Irinotecan- vs. Oxaliplatin-Based Doublets in KRASG12C-Mutated Metastatic Colorectal Cancer—A Multicentre Propensity-Score-Matched Retrospective Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
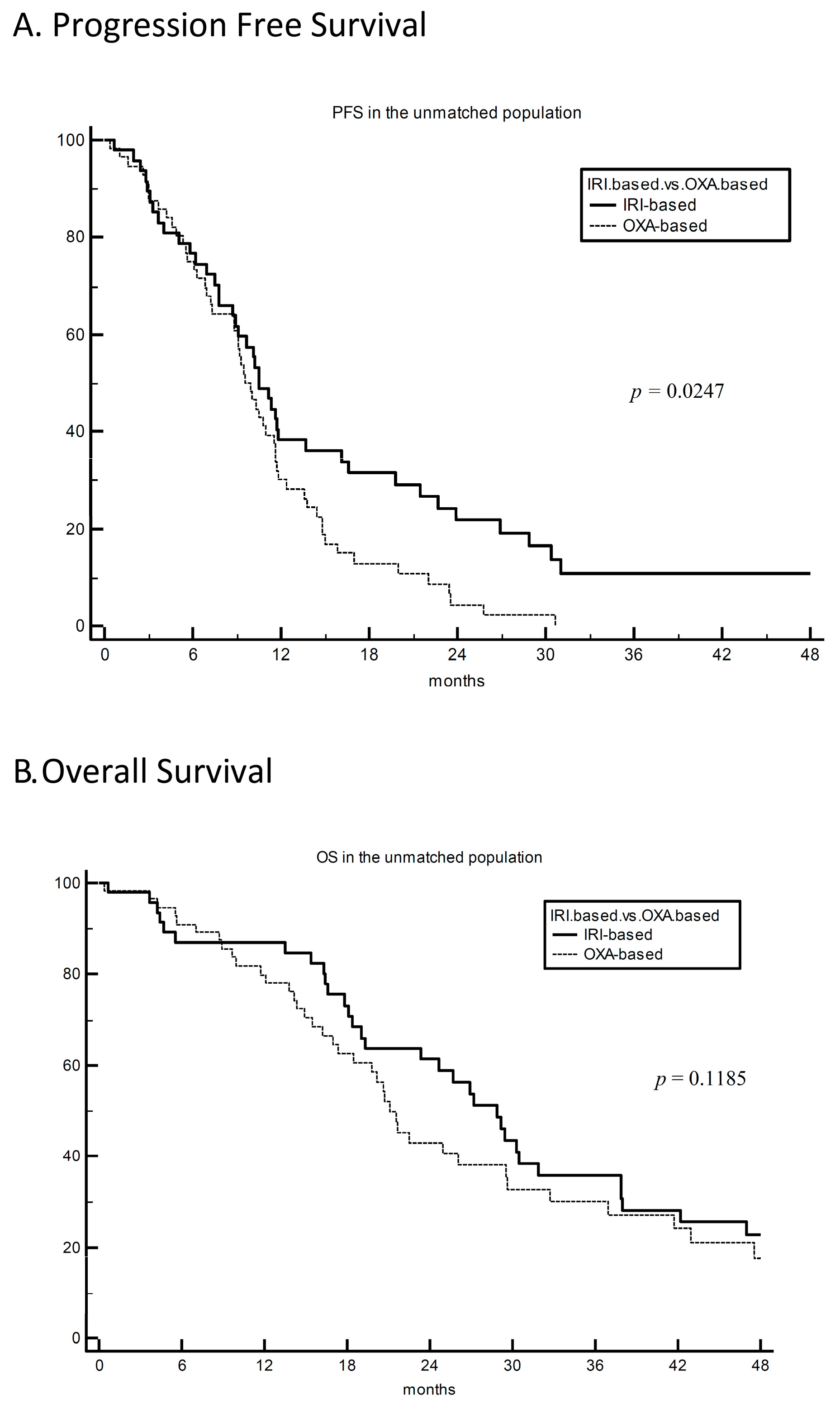
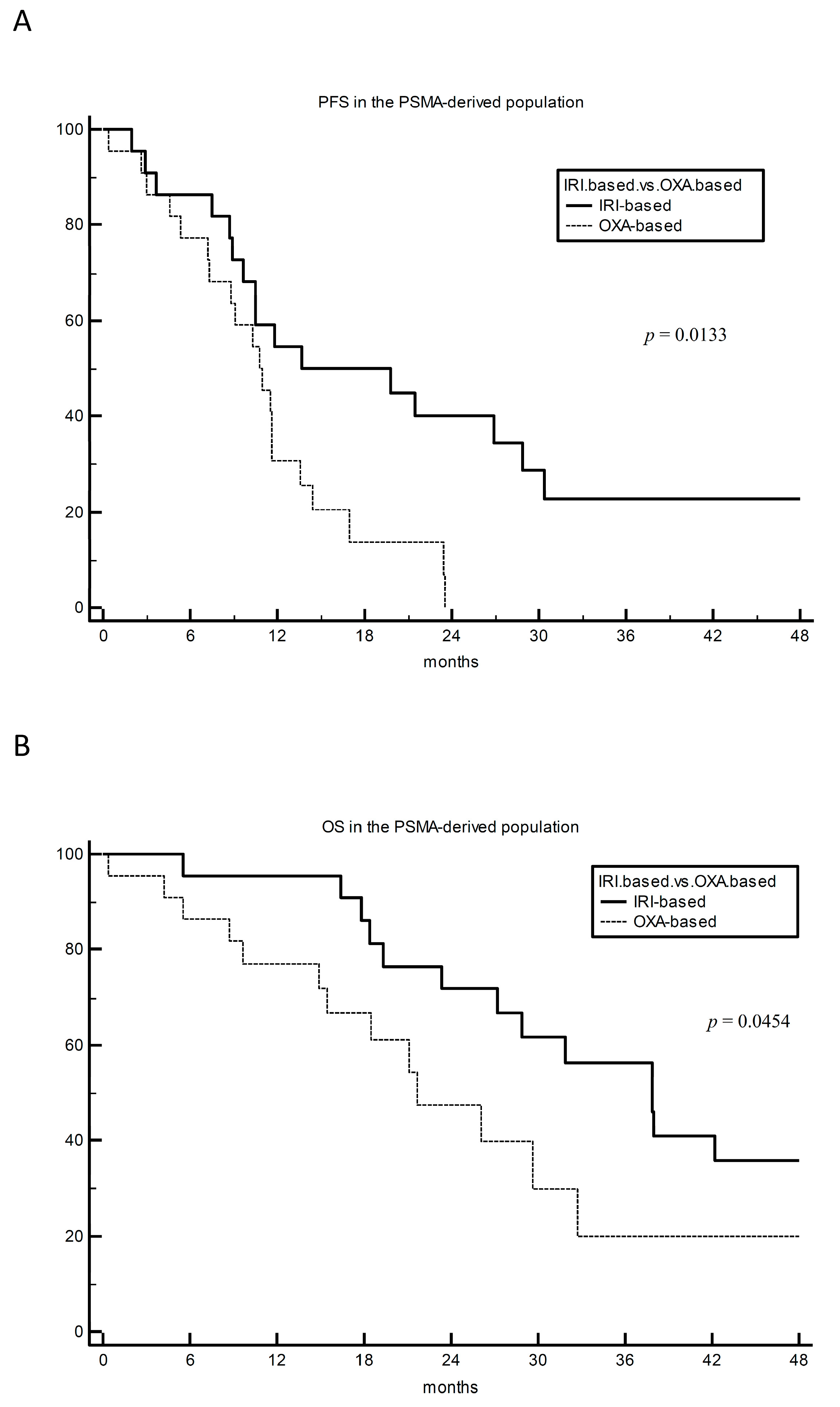
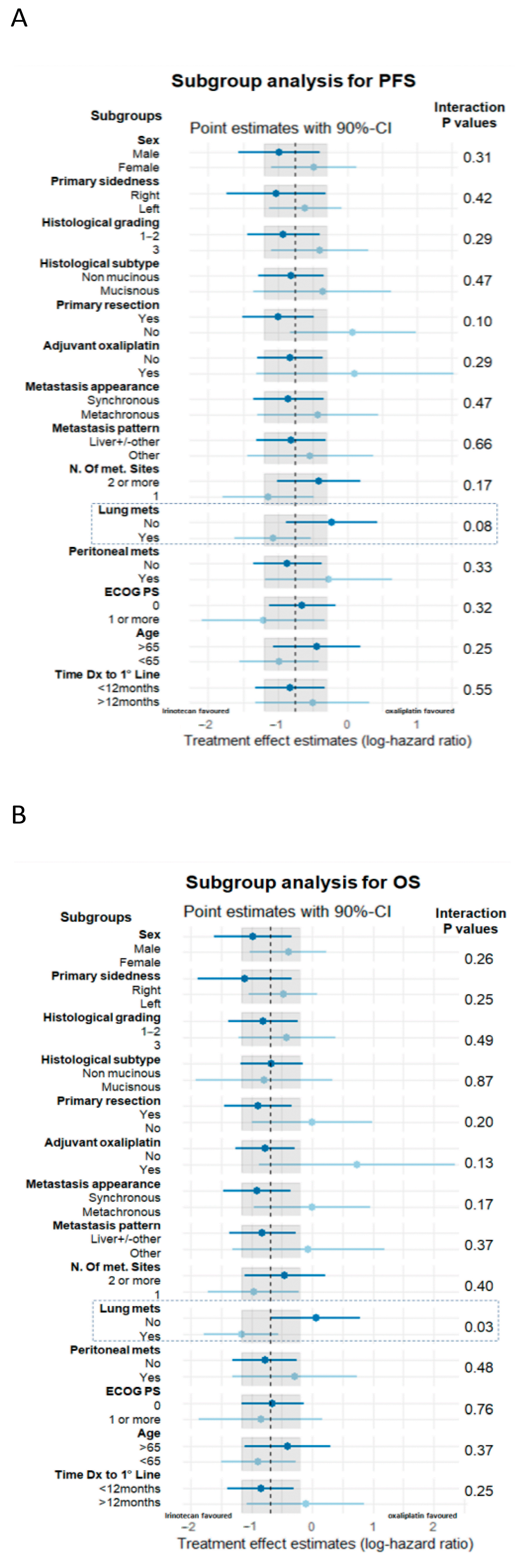
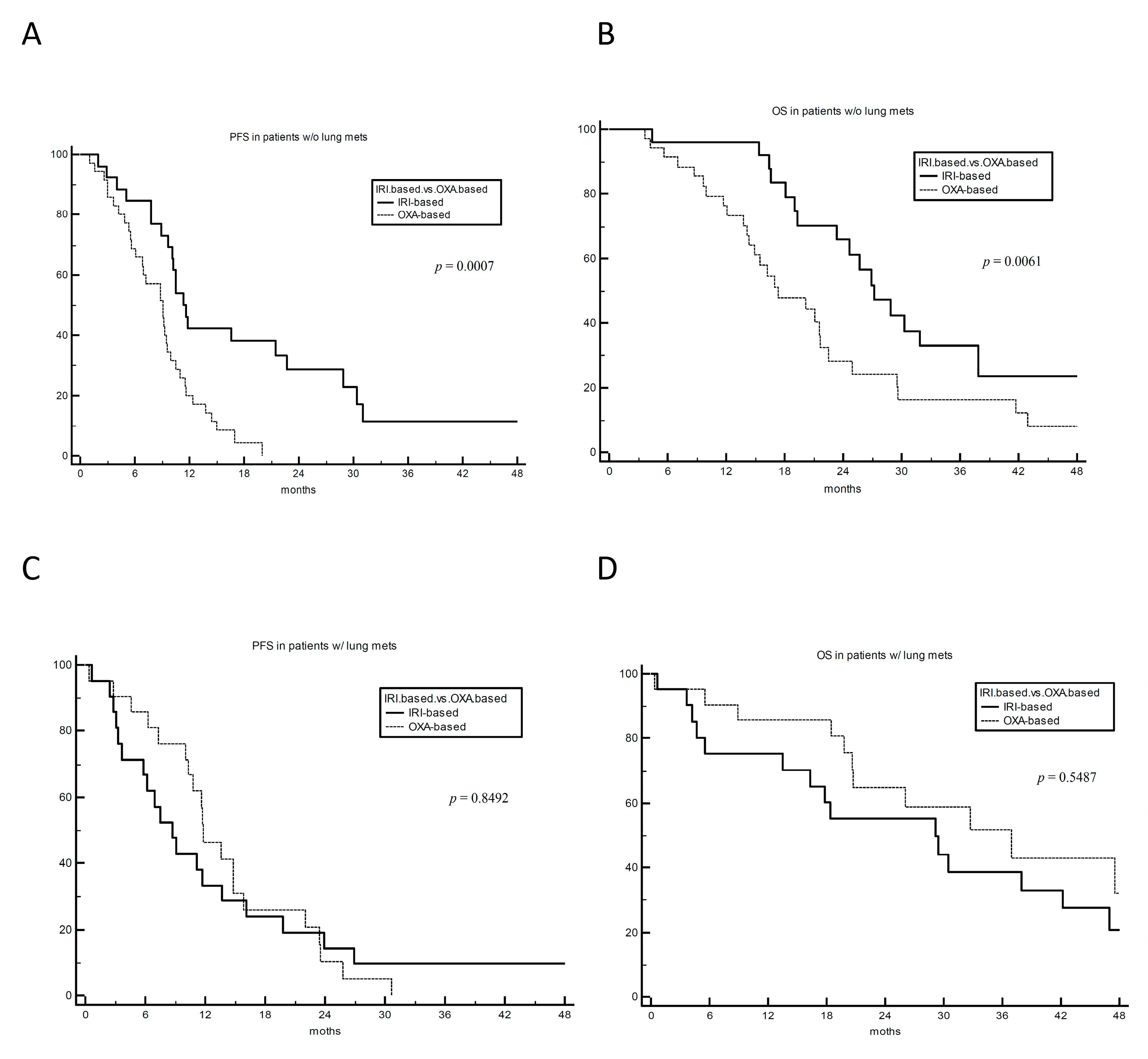
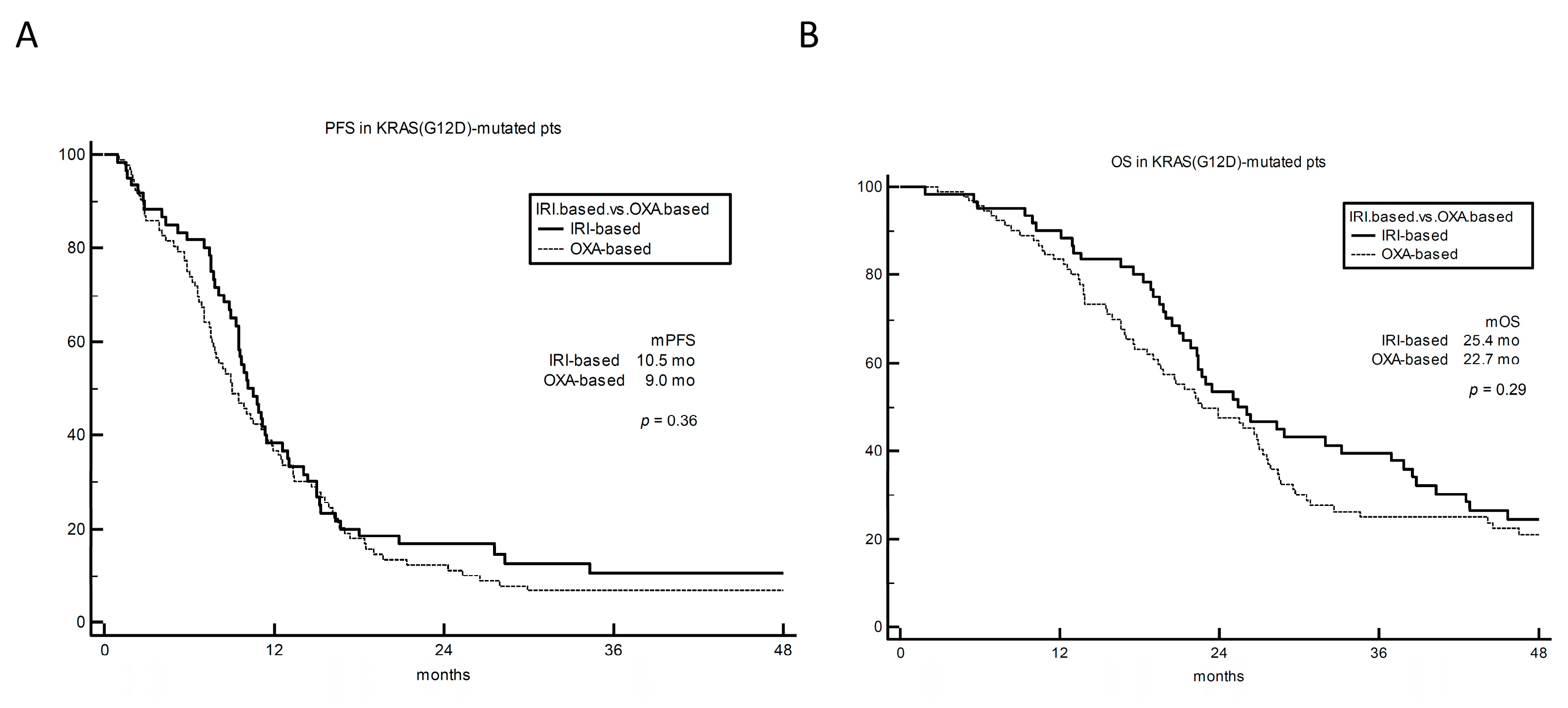
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Sauer, A.G.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Formica, V.; Sera, F.; Cremolini, C.; Riondino, S.; Morelli, C.; Arkenau, H.-T.; Roselli, M. KRAS and BRAF Mutations in Stage II and III Colon Cancer: A Systematic Review and Meta-Analysis. Gynecol. Oncol. 2022, 114, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Modest, D.P.; Ricard, I.; Heinemann, V.; Hegewisch-Becker, S.; Schmiegel, W.; Porschen, R.; Stintzing, S.; Graeven, U.; Arnold, D.; von Weikersthal, L.F.; et al. Outcome according to KRAS-, NRAS- and BRAF-mutation as well as KRAS mutation variants: Pooled analysis of five randomized trials in metastatic colorectal cancer by the AIO colorectal cancer study group. Ann. Oncol. 2016, 27, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, T.; Arnold, D.; Taniguchi, H.; Pentheroudakis, G.; Yamazaki, K.; Xu, R.