Spatial Heterogeneity in Cytoskeletal Mechanics Response to TGF-β1 and Hypoxia Mediates Partial Epithelial-to-Mesenchymal Transition in Epithelial Ovarian Cancer Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. OVCAR3 Cell Culture
2.3. TGF-β stock preparation
2.4. Polyacrylamide Substrate Synthesis (Soft and Hard)
2.5. TGF-β and Hypoxia Treatment
2.6. Cytoskeletal Inhibitors
2.7. Immunofluorescence staining
2.8. Cell Cluster Analysis
2.9. Cluster Cell and Nuclear Shape Factor Analysis
2.10. CellProfiler and MATLAB analysis
2.11. Statistics
3. Results
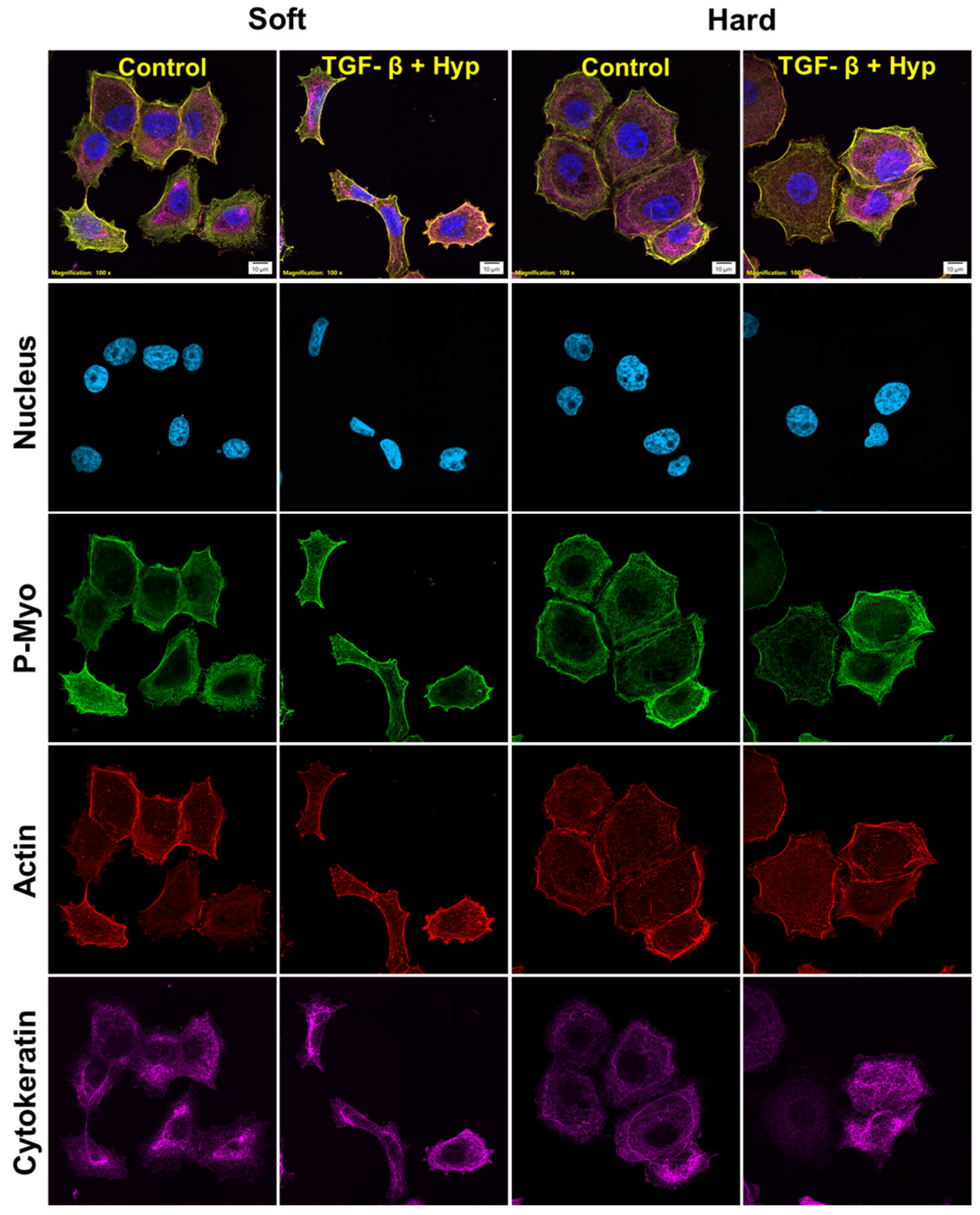
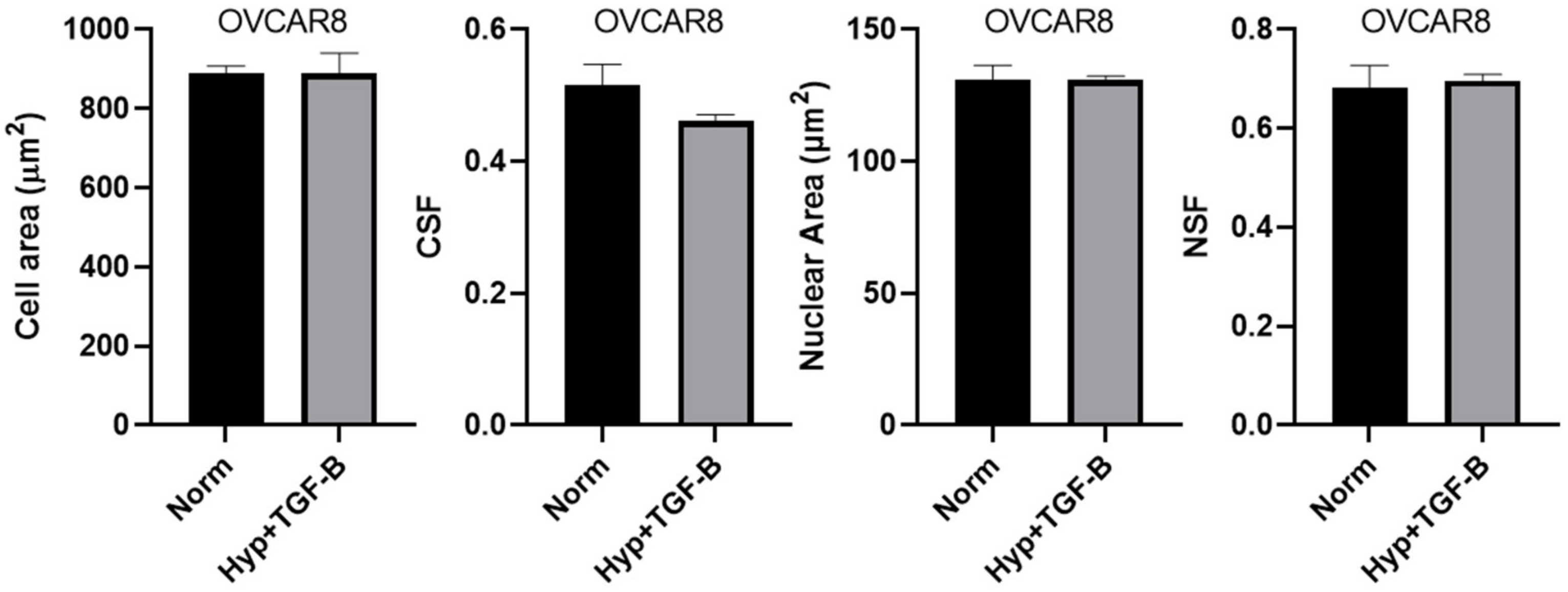
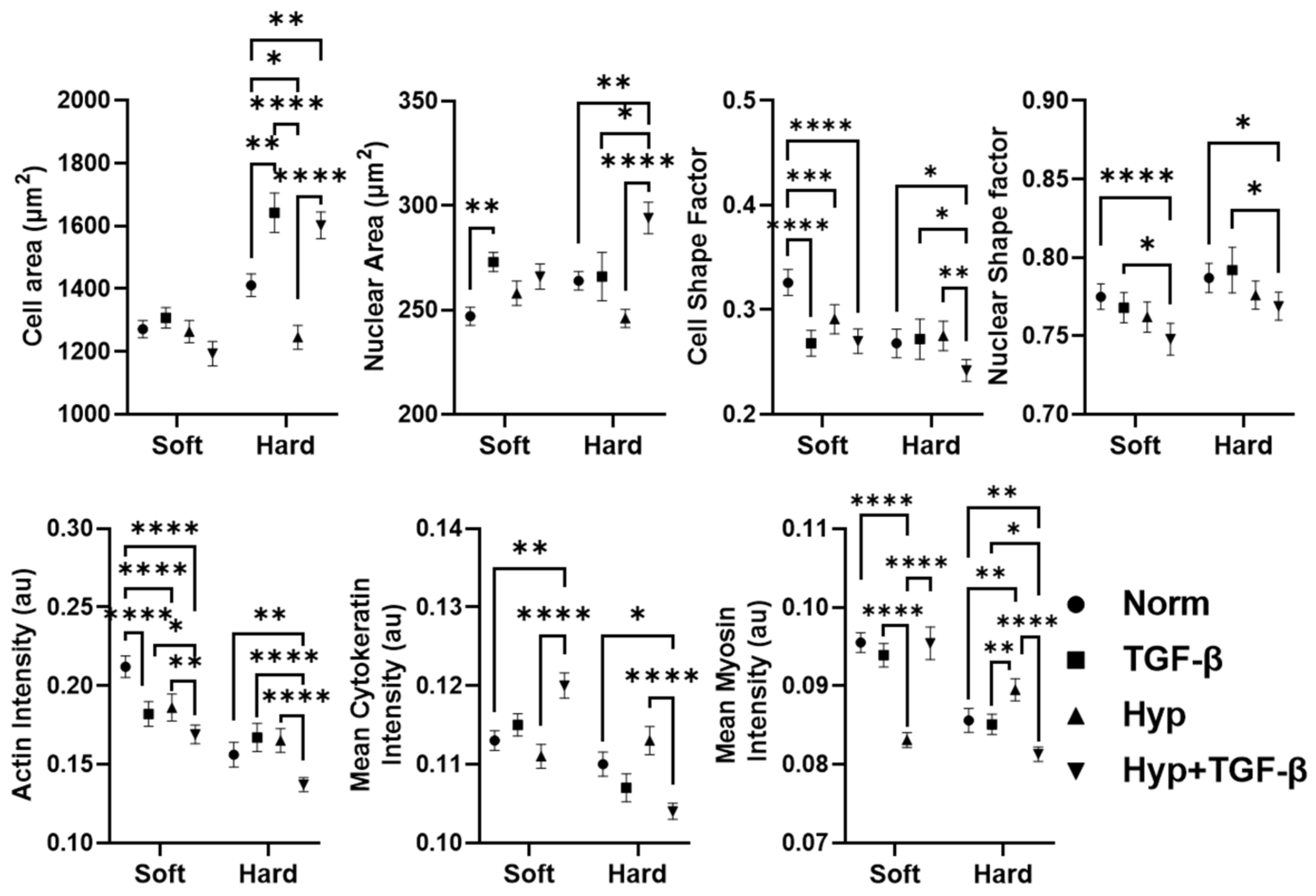
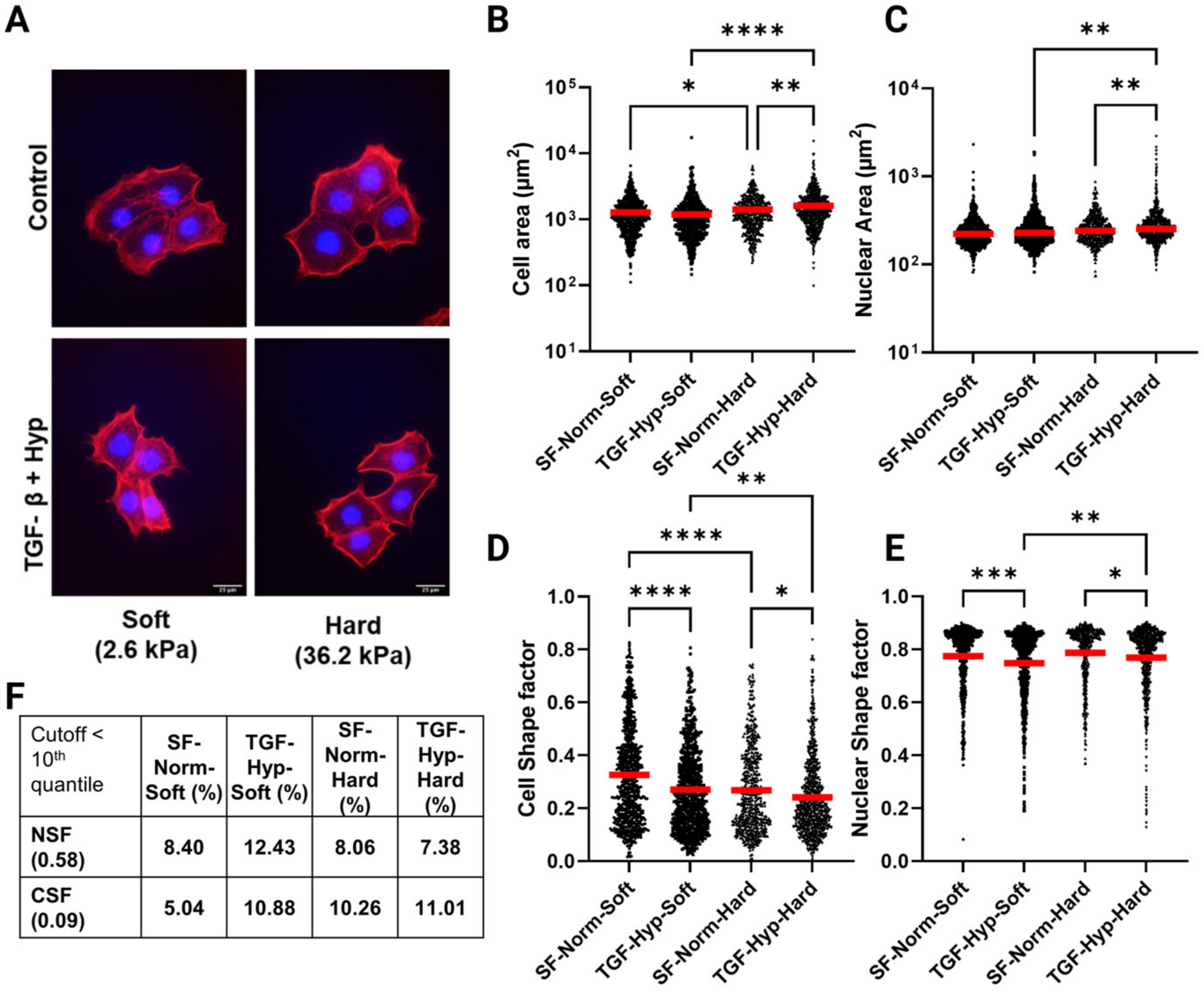
3.1. OVCAR3 Cells Exhibit Altered Cellular and Nuclear Morphologies under Mechanical and Chemical Stimuli
3.2. Reduced Size of OVCAR3 Cell Clusters under Mechanical and Chemical Stimuli
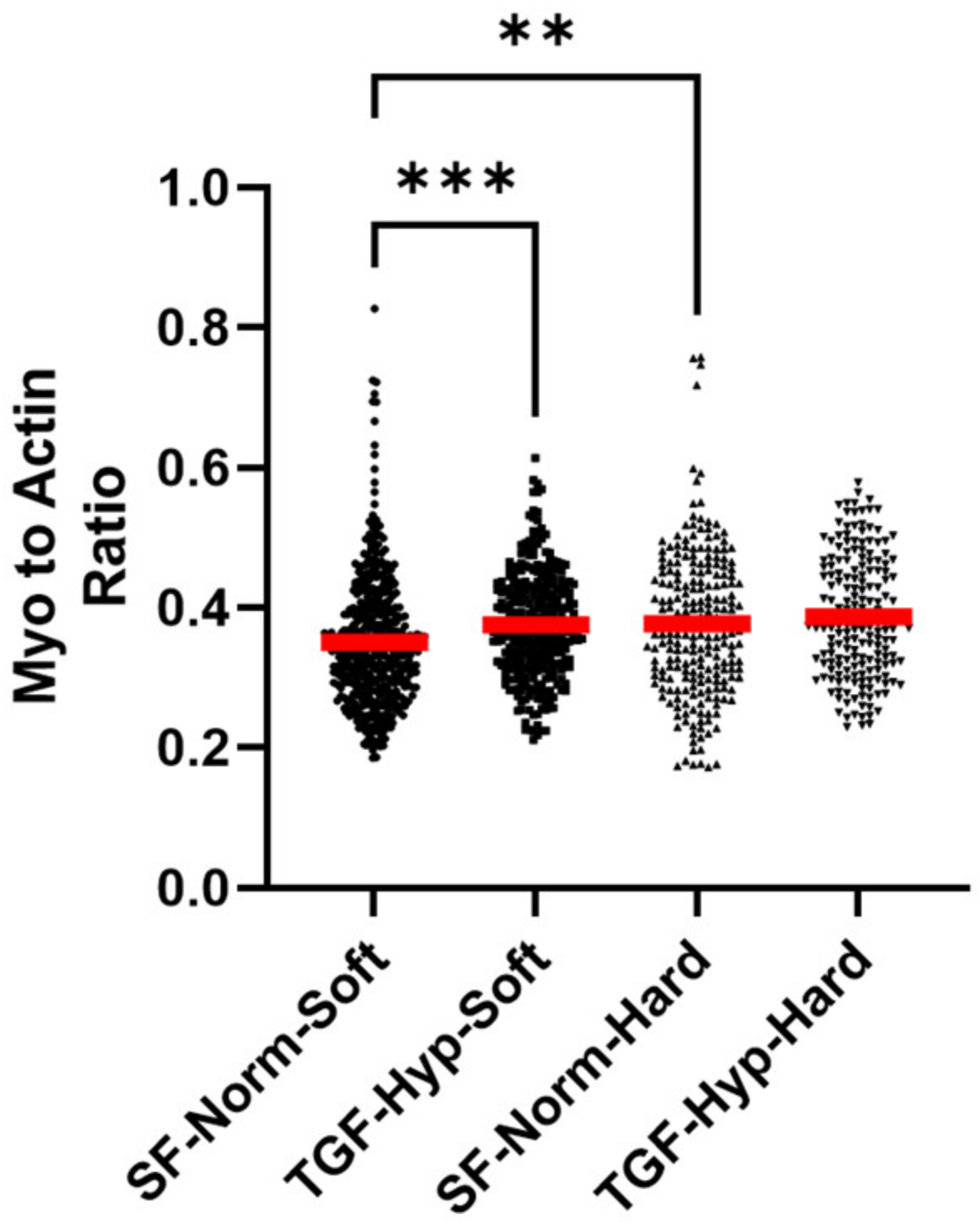
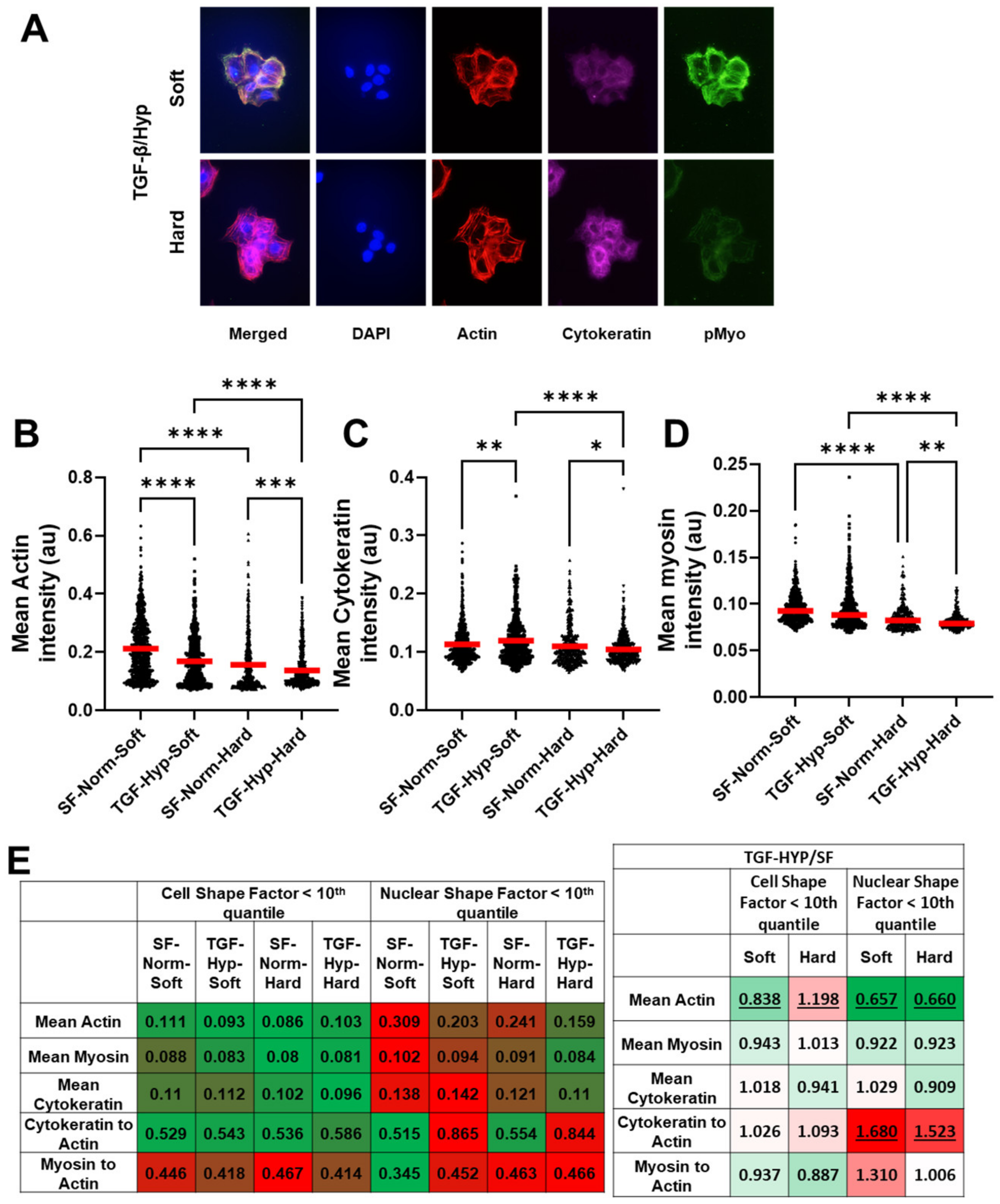
3.3. Heterogeneity in Cytoskeletal Protein Expression
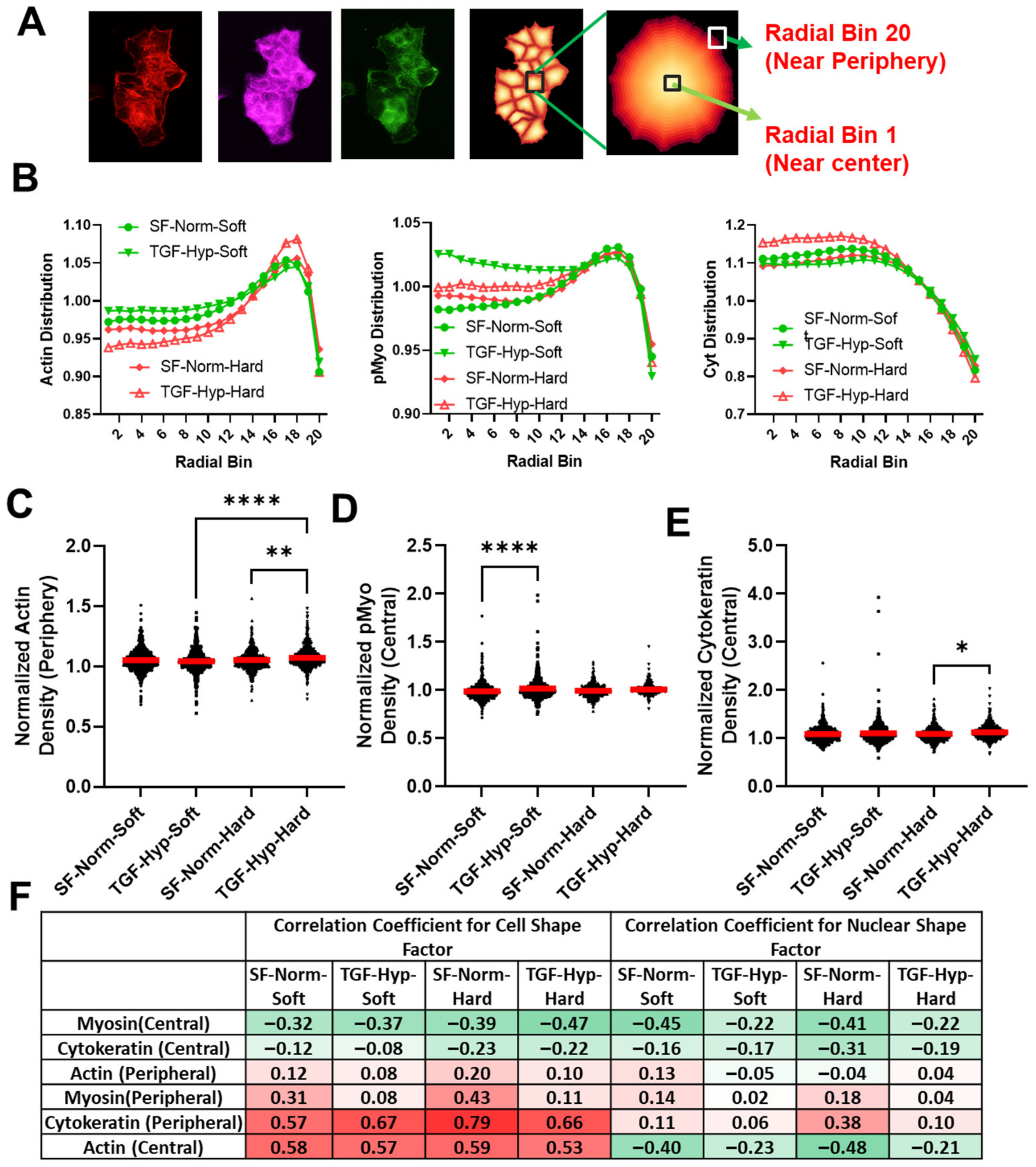
3.4. Radial Distribution in Cytoskeletal Protein Expression
3.5. Morphological Differences in Cells with High and Low Cytoskeletal Protein Expression
3.6. Cytoskeletal Inhibitors Alter Morphological Response to TGF-β1 and Hypoxia Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Methods
Appendix A.1. Cell Culture
Appendix A.2. Mechanical Testing of Polyacrylamide Gels
| Young’s Modulus | Final Acrylamide Concentration | Final Bis-Acrylamide Concentration |
|---|---|---|
| 2.6 kPa (Soft) | 10% | 0.03% |
| 36.2 kPa (Hard) | 10% | 0.30% |
Appendix A.3. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
| Marker | Forward Sequence | Reverse Sequence | |
|---|---|---|---|
| Epithelial Markers | E-Cadherin (ecad) | CTCGTAACGACGTTGCACC | CTGTGGGGTCAGTATCAGCC |
| Cytokeratin 14 (krt14) | GGAGGAGATGAATGCCCTGA | GTGAAGAACCATTCCTCGGC | |
| Cytokeratin 19 (krt19) | CGCGACTACAGCCACTACTA | AGAGCCTGTTCCGTCTCAAA | |
| Mesenchymal Markers | N-Cadherin (ncad) | CCATCAAGCCTGTGGGAATC | CTGTGGGGTCATTGTCAGCC |
| Snail (snail) | ACCACTATGCCGCGCTCTT | GGTCGTAGGGCTGCTGGAA | |
| Vimentin (vim) | CTGGATTCACTCCCTCTGGTTG | GGTCATCGTGATGCTGAGAAG | |
| ZEB1 (zeb1) | TTCAAACCCATAGTGGTTGCT | TGGGAGATACCAAACCAACTG | |
| Control | 18s rRNA | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG |
Appendix A.4. Semi-Automated Analysis of OVCAR3 Cell Images with CellProfiler
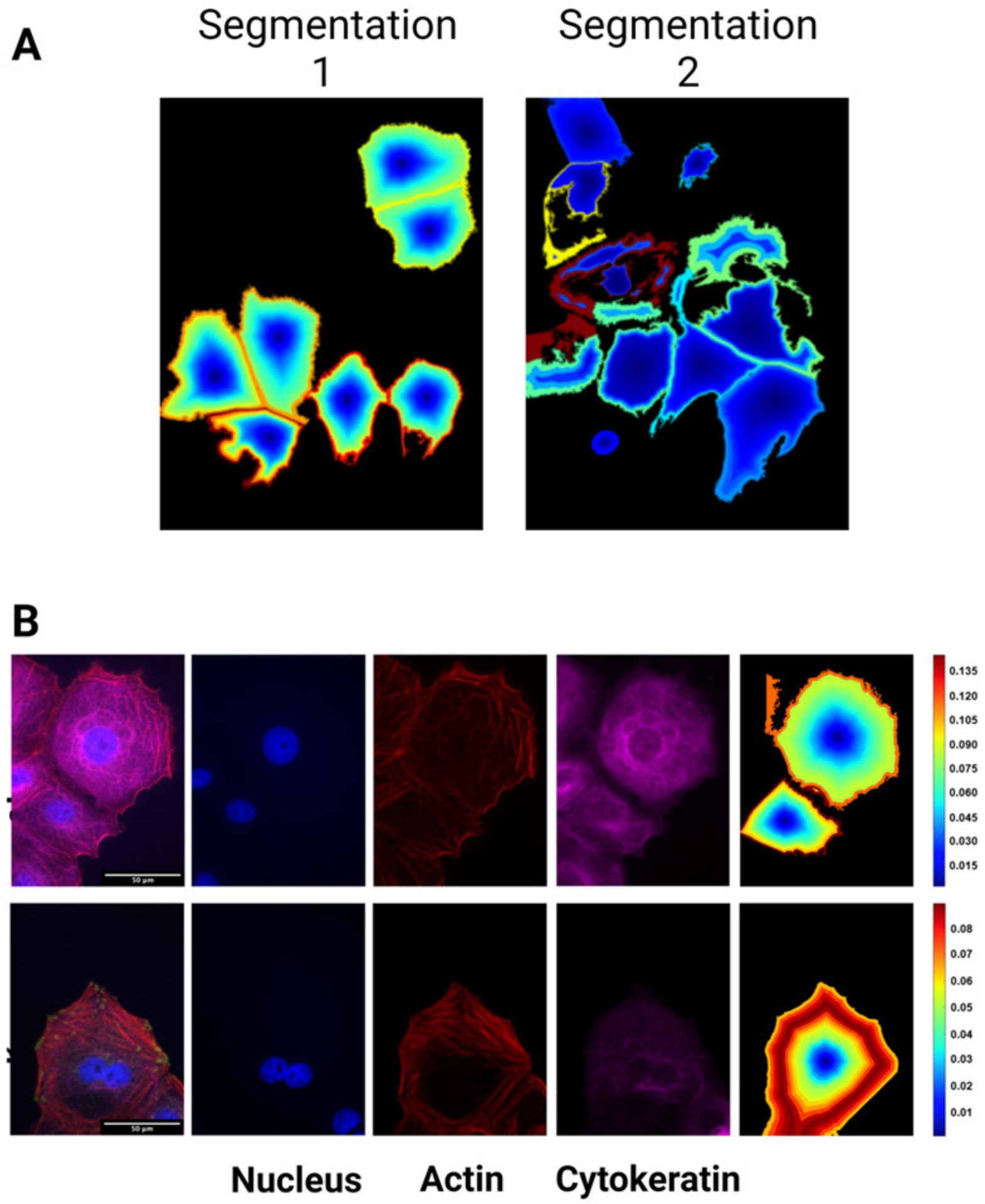
Appendix B. Data

| SF-Norm-Soft | TGF-Hyp-Soft | SF-Norm-Hard | TGF-Hyp-Hard | |
|---|---|---|---|---|
| NSF | ||||
| CV_All | 0.15 | 0.18 | 0.14 | 0.16 |
| CV_50 | 0.17 | 0.20 | 0.16 | 0.19 |
| CV_10 | 0.16 | 0.25 | 0.11 | 0.31 |
| CSF | ||||
| CV_All | 0.56 | 0.59 | 0.60 | 0.61 |
