Integrating Multi-Omics Analysis for Enhanced Diagnosis and Treatment of Glioblastoma: A Comprehensive Data-Driven Approach
Abstract
Simple Summary
Abstract
1. Introduction
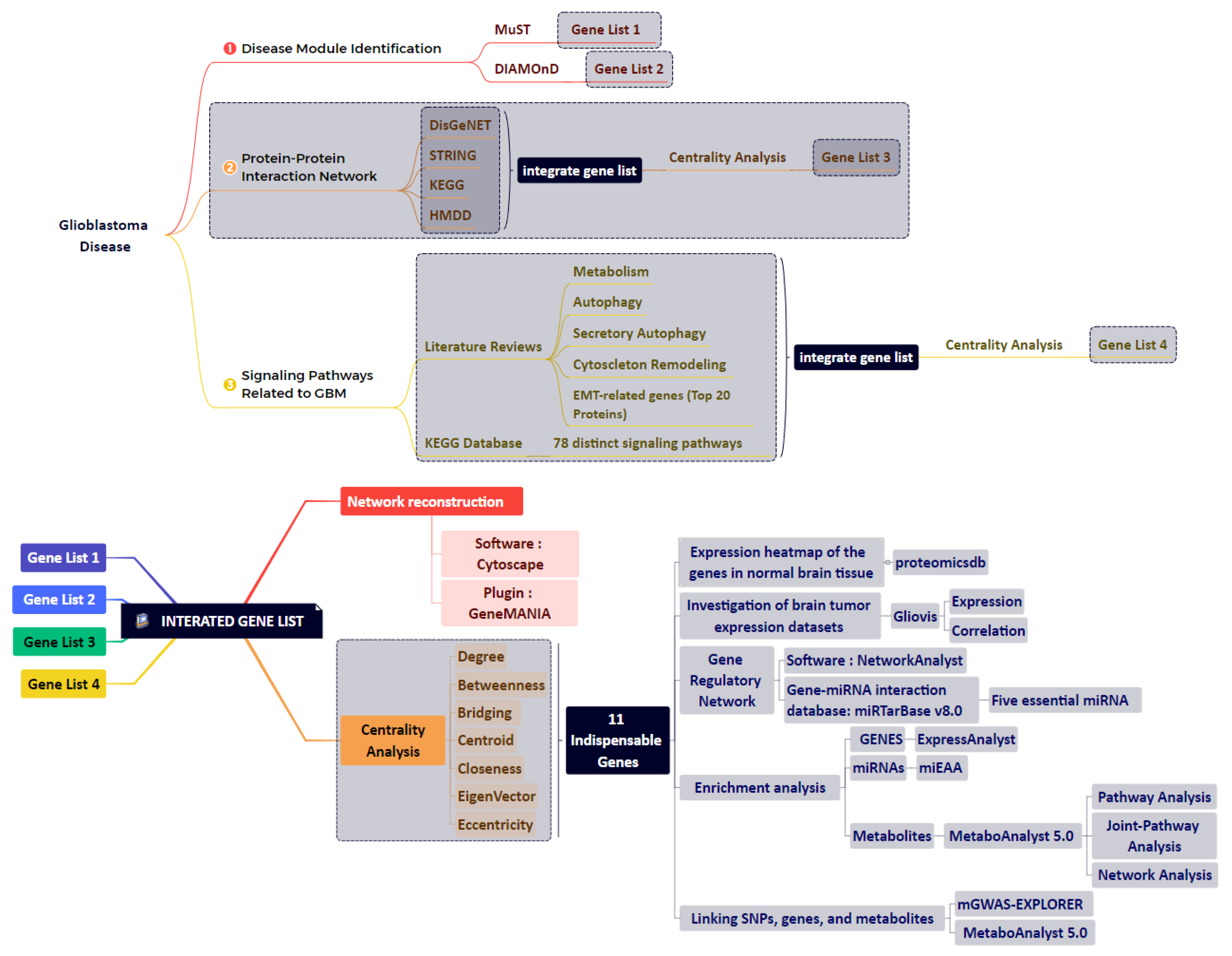
2. Materials and Methods
2.1. Data Integration Approaches
2.2. Disease Module Identification
2.3. GBM-Related Protein–Protein Interaction Network
2.4. Network Reconstruction of GBM-Related Signaling Pathways
2.5. Combining the Findings from the Aforementioned Four Stages of Research and Integrated Database
2.6. Study of Eleven Critical Proteins in Normal Brain and Brain Tumor Expression Datasets
2.7. Identification of Significant Metabolites and SNPs That Interact with Eleven Essential Genes
2.8. Enrichment Analysis
3. Results
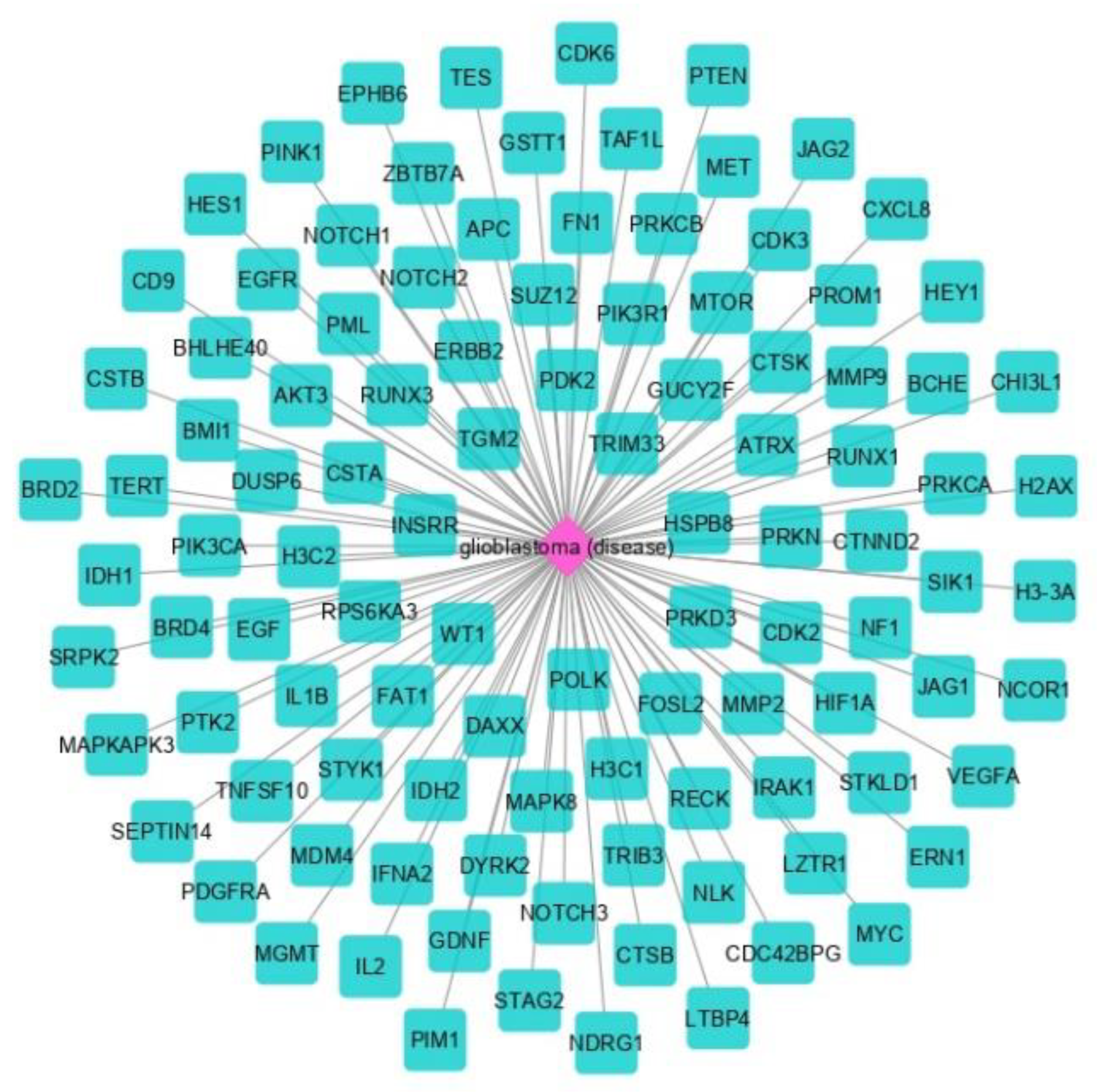
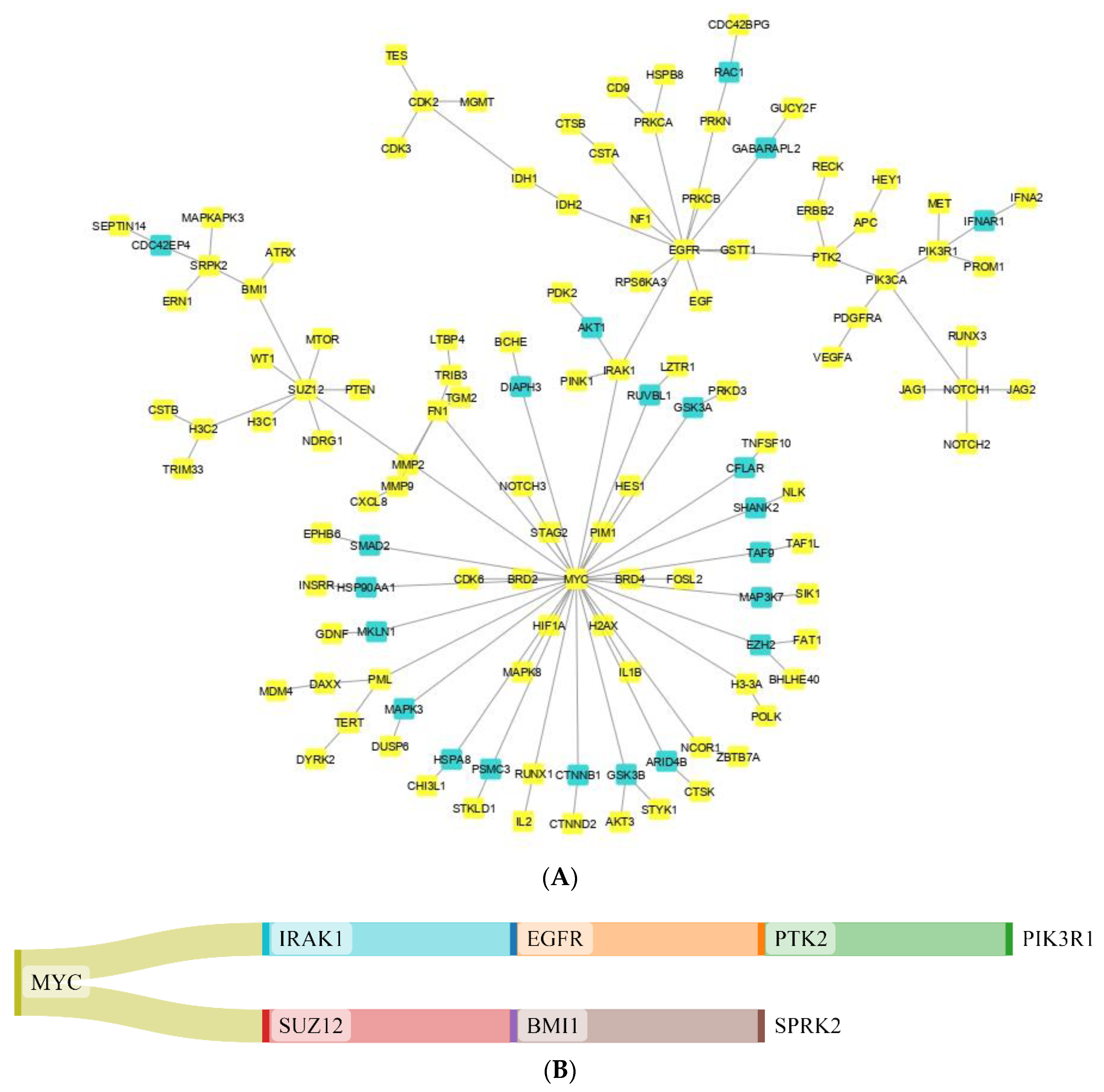
3.1. The Network Obtained from the NeDRex Plugin to Identify Disease Modules
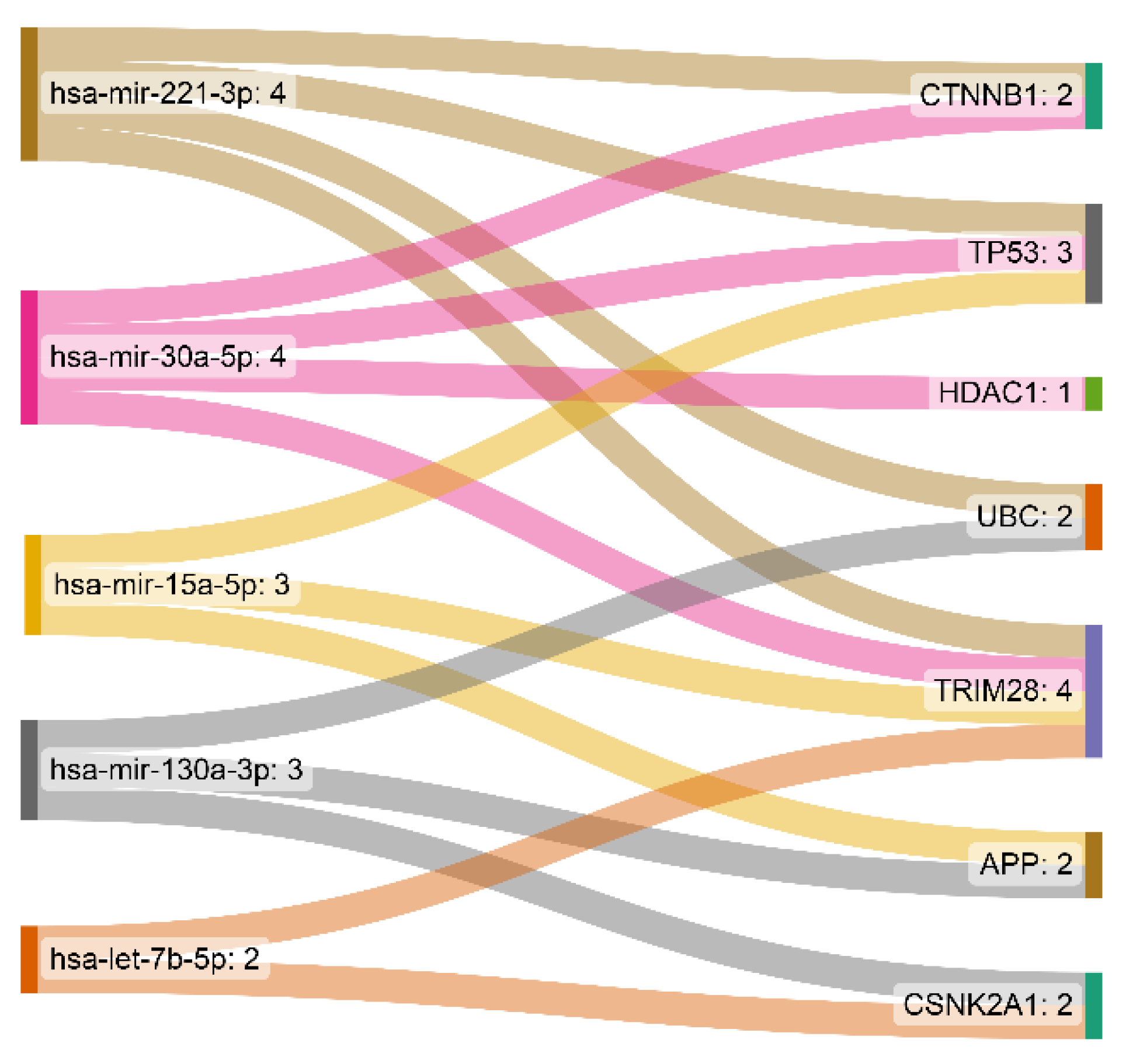
3.2. miRNA-Gene Regulatory Network Analysis
3.3. Analyzing the Status of Identified Gene Expression in Healthy and Malignant Brain Tissue
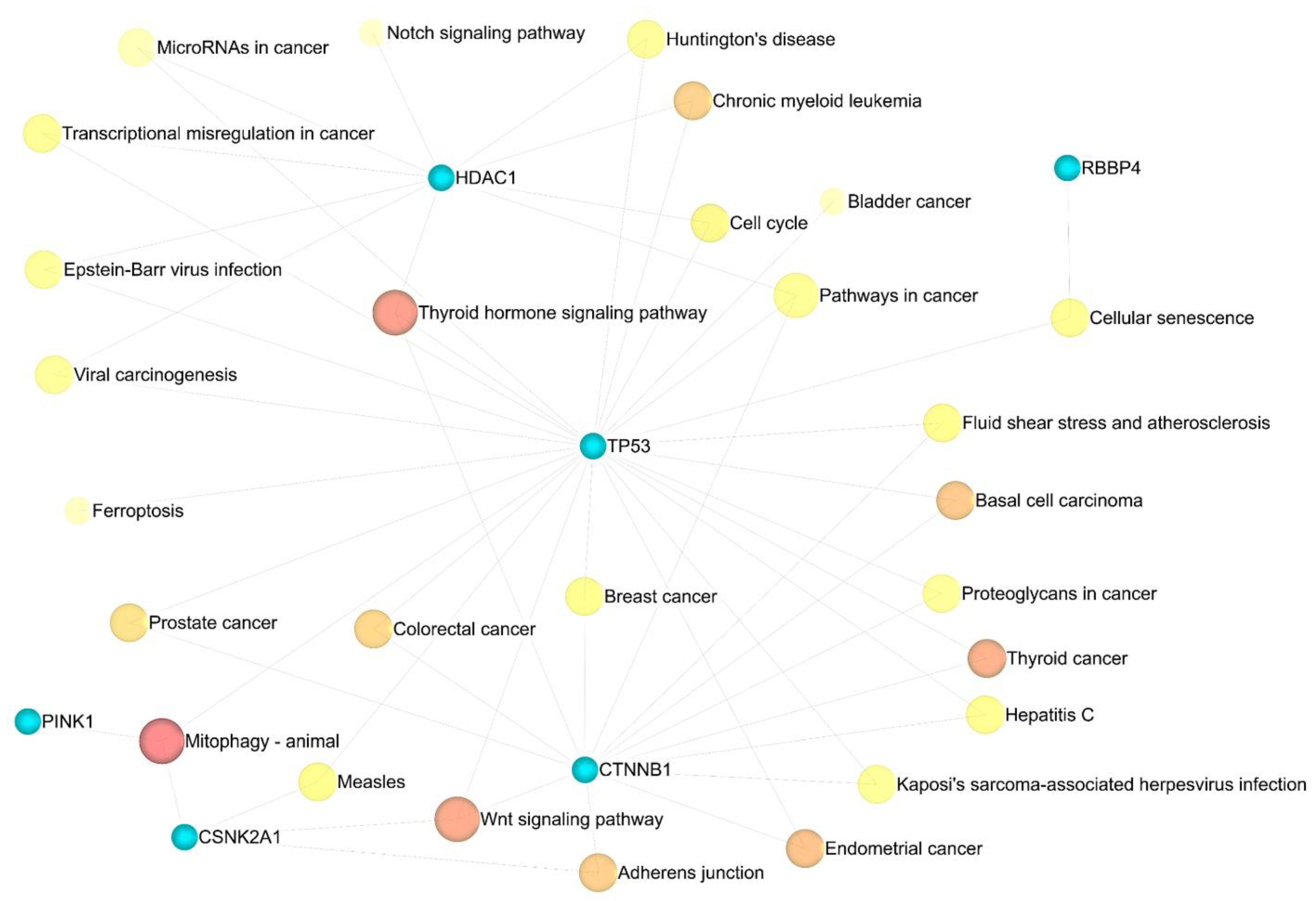
3.4. Enrichment Analysis
3.5. Metabolic Pathway Analysis
3.6. Joint Pathway Analysis
3.7. Gene–Metabolite Interaction Network
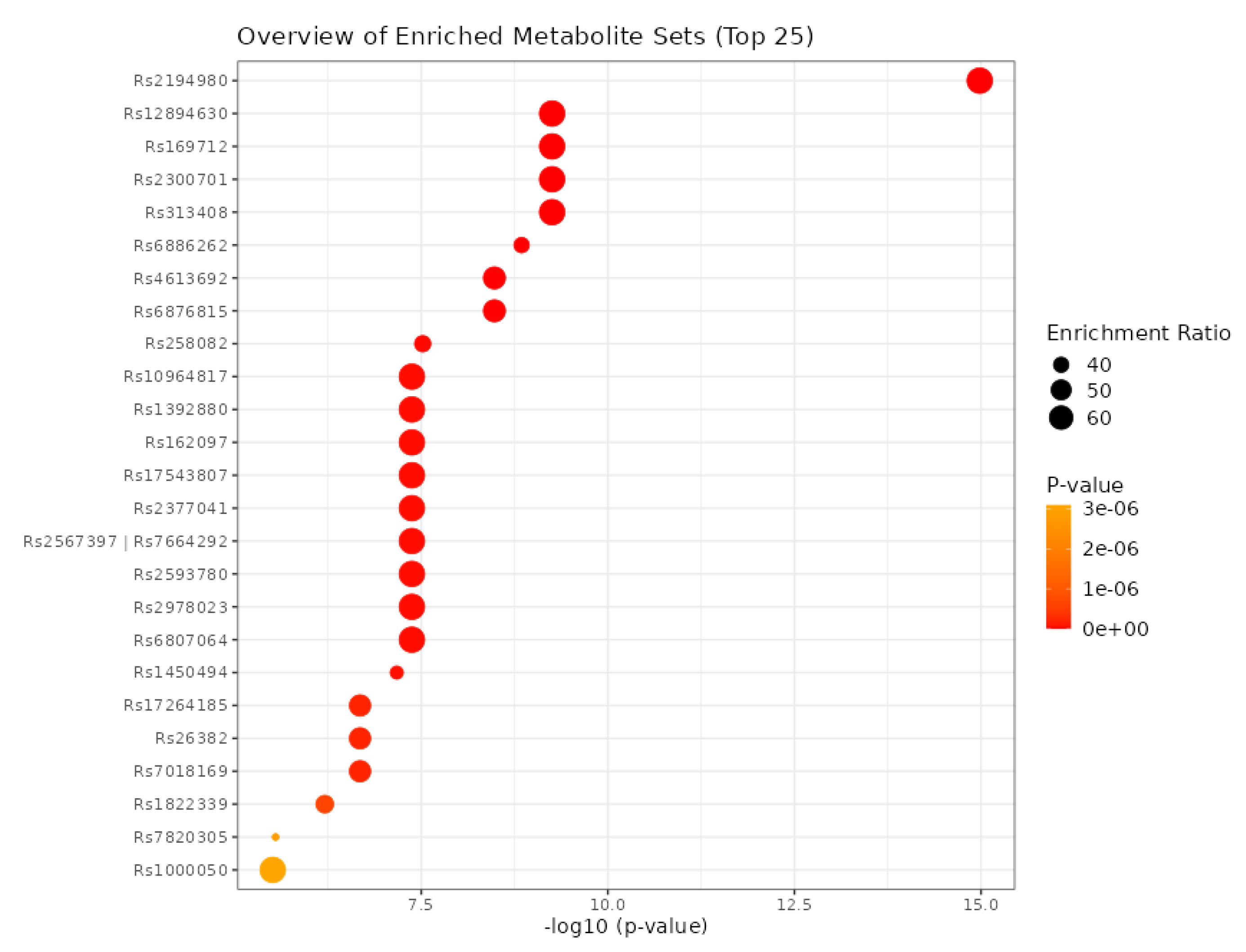
3.8. Identification of SNPs-Related Metabolites and Genes
4. Discussion
5. Conclusions and Future Direction
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fekrirad, Z.; Barzegar Behrooz, A.; Ghaemi, S.; Khosrojerdi, A.; Zarepour, A.; Zarrabi, A.; Arefian, E.; Ghavami, S. Immunology Meets Bioengineering: Improving the Effectiveness of Glioblastoma Immunotherapy. Cancers 2022, 14, 3698. [Google Scholar] [CrossRef]
- Samiei, E.; Seyfoori, A.; Toyota, B.; Ghavami, S.; Akbari, M. Investigating Programmed Cell Death and Tumor Invasion in a Three-Dimensional (3D) Microfluidic Model of Glioblastoma. Int. J. Mol. Sci. 2020, 21, 3162. [Google Scholar] [CrossRef]
- Shojaei, S.; Koleini, N.; Samiei, E.; Aghaei, M.; Cole, L.K.; Alizadeh, J.; Islam, M.I.; Vosoughi, A.R.; Albokashy, M.; Butterfield, Y.; et al. Simvastatin increases temozolomide-induced cell death by targeting the fusion of autophagosomes and lysosomes. FEBS J. 2020, 287, 1005–1034. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, S.; Alizadeh, J.; Thliveris, J.; Koleini, N.; Kardami, E.; Hatch, G.M.; Xu, F.; Hombach-Klonisch, S.; Klonisch, T.; Ghavami, S. Statins: A new approach to combat temozolomide chemoresistance in glioblastoma. J. Investig. Med. 2018, 66, 1083–1087. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.; Li, N.; Zhang, Z. Emerging therapies for glioblastoma: Current state and future directions. J. Exp. Clin. Cancer Res. 2022, 41, 142. [Google Scholar] [CrossRef]
- Hajiahmadi, S.; Lorzadeh, S.; Iranpour, R.; Karima, S.; Rajabibazl, M.; Shahsavari, Z.; Ghavami, S. Temozolomide, Simvastatin and Acetylshikonin Combination Induces Mitochondrial-Dependent Apoptosis in GBM Cells, Which Is Regulated by Autophagy. Biology 2023, 12, 302. [Google Scholar] [CrossRef]
- Sharifzad, F.; Ghavami, S.; Verdi, J.; Mardpour, S.; Mollapour Sisakht, M.; Azizi, Z.; Taghikhani, A.; Los, M.J.; Fakharian, E.; Ebrahimi, M.; et al. Glioblastoma cancer stem cell biology: Potential theranostic targets. Drug Resist. Updat. 2019, 42, 35–45. [Google Scholar] [CrossRef]
- Zhang, P.; Xia, Q.; Liu, L.; Li, S.; Dong, L. Current opinion on molecular characterization for GBM classification in guiding clinical diagnosis, prognosis, and therapy. Front. Mol. Biosci. 2020, 7, 562798. [Google Scholar] [CrossRef]
- Sharifzad, F.; Yasavoli-Sharahi, H.; Mardpour, S.; Fakharian, E.; Nikuinejad, H.; Heydari, Y.; Mardpour, S.; Taghikhani, A.; Khellat, R.; Vafaei, S.; et al. Neuropathological and genomic characterization of glioblastoma-induced rat model: How similar is it to humans for targeted therapy? J. Cell Physiol. 2019, 234, 22493–22504. [Google Scholar] [CrossRef]
- Lu, C.-H.; Wei, S.-T.; Liu, J.-J.; Chang, Y.-J.; Lin, Y.-F.; Yu, C.-S.; Chang, S.L.-Y. Recognition of a Novel Gene Signature for Human Glioblastoma. Int. J. Mol. Sci. 2022, 23, 4157. [Google Scholar] [CrossRef]
- Yabo, Y.A.; Niclou, S.P.; Golebiewska, A. Cancer cell heterogeneity and plasticity: A paradigm shift in glioblastoma. Neuro-oncology 2022, 24, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Basso, J.; Paggi, M.G.; Fortuna, A.; Vitorino, C.; Vitorino, R. Deciphering specific miRNAs in brain tumors: A5-miRNA signature in glioblastoma. Mol. Genet. Genom. 2022, 297, 507–521. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, Q.-M.; Liu, C.; Wu, S.; Nong, W.-X.; Ge, Y.-Y.; Lin, L.-N.; Li, F.; Xie, X.-X.; Luo, B. Microrna-1224-5p Is a Potential Prognostic and Therapeutic Biomarker in Glioblastoma: Integrating Bioinformatics and Clinical Analyses. Curr. Med. Sci. 2022, 42, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.; Chu, Y.; Liu, N.; Wang, Q.; Yin, Z.; Lu, Y.; Chen, Y. Joint bioinformatics analysis of underlying potential functions of hsa-let-7b-5p and core genes in human glioma. J. Transl. Med. 2019, 17, 129. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, F.; Ren, J.; Li, X.; Jiang, Y.; Ma, S. A Selective Review of Multi-Level Omics Data Integration Using Variable Selection. High Throughput 2019, 8, 4. [Google Scholar] [CrossRef]