-H.; Kim, T.; Ismail, F.; Tan, I.; Yeh, K.-H.; et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: A JSMO–ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann. Oncol. 2018, 29, 44–70. [Google Scholar] [CrossRef]
- Morris, V.K.; Kennedy, E.B.; Baxter, N.N.; Benson, A.B.; Cercek, A.; Cho, M.; Ciombor, K.K.; Cremolini, C.; Davis, A.; Deming, D.A.; et al. Treatment of Metastatic Colorectal Cancer: ASCO Guideline. J. Clin. Oncol. 2022, 41, 678–700. [Google Scholar] [CrossRef]
- Yaeger, R.; Weiss, J.; Pelster, M.S.; Spira, A.I.; Barve, M.; Ou, S.-H.I.; Leal, T.A.; Bekaii-Saab, T.S.; Paweletz, C.P.; Heavey, G.A.; et al. Adagrasib with or without Cetuximab in Colorectal Cancer with Mutated KRAS G12C. N. Engl. J. Med. 2023, 388, 44–54. [Google Scholar] [CrossRef]
- Ji, J.; Wang, C.; Fakih, M. Targeting KRASG12C-Mutated Advanced Colorectal Cancer: Research and Clinical Developments. OncoTargets Ther. 2022, 15, 747–756. [Google Scholar] [CrossRef]
- Schmiegel, W.; Reinacher-Schick, A.; Arnold, D.; Kubicka, S.; Freier, W.; Dietrich, G.; Geißler, M.; Hegewisch-Becker, S.; Tannapfel, A.; Pohl, M.; et al. Capecitabine/irinotecan or capecitabine/oxaliplatin in combination with bevacizumab is effective and safe as first-line therapy for metastatic colorectal cancer: A randomized phase II study of the AIO colorectal study group. Ann. Oncol. 2013, 24, 1580–1587. [Google Scholar] [CrossRef]