| CV_50 | 0.33 | 0.37 | 0.36 | 0.35 |
| CV_10 | 0.34 | 0.28 | 0.29 | 0.29 |
| SF-Norm-Soft | TGF-Hyp-Soft | SF-Norm-Hard | TGF-Hyp-Hard | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | CV | Mean | CV | Mean | CV | Mean | CV | |
| Mean Actin | 0.212 | 47.12 | 0.169 | 47.46 | 0.156 | 59.84 | 0.137 | 45.44 |
| Mean Myosin | 0.095 | 16.82 | 0.095 | 23.56 | 0.086 | 16.04 | 0.081 | 10.62 |
| Mean Cytokeratin | 0.113 | 28.73 | 0.120 | 32.03 | 0.110 | 30.07 | 0.104 | 23.71 |
| CV | Cell Shape Factor < 10th Quantile | Nuclear Shape Factor < 10th Quantile | ||||||
|---|---|---|---|---|---|---|---|---|
| SF-Norm-Soft | TGF-Hyp-Soft | SF-Norm-Hard | TGF-Hyp-Hard | SF-Norm-Soft | TGF-Hyp-Soft | SF-Norm-Hard | TGF-Hyp-Hard | |
| Mean Actin | 32.85 | 24.32 | 15.00 | 28.10 | 41.20 | 41.44 | 52.80 | 46.18 |
| Mean Myosin | 16.26 | 9.97 | 13.67 | 12.61 | 15.19 | 19.15 | 14.48 | 10.75 |
| Mean Cytokeratin | 31.11 | 33.32 | 37.14 | 16.37 | 25.85 | 39.50 | 39.54 | 22.68 |
| Cytokeratin to Actin | 26.58 | 28.37 | 29.67 | 40.93 | 58.23 | 74.1 | 61.85 | 42.65 |
| Myosin to Actin | 28.49 | 19.04 | 29.18 | 16.95 | 67.4 | 45.51 | 62.38 | 31.78 |
| CV | TGF-Hyp-Soft | TGF-Hyp-Hard | ||
|---|---|---|---|---|
| DMSO | H1152 | DMSO | H1152 | |
| Cell shape factor | 54.08 | 68.63 | 56.60 | 75.70 |
| Nuclear shape factor | 24.87 | 17.64 | 20.92 | 14.98 |
| Mean Actin | 30.02 | 17.11 | 22.39 | 18.90 |
| Mean Cytokeratin | 33.19 | 30.39 | 33.13 | 31.18 |
| Mean Myosin | 9.60 | 4.06 | 7.314 | 5.00 |
| Cytokeratin to Actin | 28.73 | 28.20 | 32.75 | 32.15 |
| Myosin to Actin | 21.89 | 11.72 | 17.98 | 12.84 |
| Two-Way Analysis with Four Groups | Interaction | Row Factor (Substrate Stiffness) | Column Factor (SF/TGF-β ± Hyp) |
|---|---|---|---|
| NSF | 0.62 (ns) | <0.0001 (****) | <0.0001 (****) |
| Nuclear Area | 0.0016 (**) | 0.1393 (ns) | <0.0001 (****) |
| CSF | 0.0002 (***) | <0.0001 (****) | <0.0001 (****) |
| Cell Area | <0.0001 (****) | <0.0001 (****) | <0.0001 (****) |
| Actin | <0.0001 (****) | <0.0001(****) | <0.0001 (****) |
| Cytokeratin | <0.0001 (****) | <0.0001 (****) | 0.9187 (ns) |
| Myosin | <0.0001 (****) | <0.0001 (****) | <0.0001 (****) |
| Two-way Analysis with Two Groups | Interaction | Row Factor (Substrate Stiffness) | Column Factor (SF/TGF-β + Hyp) |
| NSF | 0.3396 (ns) | <0.0005 (***) | <0.0001 (****) |
| Nuclear Area | 0.3520 (ns) | <0.0001 (****) | <0.0001 (****) |
| CSF | 0.0162 (*) | <0.0001 (****) | <0.0001 (****) |
| Cell Area | <0.0003 (***) | <0.0001 (****) | 0.1270 (ns) |
| Actin | <0.0002 (***) | <0.0001 (****) | <0.0001 (****) |
| Cytokeratin | <0.0001 (****) | <0.0001(****) | 0.7150 (ns) |
| Myosin | <0.0111 (*) | <0.0001 (****) | <0.0078 (**) |
References
