Differences in the Tumor Microenvironment of EBV-Associated Gastric Cancers Revealed Using Single-Cell Transcriptome Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources for Bulk Tumor mRNA Expression
2.2. Data Sources for Single-Cell RNA Expression
2.3. Differential Gene Expression Workflow
3. Results
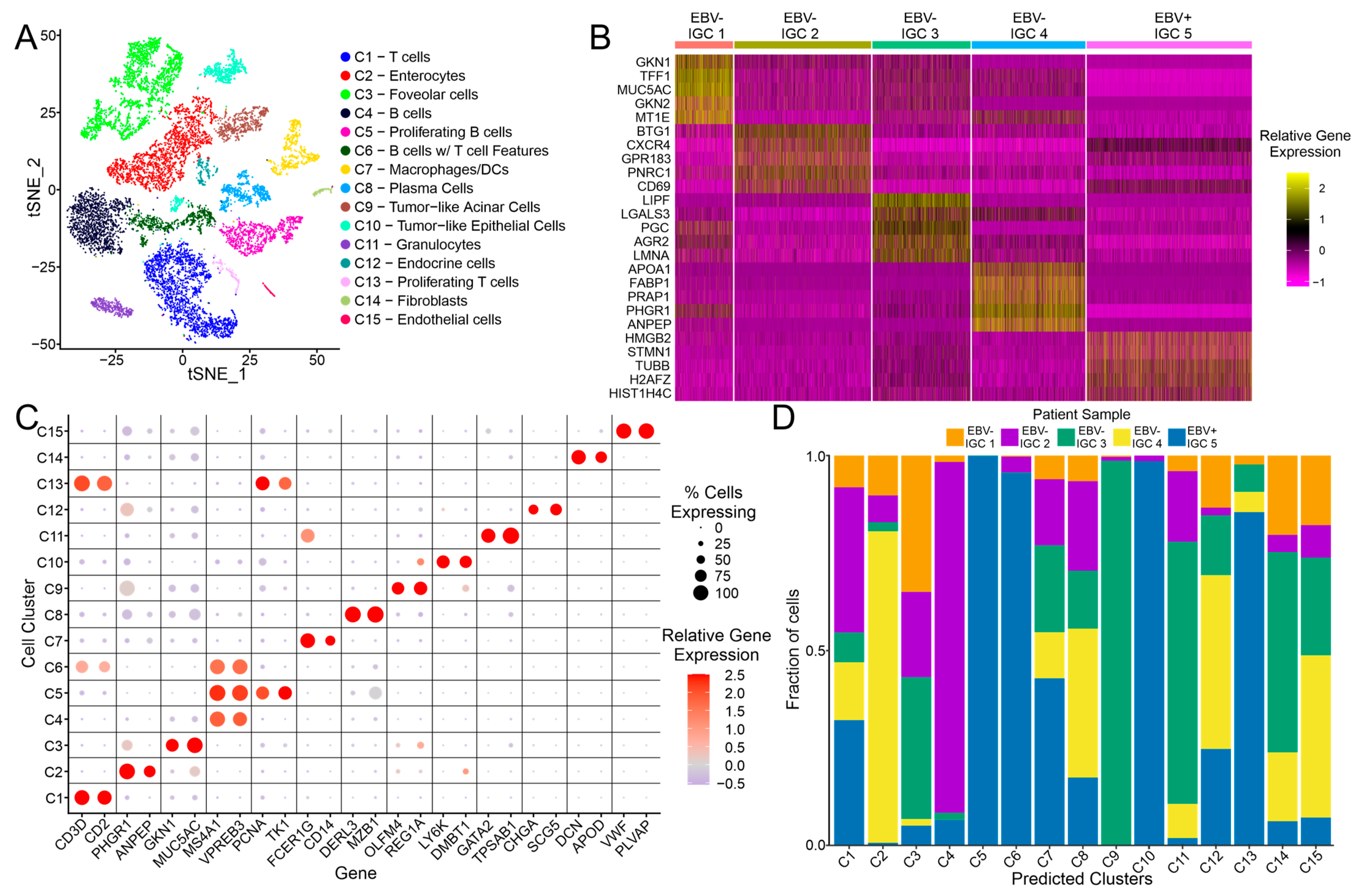
3.1. Clustering of Intestinal Gastric Cancer Samples
3.2. scRNA-Seq and Bulk Tumor Data Pipeline
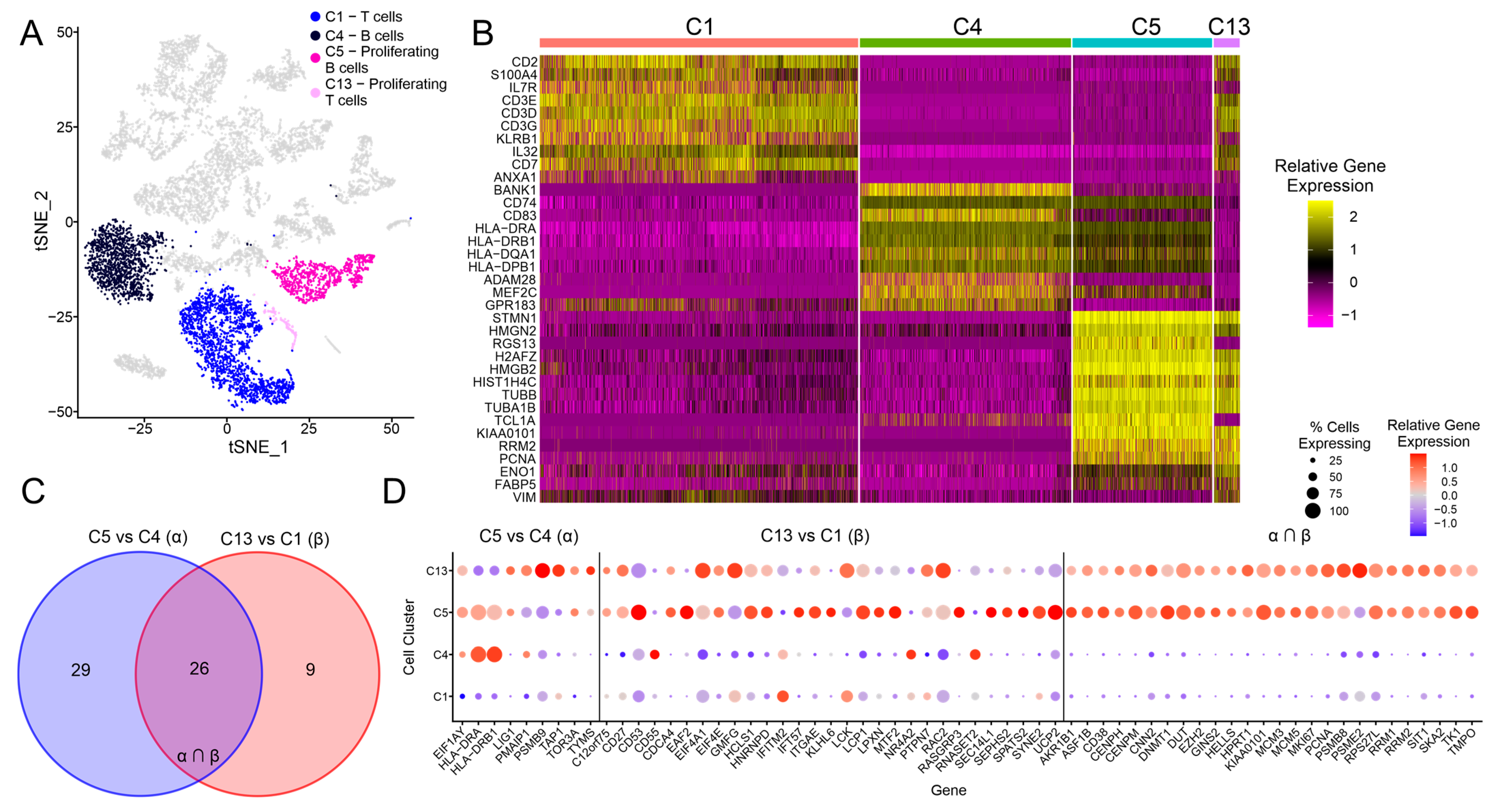
3.3. Exploration of the C5 and C13 Cell Clusters Representing Proliferating B and T Cells
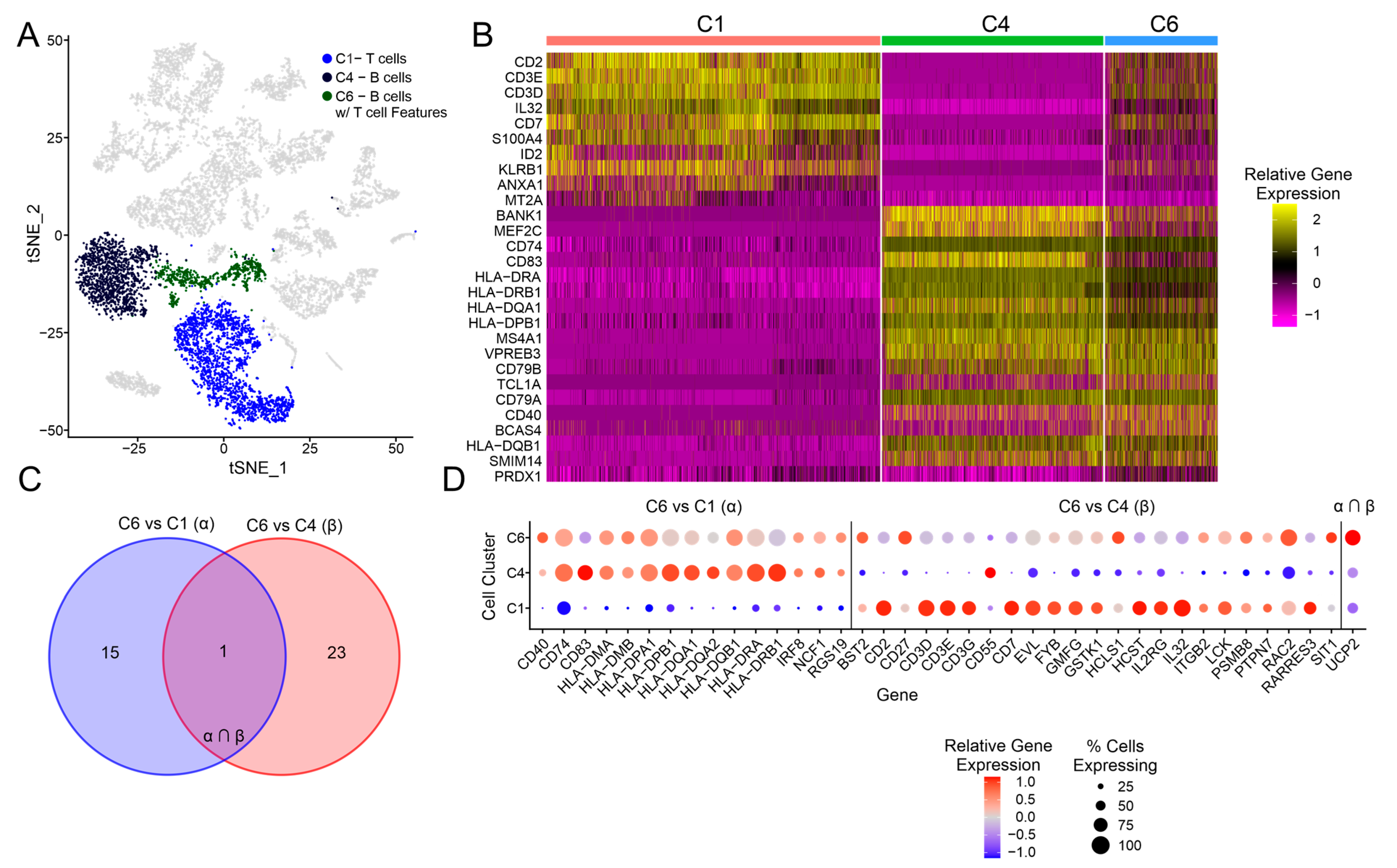
3.4. Exploration of the C6 Cluster Representing B Cells with T Cell Features
3.5. Exploration of the C10 Cluster Representing Tumor-like Epithelial Cells
3.6. Exploration of T Cells across EBV-Positive and EBV-Negative IGCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tzellos, S.; Farrell, P.J. Epstein-Barr Virus Sequence Variation-Biology and Disease. Pathogens 2012, 1, 156–174. [Google Scholar] [CrossRef]
- Eichelberg, M.R.; Welch, R.; Guidry, J.T.; Ali, A.; Ohashi, M.; Makielski, K.R.; McChesney, K.; Van Sciver, N.; Lambert, P.F.; Keleș, S.; et al. Epstein-Barr Virus Infection Promotes Epithelial Cell Growth by Attenuating Differentiation-Dependent Exit from the Cell Cycle. mBio 2019, 10, e01332-19. [Google Scholar] [CrossRef]
- Reusch, J.A.; Nawandar, D.M.; Wright, K.L.; Kenney, S.C.; Mertz, J.E. Cellular Differentiation Regulator BLIMP1 Induces Epstein-Barr Virus Lytic Reactivation in Epithelial and B Cells by Activating Transcription from Both the R and Z Promoters. J. Virol. 2015, 89, 1731–1743. [Google Scholar] [CrossRef] [PubMed]
- Raab-Traub, N. Novel Mechanisms of EBV-Induced Oncogenesis. Curr. Opin. Virol. 2012, 2, 453–458. [Google Scholar] [CrossRef] [PubMed]