- Ergun, Y.; Acikgoz, Y.; Bal, O.; Ucar, G.; Dirikoc, M.; Yildirim, E.C.; Akdeniz, N.; Uncu, D. KRAS codon 12 and 13 mutations may guide the selection of irinotecan or oxaliplatin in first-line treatment of metastatic colorectal cancer. Expert Rev. Mol. Diagn. 2019, 19, 1131–1140. [Google Scholar] [CrossRef]
- Ciardiello, D.; Chiarazzo, C.; Famiglietti, V.; Damato, A.; Pinto, C.; Zampino, M.; Castellano, G.; Gervaso, L.; Zaniboni, A.; Oneda, E.; et al. Clinical efficacy of sequential treatments in KRASG12C-mutant metastatic colorectal cancer: Findings from a real-life multicenter Italian study (CRC-KR GOIM). ESMO Open 2022, 7, 100567. [Google Scholar] [CrossRef] [PubMed]
- Fakih, M.G.; Sandhu, J.; Lim, D.; Li, S.M.; Wang, C. 320MO A phase I clinical trial of regorafenib, ipilimumab, and nivolumab (RIN) in chemotherapy resistant MSS metastatic colorectal cancer (mCRC). Ann. Oncol. 2022, 33, S684. [Google Scholar] [CrossRef]