- Landen, C.N.; Birrer, M.J.; Sood, A.K. Early Events in the Pathogenesis of Epithelial Ovarian Cancer. J. Clin. Oncol. 2008, 26, 995–1005. [Google Scholar] [CrossRef]
- Masoodi, T.; Siraj, S.; Siraj, A.K.; Azam, S.; Qadri, Z.; Parvathareddy, S.K.; Tulbah, A.; Al-Dayel, F.; AlHusaini, H.; AlOmar, O.; et al. Genetic Heterogeneity and Evolutionary History of High-Grade Ovarian Carcinoma and Matched Distant Metastases. Br. J. Cancer 2020, 122, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Winterhoff, B.J.; Maile, M.; Mitra, A.K.; Sebe, A.; Bazzaro, M.; Geller, M.A.; Abrahante, J.E.; Klein, M.; Hellweg, R.; Mullany, S.A.; et al. Single Cell Sequencing Reveals Heterogeneity within Ovarian Cancer Epithelium and Cancer Associated Stromal Cells. Gynecol. Oncol. 2017, 144, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.H.; Phillip, J.M.; Khatau, S.B.; Chen, W.C.; Stirman, J.; Rosseel, S.; Tschudi, K.; Van Patten, J.; Wong, M.; Gupta, S.; et al. Evolution of Cellular Morpho-Phenotypes in Cancer Metastasis. Sci. Rep. 2015, 5, 18437. [Google Scholar] [CrossRef] [PubMed]
- Jayatilaka, H.; Tyle, P.; Chen, J.J.; Kwak, M.; Ju, J.; Kim, H.J.; Lee, J.S.H.; Wu, P.H.; Gilkes, D.M.; Fan, R.; et al. Synergistic IL-6 and IL-8 Paracrine Signalling Pathway Infers a Strategy to Inhibit Tumour Cell Migration. Nat. Commun. 2017, 8, 15584. [Google Scholar] [CrossRef]
- Wirtz, D.; Konstantopoulos, K.; Searson, P.C. The Physics of Cancer: The Role of Physical Interactions and Mechanical Forces in Metastasis. Nat. Rev. Cancer 2011, 11, 522. [Google Scholar] [CrossRef] [PubMed]
- Jonietz, E. Mechanics: The Forces of Cancer. Nature 2012, 491, S56–S57. [Google Scholar] [CrossRef]
- Kumar, S.; Weaver, V.M. Mechanics, Malignancy, and Metastasis: The Force Journey of a Tumor Cell. Cancer Metastasis Rev. 2009, 28, 113–127. [Google Scholar] [CrossRef]
- DuFort, C.C.; Paszek, M.J.; Weaver, V.M. Balancing Forces: Architectural Control of Mechanotransduction. Nat. Rev. Mol. Cell Biol. 2011, 12, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Iskratsch, T.; Wolfenson, H.; Sheetz, M.P. Appreciating Force and Shape—The Rise of Mechanotransduction in Cell Biology. Nat. Rev. Mol. Cell Biol. 2014, 15, 825–833. [Google Scholar] [CrossRef]
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D.; et al. Tensional Homeostasis and the Malignant Phenotype. Cancer Cell 2005, 8, 241–254. [Google Scholar] [CrossRef]
- McGrail, D.J.; Kieu, Q.M.N.; Dawson, M.R. The Malignancy of Metastatic Ovarian Cancer Cells Is Increased on Soft Matrices through a Mechanosensitive Rho-ROCK Pathway. J. Cell Sci. 2014, 127, 2621–2626. [Google Scholar] [CrossRef] [PubMed]
