Delta Radiomic Analysis of Mesorectum to Predict Treatment Response and Prognosis in Locally Advanced Rectal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Image Analysis
2.3. Radiomic Analysis
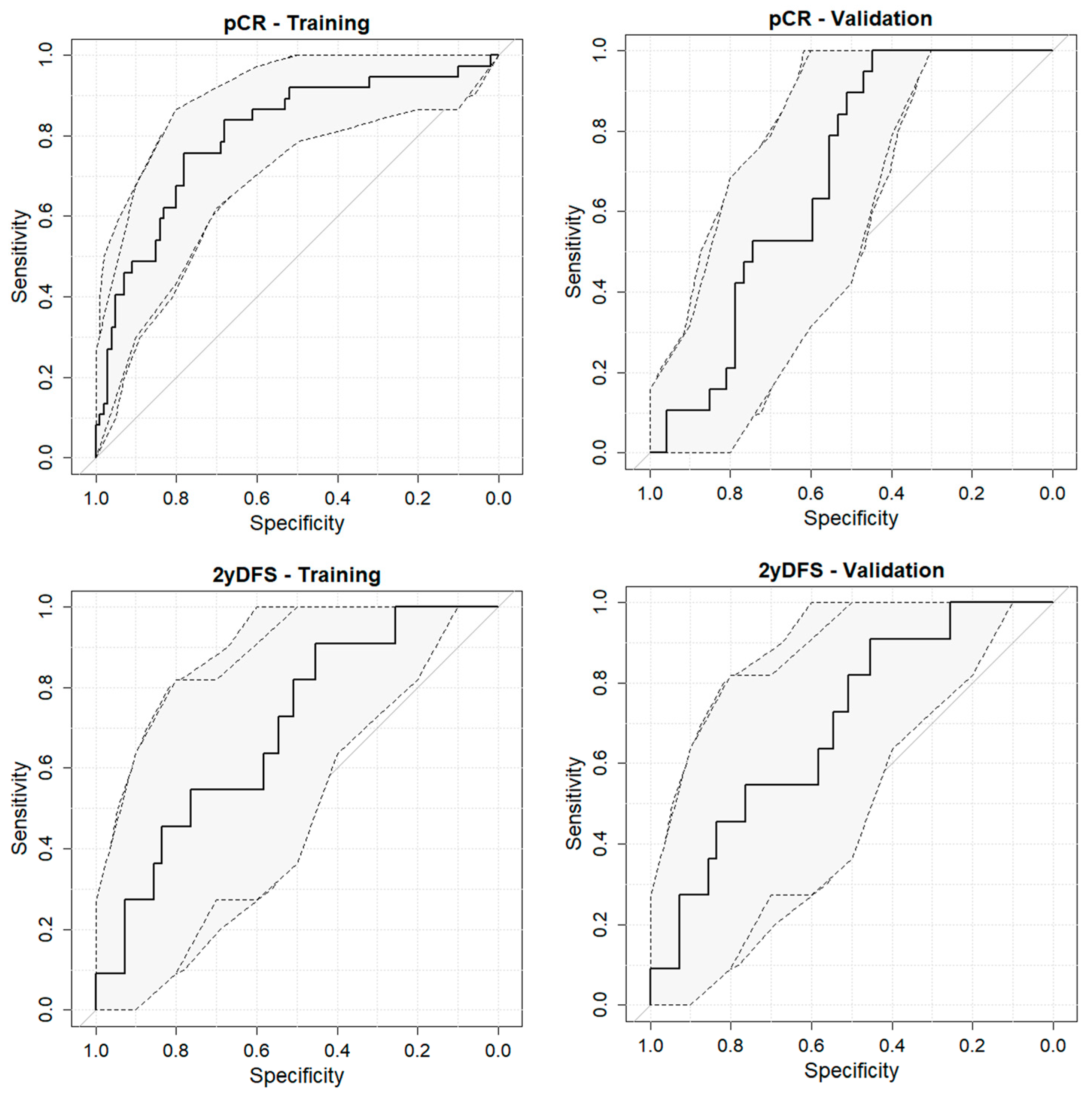
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel, C.; Cervantes, A.; Arnold, D. Rectal Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up†. Ann. Oncol. 2017, 28, iv22–iv40. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Reid, T.; Larson, C.; Knox, S.J. Locally Advanced Rectal Cancer: The Past, Present, and Future. Semin. Oncol. 2020, 47, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Kapiteijn, E.; Marijnen, C.A.; Nagtegaal, I.D.; Putter, H.; Steup, W.H.; Wiggers, T.; Rutten, H.J.; Pahlman, L.; Glimelius, B.; van Krieken, J.H.; et al. Preoperative Radiotherapy Combined with Total Mesorectal Excision for Resectable Rectal Cancer. N. Engl. J. Med. 2001, 345, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Sauer, R.; Becker, H.; Hohenberger, W.; Rödel, C.; Wittekind, C.; Fietkau, R.; Martus, P.; Tschmelitsch, J.; Hager, E.; Hess, C.F.; et al. Preoperative versus Postoperative Chemoradiotherapy for Rectal Cancer. N. Engl. J. Med. 2004, 351, 1731–1740. [Google Scholar] [CrossRef]
- Fokas, E.; Glynne-Jones, R.; Appelt, A.; Beets-Tan, R.; Beets, G.; Haustermans, K.; Marijnen, C.; Minsky, B.D.; Ludmir, E.; Quirke, P.; et al. Outcome Measures in Multimodal Rectal Cancer Trials. Lancet Oncol. 2020, 21, e252–e264. [Google Scholar] [CrossRef] [PubMed]
- Gambacorta, M.A.; Masciocchi, C.; Chiloiro, G.; Meldolesi, E.; Macchia, G.; van Soest, J.; Peters, F.; Collette, L.; Gérard, J.-P.; Ngan, S.; et al. Timing to Achieve the Highest Rate of PCR after Preoperative Radiochemotherapy in Rectal Cancer: A Pooled Analysis of 3085 Patients from 7 Randomized Trials. Radiother. Oncol. 2021, 154, 154–160. [Google Scholar] [CrossRef]
- Zorcolo, L.; Rosman, A.S.; Restivo, A.; Pisano, M.; Nigri, G.R.; Fancellu, A.; Melis, M. Complete Pathologic Response after Combined Modality Treatment for Rectal Cancer and Long-Term Survival: A Meta-Analysis. Ann. Surg. Oncol. 2012, 19, 2822–2832. [Google Scholar] [CrossRef]