- De Re, V.; Caggiari, L.; De Zorzi, M.; Fanotto, V.; Miolo, G.; Puglisi, F.; Cannizzaro, R.; Canzonieri, V.; Steffan, A.; Farruggia, P.; et al. Epstein-Barr Virus BART MicroRNAs in EBV- Associated Hodgkin Lymphoma and Gastric Cancer. Infect. Agents Cancer 2020, 15, 42. [Google Scholar] [CrossRef] [PubMed]
- Farrell, P.J. Epstein-Barr Virus and Cancer. Annu. Rev. Pathol. 2019, 14, 29–53. [Google Scholar] [CrossRef]
- Khan, G.; Hashim, M.J. Global Burden of Deaths from Epstein-Barr Virus Attributable Malignancies 1990-2010. Infect. Agents Cancer 2014, 9, 38. [Google Scholar] [CrossRef]
- Chen, J.-N.; He, D.; Tang, F.; Shao, C.-K. Epstein-Barr Virus-Associated Gastric Carcinoma: A Newly Defined Entity. J. Clin. Gastroenterol. 2012, 46, 262–271. [Google Scholar] [CrossRef]
- Tavakoli, A.; Monavari, S.H.; Solaymani Mohammadi, F.; Kiani, S.J.; Armat, S.; Farahmand, M. Association between Epstein-Barr Virus Infection and Gastric Cancer: A Systematic Review and Meta-Analysis. BMC Cancer 2020, 20, 493. [Google Scholar] [CrossRef]
- Salnikov, M.; Prusinkiewicz, M.A.; Lin, S.; Ghasemi, F.; Cecchini, M.J.; Mymryk, J.S. Tumor-Infiltrating T Cells in EBV-Associated Gastric Carcinomas Exhibit High Levels of Multiple Markers of Activation, Effector Gene Expression, and Exhaustion. Viruses 2023, 15, 176. [Google Scholar] [CrossRef]
- Ghasemi, F.; Tessier, T.M.; Gameiro, S.F.; Maciver, A.H.; Cecchini, M.J.; Mymryk, J.S. High MHC-II Expression in Epstein-Barr Virus-Associated Gastric Cancers Suggests That Tumor Cells Serve an Important Role in Antigen Presentation. Sci. Rep. 2020, 10, 14786. [Google Scholar] [CrossRef]
- Ghasemi, F.; Gameiro, S.F.; Tessier, T.M.; Maciver, A.H.; Mymryk, J.S. High Levels of Class I Major Histocompatibility Complex MRNA Are Present in Epstein-Barr Virus-Associated Gastric Adenocarcinomas. Cells 2020, 9, 499. [Google Scholar] [CrossRef] [PubMed]
- Stanland, L.J.; Luftig, M.A. The Role of EBV-Induced Hypermethylation in Gastric Cancer Tumorigenesis. Viruses 2020, 12, 1222. [Google Scholar] [CrossRef] [PubMed]