- McGrail, D.J.; Kieu, Q.M.N.; Iandoli, J.A.; Dawson, M.R. Actomyosin Tension as a Determinant of Metastatic Cancer Mechanical Tropism. Phys. Biol. 2015, 12, 026001. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P. Epithelial-Mesenchymal Transitions in Tumour Progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The Basics of Epithelial-Mesenchymal Transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Nalluri, S.M.; O’Connor, J.W.; Gomez, E.W. Cytoskeletal Signaling in TGFβ-Induced Epithelial-Mesenchymal Transition. Cytoskeleton 2015, 72, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Christofori, G. EMT, the Cytoskeleton, and Cancer Cell Invasion. Cancer Metastasis Rev. 2009, 28, 15–33. [Google Scholar] [CrossRef]
- Sun, B.; Fang, Y.; Li, Z.; Chen, Z.; Xiang, J. Role of Cellular Cytoskeleton in Epithelial-Mesenchymal Transition Process during Cancer Progression. Biomed. Rep. 2015, 3, 603. [Google Scholar] [CrossRef]
- Alibert, C.; Goud, B.; Manneville, J.B. Are Cancer Cells Really Softer than Normal Cells? Biol. Cell 2017, 109, 167–189. [Google Scholar] [CrossRef]
- Lin, H.-H.; Lin, H.-K.; Lin, I.-H.; Chiou, Y.-W.; Chen, H.-W.; Liu, C.-Y.; Harn, H.I.-C.; Chiu, W.-T.; Wang, Y.-K.; Shen, M.-R.; et al. Mechanical Phenotype of Cancer Cells: Cell Softening and Loss of Stiffness Sensing. Oncotarget 2015, 6, 20946–20958. [Google Scholar] [CrossRef] [PubMed]
- Oswald, L.; Grosser, S.; Smith, D.M.; Käs, J.A. Jamming Transitions in Cancer. J. Phys. D Appl. Phys. 2017, 50, 483001. [Google Scholar] [CrossRef] [PubMed]
- Haeger, A.; Krause, M.; Wolf, K.; Friedl, P. Cell Jamming: Collective Invasion of Mesenchymal Tumor Cells Imposed by Tissue Confinement. Biochim. Biophys. Acta-Gen. Subj. 2014, 1840, 2386–2395. [Google Scholar] [CrossRef] [PubMed]
- La Porta, C.A.M.; Zapperi, S. Phase Transitions in Cell Migration. Nat. Rev. Phys. 2020, 2, 516–517. [Google Scholar] [CrossRef]
- Saxena, K.; Jolly, M.K.; Balamurugan, K. Hypoxia, Partial EMT and Collective Migration: Emerging Culprits in Metastasis. Transl. Oncol. 2020, 13, 100845. [Google Scholar] [CrossRef]
- Kröger, C.; Afeyan, A.; Mraz, J.; Eaton, E.N.; Reinhardt, F.; Khodor, Y.L.; Thiru, P.; Bierie, B.; Ye, X.; Burge, C.B.; et al. Acquisition of a Hybrid E/M State Is Essential for Tumorigenicity of Basal Breast Cancer Cells. Proc. Natl. Acad. Sci. USA 2019, 116, 7353–7362. [Google Scholar] [CrossRef]
- Liao, T.-T.; Yang, M.-H. Hybrid Epithelial/Mesenchymal State in Cancer Metastasis: Clinical Significance and Regulatory Mechanisms. Cells 2020, 9, 623. [Google Scholar] [CrossRef] [PubMed]
- Klymenko, Y.; Johnson, J.; Bos, B.; Lombard, R.; Campbell, L.; Loughran, E.; Stack, M.S. Heterogeneous Cadherin Expression and Multicellular Aggregate Dynamics in Ovarian Cancer Dissemination. Neoplasia 2017, 19, 549. [Google Scholar] [CrossRef]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-Β-Induced Epithelial to Mesenchymal Transition. Cell Res. 2009, 19, 2767. [Google Scholar] [CrossRef] [PubMed]
