Intolerance of Uncertainty and Cognition in Breast Cancer Survivors: The Mediating Role of Anxiety
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Sample
- Female with a stage I–III breast cancer diagnosis;
- Between 1 and 4 years post-breast-cancer diagnosis;
- Post-menopausal (defined as at least 1-year post-menses);
- Aged 45–75 years;
- No diagnosis of diabetes;
- Ability to access and use internet resources, including video calls using the Zoom platform;
- Ability to read and understand English.
2.2. Recruitment and Enrollment
2.3. Data Collection
2.4. Data Analysis
3. Results
3.1. Sample Characteristics
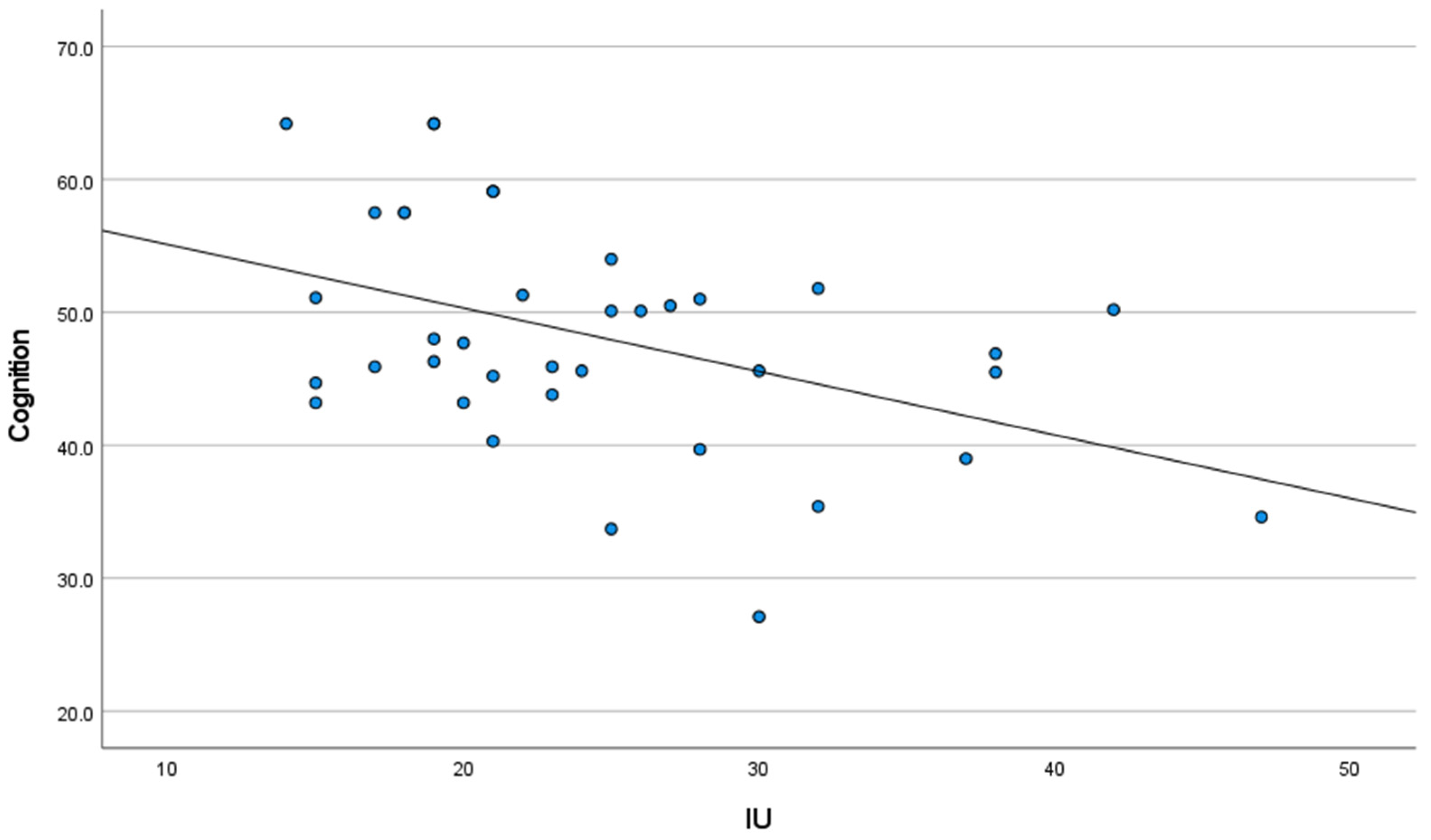
3.2. Correlation Analysis between IU, Anxiety, and Self-Reported Cognitive Function
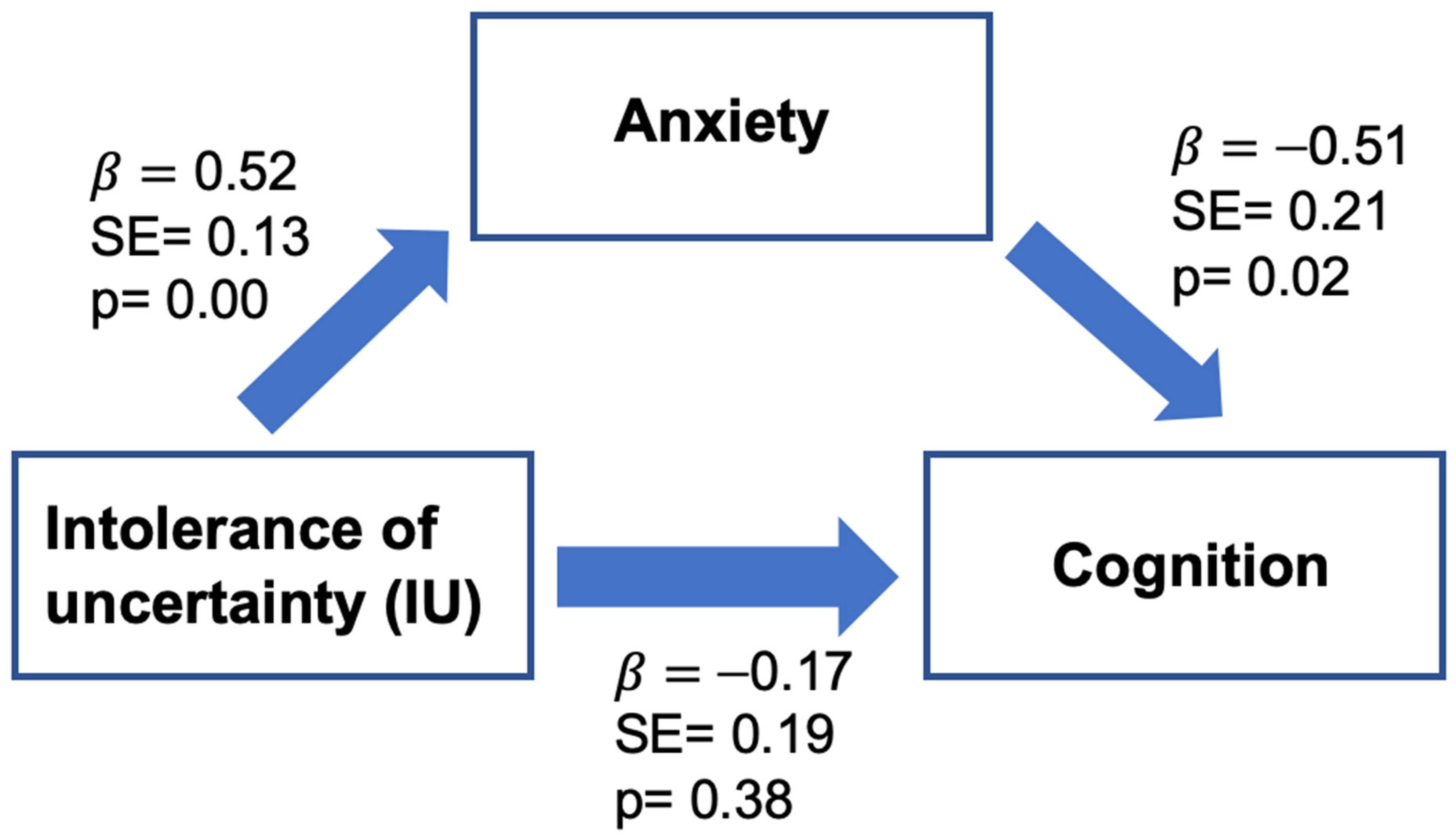
3.3. The Mediation Effect of Anxiety on the Association between IU and Self-Reported Cognitive Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Janelsins, M.C.; Kesler, S.R.; Ahles, T.A.; Morrow, G.R. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int. Rev. Psychiatry 2014, 26, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Van Dyk, K.; Ganz, P.A. Cancer-Related Cognitive Impairment in Patients With a History of Breast Cancer. JAMA 2021, 326, 1736–1737. [Google Scholar] [CrossRef] [PubMed]
- Asher, A. Cognitive dysfunction among cancer survivors. Am. J. Phys. Med. Rehabil. 2011, 90 (Suppl. S1), S16–S26. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.; Dorajoo, S.R.; Cheung, Y.T.; Lam, Y.C.; Yeo, H.L.; Shwe, M.; Gan, Y.X.; Foo, K.M.; Loh, W.-J.K.; Koo, S.-L.; et al. Distinct and heterogeneous trajectories of self-perceived cognitive impairment among Asian breast cancer survivors. Psychooncology 2018, 27, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Ahles, T.A.; Root, J.C.; Ryan, E.L. Cancer- and cancer treatment-associated cognitive change: An update on the state of the science. J. Clin. Oncol. 2012, 30, 3675–3686. [Google Scholar] [CrossRef] [PubMed]
- Koppelmans, V.; Vernooij, M.W.; Boogerd, W.; Seynaeve, C.; Ikram, M.A.; Breteler, M.M.; Schagen, S.B. Prevalence of cerebral small-vessel disease in long-term breast cancer survivors exposed to both adjuvant radiotherapy and chemotherapy. J. Clin. Oncol. 2015, 33, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Wefel, J.S.; Schagen, S.B. Chemotherapy-related cognitive dysfunction. Curr. Neurol. Neurosci. Rep. 2012, 12, 267–275. [Google Scholar] [CrossRef]