- Sadegh, S.; Skelton, J.; Anastasi, E.; Bernett, J.; Blumenthal, D.B.; Galindez, G.; Salgado-Albarrán, M.; Lazareva, O.; Flanagan, K.; Cockell, S.; et al. Network medicine for disease module identification and drug repurposing with the NeDRex platform. Nat. Commun. 2021, 12, 6848. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Ghiassian, S.D.; Menche, J.; Barabási, A.-L. A DIseAse MOdule Detection (DIAMOnD) algorithm derived from a systematic analysis of connectivity patterns of disease proteins in the human interactome. PLoS Comput. Biol. 2015, 11, e1004120. [Google Scholar] [CrossRef]
- Piñero, J.; Bravo, À.; Queralt-Rosinach, N.; Gutiérrez-Sacristán, A.; Deu-Pons, J.; Centeno, E.; García-García, J.; Sanz, F.; Furlong, L.I. DisGeNET: A comprehensive platform integrating information on human disease-associated genes and variants. Nucleic Acids Res. 2017, 45, D833–D839. [Google Scholar] [CrossRef]
- Piñero, J.; Ramírez-Anguita, J.M.; Saüch-Pitarch, J.; Ronzano, F.; Centeno, E.; Sanz, F.; Furlong, L.I. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 2020, 48, D845–D855. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P. The STRING database in 2021: Customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Shi, J.; Gao, Y.; Cui, C.; Zhang, S.; Li, J.; Zhou, Y.; Cui, Q. HMDD v3. 0: A database for experimentally supported human microRNA–disease associations. Nucleic Acids Res. 2019, 47, D1013–D1017. [Google Scholar] [CrossRef] [PubMed]
- Warde-Farley, D.; Donaldson, S.L.; Comes, O.; Zuberi, K.; Badrawi, R.; Chao, P.; Franz, M.; Grouios, C.; Kazi, F.; Lopes, C.T.; et al. The GeneMANIA prediction server: Biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010, 38 (Suppl. S2), W214–W220. [Google Scholar] [CrossRef]
- Latifi-Navid, H.; Soheili, Z.S.; Samiei, S.; Sadeghi, M.; Taghizadeh, S.; Pirmardan, E.R.; Ahmadieh, H. Network analysis and the impact of Aflibercept on specific mediators of angiogenesis in HUVEC cells. J. Cell Mol. Med. 2021, 25, 8285–8299. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Soufan, O.; Ewald, J.; Hancock, R.E.; Basu, N.; Xia, J. NetworkAnalyst 3.0: A visual analytics platform for comprehensive gene expression profiling and meta-analysis. Nucleic Acids Res. 2019, 47, W234–W241. [Google Scholar] [CrossRef]
- Xia, J.; Gill, E.E.; Hancock, R.E. NetworkAnalyst for statistical, visual and network-based meta-analysis of gene expression data. Nat. Protoc. 2015, 10, 823–844. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Lin, Y.-C.-D.; Li, J.; Huang, K.-Y.; Shrestha, S.; Hong, H.-C.; Tang, Y.; Chen, Y.-G.; Jin, C.-N.; Yu, Y.; et al. miRTarBase 2020: Updates to the experimentally validated microRNA–target interaction database. Nucleic Acids Res. 2020, 48, D148–D154. [Google Scholar] [CrossRef]
- Samaras, P.; Schmidt, T.; Frejno, M.; Gessulat, S.; Reinecke, M.; Jarzab, A.; Zecha, J.; Mergner, J.; Giansanti, P.; Ehrlich, H.-C.; et al. ProteomicsDB: A multi-omics and multi-organism resource for life science research. Nucleic Acids Res. 2020, 48, D1153–D1163. [Google Scholar] [CrossRef]
- Schmidt, T.; Samaras, P.; Frejno, M.; Gessulat, S.; Barnert, M.; Kienegger, H.; Krcmar, H.; Schlegl, J.; Ehrlich, H.-C.; Aiche, S. ProteomicsDB. Nucleic Acids Res. 2018, 46, D1271–D1281. [Google Scholar] [CrossRef]
- Bowman, R.L.; Wang, Q.; Carro, A.; Verhaak, R.G.; Squatrito, M. GlioVis data portal for visualization and analysis of brain tumor expression datasets. Neuro. Oncol. 2017, 19, 139–141. [Google Scholar] [CrossRef]
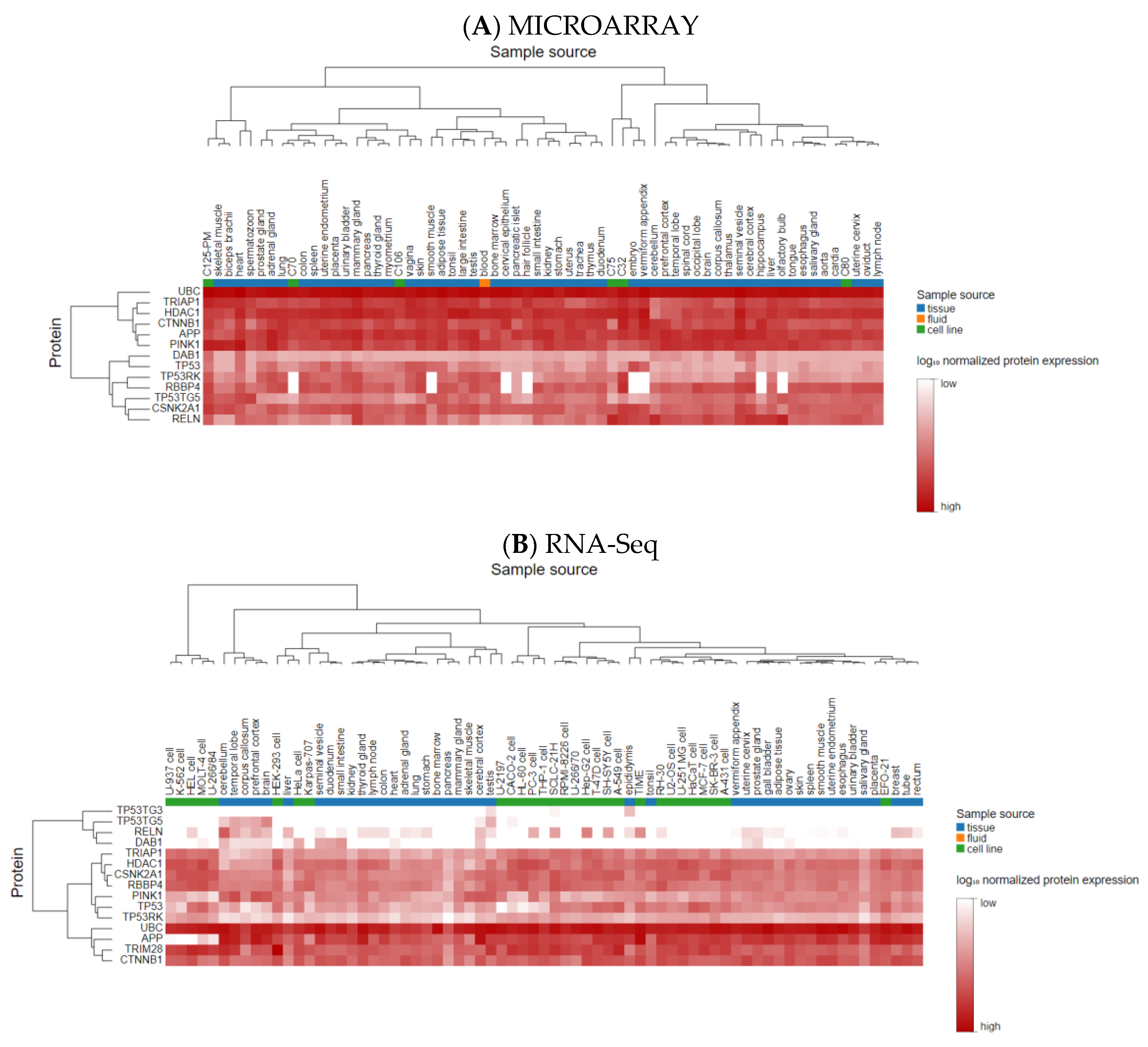
- Thul, P.J.; Lindskog, C. The human protein atlas: A spatial map of the human proteome. Protein Sci. 2018, 27, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Bastian, F.B.; Roux, J.; Niknejad, A.; Comte, A.; Fonseca Costa, S.S.; De Farias, T.M.; Moretti, S.; Parmentier, G.; De Laval, V.R.; Rosikiewicz, M. The Bgee suite: Integrated curated expression atlas and comparative transcriptomics in animals. Nucleic Acids Res. 2021, 49, D831–D847. [Google Scholar] [CrossRef] [PubMed]
- Brazma, A.; Parkinson, H.; Sarkans, U.; Shojatalab, M.; Vilo, J.; Abeygunawardena, N.; Holloway, E.; Kapushesky, M.; Kemmeren, P.; Lara, G.G.; et al. ArrayExpress—A public repository for microarray gene expression data at the EBI. Nucleic Acids Res. 2003, 31, 68–71. [Google Scholar] [CrossRef]