- Valentini, V.; Gambacorta, M.A.; Cellini, F.; Aristei, C.; Coco, C.; Barbaro, B.; Alfieri, S.; D’Ugo, D.; Persiani, R.; Deodato, F.; et al. The INTERACT Trial: Long-Term Results of a Randomised Trial on Preoperative Capecitabine-Based Radiochemotherapy Intensified by Concomitant Boost or Oxaliplatin, for CT2 (Distal)-CT3 Rectal Cancer. Radiother. Oncol. 2019, 134, 110–118. [Google Scholar] [CrossRef]
- Garcia-Aguilar, J.; Chow, O.S.; Smith, D.D.; Marcet, J.E.; Cataldo, P.A.; Varma, M.G.; Kumar, A.S.; Oommen, S.; Coutsoftides, T.; Hunt, S.R.; et al. Effect of Adding MFOLFOX6 after Neoadjuvant Chemoradiation in Locally Advanced Rectal Cancer: A Multicentre, Phase 2 Trial. Lancet Oncol. 2015, 16, 957–966. [Google Scholar] [CrossRef]
- van Gijn, W.; Marijnen, C.A.M.; Nagtegaal, I.D.; Kranenbarg, E.M.-K.; Putter, H.; Wiggers, T.; Rutten, H.J.T.; Påhlman, L.; Glimelius, B.; van de Velde, C.J.H.; et al. Preoperative Radiotherapy Combined with Total Mesorectal Excision for Resectable Rectal Cancer: 12-Year Follow-up of the Multicentre, Randomised Controlled TME Trial. Lancet Oncol. 2011, 12, 575–582. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Liu, Y.; Zhang, C.; Li, H.; Lai, B. Metastatic Patterns and Survival Outcomes in Patients with Stage IV Colon Cancer: A Population-Based Analysis. Cancer Med. 2020, 9, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Restivo, A.; Delrio, P.; Deidda, S.; Spolverato, G.; Rega, D.; Cerci, M.; Barina, A.; Perin, A.; Pace, U.; Zorcolo, L.; et al. Predictors of Early Distant Relapse in Rectal Cancer Patients Submitted to Preoperative Chemoradiotherapy. Oncol. Res. Treat. 2020, 43, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Meldolesi, E.; Chiloiro, G.; Giannini, R.; Menghi, R.; Persiani, R.; Corvari, B.; Coco, C.; Manfrida, S.; Ratto, C.; De Luca, V.; et al. The Role of Simultaneous Integrated Boost in Locally Advanced Rectal Cancer Patients with Positive Lateral Pelvic Lymph Nodes. Cancers 2022, 14, 1643. [Google Scholar] [CrossRef]
- Mayaud, A.; Bousarsar, A.; Soltani, S.; Sotton, S.; Grange, R.; Le Roy, B.; Phelip, J.-M.; Boutet, C.; Magne, N. Prognostic Factors of Pelvic MRI at the Initial Workflow in Locally Advanced Rectal Cancer: Focus on Extra Mural Venous Invasion and Tumour Deposits. Bull. Cancer 2022, 109, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- van den Ende, T.; van den Boorn, H.G.; Hoonhout, N.M.; van Etten-Jamaludin, F.S.; Meijer, S.L.; Derks, S.; de Gruijl, T.D.; Bijlsma, M.F.; van Oijen, M.G.H.; van Laarhoven, H.W.M. Priming the Tumor Immune Microenvironment with Chemo(Radio)Therapy: A Systematic Review across Tumor Types. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188386. [Google Scholar] [CrossRef]
- Dayde, D.; Tanaka, I.; Jain, R.; Tai, M.C.; Taguchi, A. Predictive and Prognostic Molecular Biomarkers for Response to Neoadjuvant Chemoradiation in Rectal Cancer. Int. J. Mol. Sci. 2017, 18, 573. [Google Scholar] [CrossRef]
- De Cecco, C.N.; Ganeshan, B.; Ciolina, M.; Rengo, M.; Meinel, F.G.; Musio, D.; De Felice, F.; Raffetto, N.; Tombolini, V.; Laghi, A. Texture Analysis as Imaging Biomarker of Tumoral Response to Neoadjuvant Chemoradiotherapy in Rectal Cancer Patients Studied with 3-T Magnetic Resonance. Invest. Radiol. 2015, 50, 239–245. [Google Scholar] [CrossRef]
- Dinapoli, N.; Barbaro, B.; Gatta, R.; Chiloiro, G.; Casà, C.; Masciocchi, C.; Damiani, A.; Boldrini, L.; Gambacorta, M.A.; Dezio, M.; et al. Magnetic Resonance, Vendor-Independent, Intensity Histogram Analysis Predicting Pathologic Complete Response After Radiochemotherapy of Rectal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 765–774. [Google Scholar] [CrossRef]
- Cusumano, D.; Dinapoli, N.; Boldrini, L.; Chiloiro, G.; Gatta, R.; Masciocchi, C.; Lenkowicz, J.; Casà, C.; Damiani, A.; Azario, L.; et al. Fractal-Based Radiomic Approach to Predict Complete Pathological Response after Chemo-Radiotherapy in Rectal Cancer. Radiol. Med. 2018, 123, 286–295. [Google Scholar] [CrossRef]
- Horvat, N.; Veeraraghavan, H.; Khan, M.; Blazic, I.; Zheng, J.; Capanu, M.; Sala, E.; Garcia-Aguilar, J.; Gollub, M.J.; Petkovska, I. MR Imaging of Rectal Cancer: Radiomics Analysis to Assess Treatment Response after Neoadjuvant Therapy. Radiology 2018, 287, 833–843. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, X.-Y.; Shi, Y.-J.; Wang, L.; Zhu, H.-T.; Tang, Z.; Wang, S.; Li, X.-T.; Tian, J.; Sun, Y.-S. Radiomics Analysis for Evaluation of Pathological Complete Response to Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer. Clin. Cancer Res. 2017, 23, 7253–7262. [Google Scholar] [CrossRef] [PubMed]