- Birrell, J.; Meares, K.; Wilkinson, A.; Freeston, M. Toward a definition of intolerance of uncertainty: A review of factor analytical studies of the Intolerance of Uncertainty Scale. Clin. Psychol. Rev. 2011, 31, 1198–1208. [Google Scholar] [CrossRef]
- Carleton, R.N. The intolerance of uncertainty construct in the context of anxiety disorders: Theoretical and practical perspectives. Expert Rev. Neurother. 2012, 12, 937–947. [Google Scholar] [CrossRef]
- Carleton, R.N.; Norton, M.A.P.J.; Asmundson, G.J.G. Fearing the unknown: A short version of the Intolerance of Uncertainty Scale. J. Anxiety Disord. 2007, 21, 105–117. [Google Scholar] [CrossRef]
- Nicholas Carleton, R.; Sharpe, D.; Asmundson, G.J.G. Anxiety sensitivity and intolerance of uncertainty: Requisites of the fundamental fears? Behav. Res. Ther. 2007, 45, 2307–2316. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, P.M.; Mahoney, A.E.J. Achieving certainty about the structure of intolerance of uncertainty in a treatment-seeking sample with anxiety and depression. J. Anxiety Disord. 2011, 25, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Greco, V.; Roger, D. Uncertainty, stress, and health. Personal. Individ. Differ. 2003, 34, 1057–1068. [Google Scholar] [CrossRef]
- Barlow, D.H. Anxiety and Its Disorders: The Nature and Treatment of Anxiety and Panic, 2nd ed.; The Guilford Press: New York, NY, USA, 2002; p. xvi, 704. [Google Scholar]
- Barlow, D.H.; Sauer-Zavala, S.; Carl, J.R.; Bullis, J.R.; Ellard, K.K. The nature, diagnosis, and treatment of neuroticism: Back to the future. Clin. Psychol. Sci. 2014, 2, 344–365. [Google Scholar] [CrossRef]
- Grupe, D.W.; Nitschke, J.B. Uncertainty and anticipation in anxiety: An integrated neurobiological and psychological perspective. Nat. Rev. Neurosci. 2013, 14, 488–501. [Google Scholar] [CrossRef]
- Anderson, E.C.; Carleton, R.N.; Diefenbach, M.; Han, P.K.J. The Relationship Between Uncertainty and Affect. Front. Psychol. 2019, 10, 2504. [Google Scholar] [CrossRef]
- Tanovic, E.; Pruessner, L.; Joormann, J. Attention and anticipation in response to varying levels of uncertain threat: An ERP study. Cogn. Affect. Behav. Neurosci. 2018, 18, 1207–1220. [Google Scholar] [CrossRef]
- Eysenck, M.W.; Derakhshan, N.; Santos, R.; Calvo, M. Anxiety and cognitive performance: Attentional control theory. Emotion 2007, 7, 336–353. [Google Scholar] [CrossRef]
- Eysenck, M.W.; Derakshan, N. New perspectives in attentional control theory. Personal. Individ. Differ. 2011, 50, 955–960. [Google Scholar] [CrossRef]
- Maloney, E.A.; Sattizahn, J.R.; Beilock, S.L. Anxiety and cognition. Wiley Interdiscip. Rev. Cogn. Sci. 2014, 5, 403–411. [Google Scholar] [CrossRef]