- Mettu, N.B.; Ou, F.-S.; Zemla, T.J.; Halfdanarson, T.R.; Lenz, H.-J.; Breakstone, R.A.; Boland, P.M.; Crysler, O.V.; Wu, C.; Nixon, A.B.; et al. Assessment of Capecitabine and Bevacizumab with or without Atezolizumab for the Treatment of Refractory Metastatic Colorectal Cancer. JAMA Netw. Open 2022, 5, e2149040. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sandhu, J.; Ouyang, C.; Ye, J.; Lee, P.P.; Fakih, M. Clinical Response to Immunotherapy Targeting Programmed Cell Death Receptor 1/Programmed Cell Death Ligand 1 in Patients with Treatment-Resistant Microsatellite Stable Colorectal Cancer with and without Liver Metastases. JAMA Netw. Open 2021, 4, e2118416. [Google Scholar] [CrossRef]
- Formica, V.; Cereda, V.; Nardecchia, A.; Tesauro, M.; Roselli, M. Immune reaction and colorectal cancer: Friends or foes? World J. Gastroenterol. 2014, 20, 12407–12419. [Google Scholar] [CrossRef]
- Chen, H.-N.; Shu, Y.; Liao, F.; Liao, X.; Zhang, H.; Qin, Y.; Wang, Z.; Luo, M.; Liu, Q.; Xue, Z.; et al. Genomic evolution and diverse models of systemic metastases in colorectal cancer. Gut 2022, 71, 322–332. [Google Scholar] [CrossRef]
- Tie, J.; Lipton, L.; Desai, J.; Gibbs, P.; Jorissen, R.N.; Christie, M.; Drummond, K.J.; Thomson, B.N.; Usatoff, V.; Evans, P.M.; et al. KRAS Mutation Is Associated with Lung Metastasis in Patients with Curatively Resected Colorectal Cancer. Clin. Cancer Res. 2011, 17, 1122–1130. [Google Scholar] [CrossRef]