- Enkhbaatar, N.-E.; Inoue, S.; Yamamuro, H.; Kawada, S.; Miyaoka, M.; Nakamura, N.; Sadahiro, S.; Imai, Y. MR Imaging with Apparent Diffusion Coefficient Histogram Analysis: Evaluation of Locally Advanced Rectal Cancer after Chemotherapy and Radiation Therapy. Radiology 2018, 288, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yang, X.; Shi, Z.; Yang, Z.; Du, X.; Zhao, Z.; Cheng, X. Radiomics Analysis of Multiparametric MRI for Prediction of Pathological Complete Response to Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer. Eur. Radiol. 2019, 29, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Cusumano, D.; Meijer, G.; Lenkowicz, J.; Chiloiro, G.; Boldrini, L.; Masciocchi, C.; Dinapoli, N.; Gatta, R.; Casà, C.; Damiani, A.; et al. A Field Strength Independent MR Radiomics Model to Predict Pathological Complete Response in Locally Advanced Rectal Cancer. Radiol. Med. 2021, 126, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Chiloiro, G.; Rodriguez-Carnero, P.; Lenkowicz, J.; Casà, C.; Masciocchi, C.; Boldrini, L.; Cusumano, D.; Dinapoli, N.; Meldolesi, E.; Carano, D.; et al. Delta Radiomics Can Predict Distant Metastasis in Locally Advanced Rectal Cancer: The Challenge to Personalize the Cure. Front. Oncol. 2020, 10, 595012. [Google Scholar] [CrossRef] [PubMed]
- Cusumano, D.; Boldrini, L.; Yadav, P.; Yu, G.; Musurunu, B.; Chiloiro, G.; Piras, A.; Lenkowicz, J.; Placidi, L.; Romano, A.; et al. Delta Radiomics for Rectal Cancer Response Prediction Using Low Field Magnetic Resonance Guided Radiotherapy: An External Validation. Phys. Med. 2021, 84, 186–191. [Google Scholar] [CrossRef]
- Fiorino, C.; Passoni, P.; Palmisano, A.; Gumina, C.; Cattaneo, G.M.; Broggi, S.; Di Chiara, A.; Esposito, A.; Mori, M.; Ronzoni, M.; et al. Accurate Outcome Prediction after Neo-Adjuvant Radio-Chemotherapy for Rectal Cancer Based on a TCP-Based Early Regression Index. Clin. Transl. Radiat. Oncol. 2019, 19, 12–16. [Google Scholar] [CrossRef]
- Fiorino, C.; Gumina, C.; Passoni, P.; Palmisano, A.; Broggi, S.; Cattaneo, G.M.; Di Chiara, A.; Esposito, A.; Mori, M.; Raso, R.; et al. A TCP-Based Early Regression Index Predicts the Pathological Response in Neo-Adjuvant Radio-Chemotherapy of Rectal Cancer. Radiother. Oncol. 2018, 128, 564–568. [Google Scholar] [CrossRef]