- Moran, T.P. Anxiety and working memory capacity: A meta-analysis and narrative review. Psychol. Bull. 2016, 142, 831–864. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.L.; Mishel, M.H.; Germino, B.B. Living with cancer-related uncertainty: Associations with fatigue, insomnia, and affect in younger breast cancer survivors. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2014, 22, 2489–2495. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, J.P.; Choppala, S.; Hamann, H.A.; Gjerde, J. Uncertainty during the transition from cancer patient to survivor. Cancer Nurs. 2009, 32, E8–E14. [Google Scholar] [CrossRef] [PubMed]
- Krok-Schoen, J.L.; Naughton, M.J.; Bernardo, B.M.; Young, G.S.; Paskett, E.D. Fear of recurrence among older breast, ovarian, endometrial, and colorectal cancer survivors: Findings from the WHI LILAC study. Psychooncology 2018, 27, 1810–1815. [Google Scholar] [CrossRef]
- Yang, Y.; Li, W.; Wen, Y.; Wang, H.; Sun, H.; Liang, W.; Zhang, B.; Humphris, G. Fear of cancer recurrence in adolescent and young adult cancer survivors: A systematic review of the literature. Psychooncology 2019, 28, 675–686. [Google Scholar] [CrossRef]
- Dijkshoorn, A.B.C.; van Stralen, H.E.; Sloots, M.; Schagen, S.B.; Visser-Meily, J.M.A.; Schepers, V.P.M. Prevalence of cognitive impairment and change in patients with breast cancer: A systematic review of longitudinal studies. Psychooncology 2021, 30, 635–648. [Google Scholar] [CrossRef]
- Whittaker, A.L.; George, R.P.; O’Malley, L. Prevalence of cognitive impairment following chemotherapy treatment for breast cancer: A systematic review and meta-analysis. Sci. Rep. 2022, 12, 2135. [Google Scholar] [CrossRef]
- Cella, D.; Riley, W.; Stone, A.; Rothrock, N.; Reeve, B.; Yount, S.; Amtmann, D.; Bode, R.; Buysse, D.; Choi, S.; et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J. Clin. Epidemiol. 2010, 63, 1179–1194. [Google Scholar] [CrossRef]
- Schalet, B.D.; Cook, K.F.; Choi, S.W.; Cella, D. Establishing a common metric for self-reported anxiety: Linking the MASQ, PANAS, and GAD-7 to PROMIS Anxiety. J. Anxiety Disord. 2014, 28, 88–96. [Google Scholar] [CrossRef]
- Cella, D.; Lai, J.-S.; Nowinski, C.J.; Victorson, D.; Peterman, A.; Miller, D.; Bethoux, F.; Heinemann, A.; Rubin, S.; Cavazos, J.E.; et al. Neuro-QOL: Brief measures of health-related quality of life for clinical research in neurology. Neurology 2012, 78, 1860–1867. [Google Scholar] [CrossRef]