- Camargo, M.C.; Kim, W.-H.; Chiaravalli, A.M.; Kim, K.-M.; Corvalan, A.H.; Matsuo, K.; Yu, J.; Sung, J.J.Y.; Herrera-Goepfert, R.; Meneses-Gonzalez, F.; et al. Improved Survival of Gastric Cancer with Tumour Epstein-Barr Virus Positivity: An International Pooled Analysis. Gut 2014, 63, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hu, S.; Min, M.; Ni, Y.; Lu, Z.; Sun, X.; Wu, J.; Liu, B.; Ying, X.; Liu, Y. Dissecting Transcriptional Heterogeneity in Primary Gastric Adenocarcinoma by Single Cell RNA Sequencing. Gut 2021, 70, 464–475. [Google Scholar] [CrossRef]
- McGinnis, C.S.; Murrow, L.M.; Gartner, Z.J. DoubletFinder: Doublet Detection in Single-Cell RNA Sequencing Data Using Artificial Nearest Neighbors. Cell Syst. 2019, 8, 329–337.e4. [Google Scholar] [CrossRef]
- Butler, A.; Hoffman, P.; Smibert, P.; Papalexi, E.; Satija, R. Integrating Single-Cell Transcriptomic Data across Different Conditions, Technologies, and Species. Nat. Biotechnol. 2018, 36, 411–420. [Google Scholar] [CrossRef]
- Thul, P.J.; Lindskog, C. The Human Protein Atlas: A Spatial Map of the Human Proteome. Protein Sci. 2018, 27, 233–244. [Google Scholar] [CrossRef]
- Crotty, S. T Follicular Helper Cell Differentiation, Function, and Roles in Disease. Immunity 2014, 41, 529–542. [Google Scholar] [CrossRef]
- Meng, X.; Yu, X.; Dong, Q.; Xu, X.; Li, J.; Xu, Q.; Ma, J.; Zhou, C. Distribution of Circulating Follicular Helper T Cells and Expression of Interleukin-21 and Chemokine C-X-C Ligand 13 in Gastric Cancer. Oncol. Lett. 2018, 16, 3917–3922. [Google Scholar] [CrossRef]
- Laurent, C.; Fazilleau, N.; Brousset, P. A Novel Subset of T-Helper Cells: Follicular T-Helper Cells and Their Markers. Haematologica 2010, 95, 356–358. [Google Scholar] [CrossRef]
- Ioannidou, K.; Ndiaye, D.-R.; Noto, A.; Fenwick, C.; Fortis, S.P.; Pantaleo, G.; Petrovas, C.; de Leval, L. In Situ Characterization of Follicular Helper CD4 T Cells Using Multiplexed Imaging. Front. Immunol. 2020, 11, 607626. [Google Scholar] [CrossRef]