- Cusumano, D.; Boldrini, L.; Yadav, P.; Yu, G.; Musurunu, B.; Chiloiro, G.; Piras, A.; Lenkowicz, J.; Placidi, L.; Broggi, S.; et al. External Validation of Early Regression Index (ERITCP) as Predictor of Pathologic Complete Response in Rectal Cancer Using Magnetic Resonance-Guided Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 1347–1356. [Google Scholar] [CrossRef]
- Cusumano, D.; Catucci, F.; Romano, A.; Boldrini, L.; Piras, A.; Broggi, S.; Votta, C.; Placidi, L.; Nardini, M.; Chiloiro, G.; et al. Evaluation of an Early Regression Index (ERITCP) as Predictor of Pathological Complete Response in Cervical Cancer: A Pilot-Study. Appl. Sci. 2020, 10, 8001. [Google Scholar] [CrossRef]
- Shaish, H.; Aukerman, A.; Vanguri, R.; Spinelli, A.; Armenta, P.; Jambawalikar, S.; Makkar, J.; Bentley-Hibbert, S.; Del Portillo, A.; Kiran, R.; et al. Radiomics of MRI for Pretreatment Prediction of Pathologic Complete Response, Tumor Regression Grade, and Neoadjuvant Rectal Score in Patients with Locally Advanced Rectal Cancer Undergoing Neoadjuvant Chemoradiation: An International Multicenter Study. Eur. Radiol. 2020, 30, 6263–6273. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasam, V.S.; Paroder, V.; Gibbs, P.; Bajwa, R.; Gangai, N.; Sosa, R.E.; Petkovska, I.; Golia Pernicka, J.S.; Fuqua, J.L.; Bates, D.D.B.; et al. MRI Radiomics Features of Mesorectal Fat Can Predict Response to Neoadjuvant Chemoradiation Therapy and Tumor Recurrence in Patients with Locally Advanced Rectal Cancer. Eur. Radiol. 2022, 32, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Valentini, V.; Gambacorta, M.A.; Barbaro, B.; Chiloiro, G.; Coco, C.; Das, P.; Fanfani, F.; Joye, I.; Kachnic, L.; Maingon, P.; et al. International Consensus Guidelines on Clinical Target Volume Delineation in Rectal Cancer. Radiother. Oncol. 2016, 120, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Rödel, C.; Graeven, U.; Fietkau, R.; Hohenberger, W.; Hothorn, T.; Arnold, D.; Hofheinz, R.-D.; Ghadimi, M.; Wolff, H.A.; Lang-Welzenbach, M.; et al. Oxaliplatin Added to Fluorouracil-Based Preoperative Chemoradiotherapy and Postoperative Chemotherapy of Locally Advanced Rectal Cancer (the German CAO/ARO/AIO-04 Study): Final Results of the Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2015, 16, 979–989. [Google Scholar] [CrossRef]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef]