- Hayes, A.F.; Rockwood, N.J. Conditional process analysis: Concepts, computation, and advances in the modeling of the contingencies of mechanisms. Am. Behav. Sci. 2020, 64, 19–54. [Google Scholar] [CrossRef]
- Shrout, P.E.; Bolger, N. Mediation in experimental and nonexperimental studies: New procedures and recommendations. Psychol. Methods 2002, 7, 422–445. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach; The Guilford Press: New York, NY, USA, 2022. [Google Scholar]
- Hayes, A.F.; Scharkow, M. The relative trustworthiness of inferential tests of the indirect effect in statistical mediation analysis: Does method really matter? Psychol. Sci. 2013, 24, 1918–1927. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.D. How do you feel—Now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009, 10, 59–70. [Google Scholar] [CrossRef]
- Paulus, M.P.; Stein, M.B. An insular view of anxiety. Biol. Psychiatry 2006, 60, 383–387. [Google Scholar] [CrossRef]
- Gorka, S.M.; Huggins, A.A.; Fitzgerald, D.A.; Nelson, B.D.; Phan, K.L.; Shankman, S.A. Neural response to reward anticipation in those with depression with and without panic disorder. J. Affect. Disord. 2014, 164, 50–56. [Google Scholar] [CrossRef]
- Straube, T.; Mentzel, H.J.; Miltner, W.H. Waiting for spiders: Brain activation during anticipatory anxiety in spider phobics. Neuroimage 2007, 37, 1427–1436. [Google Scholar] [CrossRef]
- Khorrami, K.J.; Manzler, C.A.; Kreutzer, K.A.; Gorka, S.M. Neural and Self-report Measures of Sensitivity to Uncertainty as Predictors of COVID-Related Negative Affect. Psychiatry Res. Neuroimaging 2022, 319, 111414. [Google Scholar] [CrossRef]
- Gorka, S.M.; Nelson, B.D.; Phan, K.L.; Shankman, S.A. Intolerance of uncertainty and insula activation during uncertain reward. Cogn. Affect. Behav. Neurosci. 2016, 16, 929–939. [Google Scholar] [CrossRef]
- Haase, L.; Thom, N.J.; Shukla, A.; Davenport, P.W.; Simmons, A.N.; Stanley, E.A.; Paulus, M.P.; Johnson, D.C. Mindfulness-based training attenuates insula response to an aversive interoceptive challenge. Soc. Cogn. Affect. Neurosci. 2014, 11, 182–190. [Google Scholar] [CrossRef]
- Menon, V.; Uddin, L.Q. Saliency, switching, attention and control: A network model of insula function. Brain Struct. Funct. 2010, 214, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Joly, F.; Vardy, J.; Ahles, T.; Dubois, M.; Tron, L.; Winocur, G.; De Ruiter, M.B.; Castel, H. Cancer-related cognitive impairment: An update on state of the art, detection, and management strategies in cancer survivors. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1925–1940. [Google Scholar] [CrossRef] [PubMed]