- Wendt, M.K.; Allington, T.M.; Schiemann, W.P. Mechanisms of the Epithelial–Mesenchymal Transition by TGF-β. Future Oncol. 2009, 5, 1145–1168. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.; Glowacka, E.; Szpakowski, M.; Szyllo, K.; Malinowski, A.; Kulig, A.; Tchorzewski, H.; Wilczynski, J. Proinflammatory and Immunosuppressive Serum, Ascites and Cyst Fluid Cytokines in Patients with Early and Advanced Ovarian Cancer and Benign Ovarian Tumors. Neuroendocr. Lett 2010, 31, 375–383. [Google Scholar]
- Papageorgis, P.; Stylianopoulos, T. Role of TGFβ in Regulation of the Tumor Microenvironment and Drug Delivery (Review). Int. J. Oncol. 2015, 46, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Zhang, Y.E. Smad-Dependent and Smad-Independent Pathways in TGF-β Family Signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef]
- Boyle, S.T.; Samuel, M.S. Mechano-Reciprocity Is Maintained between Physiological Boundaries by Tuning Signal Flux through the Rho-Associated Protein Kinase. Small GTPases 2016, 7, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, K.; Chen, Y.; Chen, H.; Nice, E.C.; Huang, C. Redox Regulation in Tumor Cell Epithelial–Mesenchymal Transition: Molecular Basis and Therapeutic Strategy. Signal Transduct. Target. Ther. 2017, 2, 17036. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, R.; Chatterjee, J. ROS and Oncogenesis with Special Reference to EMT and Stemness. Eur. J. Cell Biol. 2020, 99, 151073. [Google Scholar] [CrossRef] [PubMed]
- Cannito, S.; Novo, E.; Di Bonzo, L.V.; Busletta, C.; Colombatto, S.; Parola, M. Epithelial-Mesenchymal Transition: From Molecular Mechanisms, Redox Regulation to Implications in Human Health and Disease. Antioxid. Redox Signal. 2010, 12, 1383–1430. [Google Scholar] [CrossRef]
- Giannoni, E.; Parri, M.; Chiarugi, P. EMT and Oxidative Stress: A Bidirectional Interplay Affecting Tumor Malignancy. Antioxid. Redox Signal. 2012, 16, 1248–1263. [Google Scholar] [CrossRef] [PubMed]
- Mallikarjuna, P.; Raviprakash, T.S.; Aripaka, K.; Ljungberg, B.; Landström, M. Interactions between TGF-β Type I Receptor and Hypoxia-Inducible Factor-α Mediates a Synergistic Crosstalk Leading to Poor Prognosis for Patients with Clear Cell Renal Cell Carcinoma. Cell Cycle 2019, 18, 2141–2156. [Google Scholar] [CrossRef] [PubMed]
- Tam, S.Y.; Wu, V.W.C.; Law, H.K.W. Hypoxia-Induced Epithelial-Mesenchymal Transition in Cancers: HIF-1α and Beyond. Front. Oncol. 2020, 10, 486. [Google Scholar] [CrossRef]