- Mandard, A.M.; Dalibard, F.; Mandard, J.C.; Marnay, J.; Henry-Amar, M.; Petiot, J.F.; Roussel, A.; Jacob, J.H.; Segol, P.; Samama, G. Pathologic Assessment of Tumor Regression after Preoperative Chemoradiotherapy of Esophageal Carcinoma. Clinicopathologic Correlations. Cancer 1994, 73, 2680–2686. [Google Scholar] [CrossRef]
- Chiloiro, G.; Cusumano, D.; de Franco, P.; Lenkowicz, J.; Boldrini, L.; Carano, D.; Barbaro, B.; Corvari, B.; Dinapoli, N.; Giraffa, M.; et al. Does Restaging MRI Radiomics Analysis Improve Pathological Complete Response Prediction in Rectal Cancer Patients? A Prognostic Model Development. Radiol. Med. 2022, 127, 11–20. [Google Scholar] [CrossRef]
- Dinapoli, N.; Alitto, A.R.; Vallati, M.; Gatta, R.; Autorino, R.; Boldrini, L.; Damiani, A.; Valentini, V. Moddicom: A Complete and Easily Accessible Library for Prognostic Evaluations Relying on Image Features. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2015, 2015, 771–774. [Google Scholar] [CrossRef]
- Gatta, R.; Vallati, M.; Dinapoli, N.; Masciocchi, C.; Lenkowicz, J.; Cusumano, D.; Casá, C.; Farchione, A.; Damiani, A.; van Soest, J.; et al. Towards a Modular Decision Support System for Radiomics: A Case Study on Rectal Cancer. Artif. Intell. Med. 2019, 96, 145–153. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, Y.; Dong, D.; Li, C.; Liang, X.; Zhang, C.; Wan, L.; Zhao, X.; Xu, K.; Zhou, C.; et al. Novel Radiomic Signature as a Prognostic Biomarker for Locally Advanced Rectal Cancer. J. Magn. Reson. Imaging 2018, 48, 605–614. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G.M. Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD). Circulation 2015, 131, 211–219. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Yaparpalvi, R.; Hong, L.; Mah, D.; Shen, J.; Mutyala, S.; Spierer, M.; Garg, M.; Guha, C.; Kalnicki, S. ICRU Reference Dose in an Era of Intensity-Modulated Radiation Therapy Clinical Trials: Correlation with Planning Target Volume Mean Dose and Suitability for Intensity-Modulated Radiation Therapy Dose Prescription. Radiother. Oncol. 