- Gorka, S.M.; Kreutzer, K.A.; Petrey, K.M.; Radoman, M.; Phan, K.L. Behavioral and neural sensitivity to uncertain threat in individuals with alcohol use disorder: Associations with drinking behaviors and motives. Addict. Biol. 2020, 25, e12774. [Google Scholar] [CrossRef]
- Radoman, M.; Lieberman, L.; Jimmy, J.; Gorka, S.M. Shared and unique neural circuitry underlying temporally unpredictable threat and reward processing. Soc. Cogn. Affect. Neurosci. 2021, 16, 370–382. [Google Scholar] [CrossRef]
- Schmitz, A.; Grillon, C. Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test). Nat. Protoc. 2012, 7, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Klumpp, H.; Angstadt, M.; Phan, K.L. Insula reactivity and connectivity to anterior cingulate cortex when processing threat in generalized social anxiety disorder. Biol. Psychol. 2012, 89, 273–276. [Google Scholar] [CrossRef]
- Morriss, J. What do I do now? Intolerance of uncertainty is associated with discrete patterns of anticipatory physiological responding to different contexts. Psychophysiology 2019, 56, e13396. [Google Scholar] [CrossRef]
- Bennett, K.P.; Dickmann, J.S.; Larson, C.L. If or when? Uncertainty’s role in anxious anticipation. Psychophysiology 2018, 55, e13066. [Google Scholar] [CrossRef]
- Kucherer, S.; Ferguson, R.J. Cognitive behavioral therapy for cancer-related cognitive dysfunction. Curr. Opin. Support Palliat. Care 2017, 11, 46–51. [Google Scholar] [CrossRef]
- Ladouceur, R.; Dugas, M.J.; Freeston, M.H.; Léger, E.; Gagnon, F.; Thibodeau, N. Efficacy of a cognitive-behavioral treatment for generalized anxiety disorder: Evaluation in a controlled clinical trial. J. Consult. Clin. Psychol. 2000, 68, 957–964. [Google Scholar] [CrossRef]
- van der Heiden, C.; Muris, P.; van der Molen, H.T. Randomized controlled trial on the effectiveness of metacognitive therapy and intolerance-of-uncertainty therapy for generalized anxiety disorder. Behav. Res. Ther. 2012, 50, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Lien, C.Y.; Lin, H.R.; Kuo, I.T.; Chen, M.L. Perceived uncertainty, social support and psychological adjustment in older patients with cancer being treated with surgery. J. Clin. Nurs. 2009, 18, 2311–2319. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Chen, X.; Yi, H.; Luo, Y.; Yan, Q.; Feng, T.; He, Q.; Lei, X.; Qiu, J.; Chen, H. Stronger functional network connectivity and social support buffer against negative affect during the COVID-19 outbreak and after the pandemic peak. Neurobiol. Stress 2021, 15, 100418. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Sun, D.; Sun, J. Social Support and Fear of Cancer Recurrence Among Chinese Breast Cancer Survivors: The Mediation Role of Illness Uncertainty. Front. Psychol. 2022, 13, 864129. [Google Scholar] [CrossRef]
