Metaplastic Breast Carcinoma in U.S. Population: Racial Disparities, Survival Benefit of Adjuvant Chemoradiation and Future Personalized Treatment with Genomic Landscape
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Demographic Data and Tumor Characteristics
2.2. Distant Metastasis and Lymph Node Status at the Time of Diagnosis of Metaplastic Carcinoma of the Breast
2.3. Treatment Characteristics
2.4. Outcomes and Survival Analysis
2.5. Survival Analysis by Race
2.6. Survival Difference by Stage for Race and Treatment
2.7. Multivariable Analysis
3. Mutations Associated with Metaplastic Breast Carcinoma in the COSMIC Database
4. Discussion
Immunotherapy and Molecular Targets
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reddy, T.P.; Rosato, R.R.; Li, X.; Moulder, S.; Piwnica-Worms, H.; Chang, J.C. A comprehensive overview of metaplastic breast cancer: Clinical features and molecular aberrations. Breast Cancer Res. 2020, 22, 121. [Google Scholar] [CrossRef]
- McKinnon, E.; Xiao, P. Metaplastic carcinoma of the breast. Arch. Pathol. Lab. Med. 2015, 139, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Al Sayed, A.D.; El Weshi, A.N.; Tulbah, A.M.; Rahal, M.M.; Ezzat, A.A. Metaplastic carcinoma of the breast Clinical presentation, treatment results and prognostic factors. Acta Oncol. 2009, 45, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Weigelt, B.; Eberle, C.; Cowell, C.F.; Ng, C.K.Y.; Reis-Filho, J.S. Metaplastic breast carcinoma: More than a special type. Nat. Rev. Cancer 2014, 14, 147–148. [Google Scholar] [CrossRef] [PubMed]
- González-Martínez, S.; Pérez-Mies, B.; Carretero-Barrio, I.; Palacios-Berraquero, M.L.; Perez-García, J.; Cortés, J.; Palacios, J. Molecular Features of Metaplastic Breast Carcinoma: An Infrequent Subtype of Triple Negative Breast Carcinoma. Cancers 2020, 12, 1832. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.T.; Campbell, B.M.; Thomas, S.M.; Greenup, R.A.; Plichta, J.K.; Rosenberger, L.H.; Force, J.; Hall, A.; Hyslop, T.; Hwang, E.S.; et al. Metaplastic Breast Cancer Treatment and Outcomes in 2500 Patients: A Retrospective Analysis of a National Oncology Database. Ann. Surg. Oncol. 2018, 25, 2249–2260. [Google Scholar] [CrossRef]
- Nelson, R.A.; Guye, M.L.; Luu, T.; Lai, L.L. Survival Outcomes of Metaplastic Breast Cancer Patients: Results from a US Population-based Analysis. Ann. Surg. Oncol. 2015, 22, 24–31. [Google Scholar] [CrossRef]
- Pezzi, C.M.; Patel-Parekh, L.; Cole, K.; Franko, J.; Klimberg, V.S.; Bland, K. Characteristics and Treatment of Metaplastic Breast Cancer: Analysis of 892 Cases from the National Cancer Data Base. Ann. Surg. Oncol. 2007, 14, 166–173. [Google Scholar] [CrossRef]
- Beatty, J.D.; Atwood, M.; Tickman, R.; Reiner, M. Metaplastic breast cancer: Clinical significance. Am. J. Surg. 2006, 191, 657–664. [Google Scholar] [CrossRef]
- Giroux, V.; Rustgi, A.K. Metaplasia: Tissue injury adaptation and a precursor to the dysplasia–cancer sequence. Nat. Rev. Cancer 2017, 17, 594–604. [Google Scholar] [CrossRef]
- Tse, G.M.; Tan, P.H.; Putti, T.C.; Lui, P.C.W.; Chaiwun, B.; Law, B.K.B. Metaplastic carcinoma of the breast: A clinicopathological review. J. Clin. Pathol. 2006, 59, 1079–1083. [Google Scholar] [CrossRef]
- WHO. WHO Classification of Tumours of the Breast. World Health Organization Classification of Tumours, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2012. [Google Scholar]