- Tse, J.R.; Engler, A.J. Preparation of Hydrogel Substrates with Tunable Mechanical Properties. Curr. Protoc. Cell Biol 2010, 47, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Estabrook, I.D.; Thiam, H.R.; Piel, M.; Hawkins, R.J. Calculation of the Force Field Required for Nucleus Deformation during Cell Migration through Constrictions. PLOS Comput. Biol. 2021, 17, e1008592. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wen, J.; Xiao, Y.; Liu, J.; Hopyan, S.; Radisic, M.; Simmons, C.A.; Sun, Y. In Situ Mechanical Characterization of the Cell Nucleus by Atomic Force Microscopy. ACS Nano 2014, 8, 3821–3828. [Google Scholar] [CrossRef] [PubMed]
- Lammerding, J. Mechanics of the Nucleus. Compr. Physiol. 2011, 1, 783–807. [Google Scholar] [CrossRef]
- Choi, Y.P.; Shim, H.S.; Gao, M.-Q.; Kang, S.; Cho, N.H. Molecular Portraits of Intratumoral Heterogeneity in Human Ovarian Cancer. Cancer Lett. 2011, 307, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Kossaï, M.; Leary, A.; Scoazec, J.Y.; Genestie, C. Ovarian Cancer: A Heterogeneous Disease. Pathobiology 2018, 85, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian Cancer Statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, A.J.; Hicks, S.R.; Svec, K.V.; Naughton, H.; Edmunds, Z.L.; Howe, A.K. The Mechanical Microenvironment Regulates Ovarian Cancer Cell Morphology, Migration, and Spheroid Disaggregation. Sci. Rep. 2018, 8, 7228. [Google Scholar] [CrossRef] [PubMed]
- Strauss, R.; Li, Z.Y.; Liu, Y.; Beyer, I.; Persson, J.; Sova, P.; Möller, T.; Pesonen, S.; Hemminki, A.; Hamerlik, P.; et al. Analysis of Epithelial and Mesenchymal Markers in Ovarian Cancer Reveals Phenotypic Heterogeneity and Plasticity. PLoS ONE 2011, 6, e16186. [Google Scholar] [CrossRef]
- Kurman, R.J.; Shih, I.M. The Origin and Pathogenesis of Epithelial Ovarian Cancer: A Proposed Unifying Theory. Am. J. Surg. Pathol. 2010, 34, 433–443. [Google Scholar] [CrossRef]
- Weidle, U.H.; Birzele, F.; Kollmorgen, G.; Rueger, R. Mechanisms and Targets Involved in Dissemination of Ovarian Cancer. Cancer Genom. Proteom. 2016, 13, 407. [Google Scholar] [CrossRef]
- Thibault, B.; Castells, M.; Delord, J.P.; Couderc, B. Ovarian Cancer Microenvironment: Implications for Cancer Dissemination and Chemoresistance Acquisition. Cancer Metastasis Rev. 2014, 33, 17–39. [Google Scholar] [CrossRef]
- Yang, M.H.; Wu, M.Z.; Chiou, S.H.; Chen, P.M.; Chang, S.Y.; Liu, C.J.; Teng, S.C.; Wu, K.J. Direct Regulation of TWIST by HIF-1α Promotes Metastasis. Nat. Cell Biol. 2008, 10, 295–305. [Google Scholar] [CrossRef]
- Sánchez-Elsner, T.; Botella, L.M.; Velasco, B.; Corbí, A.; Attisano, L.; Bernabéu, C. Synergistic Cooperation between Hypoxia and Transforming Growth Factor-β Pathways on Human Vascular Endothelial Growth Factor Gene Expression. J. Biol. Chem. 2001, 276, 38527–38535. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, S.; Tanaka, F.; Morita, H.; Hiraki, A.; Hashimoto, S. Hypoxia-Induced HIF-1α and ZEB1 Are Critical for the Malignant Transformation of Ameloblastoma via TGF-β-Dependent EMT. Cancer Med. 2019, 8, 7822–7832. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhu, Y.; Nilsson, M.; Sundfeldt, K. TGF-β Isoforms Induce EMT Independent Migration of Ovarian Cancer Cells. Cancer Cell Int. 2014, 14, 72. [Google Scholar] [CrossRef] [PubMed]