- Sun, Z.; Li, Y.; Zhang, Z.; Fu, Y.; Han, X.; Hu, Q.; Ding, H.; Shang, H.; Jiang, Y. CD160 Promotes NK Cell Functions by Upregulating Glucose Metabolism and Negatively Correlates With HIV Disease Progression. Front. Immunol. 2022, 13, 854432. [Google Scholar] [CrossRef]
- Crombie, J.L.; LaCasce, A.S. Epstein Barr Virus Associated B-Cell Lymphomas and Iatrogenic Lymphoproliferative Disorders. Front. Oncol. 2019, 9, 109. [Google Scholar] [CrossRef]
- Lv, K.; Yin, T.; Yu, M.; Chen, Z.; Zhou, Y.; Li, F. Treatment Advances in EBV Related Lymphoproliferative Diseases. Front. Oncol. 2022, 12, 838817. [Google Scholar] [CrossRef]
- Dojcinov, S.D.; Fend, F.; Quintanilla-Martinez, L. EBV-Positive Lymphoproliferations of B- T- and NK-Cell Derivation in Non-Immunocompromised Hosts. Pathogens 2018, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Mrozek-Gorska, P.; Buschle, A.; Pich, D.; Schwarzmayr, T.; Fechtner, R.; Scialdone, A.; Hammerschmidt, W. Epstein-Barr Virus Reprograms Human B Lymphocytes Immediately in the Prelatent Phase of Infection. Proc. Natl. Acad. Sci. USA 2019, 116, 16046–16055. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Robertson, E.S. Mechanisms of B-Cell Oncogenesis Induced by Epstein-Barr Virus. J. Virol. 2019, 93, e00238-19. [Google Scholar] [CrossRef]
- De Mel, S.; Tan, J.Z.-C.; Jeyasekharan, A.D.; Chng, W.-J.; Ng, S.-B. Transcriptomic Abnormalities in Epstein Barr Virus Associated T/NK Lymphoproliferative Disorders. Front. Pediatr. 2018, 6, 405. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Yu, Q.; Chen, G.; Wang, M.; Zhao, Z.; Zhang, Y.; Qiu, L. Altered Ratio of Circulating Follicular Regulatory T Cells and Follicular Helper T Cells during Primary EBV Infection. Clin. Exp. Med. 2020, 20, 373–380. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Y.; Yu, Q.; Zhao, Z.; Wang, H.; Luo, X.; Chen, Y.; Zhu, Z.; Chen, G.; Wu, M.; et al. Higher Frequency of CD4+CXCR5+ICOS+PD1+ T Follicular Helper Cells in Patients With Infectious Mononucleosis. Medicine 2015, 94, e2061. [Google Scholar] [CrossRef] [PubMed]