- Edgar, R.; Domrachev, M.; Lash, A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Brägelmann, J.; Kryukov, I.; Saraiva-Agostinho, N.; Perner, S. FirebrowseR: An R client to the Broad Institute’s Firehose Pipeline. Database 2017, 2017, baw160. [Google Scholar] [CrossRef] [PubMed]
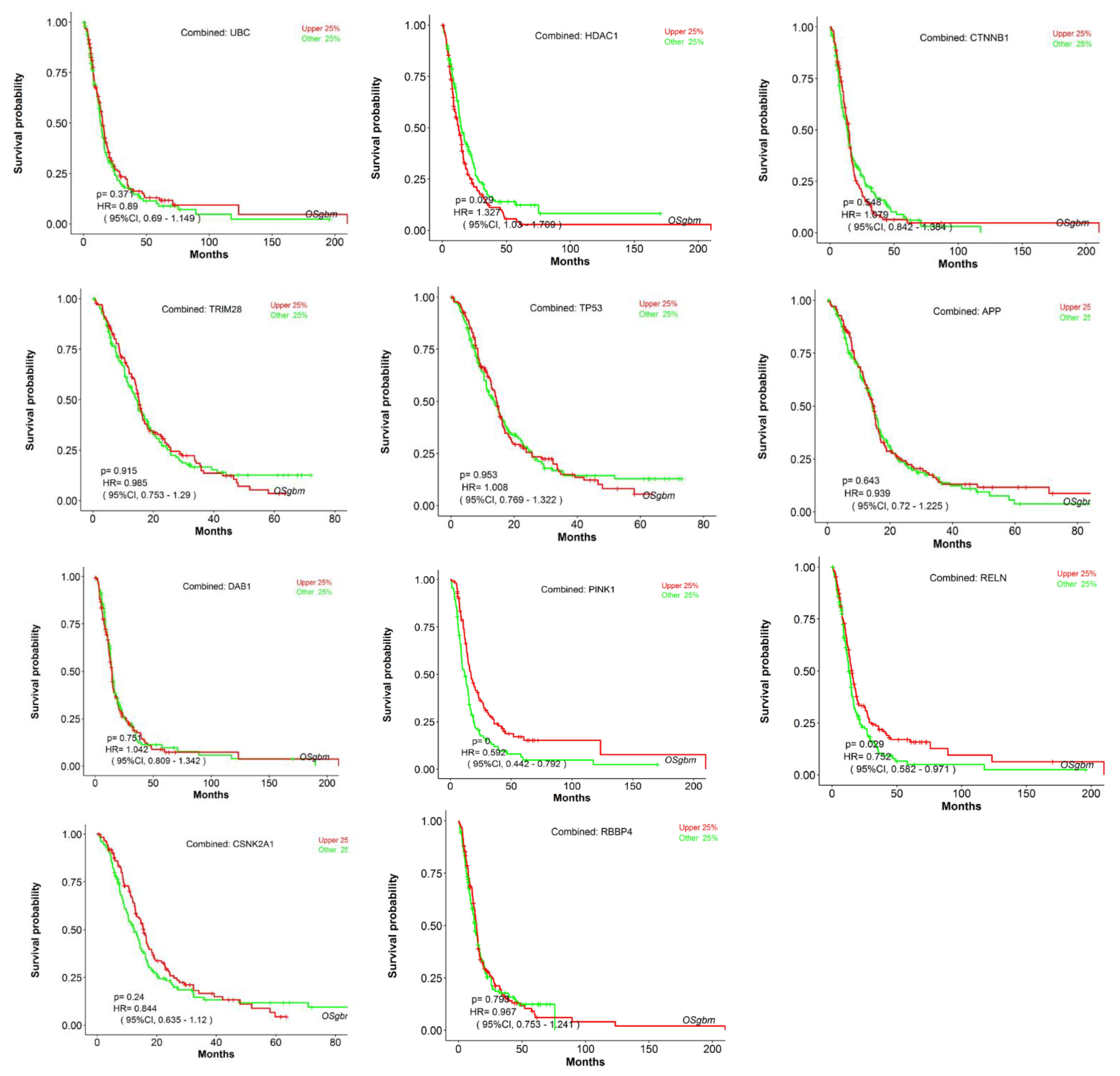
- Dong, H.; Wang, Q.; Li, N.; Lv, J.; Ge, L.; Yang, M.; Zhang, G.; An, Y.; Wang, F.; Xie, L.; et al. OSgbm: An Online Consensus Survival Analysis Web Server for Glioblastoma. Front. Genet. 2019, 10, 1378. [Google Scholar] [CrossRef]
- Chang, L.; Zhou, G.; Ou, H.; Xia, J. mGWAS-Explorer: Linking SNPs, Genes, Metabolites, and Diseases for Functional Insights. Metabolites 2022, 12, 526. [Google Scholar] [CrossRef]
- Deshmukh, R.; Allega, M.F.; Tardito, S. A map of the altered glioma metabolism. Trends Mol. Med. 2021, 27, 1045–1059. [Google Scholar] [CrossRef]
- Jaroch, K.; Modrakowska, P.; Bojko, B. Glioblastoma Metabolomics—In Vitro Studies. Metabolites 2021, 11, 315. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Zhou, G.; Ewald, J.; Chang, L.; Hacariz, O.; Basu, N.; Xia, J. Using MetaboAnalyst 5.0 for LC–HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat. Protoc. 2022, 17, 1735–1761. [Google Scholar] [CrossRef] [PubMed]
- Backes, C.; Khaleeq, Q.T.; Meese, E.; Keller, A. miEAA: microRNA enrichment analysis and annotation. Nucleic Acids Res. 2016, 44, W110–W116. [Google Scholar] [CrossRef] [PubMed]
- Kern, F.; Fehlmann, T.; Solomon, J.; Schwed, L.; Grammes, N.; Backes, C.; Van Keuren-Jensen, K.; Craig, D.W.; Meese, E.; Keller, A. miEAA 2.0: Integrating multi-species microRNA enrichment analysis and workflow management systems. Nucleic Acids Res. 2020, 48, W521–W528. [Google Scholar] [CrossRef] [PubMed]
- Jewison, T.; Su, Y.; Disfany, F.M.; Liang, Y.; Knox, C.; Maciejewski, A.; Poelzer, J.; Huynh, J.; Zhou, Y.; Arndt, D.; et al. SMPDB 2.0: Big improvements to the Small Molecule Pathway Database. Nucleic Acids Res. 2014, 42, D478–D484. [Google Scholar] [CrossRef]
- Clarke, J.; Penas, C.; Pastori, C.; Komotar, R.J.; Bregy, A.; Shah, A.H.; Wahlestedt, C.; Ayad, N.G. Epigenetic pathways and glioblastoma treatment. Epigenetics 2013, 8, 785–795. [Google Scholar] [CrossRef]
- Barzegar Behrooz, A.; Talaie, Z.; Jusheghani, F.; Łos, M.J.; Klonisch, T.; Ghavami, S. Wnt and PI3K/Akt/mTOR survival pathways as therapeutic targets in glioblastoma. Int. J. Mol. Sci. 2022, 23, 1353. [Google Scholar] [CrossRef]
- Ruiz-Pérez, M.V.; Henley, A.B.; Arsenian-Henriksson, M. The MYCN protein in health and disease. Genes 2017, 8, 113. [Google Scholar] [CrossRef]
- Orian, J.; Vasilopoulos, K.; Yoshida, S.; Kaye, A.; Chow, C.; Gonzales, M. Overexpression of multiple oncogenes related to histological grade of astrocytic glioma. Br. J. Cancer 1992, 66, 106–112. [Google Scholar] [CrossRef]
- Herms, J.W.; von Loewenich, F.D.; Behnke, J.; Markakis, E.; Kretzschmar, H.A. c-Myc oncogene family expression in glioblastoma and survival. Surg. Neurol. 1999, 51, 536–542. [Google Scholar] [CrossRef]
- Annibali, D.; Whitfield, J.R.; Favuzzi, E.; Jauset, T.; Serrano, E.; Cuartas, I.; Redondo-Campos, S.; Folch, G.; Gonzàlez-Juncà, A.; Sodir, N.M.; et al. Myc inhibition is effective against glioma and reveals a role for Myc in proficient mitosis. Nat. Commun. 2014, 5, 4632. [Google Scholar] [CrossRef] [PubMed]
- Alkema, M.; Wiegant, J.; Raap, A.K.; Bems, A.; van Lohuizen, M. Characterization and chromosomal localization of the human proto-oncogene BMI-1. Hum. Mol. Genet. 1993, 2, 1597–1603. [Google Scholar] [CrossRef] [PubMed]
- Bracken, A.P.; Dietrich, N.; Pasini, D.; Hansen, K.H.; Helin, K. Genome-wide mapping of Polycomb target genes unravels their roles in cell fate transitions. Genes Dev. 2006, 20, 1123–1136. [Google Scholar] [CrossRef]
- Sauvageau, M.; Sauvageau, G. Polycomb group proteins: Multi-faceted regulators of somatic stem cells and cancer. Cell Stem Cell 2010, 7, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Schuringa, J.J.; Vellenga, E. Role of the polycomb group gene BMI1 in normal and leukemic hematopoietic stem and progenitor cells. Curr. Opin. Hematol. 2010, 17, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Valk-Lingbeek, M.E.; Bruggeman, S.W.; van Lohuizen, M. Stem cells and cancer: The polycomb connection. Cell 2004, 118, 409–418. [Google Scholar] [CrossRef]
- Jin, X.; Kim, L.J.; Wu, Q.; Wallace, L.C.; Prager, B.C.; Sanvoranart, T.; Gimple, R.C.; Wang, X.; Mack, S.C.; Miller, T.E. Targeting glioma stem cells through combined BMI1 and EZH2 inhibition. Nat. Med. 2017, 23, 1352–1361. [Google Scholar] [CrossRef]
- Xu, H.; Zong, H.; Ma, C.; Ming, X.; Shang, M.; Li, K.; He, X.; Du, H.; Cao, L. Epidermal growth factor receptor in glioblastoma. Oncol. Lett. 2017, 14, 512–516. [Google Scholar] [CrossRef]