- Jo, P.; Bernhardt, M.; Nietert, M.; König, A.; Azizian, A.; Schirmer, M.A.; Grade, M.; Kitz, J.; Reuter-Jessen, K.; Ghadimi, M.; et al. KRAS mutation status concordance between the primary tumor and the corresponding metastasis in patients with rectal cancer. PLoS ONE 2020, 15, e0239806. [Google Scholar] [CrossRef]
- Roselli, M.; Formica, V.; Cereda, V.; Jochems, C.; Richards, J.; Grenga, I.; Orlandi, A.; Ferroni, P.; Guadagni, F.; Schlom, J. The association of clinical outcome and peripheral T-cell subsets in metastatic colorectal cancer patients receiving first-line FOLFIRI plus bevacizumab therapy. Oncoimmunology 2016, 5, e1188243. [Google Scholar] [CrossRef]
- Formica, V.; Cereda, V.; di Bari, M.-G.; Grenga, I.; Tesauro, M.; Raffaele, P.; Ferroni, P.; Guadagni, F.; Roselli, M. Peripheral CD45RO, PD-1, and TLR4 expression in metastatic colorectal cancer patients treated with bevacizumab, fluorouracil, and irinotecan (FOLFIRI-B). Med Oncol. 2013, 30, 1–8. [Google Scholar] [CrossRef]
- Masuishi, T.; Kuboki, Y.; Fakih, M.; Strickler, J.; Furqan, M.; Kim, E.; Cardona, P.; Tran, Q.; Chan, E.; Hong, D. 444TiP Trial in progress: A phase Ib study of sotorasib, a selective KRAS G12C inhibitor, in combination with panitumumab and FOLFIRI in treatment naïve and previously treated metastatic colorectal cancer (CodeBreaK 101). Ann. Oncol. 2022, 33, S737–S738. [Google Scholar] [CrossRef]