2008, 89, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Chiloiro, G.; Boldrini, L.; Preziosi, F.; Cusumano, D.; Yadav, P.; Romano, A.; Placidi, L.; Lenkowicz, J.; Dinapoli, N.; Bassetti, M.F.; et al. A Predictive Model of 2yDFS During MR-Guided RT Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer Patients. Front. Oncol. 2022, 12, 831712. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.D.B.; Homsi, M.E.; Chang, K.J.; Lalwani, N.; Horvat, N.; Sheedy, S.P. MRI for Rectal Cancer: Staging, MrCRM, EMVI, Lymph Node Staging and Post-Treatment Response. Clin. Color. Cancer 2022, 21, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Lord, A.C.; D’Souza, N.; Shaw, A.; Rokan, Z.; Moran, B.; Abulafi, M.; Rasheed, S.; Chandramohan, A.; Corr, A.; Chau, I.; et al. MRI-Diagnosed Tumor Deposits and EMVI Status Have Superior Prognostic Accuracy to Current Clinical TNM Staging in Rectal Cancer. Ann. Surg. 2022, 276, 334–344. [Google Scholar] [CrossRef]
- Tudyka, V.; Blomqvist, L.; Beets-Tan, R.G.H.; Boelens, P.G.; Valentini, V.; van de Velde, C.J.; Dieguez, A.; Brown, G. EURECCA Consensus Conference Highlights about Colon & Rectal Cancer Multidisciplinary Management: The Radiology Experts Review. Eur. J. Surg. Oncol. 2014, 40, 469–475. [Google Scholar] [CrossRef]
- Yang, Y.-S.; Feng, F.; Qiu, Y.-J.; Zheng, G.-H.; Ge, Y.-Q.; Wang, Y.-T. High-Resolution MRI-Based Radiomics Analysis to Predict Lymph Node Metastasis and Tumor Deposits Respectively in Rectal Cancer. Abdom. Radiol. 2021, 46, 873–884. [Google Scholar] [CrossRef]
- Yu, X.; Song, W.; Guo, D.; Liu, H.; Zhang, H.; He, X.; Song, J.; Zhou, J.; Liu, X. Preoperative Prediction of Extramural Venous Invasion in Rectal Cancer: Comparison of the Diagnostic Efficacy of Radiomics Models and Quantitative Dynamic Contrast-Enhanced Magnetic Resonance Imaging. Front. Oncol. 2020, 10, 459. [Google Scholar] [CrossRef]
- Chiloiro, G.; Romano, A.; Mariani, S.; Macchia, G.; Giannarelli, D.; Caravatta, L.; Franco, P.; Boldrini, L.; Arcelli, A.; Bacigalupo, A.; et al. Predictive and Prognostic Value of Inflammatory Markers in Locally Advanced Rectal Cancer (PILLAR)—A Multicentric Analysis by the Italian Association of Radiotherapy and Clinical Oncology (AIRO) Gastrointestinal Study Group. Clin. Transl. Radiat. Oncol. 2023, 39, 100579. [Google Scholar] [CrossRef]
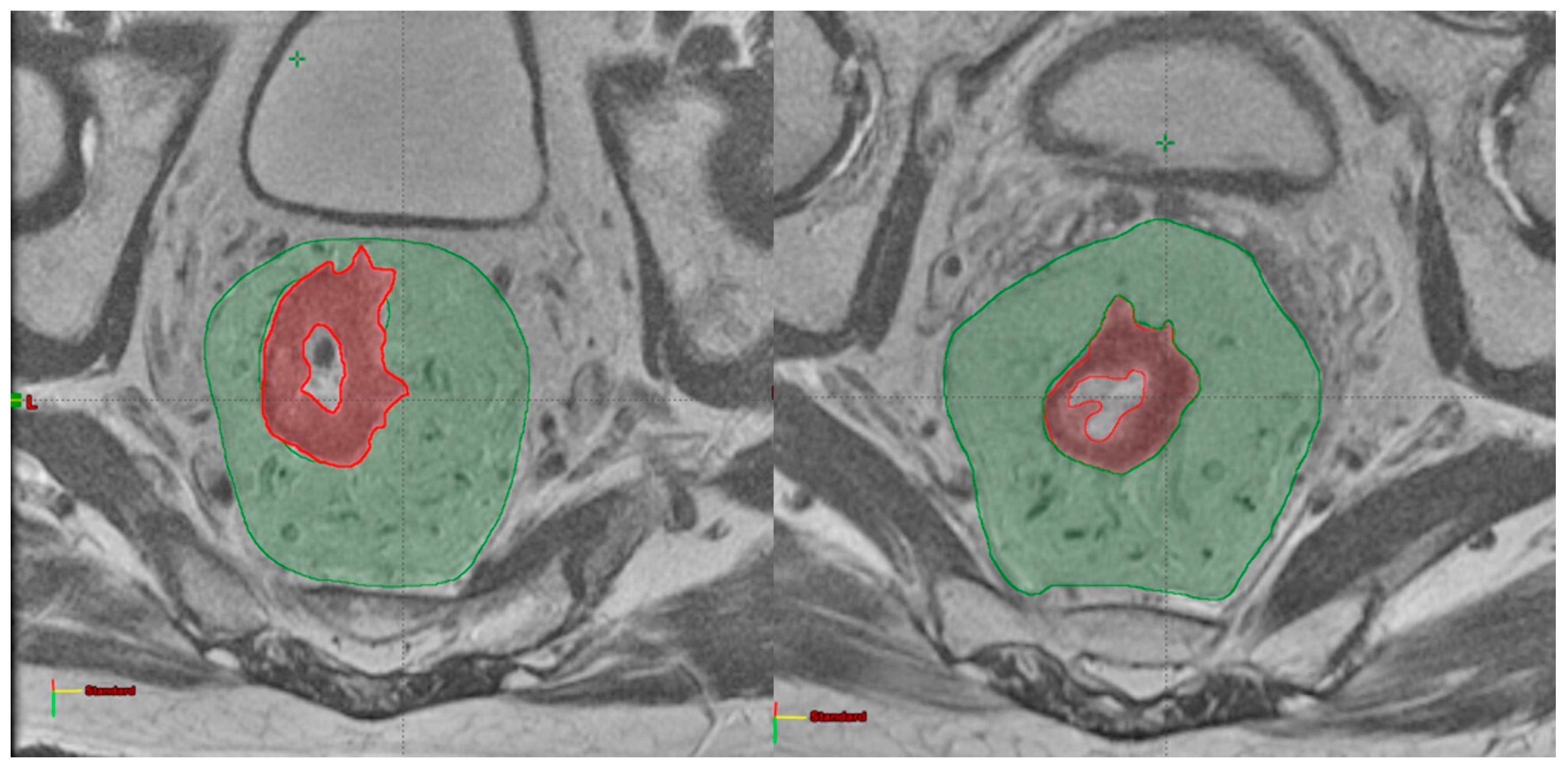