- Cox, B.S.; Alharshawi, K.; Mena-Palomo, I.; Lafuse, W.P.; Ariza, M.E. EBV/HHV-6A DUTPases Contribute to Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Pathophysiology by Enhancing TFH Cell Differentiation and Extrafollicular Activities. JCI Insight 2022, 7, e158193. [Google Scholar] [CrossRef] [PubMed]
- Baumjohann, D.; Brossart, P. T Follicular Helper Cells: Linking Cancer Immunotherapy and Immune-Related Adverse Events. J. Immunother. Cancer 2021, 9, e002588. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Melo, N.; Baumjohann, D. T Follicular Helper Cells in Cancer. Trends Cancer 2023, 9, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Szabo, P.A.; Levitin, H.M.; Miron, M.; Snyder, M.E.; Senda, T.; Yuan, J.; Cheng, Y.L.; Bush, E.C.; Dogra, P.; Thapa, P.; et al. Single-Cell Transcriptomics of Human T Cells Reveals Tissue and Activation Signatures in Health and Disease. Nat. Commun. 2019, 10, 4706. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Zhu, B. T-Cell Exhaustion in the Tumor Microenvironment. Cell Death Dis. 2015, 6, e1792. [Google Scholar] [CrossRef]
- Cho, J.; Kang, M.-S.; Kim, K.-M. Epstein-Barr Virus-Associated Gastric Carcinoma and Specific Features of the Accompanying Immune Response. J. Gastric Cancer 2016, 16, 1–7. [Google Scholar] [CrossRef]
- Barros, M.H.M.; Vera-Lozada, G.; Segges, P.; Hassan, R.; Niedobitek, G. Revisiting the Tissue Microenvironment of Infectious Mononucleosis: Identification of EBV Infection in T Cells and Deep Characterization of Immune Profiles. Front. Immunol. 2019, 10, 146. [Google Scholar] [CrossRef]