- Ding, J.; Li, X.; Khan, S.; Zhang, C.; Gao, F.; Sen, S.; Wasylishen, A.R.; Zhao, Y.; Lozano, G.; Koul, D.; et al. EGFR suppresses p53 function by promoting p53 binding to DNA-PKcs: A noncanonical regulatory axis between EGFR and wild-type p53 in glioblastoma. Neuro. Oncol. 2022, 24, 1712–1725. [Google Scholar] [CrossRef]
- Tanaka, S.; Batchelor, T.T.; Iafrate, A.J.; Dias-Santagata, D.; Borger, D.R.; Ellisen, L.W.; Yang, D.; Louis, D.N.; Cahill, D.P.; Chi, A.S. PIK3CA activating mutations are associated with more disseminated disease at presentation and earlier recurrence in glioblastoma. Acta Neuropathol. Commun. 2019, 7, 66. [Google Scholar] [CrossRef]
- Jones, G.; Machado, J., Jr.; Tolnay, M.; Merlo, A. PTEN-independent induction of caspase-mediated cell death and reduced invasion by the focal adhesion targeting domain (FAT) in human astrocytic brain tumors which highly express focal adhesion kinase (FAK). Cancer Res. 2001, 61, 5688–5691. [Google Scholar]
- Knobbe, C.B.; Reifenberger, G. Genetic alterations and aberrant expression of genes related to the phosphatidyl-lnositol-3′-kinase/protein kinase B (Akt) signal transduction pathway in glioblastomas. Brain Pathol. 2003, 13, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Scholz, N.; Kurian, K.M.; Siebzehnrubl, F.A.; Licchesi, J.D. Targeting the ubiquitin system in glioblastoma. Front. Oncol. 2020, 10, 574011. [Google Scholar] [CrossRef] [PubMed]
- Delle Donne, R.; Iannucci, R.; Rinaldi, L.; Roberto, L.; Oliva, M.A.; Senatore, E.; Borzacchiello, D.; Lignitto, L.; Giurato, G.; Rizzo, F.; et al. Targeted inhibition of ubiquitin signaling reverses metabolic reprogramming and suppresses glioblastoma growth. Commun. Biol. 2022, 5, 780. [Google Scholar] [CrossRef]
- Li, J.; Sun, Y.; Ma, Y.; Zhao, X.; Sun, X.; Wang, Y.; Zhang, X. Comprehensive Pan-Cancer Analysis of IRAK Family Genes Identifies IRAK1 as a Novel Oncogene in Low-Grade Glioma. J. Oncol. 2022, 2022, 6497241. [Google Scholar] [CrossRef]
- Lehrer, S. Glioblastoma and dementia may share a common cause. Med. Hypotheses 2010, 75, 67–68. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, X.; Mao, L.; Zahid, K.R.; Wen, J.; Zhang, L.; Zhang, M.; Duan, J.; Duan, J.; Yin, X.; et al. Histone deacetylase 1 promotes glioblastoma cell proliferation and invasion via activation of PI3K/AKT and MEK/ERK signaling pathways. Brain Res. 2018, 1692, 154–162. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Chen, J.; Tan, Q.; Xie, C.; Li, C.; Zhan, W.; Wang, M. Silencing of histone deacetylase 2 suppresses malignancy for proliferation, migration, and invasion of glioblastoma cells and enhances temozolomide sensitivity. Cancer Chemother. Pharmacol. 2016, 78, 1289–1296. [Google Scholar] [CrossRef]
- Zhong, S.; Fan, Y.; Wu, B.; Wang, Y.; Jiang, S.; Ge, J.; Hua, C.; Zhao, G.; Chen, Y.; Xu, H. HDAC3 expression correlates with the prognosis and grade of patients with glioma: A diversification analysis based on transcriptome and clinical evidence. World Neurosurg. 2018, 119, e145–e158. [Google Scholar] [CrossRef]
- Chang, H.H.; Chang, Y.-Y.; Tsai, B.-C.; Chen, L.-J.; Chang, A.-C.; Chuang, J.-Y.; Gean, P.-W.; Hsueh, Y.-S. A Selective Histone Deacetylase Inhibitor Induces Autophagy and Cell Death via SCNN1A Downregulation in Glioblastoma Cells. Cancers 2022, 14, 4537. [Google Scholar] [CrossRef]
- Fan, Y.; Peng, X.; Wang, Y.; Li, B.; Zhao, G. Comprehensive analysis of HDAC family Identifies HDAC1 as A Prognostic and Immune Infiltration Indicator and HDAC1-related Signature for Prognosis Estimation in Glioma. Front. Mol. Biosci. 2021, 8, 737. [Google Scholar] [CrossRef]
- Zhang, Y.; Dube, C.; Gibert, M., Jr.; Cruickshanks, N.; Wang, B.; Coughlan, M.; Yang, Y.; Setiady, I.; Deveau, C.; Saoud, K.; et al. The p53 pathway in glioblastoma. Cancers 2018, 10, 297. [Google Scholar] [CrossRef]
- Sukumar, U.K.; Massoud, T.F.; Paulmurugan, R. p53 supplementation as a targeted cancer gene therapy for glioblastoma. In Glioblastoma Resistance to Chemotherapy: Molecular Mechanisms and Innovative Reversal Strategies; Elsevier: Amsterdam, The Netherlands, 2021; pp. 773–786. [Google Scholar]
- Nàger, M.; Sallán, M.C.; Visa, A.; Pushparaj, C.; Santacana, M.; Macià, A.; Yeramian, A.; Cantí, C.; Herreros, J. Inhibition of WNT-CTNNB1 signaling upregulates SQSTM1 and sensitizes glioblastoma cells to autophagy blockers. Autophagy 2018, 14, 619–636. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, J.; Kavoosi, M.; Singh, N.; Lorzadeh, S.; Ravandi, A.; Kidane, B.; Ahmed, N.; Mraiche, F.; Mowat, M.R.; Ghavami, S. Regulation of Autophagy via Carbohydrate and Lipid Metabolism in Cancer. Cancers 2023, 15, 2195. [Google Scholar] [CrossRef]
- Lorzadeh, S.; Kohan, L.; Ghavami, S.; Azarpira, N. Autophagy and the Wnt signaling pathway: A focus on Wnt/beta-catenin signaling. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118926. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.-H.; Cao, Y.-T.; Zhang, L.-L.; Huang, F.; Yi, C. The role of mitochondrial dynamics and mitophagy in carcinogenesis, metastasis and therapy. Front. Cell Dev. Biol. 2020, 8, 413. [Google Scholar] [CrossRef]