- Eisenberger, N.I. An empirical review of the neural underpinnings of receiving and giving social support: Implications for health. Psychosom. Med. 2013, 75, 545–556. [Google Scholar] [CrossRef]


| n | % | |
|---|---|---|
| Race/Ethnicity | ||
| Non-Hispanic White | 35 | 92 |
| Black or African American | 3 | 8 |
| Education | ||
| Associate degree | 5 | 13 |
| Some college course work completed | 3 | 8 |
| Bachelor’s degree | 14 | 37 |
| Advanced degree (master, doctorate, medical, etc.) | 16 | 42 |
| Marital status | ||
| Single (unmarried, divorced, widowed, etc.) | 10 | 26 |
| Married or cohabiting with partner | 28 | 74 |
| Employment status | ||
| Work 40+ hours a week | 17 | 45 |
| Work fewer than 40 h a week | 9 | 24 |
| Homemaker | 1 | 2 |
| Retired | 11 | 29 |
| Cancer stage | ||
| I | 20 | 53 |
| II | 9 | 24 |
| III | 8 | 21 |
| Unknown/I do not know | 1 | 2 |
| Cancer treatment (select all that apply) | ||
| Surgery—lumpectomy/partial mastectomy | 18 | 47 |
| Surgery—total (simple) mastectomy | 13 | 34 |
| Surgery—modified radical mastectomy | 7 | 18 |
| Radiation | 25 | 66 |
| Chemotherapy | 22 | 58 |
| Anti-hormone therapy | 27 | 71 |
| Targeted therapy | 7 | 18 |
| Immunotherapy | 1 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Gorka, S.M.; Pennell, M.L.; Weinhold, K.; Orchard, T. Intolerance of Uncertainty and Cognition in Breast Cancer Survivors: The Mediating Role of Anxiety. Cancers 2023, 15, 3105. https://doi.org/10.3390/cancers15123105
Yang Y, Gorka SM, Pennell ML, Weinhold K, Orchard T. Intolerance of Uncertainty and Cognition in Breast Cancer Survivors: The Mediating Role of Anxiety. Cancers. 2023; 15(12):3105. https://doi.org/10.3390/cancers15123105
Chicago/Turabian StyleYang, Yesol, Stephanie M. Gorka, Michael L. Pennell, Kellie Weinhold, and Tonya Orchard. 2023. "Intolerance of Uncertainty and Cognition in Breast Cancer Survivors: The Mediating Role of Anxiety" Cancers 15, no. 12: 3105. https://doi.org/10.3390/cancers15123105
APA StyleYang, Y., Gorka, S. M., Pennell, M. L., Weinhold, K., & Orchard, T. (2023). Intolerance of Uncertainty and Cognition in Breast Cancer Survivors: The Mediating Role of Anxiety. Cancers, 15(12), 3105. https://doi.org/10.3390/cancers15123105