- Hu, Q.; Chen, W.-X.; Zhong, S.-L.; Li, J.; Luo, Z.; Tang, J.-H.; Zhao, J.-H. Current Progress in the Treatment of Metaplastic Breast Carcinoma. Asian Pac. J. Cancer Prev. 2013, 14, 6221–6225. [Google Scholar] [CrossRef]
- Chen, I.C.; Lin, C.H.; Huang, C.S.; Lien, H.C.; Hsu, C.; Kuo, W.H.; Lu, Y.S.; Cheng, A.L. Lack of efficacy to systemic chemotherapy for treatment of metaplastic carcinoma of the breast in the modern era. Breast Cancer Res. Treat. 2011, 130, 345–351. [Google Scholar] [CrossRef]
- Osei-Twum, J.-A.; Gedleh, S.; Lofters, A.; Nnorom, O. Differences in Breast Cancer Presentation at Time of Diagnosis for Black and White Women in High Resource Settings. J. Immigr. Minor. Health 2021, 23, 1305–1342. [Google Scholar] [CrossRef]
- Ren, J.-X.; Gong, Y.; Ling, H.; Hu, X.; Shao, Z.-M. Racial/ethnic differences in the outcomes of patients with metastatic breast cancer: Contributions of demographic, socioeconomic, tumor and metastatic characteristics. Breast Cancer Res. Treat. 2019, 173, 225–237. [Google Scholar] [CrossRef]
- Ellis, L.; Canchola, A.J.; Spiegel, D.; Ladabaum, U.; Haile, R.; Gomez, S.L. Racial and Ethnic Disparities in Cancer Survival: The Contribution of Tumor, Sociodemographic, Institutional, and Neighborhood Characteristics. J. Clin. Oncol. 2018, 36, 25–33. [Google Scholar] [CrossRef]
- DeSantis, C.; Jemal, A.; Ward, E. Disparities in breast cancer prognostic factors by race, insurance status, and education. Cancer Causes Control. 2010, 21, 1445–1450. [Google Scholar] [CrossRef]
- Monnat, S.M. Race/ethnicity and the socioeconomic status gradient in women’s cancer screening utilization: A case of diminishing returns? J. Health Care Poor Underserved 2014, 25, 332–356. [Google Scholar] [CrossRef]
- Khrouf, S.; Ksontini, F.L.; Ayadi, M.; Rais, H.B.A.; Mezlini, A. Breast cancer screening: A dividing controversy. La Tunis. Med. 2020, 98, 22–34. [Google Scholar]
- Roth, M.Y.; Elmore, J.G.; Yi-Frazier, J.P.; Reisch, L.M.; Oster, N.V.; Miglioretti, D.L. Self-Detection Remains a Key Method of Breast Cancer Detection for U.S. Women. J. Women’s Health 2011, 20, 1135–1139. [Google Scholar] [CrossRef]
- Schroeder, M.C.; Rastogi, P.; Geyer, C.E., Jr.; Miller, L.D.; Thomas, A. Early and Locally Advanced Metaplastic Breast Cancer: Presentation and Survival by Receptor Status in Surveillance, Epidemiology, and End Results (SEER) 2010–2014. Oncologist 2018, 23, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Anders, C.K.; Johnson, R.; Litton, J.; Phillips, M.; Bleyer, A. Breast Cancer Before Age 40 Years. Semin. Oncol. 2009, 36, 237–249. [Google Scholar] [CrossRef]
- Song, Y.; Liu, X.; Zhang, G.; Song, H.; Ren, Y.; He, X.; Wang, Y.; Zhang, J.; Zhang, Y.; Sun, S.; et al. Unique clinicopathological features of metaplastic breast carcinoma compared with invasive ductal carcinoma and poor prognostic indicators. World J. Surg. Oncol. 2013, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.M.; Cagney, D.N.; Catalano, P.J.; Warren, L.E.; Bellon, J.R.; Punglia, R.S.; Claus, E.B.; Lee, E.Q.; Wen, P.Y.; Haas-Kogan, D.A.; et al. Brain Metastases in Newly Diagnosed Breast Cancer: A Population-Based Study. JAMA Oncol. 2017, 3, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Tzanninis, I.-G.; Kotteas, E.A.; Ntanasis-Stathopoulos, I.; Kontogianni, P.; Fotopoulos, G. Management and Outcomes in Metaplastic Breast Cancer. Clin. Breast Cancer 2016, 16, 437–443. [Google Scholar] [CrossRef]