- Lv, D.; Gimple, R.C.; Zhong, C.; Wu, Q.; Yang, K.; Prager, B.C.; Godugu, B.; Qiu, Z.; Zhao, L.; Zhang, G.; et al. PDGF signaling inhibits mitophagy in glioblastoma stem cells through N6-methyladenosine. Dev. Cell 2022, 57, 1466–1481. [Google Scholar] [CrossRef]
- Zhang, C.-Z.; Zhang, J.-X.; Zhang, A.-L.; Shi, Z.-D.; Han, L.; Jia, Z.-F.; Yang, W.-D.; Wang, G.-X.; Jiang, T.; You, Y.-P.; et al. MiR-221 and miR-222 target PUMA to induce cell survival in glioblastoma. Mol. Cancer 2010, 9, 229. [Google Scholar] [CrossRef]
- Quintavalle, C.; Mangani, D.; Roscigno, G.; Romano, G.; Diaz-Lagares, A.; Iaboni, M.; Donnarumma, E.; Fiore, D.; De Marinis, P.; Soini, Y.; et al. MiR-221/222 target the DNA methyltransferase MGMT in glioma cells. PLoS ONE 2013, 8, e74466. [Google Scholar] [CrossRef]
- Areeb, Z.; Stuart, S.F.; West, A.J.; Gomez, J.; Nguyen, H.; Paradiso, L.; Zulkifli, A.; Jones, J.; Kaye, A.H.; Morokoff, A.P.; et al. Reduced EGFR and increased miR-221 is associated with increased resistance to temozolomide and radiotherapy in glioblastoma. Sci. Rep. 2020, 10, 17768. [Google Scholar] [CrossRef]
- Wang, K.; Jia, Z.; Zou, J.; Zhang, A.; Wang, G.; Hao, J.; Wang, Y.; Yang, S.; Pu, P. Analysis of hsa-miR-30a-5p expression in human gliomas. Pathol. Oncol. Res. 2013, 19, 405–411. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, X.; Chen, Y.; Sun, C.; Zhu, Q.; Zhao, H.; Liu, G.; Huang, Q.; Lan, Q. MiR-30a-5p is induced by Wnt/β-catenin pathway and promotes glioma cell invasion by repressing NCAM. Biochem. Biophys. Res. Commun. 2015, 465, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Isobe, T.; Hisamori, S.; Hogan, D.J.; Zabala, M.; Hendrickson, D.G.; Dalerba, P.; Cai, S.; Scheeren, F.; Kuo, A.H.; Sikandar, S.S. miR-142 regulates the tumorigenicity of human breast cancer stem cells through the canonical WNT signaling pathway. Elife 2014, 3, e01977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, W.; Kang, Y.; Zhang, J.; Yuan, H. SynCAM, a novel putative tumor suppressor, suppresses growth and invasiveness of glioblastoma. Mol. Biol. Rep. 2013, 40, 5469–5475. [Google Scholar] [CrossRef]
- McManus, E.J.; Frampton, C.; Tan, A.; Phillips, M.C. Metabolics risk factors in a New Zealand glioblastoma cohort. Neuro-Oncol. Pract. 2022, 9, 43–49. [Google Scholar] [CrossRef]
- Liang, R.; Li, J.; Li, M.; Yang, Y.; Wang, X.; Mao, Q.; Liu, Y. Clinical significance of pre-surgical serum lipid levels in patients with glioblastoma. Oncotarget 2017, 8, 85940. [Google Scholar] [CrossRef]
- Pirmoradi, L.; Seyfizadeh, N.; Ghavami, S.; Zeki, A.A.; Shojaei, S. Targeting cholesterol metabolism in glioblastoma: A new therapeutic approach in cancer therapy. J. Investig. Med. 2019, 67, 715–719. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, S.; Yang, Z.; Li, Z.-A.; Hu, W.; Dai, L.; Liang, W.; Wang, X. Cholesterol metabolism and its implication in glioblastoma therapy. J. Cancer 2022, 13, 1745. [Google Scholar] [CrossRef]
- Villa, G.R.; Hulce, J.J.; Zanca, C.; Bi, J.; Ikegami, S.; Cahill, G.L.; Gu, Y.; Lum, K.M.; Masui, K.; Yang, H.; et al. An LXR-cholesterol axis creates a metabolic co-dependency for brain cancers. Cancer Cell 2016, 30, 683–693. [Google Scholar] [CrossRef]
- Taïb, B.; Aboussalah, A.M.; Moniruzzaman, M.; Chen, S.; Haughey, N.J.; Kim, S.F.; Ahima, R.S. Lipid accumulation and oxidation in glioblastoma multiforme. Sci. Rep. 2019, 9, 19593. [Google Scholar] [CrossRef] [PubMed]
- Sperry, J.; Condro, M.C.; Guo, L.; Braas, D.; Vanderveer-Harris, N.; Kim, K.K.; Pope, W.B.; Divakaruni, A.S.; Lai, A.; Christofk, H.; et al. Glioblastoma utilizes fatty acids and ketone bodies for growth allowing progression during ketogenic diet therapy. Iscience 2020, 23, 101453. [Google Scholar] [CrossRef] [PubMed]
- So, J.-S.; Kim, H.; Han, K.-S. Mechanisms of Invasion in Glioblastoma: Extracellular Matrix, Ca2+ Signaling, and Glutamate. Front. Cell Neurosci. 2021, 15, 663092. [Google Scholar] [CrossRef]
- Cappelletti, P.; Tallarita, E.; Rabattoni, V.; Campomenosi, P.; Sacchi, S.; Pollegioni, L. Proline oxidase controls proline, glutamate, and glutamine cellular concentrations in a U87 glioblastoma cell line. PLoS ONE 2018, 13, e0196283. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Sasayama, T.; Nagashima, H.; Irino, Y.; Takahashi, M.; Izumi, Y.; Uno, T.; Satoh, N.; Kitta, A.; Kyotani, K.; et al. Glioma cells require one-carbon metabolism to survive glutamine starvation. Acta Neuropathol. Commun. 2021, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhao, J.; Cui, X.; Zhan, Q.; Yi, K.; Wang, Q.; Xiao, M.; Tan, Y.; Hong, B.; Fang, C. TCA-phospholipid-glycolysis targeted triple therapy effectively suppresses ATP production and tumor growth in glioblastoma. Theranostics 2022, 12, 7032–7050. [Google Scholar] [CrossRef] [PubMed]