- Haeger, A.; Wolf, K.; Zegers, M.M.; Friedl, P. Collective Cell Migration: Guidance Principles and Hierarchies. Trends Cell Biol. 2015, 25, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P.; Mayor, R. Tuning Collective Cell Migration by Cell–Cell Junction Regulation. Cold Spring Harb. Perspect. Biol. 2017, 9, a029199. [Google Scholar] [CrossRef]
- McGrail, D.J.; Ghosh, D.; Quach, N.D.; Dawson, M.R. Differential Mechanical Response of Mesenchymal Stem Cells and Fibroblasts to Tumor-Secreted Soluble Factors. PLoS ONE 2012, 7, e33248. [Google Scholar] [CrossRef]

| A | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cell Form Factor | Nuclear Form Factor | |||||||
| SF-Norm-Soft | TGF-Hyp-Soft | SF-Norm-Hard | TGF-Hyp-Hard | SF-Norm-Soft | TGF-Hyp-Soft | SF-Norm-Hard | TGF-Hyp-Hard | |
| Actin-Lo | 0.15 | 0.136 | 0.127 | 0.173 | 0.827 | 0.816 | 0.844 | 0.752 |
| Actin-Hi | 0.442 | 0.394 | 0.421 | 0.417 | 0.712 | 0.686 | 0.679 | 0.699 |
| Myosin Lo | 0.355 | 0.404 | 0.402 | 0.486 | 0.857 | 0.807 | 0.817 | 0.802 |
| Myosin Hi | 0.634 | 0.723 | 0.62 | 0.498 | 0.755 | 0.752 | 0.707 | 0.663 |
| Cytokeratin-Lo | 0.239 | 0.346 | 0.3 | 0.203 | 0.827 | 0.762 | 0.796 | 0.798 |
| Cytokeratin-Hi | 0.341 | 0.366 | 0.315 | 0.235 | 0.673 | 0.708 | 0.745 | 0.728 |
| Cytokeratin to Actin-Lo | 0.487 | 0.404 | 0.445 | 0.421 | 0.742 | 0.709 | 0.659 | 0.736 |
| Cytokeratin to Actin-Hi | 0.19 | 0.168 | 0.153 | 0.195 | 0.753 | 0.749 | 0.822 | 0.762 |
| Myosin to Actin-Lo | 0.489 | 0.436 | 0.444 | 0.43 | 0.694 | 0.672 | 0.662 | 0.697 |
| Myosin to Actin-Hi | 0.786 | 0.767 | 0.777 | 0.788 | 0.816 | 0.767 | 0.796 | 0.788 |
| B | ||||||||
| TGF-HYP/SF | ||||||||
| Cell Form Factor | Nuclear Form Factor | |||||||
| Soft | Hard | Soft | Hard | |||||
| Actin-Lo | 0.906667 | 1.362205 | 0.986699 | 0.890995 | ||||
| Actin-Hi | 0.891403 | 0.990499 | 0.963483 | 1.029455 | ||||
| Myosin Lo | 1.138028 | 1.208955 | 0.941657 | 0.98164 | ||||
| Myosin Hi | 1.140379 | 0.803226 | 0.996026 | 0.937765 | ||||
| Cytokeratin-Lo | 1.447699 | 0.676667 | 0.921403 | 1.002513 | ||||
| Cytokeratin-Hi | 1.073314 | 0.746032 | 1.052006 | 0.977181 | ||||
| Cytokeratin to Actin-Lo | 0.829569 | 0.946067 | 0.955526 | 1.116844 | ||||
| Cytokeratin to Actin-Hi | 0.884211 | 1.27451 | 0.994688 | 0.927007 | ||||
| Myosin to Actin-Lo | 0.891616 | 0.968468 | 0.9683 | 1.05287 | ||||
| Myosin to Actin-Hi | 0.975827 | 1.014157 | 0.939951 | 0.98995 | ||||