| Characteristics | % (N = 104) |
|---|---|
| Sex | |
| Male | 40% (42) |
| Female | 60% (62) |
| Age | |
| <65 | 54% (56) |
| ≥65 | 46% (48) |
| Primary tumour sidedness | |
| Right | 37% (38) |
| Left | 63% (66) |
| Mucinous histology | |
| Yes | 14% (15) |
| No | 86% (89) |
| Metastasis at diagnosis | |
| Yes | 69% (72) |
| No | 31% (32) |
| Number of metastatic sites | |
| 1 | 41% (43) |
| ≥2 | 59% (61) |
| Metastatic sites | |
| Liver | 77% (80) |
| Lung | 41% (43) |
| Peritoneum | 22% (23) |
| First-line treatment | |
| Oxaliplatin-based doublet | 55% (57) |
| Irinotecan-based doublet | 45% (47) |
| Bevacizumab use in first line | |
| Yes | 75% (78) |
| No | 25% (26) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Formica, V.; Morelli, C.; Conca, V.; Calegari, M.A.; Lucchetti, J.; Dell’Aquila, E.; Schirripa, M.; Messina, M.; Salvatore, L.; Lo Prinzi, F.; et al. Irinotecan- vs. Oxaliplatin-Based Doublets in KRASG12C-Mutated Metastatic Colorectal Cancer—A Multicentre Propensity-Score-Matched Retrospective Analysis. Cancers 2023, 15, 3064. https://doi.org/10.3390/cancers15113064