- Stanke, K.M.; Wilson, C.; Kidambi, S. High Expression of Glycolytic Genes in Clinical Glioblastoma Patients Correlates with Lower Survival. Front. Mol. Biosci. 2021, 8, 1281. [Google Scholar] [CrossRef] [PubMed]
- Firdous, S.; Abid, R.; Nawaz, Z.; Bukhari, F.; Anwer, A.; Cheng, L.L.; Sadaf, S. Dysregulated alanine as a potential predictive marker of glioma—An insight from untargeted HRMAS-NMR and machine learning data. Metabolites 2021, 11, 507. [Google Scholar] [CrossRef]
- Ijare, O.; Baskin, D.; Pichumani, K. Cbmt-01. Alanine Fuels Energy Metabolism of Glioblastoma Cells. Neuro. Oncol. 2019, 21 (Suppl. S6), vi32. [Google Scholar] [CrossRef]
- Mohan, A.A.; Tomaszewski, W.H.; Haskell-Mendoza, A.P.; Hotchkiss, K.M.; Singh, K.; Reedy, J.L.; Fecci, P.E.; Sampson, J.H.; Khasraw, M. Targeting Immunometabolism in Glioblastoma. Front. Oncol. 2021, 11, 2260. [Google Scholar] [CrossRef]
- Rath, M.; Müller, I.; Kropf, P.; Closs, E.I.; Munder, M. Metabolism via arginase or nitric oxide synthase: Two competing arginine pathways in macrophages. Front. Immunol. 2014, 5, 532. [Google Scholar] [CrossRef]
- Kobayashi, K.; Ohnishi, A.; Promsuk, J.; Shimizu, S.; Kanai, Y.; Shiokawa, Y.; Nagane, M. Enhanced tumor growth elicited by L-type amino acid transporter 1 in human malignant glioma cells. Neurosurgery 2008, 62, 493–504. [Google Scholar] [CrossRef]
- Chinnaiyan, P.; Kensicki, E.; Bloom, G.; Prabhu, A.; Sarcar, B.; Kahali, S.; Eschrich, S.; Qu, X.; Forsyth, P.; Gillies, R. The metabolomic signature of malignant glioma reflects accelerated anabolic metabolism. Cancer Res. 2012, 72, 5878–5888. [Google Scholar] [CrossRef] [PubMed]
- Khoury, O.; Ghazale, N.; Stone, E.; El-Sibai, M.; Frankel, A.E.; Abi-Habib, R.J. Human recombinant arginase I (Co)-PEG5000 [HuArgI (Co)-PEG5000]-induced arginine depletion is selectively cytotoxic to human glioblastoma cells. J. Neuro-Oncol. 2015, 122, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, M.A.; Ijare, O.B.; Baskin, D.S.; Baskin, A.M.; Baskin, B.N.; Pichumani, K. The leloir cycle in glioblastoma: Galactose scavenging and metabolic remodeling. Cancers 2021, 13, 1815. [Google Scholar] [CrossRef]





| Database Name | Type of Data | Purpose |
|---|---|---|
| The Cancer Genome Atlas Research Network (TCGA) | Genomic, Epigenomic, Transcriptomic | Investigate the genetic profile and molecular subtypes of GBM |
| NeDRex plugin version 1.0.0 | Disease Module Detection | Find GBM-related disease modules in the Cytoscape platform |
| MuST Algorithm | Approximate Steiner Tree Calculation | Extract a connected subnetwork engaged in the disease pathways |
| DIAMOnD Algorithm | Disease Module Detection | Determine the disease module surrounding a set of known disease genes or proteins |
| STRING | Proteins | Protein–protein Interaction Networks |
| KEGG | Proteins | Find GBM-related proteins |
| HMDD | miRNAs and proteins | GBM-related miRNAs-proteins Interaction network |
| GlioVis | Over 6500 tumor samples of approximately 50 expression datasets of a large collection of brain tumor entities (mostly gliomas), both adult and pediatric | To analyze the correlation between identified genes based on the TCGA database |
| OSgbm | Transcriptome profiles and clinical information from The Cancer Genome Atlas (TCGA), Gene Expression Omnibus (GEO), and Chinese Glioma Genome Atlas (CGGA). | An online consensus survival analysis web server |
| 1 | VEGF signaling pathway-hsa04370 | 40 | TNF signaling pathway-hsa04668 |
| 2 | PI3K-Akt signaling pathway-hsa04151 | 41 | Citrate cycle (T.C.A. cycle)-hsa00020 |
| 3 | Ras signaling pathway- hsa04014 | 42 | Glycolysis/Gluconeogenesis-hsa00010 |
| 4 | TGF-beta signaling pathway-hsa04350 | 43 | Oxidative phosphorylation-hsa00190 |
| 5 | HIF-1 signaling pathway-hsa04066 | 44 | Starch and sucrose metabolism-hsa00500 |
| 6 | AMPK signaling pathway-hsa04152 | 45 | Pentose phosphate pathway-hsa00030 |
| 7 | MAPK signaling pathway-hsa04010 | 46 | Pyruvate metabolism-hsa00620 |
| 8 | Rap1 signaling pathway-hsa04015 | 47 | Insulin signaling pathway-hsa04910 |
| 9 | Wnt signaling pathway-hsa04310 | 48 | Lysosome-hsa04142 |
| 10 | Notch signaling pathway-hsa04330 | 49 | Phospholipase D signaling pathway-hsa04072 |
| 11 | Hedgehog signaling pathway-hsa04340 | 50 | Mitophagy- hsa04137 |
| 12 | Hippo signaling pathway-hsa04390 | 51 | Signaling pathways regulating pluripotency of stem cells- hsa04550 |
| 13 | JAK-STAT signaling pathway-hsa04630 | 52 | Cell adhesion molecules-hsa04514 |
| 14 | Apelin signaling pathway-hsa04371 | 53 | Cell cycle -hsa04110 |
| 15 | NF-kappa B signaling pathway-hsa04064 | 54 | ECM-receptor interaction-hsa04512 |