| Number of Patients (%) | |
|---|---|
| Median age (range) | 65 (26–83) |
| Gender | |
| Male | 132 (65) |
| Female | 71 (35) |
| cT | |
| 2 | 14 (6.9) |
| 3 | 119 (58.6) |
| 4 | 70 (34.4) |
| cN | |
| 0 | 13 (6.4) |
| 1–2 | 190 (93.6) |
| Stage | |
| II | 13 (6.4) |
| III | 190 (93.6) |
| Median RT dose [Gy] (range) | 55 (50–59.4) |
| ypT0/in situ | 58 (28.6) |
| 1 | 3 (1.48) |
| 2 | 60 (29.55) |
| 3 | 76 (37.44) |
| 4 | 6 (2.95) |
| ypN | |
| 0 | 151 (74.38) |
| 1–2 | 52 (25.62) |
| pCR/cCR | |
| Yes | 54 (26.6) |
| No | 149 (73.4) |
| 2yDFS | |
| Yes | 171 (84.3) |
| No | 32 (16.7) |
| Outcome | Dataset | Sensitivity | Specificity | AUC |
|---|---|---|---|---|
| pCR | Training | 75.7 | 78.0 | 80.2 (71.4–88.9) |
| Validation | 100.0 | 44.7 | 68.6 (56.0–81.2) | |
| 2yDFS | Training | 61.9 | 87.9 | 79.2 (68.7–89.6) |
| Validation | 90.9 | 45.5 | 69.6 (53.5–85.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiloiro, G.; Cusumano, D.; Romano, A.; Boldrini, L.; Nicolì, G.; Votta, C.; Tran, H.E.; Barbaro, B.; Carano, D.; Valentini, V.; et al. Delta Radiomic Analysis of Mesorectum to Predict Treatment Response and Prognosis in Locally Advanced Rectal Cancer. Cancers 2023, 15, 3082. https://doi.org/10.3390/cancers15123082
Chiloiro G, Cusumano D, Romano A, Boldrini L, Nicolì G, Votta C, Tran HE, Barbaro B, Carano D, Valentini V, et al. Delta Radiomic Analysis of Mesorectum to Predict Treatment Response and Prognosis in Locally Advanced Rectal Cancer. Cancers. 2023; 15(12):3082. https://doi.org/10.3390/cancers15123082
Chicago/Turabian StyleChiloiro, Giuditta, Davide Cusumano, Angela Romano, Luca Boldrini, Giuseppe Nicolì, Claudio Votta, Huong Elena Tran, Brunella Barbaro, Davide Carano, Vincenzo Valentini, and et al. 2023. "Delta Radiomic Analysis of Mesorectum to Predict Treatment Response and Prognosis in Locally Advanced Rectal Cancer" Cancers 15, no. 12: 3082. https://doi.org/10.3390/cancers15123082
APA StyleChiloiro, G., Cusumano, D., Romano, A., Boldrini, L., Nicolì, G., Votta, C., Tran, H. E., Barbaro, B., Carano, D., Valentini, V., & Gambacorta, M. A. (2023). Delta Radiomic Analysis of Mesorectum to Predict Treatment Response and Prognosis in Locally Advanced Rectal Cancer. Cancers, 15(12), 3082. https://doi.org/10.3390/cancers15123082