- Hoennscheidt, C.; Max, D.; Richter, N.; Staege, M.S. Expression of CD4 on Epstein-Barr Virus-Immortalized B Cells. Scand. J. Immunol. 2009, 70, 216–225. [Google Scholar] [CrossRef]
- Ahmed, R.; Omidian, Z.; Giwa, A.; Cornwell, B.; Majety, N.; Bell, D.R.; Lee, S.; Zhang, H.; Michels, A.; Desiderio, S.; et al. A Public BCR Present in a Unique Dual-Receptor-Expressing Lymphocyte from Type 1 Diabetes Patients Encodes a Potent T Cell Autoantigen. Cell 2019, 177, 1583–1599.e16. [Google Scholar] [CrossRef]
- Japp, A.S.; Meng, W.; Rosenfeld, A.M.; Perry, D.J.; Thirawatananond, P.; Bacher, R.L.; Liu, C.; Gardner, J.S.; HPAP Consortium; Atkinson, M.A.; et al. TCR+/BCR+ Dual-Expressing Cells and Their Associated Public BCR Clonotype Are Not Enriched in Type 1 Diabetes. Cell 2021, 184, 827–839.e14. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Omidian, Z.; Giwa, A.; Donner, T.; Jie, C.; Hamad, A.R.A. A Reply to “TCR+/BCR+ Dual-Expressing Cells and Their Associated Public BCR Clonotype Are Not Enriched in Type 1 Diabetes”. Cell 2021, 184, 840–843. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.; Sahnane, N.; Tibiletti, M.G.; Magnoli, F.; Vanoli, A.; Sessa, F.; Chiaravalli, A.M. EBV⁺ and MSI Gastric Cancers Harbor High PD-L1/PD-1 Expression and High CD8⁺ Intratumoral Lymphocytes. Cancers 2018, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Camps, J.; Fan, B.; Jiang, H.; Ibrahim, M.M.; Hu, X.; Qin, S.; Kirchhoff, D.; Chiang, D.Y.; Wang, S.; et al. Parallel Single-Cell and Bulk Transcriptome Analyses Reveal Key Features of the Gastric Tumor Microenvironment. Genome Biol. 2022, 23, 265. [Google Scholar] [CrossRef]
- Salnikov, M.Y.; Wang, E.; Christensen, E.; Prusinkiewicz, M.A.; Shooshtari, P.; Mymryk, J.S. The EBV Gastric Cancer Resource (EBV-GCR): A Suite of Tools for Investigating EBV-Associated Human Gastric Carcinogenesis. Viruses 2023, 15, 853. [Google Scholar] [CrossRef]