| 16 | TNF signaling pathway-hsa04668 | 55 | PD-L1 expression and PD-1 checkpoint pathway in cancer- hsa05235 |
| 17 | FoxO signaling pathway-hsa04068 | 56 | Pathways in cancer-hsa05200 |
| 18 | Phosphatidylinositol signaling system-hsa04070 | 57 | Transcriptional misregulation in cancer-hsa05202 |
| 19 | mTOR signaling pathway-hsa04150 | 58 | Central carbon metabolism in cancer-hsa05230 |
| 20 | p53 signaling pathway-hsa04115 | 59 | IL-17 signaling pathway-hsa04657 |
| 21 | Apoptosis-hsa04210 | 60 | Necroptosis-hsa04217 |
| 22 | Ubiquitin-mediated proteolysis-hsa04120 | 61 | Cellular senescence-hsa04218 |
| 23 | Cell cycle-hsa04110 | 62 | Chemokine signaling pathway-hsa04062 |
| 24 | Regulation of actin cytoskeleton-hsa04810 | 63 | Transcriptional misregulation in cancer-hsa05202 |
| 25 | Calcium signaling pathway-hsa04020 | 64 | ECM-receptor interaction-hsa04512 |
| 26 | T cell receptor signaling pathway-hsa04660 | 65 | Proteoglycans in cancer-hsa05205 |
| 27 | Focal adhesion-hsa04510 | 66 | Choline metabolism in cancer-hsa05231 |
| 28 | Adherens junction-hsa04520 | 67 | PD-L1 expression and PD-1 checkpoint pathway in cancer-hsa05235 |
| 29 | Gap junction-hsa04540 | 68 | Ferroptosis-hsa04216 |
| 30 | Tight junction-hsa04530 | 69 | Cholesterol metabolism-map04979 |
| 31 | Arachidonic acid metabolism-hsa00590 | 70 | Lipid and atherosclerosis-map05417 |
| 32 | Autophagy-hsa04140 | 71 | Fat digestion and absorption-map04975 |
| 33 | Regulation of lipolysis in adipocytes-hsa04923 | 72 | Vitamin digestion and absorption-map04977 |
| 34 | Cytokine-cytokine receptor interaction-hsa04060 | 73 | Aldosterone synthesis and secretion-map04925 |
| 35 | Proteasome- hsa03050 | 74 | Primary bile acid biosynthesis-map00120 |
| 36 | B cell receptor signaling pathway-hsa04662 | 75 | Cortisol synthesis and secretion-map04927 |
| 37 | Complement and coagulation cascades-hsa04610 | 76 | Bile secretion-map04976 |
| 38 | Toll-like receptor signaling pathway-hsa04620 | 77 | Ovarian steroidogenesis-map04913 |
| 39 | RIG-I-like receptor signaling pathway-hsa04622 | 78 | Steroid biosynthesis-map00100 |
| Gene Name | Description | Deg | Bet | Bridg | Cent | Close | EiVe |
|---|---|---|---|---|---|---|---|
| UBC | Ubiquitin C [Source: HGNC Symbol; Acc: HGNC:12468 | + | + | -- | -- | + | + |
| HDAC1 | Histone deacetylase 1 [Source: HGNC Symbol; Acc: HGNC:4852 | + | -- | -- | -- | + | + |
| CTNNB1 | Catenin beta 1 [Source: HGNC Symbol; Acc: HGNC:2514 | + | -- | -- | -- | + | + |
| TRIM28 | Tripartite motif-containing 28 [Source: HGNC Symbol; Acc: HGNC:16384 | -- | + | -- | -- | + | + |
| CSNK2A1 | casein kinase two alpha 1 [Source: HGNC Symbol; Acc: HGNC:2457 | -- | -- | -- | -- | + | + |
| RBBP4 | RB binding protein 4, chromatin remodeling factor [Source: HGNC Symbol; Acc: HGNC:9887 | + | -- | -- | -- | -- | -- |
| TP53 | Tumor protein p53 [Source:HGNC Symbol;Acc:HGNC:11998 | + | -- | -- | -- | -- | -- |
| APP | Amyloid beta precursor protein [Source: HGNC Symbol; Acc: HGNC:620 | -- | + | -- | -- | -- | -- |
| DAB1 | DAB1, reelin adaptor protein [Source: HGNC Symbol; Acc: HGNC:2661 | -- | + | -- | -- | -- | -- |
| PINK1 | PTEN-induced putative kinase 1 [Source: HGNC Symbol; Acc: HGNC:14581 | -- | + | -- | -- | -- | -- |
| RELN | Reelin | literature review + miRNA-gene regulatory network | |||||
| Label | Degree | Betweenness |
|---|---|---|
| hsa-mir-221-3p | 4 | 5682.13 |
| hsa-mir-30a-5p | 4 | 2373.43 |
| hsa-mir-15a-5p | 3 | 3710.08 |
| hsa-mir-130a-3p | 3 | 3589.18 |
| hsa-let-7b-5p | 2 | 2523.74 |
| Gene Name | Non-Tumor | GBM | Pairwise t-Test (GBM-Non-Tumor) p.adj (p-Value with Bonferroni Correction) | Primary | Secondary | Recurrent |
|---|---|---|---|---|---|---|
| UBC | -- | + | 1.8 × 10−3 | + | -- | -- |
| HDAC 1 | -- | + | 7.8 × 10−18 | + | -- | -- |
| CTNNB1 | -- | + | 6.0 × 10−3 | + | -- | -- |
| TRIM28 | -- | + | 1.1 × 10−3 | + | -- | -- |
| CSNK2A1 | -- | + | 6.9 × 10−1 (ns) | + | -- | -- |
| RBBP4 | -- | + | 3.2 × 10−5 | + | -- | -- |
| TP53 | -- | + | 1.6 × 10−13 | + | -- | -- |
| APP | + | -- | 1.2 × 10−3 | + | -- | -- |
| DAB1 | + | -- | 4.0 × 10−4 | + | -- | -- |
| PINK1 | + | -- | 2.9 × 10−10 | + | -- | -- |
| RELN | + | -- | 5.7 × 10−8 | + | -- | -- |
| Result | Visualization Methods | Enrichment Method | Topology Analysis | Reference Metabolome | Pathway Library |
|---|---|---|---|---|---|
| 1 | Scatter plot | Hypergeometric test | Relative-betweenness centrality R-b C | All compounds in the selected pathway library | Homo sapiens (KEGG) |
| 2 | Scatter plot | Hypergeometric test | Out-degree Centrality O-d C | All combinations in the selected pathway library | Homo sapiens (KEGG) |
| 3 | Scatter plot | Hypergeometric test | Relative-betweenness centrality | All compounds in the selected pathway library | Homo sapiens (SMPDB) |
