites. Gut 2022, 71, 2439–2450. [Google Scholar] [CrossRef]
- Lo Sasso, G.; Phillips, B.W.; Sewer, A.; Battey, J.N.D.; Kondylis, A.; Talikka, M.; Titz, B.; Guedj, E.; Peric, D.; Bornand, D.; et al. The reduction of DSS-induced colitis severity in mice exposed to cigarette smoke is linked to immune modulation and microbial shifts. Sci. Rep. 2020, 10, 3829. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, L.; Brülisauer, K.; Zeitz, J.; Frei, P.; Scharl, M.; Vavricka, S.R.; Fried, M.; Loessner, M.J.; Rogler, G.; Schuppler, M. Smoking cessation alters intestinal microbiota: Insights from quantitative investigations on human fecal samples using FISH. Inflamm. Bowel Dis. 2014, 20, 1496–1501. [Google Scholar] [CrossRef]
- Cosnes, J.; Beaugerie, L.; Carbonnel, F.; Gendre, J.P. Smoking cessation and the course of Crohn’s disease: An intervention study. Gastroenterology 2001, 120, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez-Mena, J.M.; Walter, V.; Schöttker, B.; Jenab, M.; O’Doherty, M.G.; Kee, F.; Bueno-de-Mesquita, B.; Peeters, P.H.M.; Stricker, B.H.; Ruiter, R.; et al. Impact of prediagnostic smoking and smoking cessation on colorectal cancer prognosis: A meta-analysis of individual patient data from cohorts within the CHANCES consortium. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, H.; Goldenberg, K.; Ratiner, K.; Elinav, E. Smoking-induced microbial dysbiosis in health and disease. Clin. Sci. 2022, 136, 1371–1387. [Google Scholar] [CrossRef]
| Molecular Pathway | Role on Carcinogenesis | Involved Bacteria on the Pathway |
|---|---|---|
| IL-17/IL-22 | Anti-carcinogenic | Promoted by Lactobacillus spp. [26,27,33,34,40] |
| TNFα | Pro-carcinogenic | Prevented by L. plantarum [53], B. Lactis [69], C. butyricum [74] Promoted or reduced by A. muciniphila [105,106] (?) |
| IL-1β | Pro-carcinogenic | Prevented by L. plantarum [53] Promoted or reduced by A. muciniphila [105,106] (?) |
| IL-6 | Pro-carcinogenic | Prevented by L. plantarum [53], B. Lactis [69], C. butyricum [74] Promoted or reduced by A. muciniphila [105,106] (?) |
| TLR-2/TLR-9 | Anti-carcinogenic | Promoted by Bifidobacteria spp. [64,65] |
| NF-kB | Pro-carcinogenic | Prevented by C. butyricum [74] Promoted by ETBF [84] |
| WNT/β catenin | Pro-carcinogenic | Promoted by ETBF [84] Promoted by F. nucleatum [89] |
| MAPK | Pro-carcinogenic | Promoted by ETBF [84] |
| NLRP3 | Pro-carcinogenic | Prevented by B. fragilis [77] |
| PD1-PDL1 | Pro-carcinogenic | Promoted or reduced by F. nucleatum [96,98] (?) |
| Environmental Factor | Involved Molecular Pathways | Involved Bacteria | Role on Carcinogenesis |
|---|---|---|---|
| Diabetes Mellitus II/Obesity | Increased IGF-1 levels Lower adiponectin and resistin levels Higher leptin levels Fatty Acid Synthase overexpression Increased TNF alfa Increased IL-1B Increased NOX4 Enhanced NF-kB activation | Increased Firmicutes/Bacteroides ratio Increased Enterobacteriacea, Increased Actinobacteria, Reduced F. prausnitzii, Clostridia, Verrucomicrobia | Pro-carcinogenic |
| Red Meat | Heme compounds N-nitroso compounds Heterocyclic aromatic amines Undigested proteins | Increased Bacteroidetes, Proteobacteria, Alistipes Reduced Firmicutes, Lachnospiraceae, Faecalibaculum, Blautia, Dubosiella | Pro-carcinogenic |
| Smoking | Altered gut barrier function Activation of MAPK/ERK signalling Higher IL-17 and TNF levels | Increased Bacteroides-Prevotella, Escherichia, Shigella, Klebsiella, Lactobacillus, Eggerthella Lenta, Akkermansia, Intestinomonas Reduced Bifidobacteria, Prevotella, Neisseria, Parabacteroides distasonis, Alistipes | Pro-carcinogenic |
| Vitamin A | Protective: Increased IL-10 T cells differentiation in T reg cells Reduced IL-6 levels Pro-inflammatory: increased IL-17, IFN-γ Reduced anti-tumour action CD8+ T cells, reduced Il-10 | Increased Akkermansia, Prevotella, Lactobacillus Reduced Bacteroides, Escherichia, Shigella | Debated |
| Vitamin D | Downregulation of Th1 and Th17 Increased gut barrier function Reduced TNF-α, increased IL-10 Interference WNT/b catenin pathway | Increased Actinobacteria, Prevotella Reduced Bifidobacterium | Anti-carcinogenic |
| Vitamin E | Reduced IL-1β GM-CSF and MCP-1 inhibition Increased gut barrier function | Increased Lactococcus lactis subsp, Cremoris, Bacteroides fragilis, Roseburia Reduced Firmicutes/bacteroides ratio | Anti-carcinogenic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mignini, I.; Ainora, M.E.; Di Francesco, S.; Galasso, L.; Gasbarrini, A.; Zocco, M.A. Tumorigenesis in Inflammatory Bowel Disease: Microbiota-Environment Interconnections. Cancers 2023, 15, 3200. https://doi.org/10.3390/cancers15123200
Mignini I, Ainora ME, Di Francesco S, Galasso L, Gasbarrini A, Zocco MA. Tumorigenesis in Inflammatory Bowel Disease: Microbiota-Environment Interconnections. Cancers. 2023; 15(12):3200. https://doi.org/10.3390/cancers15123200
Chicago/Turabian StyleMignini, Irene, Maria Elena Ainora, Silvino Di Francesco, Linda Galasso, Antonio Gasbarrini, and Maria Assunta Zocco. 2023. "Tumorigenesis in Inflammatory Bowel Disease: Microbiota-Environment Interconnections" Cancers 15, no. 12: 3200. https://doi.org/10.3390/cancers15123200
APA StyleMignini, I., Ainora, M. E., Di Francesco, S., Galasso, L., Gasbarrini, A., & Zocco, M. A. (2023). Tumorigenesis in Inflammatory Bowel Disease: Microbiota-Environment Interconnections. Cancers, 15(12), 3200. https://doi.org/10.3390/cancers15123200