| A | ||||||||
| TGF-Hyp-Soft | TGF-Hyp-Hard | |||||||
| DMSO | ML7 | H1152 | CCG1423 | DMSO | ML7 | H1152 | CCG1423 | |
| Cell form factor | 0.298 | 0.306 | 0.217 | 0.324 | 0.334 | 0.329 | 0.234 | 0.332 |
| Nuclear form factor | 0.617 | 0.66 | 0.748 | 0.652 | 0.677 | 0.714 | 0.772 | 0.7 |
| Mean Actin | 0.148 | 0.134 | 0.099 | 0.117 | 0.133 | 0.132 | 0.097 | 0.123 |
| Mean Cytokeratin | 0.132 | 0.116 | 0.122 | 0.167 | 0.131 | 0.123 | 0.12 | 0.156 |
| Mean Myosin | 0.074 | 0.07 | 0.067 | 0.073 | 0.073 | 0.069 | 0.068 | 0.073 |
| Cytokeratin to Actin | 1 | 0.888 | 1.247 | 1.341 | 1.012 | 0.941 | 1.261 | 1.361 |
| Myosin to Actin | 0.502 | 0.541 | 0.688 | 0.715 | 0.572 | 0.58 | 0.712 | 0.598 |
| B | ||||||||
| TGF-Hyp-Soft (Normalized to DMSO) | TGF-Hyp-Hard (Normalized to DMSO) | |||||||
| ML7 | H1152 | CCG1423 | ML7 | H1152 | CCG1423 | |||
| Cell form factor | 1.027 | 0.728 | 1.087 | 0.985 | 0.701 | 0.994 | ||
| Nuclear form factor | 1.070 | 1.212 | 1.057 | 1.055 | 1.140 | 1.034 | ||
| Mean Actin | 0.905 | 0.669 | 0.791 | 0.992 | 0.729 | 0.925 | ||
| Mean Cytokeratin | 0.879 | 0.924 | 1.265 | 0.939 | 0.916 | 1.191 | ||
| Mean Myosin | 0.946 | 0.905 | 0.986 | 0.945 | 0.932 | 1.000 | ||
| Cytokeratin to Actin | 0.888 | 1.247 | 1.341 | 0.930 | 1.246 | 1.345 | ||
| Myosin to Actin | 1.078 | 1.371 | 1.424 | 1.014 | 1.245 | 1.045 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, D.; Hsu, J.; Soriano, K.; Peña, C.M.; Lee, A.H.; Dizon, D.S.; Dawson, M.R. Spatial Heterogeneity in Cytoskeletal Mechanics Response to TGF-β1 and Hypoxia Mediates Partial Epithelial-to-Mesenchymal Transition in Epithelial Ovarian Cancer Cells. Cancers 2023, 15, 3186. https://doi.org/10.3390/cancers15123186
Ghosh D, Hsu J, Soriano K, Peña CM, Lee AH, Dizon DS, Dawson MR. Spatial Heterogeneity in Cytoskeletal Mechanics Response to TGF-β1 and Hypoxia Mediates Partial Epithelial-to-Mesenchymal Transition in Epithelial Ovarian Cancer Cells. Cancers. 2023; 15(12):3186. https://doi.org/10.3390/cancers15123186
Chicago/Turabian StyleGhosh, Deepraj, Jeffrey Hsu, Kylen Soriano, Carolina Mejia Peña, Amy H. Lee, Don S. Dizon, and Michelle R. Dawson. 2023. "Spatial Heterogeneity in Cytoskeletal Mechanics Response to TGF-β1 and Hypoxia Mediates Partial Epithelial-to-Mesenchymal Transition in Epithelial Ovarian Cancer Cells" Cancers 15, no. 12: 3186. https://doi.org/10.3390/cancers15123186
APA StyleGhosh, D., Hsu, J., Soriano, K., Peña, C. M., Lee, A. H., Dizon, D. S., & Dawson, M. R. (2023). Spatial Heterogeneity in Cytoskeletal Mechanics Response to TGF-β1 and Hypoxia Mediates Partial Epithelial-to-Mesenchymal Transition in Epithelial Ovarian Cancer Cells. Cancers, 15(12), 3186. https://doi.org/10.3390/cancers15123186