| 4 | Scatter plot | Hypergeometric test | Out-degree Centrality | All combinations in the selected pathway library | Homo sapiens (SMPDB) |
| KEGG Database | SMPDB Database | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Result 1 | R-b C Impact | FDR | Result 2 | O-d C Impact | FDR | Result 3 | R-b C Impact | FDR | Result 4 | O-d C Impact | FDR |
| Final Decision (FD) | Final Decision (FD) | Final Decision (FD) | Final Decision (FD) | ||||||||
| Nitrogen metabolism | 1 | 0.043213 | Arginine biosynthesis | 0.8125 | 1.90 × 10−7 | Alanine metabolism | 1 | 0.010641 | Malate-aspartate shuttle | 0.63333 | 0.013128 |
| FD: + | FD: -- | FD: + | FD: + | ||||||||
| Phenylalanine, tyrosine, and tryptophan biosynthesis | 1 | 0.12885 | Alanine, aspartate, and glutamate metabolism | 0.75 | 1.73 × 10−7 | Trehalose degradation | 0.84211 | 0.18355 | Phosphatidylcholine biosynthesis | 0.56707 | 0.00011577 |
| FD: -- | FD: + | FD: -- | FD: -- | ||||||||
| Synthesis and degradation of ketone bodies | 0.86667 | 0.18716 | Valine, leucine, and isoleucine biosynthesis | 0.75 | 8.82 × 10−5 | Aspartate metabolism | 0.8 | 0.0044894 | Transfer of acetyl groups into mitochondria | 0.54167 | 0.010641 |
| FD: -- | FD: -- | FD: + | FD: -- | ||||||||
| Alanine, aspartate, and glutamate metabolism | 0.81732 | 1.73 × 10−7 | Nitrogen metabolism | 0.75 | 0.043213 | Glycerol phosphate shuttle | 0.7619 | 0.3023 | Ammonia recycling | 0.49306 | 0.00011577 |
| FD: + | FD: + | FD: -- | FD: -- | ||||||||
| One-carbon pool by folate | 0.80793 | 0.46957 | Phenylalanine, tyrosine, and tryptophan biosynthesis | 0.75 | 0.12885 | Malate-Aspartate Shuttle | 0.71429 | 0.013128 | Cardiolipin biosynthesis | 0.49057 | 0.013128 |
| FD: -- | FD: -- | FD: + | FD: -- | ||||||||
| Result | Enrichment Method | Topology Measure | Integration Method |
|---|---|---|---|
| 1 | Hypergeometric test | Degree centrality | Combined score |
| 2 | Betweenness centrality | ||
| 3 | Closeness centrality |
| Title | Degree | Betweenness | Closeness |
|---|---|---|---|
| Alanine, aspartate and glutamate metabolism | + | + | -- |
| Citrate cycle (TCA cycle) | + | + | + |
| Arginine biosynthesis | + | + | + |
| Synthesis and degradation of ketone bodies | + | -- | + |
| Pyruvate metabolism | + | -- | + |
| Purine metabolism | + | + | -- |
| Glutathione metabolism | + | + | -- |
| Pyrimidine metabolism | + | + | -- |
| Glycolysis or gluconeogenesis | -- | + | + |
| Enrichment Analysis | Pathway Analysis | Joint Pathway Analysis | ||
|---|---|---|---|---|
| Eleven Genes | Five miRNAs | 182 Metabolites | ||
| Mitophagy | Fatty acid biosynthesis | Aminoacyl-tRNA biosynthesis | Nitrogen metabolism | Citrate cycle (TCA cycle) |
| Wnt signaling pathway | Galactose metabolism | Arginine biosynthesis | Alanine, aspartate and glutamate metabolism | Arginine biosynthesis |
| Mucin-type O-glycan biosynthesis | Alanine, aspartate and glutamate metabolism | Malate-aspartate shuttle | ||
| Autophagy | Glutamate metabolism | |||
| Urea cycle | ||||
| Arginine and proline metabolism | ||||
| Metabolite | SNP |
|---|---|
| HDL | rs111929233, rs7298751 |
| N6-acetyllysine | rs12602273, rs12603869, rs12945970, rs12947788, rs12949655, rs12951053, rs1642782, rs17881556, rs1794284, rs2078486, rs5819163 |
| Cholesterol | rs35608584, rs111929233 |
| Formate | rs17520463 |
| N, N-Dimethylglycine/Xylose | rs41450451 |
| X2.piperidinone | rs75787097, rs75524270, rs79232054, rs145435197, rs74901488, rs117235978 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barzegar Behrooz, A.; Latifi-Navid, H.; da Silva Rosa, S.C.; Swiat, M.; Wiechec, E.; Vitorino, C.; Vitorino, R.; Jamalpoor, Z.; Ghavami, S. Integrating Multi-Omics Analysis for Enhanced Diagnosis and Treatment of Glioblastoma: A Comprehensive Data-Driven Approach. Cancers 2023, 15, 3158. https://doi.org/10.3390/cancers15123158
Barzegar Behrooz A, Latifi-Navid H, da Silva Rosa SC, Swiat M, Wiechec E, Vitorino C, Vitorino R, Jamalpoor Z, Ghavami S. Integrating Multi-Omics Analysis for Enhanced Diagnosis and Treatment of Glioblastoma: A Comprehensive Data-Driven Approach. Cancers. 2023; 15(12):3158. https://doi.org/10.3390/cancers15123158
Chicago/Turabian StyleBarzegar Behrooz, Amir, Hamid Latifi-Navid, Simone C. da Silva Rosa, Maciej Swiat, Emilia Wiechec, Carla Vitorino, Rui Vitorino, Zahra Jamalpoor, and Saeid Ghavami. 2023. "Integrating Multi-Omics Analysis for Enhanced Diagnosis and Treatment of Glioblastoma: A Comprehensive Data-Driven Approach" Cancers 15, no. 12: 3158. https://doi.org/10.3390/cancers15123158
APA StyleBarzegar Behrooz, A., Latifi-Navid, H., da Silva Rosa, S. C., Swiat, M., Wiechec, E., Vitorino, C., Vitorino, R., Jamalpoor, Z., & Ghavami, S. (2023). Integrating Multi-Omics Analysis for Enhanced Diagnosis and Treatment of Glioblastoma: A Comprehensive Data-Driven Approach. Cancers, 15(12), 3158. https://doi.org/10.3390/cancers15123158