- Murata, T.; Sugimoto, A.; Inagaki, T.; Yanagi, Y.; Watanabe, T.; Sato, Y.; Kimura, H. Molecular Basis of Epstein-Barr Virus Latency Establishment and Lytic Reactivation. Viruses 2021, 13, 2344. [Google Scholar] [CrossRef]
- Shinozaki-Ushiku, A.; Kunita, A.; Fukayama, M. Update on Epstein-Barr Virus and Gastric Cancer (Review). Int. J. Oncol. 2015, 46, 1421–1434. [Google Scholar] [CrossRef]
- Nakamura, S.; Ueki, T.; Yao, T.; Ueyama, T.; Tsuneyoshi, M. Epstein-Barr Virus in Gastric Carcinoma with Lymphoid Stroma. Special Reference to Its Detection by the Polymerase Chain Reaction and in Situ Hybridization in 99 Tumors, Including a Morphologic Analysis. Cancer 1994, 73, 2239–2249. [Google Scholar] [CrossRef]
- Oda, K.; Tamaru, J.; Takenouchi, T.; Mikata, A.; Nunomura, M.; Saitoh, N.; Sarashina, H.; Nakajima, N. Association of Epstein-Barr Virus with Gastric Carcinoma with Lymphoid Stroma. Am. J. Pathol. 1993, 143, 1063–1071. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salnikov, M.Y.; Fonseca, G.J.; Mymryk, J.S. Differences in the Tumor Microenvironment of EBV-Associated Gastric Cancers Revealed Using Single-Cell Transcriptome Analysis. Cancers 2023, 15, 3178. https://doi.org/10.3390/cancers15123178
Salnikov MY, Fonseca GJ, Mymryk JS. Differences in the Tumor Microenvironment of EBV-Associated Gastric Cancers Revealed Using Single-Cell Transcriptome Analysis. Cancers. 2023; 15(12):3178. https://doi.org/10.3390/cancers15123178
Chicago/Turabian StyleSalnikov, Mikhail Y., Gregory J. Fonseca, and Joe S. Mymryk. 2023. "Differences in the Tumor Microenvironment of EBV-Associated Gastric Cancers Revealed Using Single-Cell Transcriptome Analysis" Cancers 15, no. 12: 3178. https://doi.org/10.3390/cancers15123178
APA StyleSalnikov, M. Y., Fonseca, G. J., & Mymryk, J. S. (2023). Differences in the Tumor Microenvironment of EBV-Associated Gastric Cancers Revealed Using Single-Cell Transcriptome Analysis. Cancers, 15(12), 3178. https://doi.org/10.3390/cancers15123178