Formica V, Morelli C, Conca V, Calegari MA, Lucchetti J, Dell’Aquila E, Schirripa M, Messina M, Salvatore L, Lo Prinzi F, et al. Irinotecan- vs. Oxaliplatin-Based Doublets in KRASG12C-Mutated Metastatic Colorectal Cancer—A Multicentre Propensity-Score-Matched Retrospective Analysis. Cancers. 2023; 15(11):3064. https://doi.org/10.3390/cancers15113064
Chicago/Turabian StyleFormica, Vincenzo, Cristina Morelli, Veronica Conca, Maria Alessandra Calegari, Jessica Lucchetti, Emanuela Dell’Aquila, Marta Schirripa, Marco Messina, Lisa Salvatore, Federica Lo Prinzi, and et al. 2023. "Irinotecan- vs. Oxaliplatin-Based Doublets in KRASG12C-Mutated Metastatic Colorectal Cancer—A Multicentre Propensity-Score-Matched Retrospective Analysis" Cancers 15, no. 11: 3064. https://doi.org/10.3390/cancers15113064
APA StyleFormica, V., Morelli, C., Conca, V., Calegari, M. A., Lucchetti, J., Dell’Aquila, E., Schirripa, M., Messina, M., Salvatore, L., Lo Prinzi, F., Dima, G., Trovato, G., Riondino, S., Roselli, M., Skoulidis, F., Arkenau, H.-T., & Cremolini, C. (2023). Irinotecan- vs. Oxaliplatin-Based Doublets in KRASG12C-Mutated Metastatic Colorectal Cancer—A Multicentre Propensity-Score-Matched Retrospective Analysis. Cancers, 15(11), 3064. https://doi.org/10.3390/cancers15113064