- Hu, J.; Tan, J.; Dong, F.; Zhang, X.; Ming, J.; Huang, T. The Effect of Post-Mastectomy Radiotherapy in Patients with Metaplastic Breast Cancer: A Propensity Score-Matched Analysis of the SEER Database. Front. Oncol. 2021, 11, 5732. [Google Scholar] [CrossRef]
- Tray, N.; Taff, J.; Adams, S. Therapeutic landscape of metaplastic breast cancer. Cancer Treat. Rev. 2019, 79, 101888. [Google Scholar] [CrossRef]
- Stefansson, O.A.; Villanueva, A.; Vidal, A.; Martí, L.; Esteller, M. BRCA1 epigenetic inactivation predicts sensitivity to platinum-based chemotherapy in breast and ovarian cancer. Epigenetics 2012, 7, 1225–1229. [Google Scholar] [CrossRef]
- Bataillon, G.; Fuhrmann, L.; Girard, E.; Menet, E.; Laé, M.; Capovilla, M.; Treilleux, I.; Arnould, L.; Penault-Llorca, F.; Rouzier, R.; et al. High rate of PIK3CA mutations but no TP53 mutations in low-grade adenosquamous carcinoma of the breast. Histopathology 2018, 73, 273–283. [Google Scholar] [CrossRef]
- Hennessy, B.T.; Gonzalez-Angulo, A.-M.; Stemke-Hale, K.; Gilcrease, M.Z.; Krishnamurthy, S.; Lee, J.-S.; Fridlyand, J.; Sahin, A.; Agarwal, R.; Joy, C.; et al. Characterization of a naturally occurring breast cancer subset enriched in epithelial-to-mesenchymal transition and stem cell characteristics. Cancer Res. 2009, 69, 4116–4124. [Google Scholar] [CrossRef]
- Moulder, S.; Moroney, J.; Helgason, T.; Wheler, J.; Booser, D.; Albarracin, C.; Morrow, P.K.; Koenig, K.; Kurzrock, R. Responses to Liposomal Doxorubicin, Bevacizumab, and Temsirolimus in Metaplastic Carcinoma of the Breast: Biologic Rationale and Implications for Stem-Cell Research in Breast Cancer. J. Clin. Oncol. 2011, 29, e572–e575. [Google Scholar] [CrossRef]
- Basho, R.K.; Yam, C.; Gilcrease, M.; Murthy, R.K.; Helgason, T.; Karp, D.D.; Meric-Bernstam, F.; Hess, K.R.; Valero, V.; Albarracin, C.; et al. Comparative Effectiveness of an mTOR-Based Systemic Therapy Regimen in Advanced, Metaplastic and Nonmetaplastic Triple-Negative Breast Cancer. Oncologist 2018, 23, 1300–1309. [Google Scholar] [CrossRef]
- Qiu, S.Q.; Waaijer SJ, H.; Zwager, M.C.; de Vries, E.G.E.; van der Vegt, B.; Schröder, C.P. Tumor-associated macrophages in breast cancer: Innocent bystander or important player? Cancer Treat. Rev. 2018, 70, 178–189. [Google Scholar] [CrossRef]
- Montemurro, N.; Pahwa, B.; Tayal, A.; Shukla, A.; Encarnacion, M.D.J.; Ramirez, I.; Nurmukhametov, R.; Chavda, V.; De Carlo, A. Macrophages in Recurrent Glioblastoma as a Prognostic Factor in the Synergistic System of the Tumor Microenvironment. Neurol. Int. 2023, 15, 595–608. [Google Scholar] [CrossRef]
- Mani, S.A.; Guo, W.; Liao, M.-J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The Epithelial-Mesenchymal Transition Generates Cells with Properties of Stem Cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef]
- Oon, M.L.; Thike, A.A.; Tan, S.Y.; Tan, P.H. Cancer stem cell and epithelial–mesenchymal transition markers predict worse outcome in metaplastic carcinoma of the breast. Breast Cancer Res. Treat. 2015, 150, 31–41. [Google Scholar] [CrossRef]
- Zhang, Y.; Toy, K.A.; Kleer, C.G. Metaplastic breast carcinomas are enriched in markers of tumor-initiating cells and epithelial to mesenchymal transition. Mod. Pathol. 2012, 25, 178–184. [Google Scholar] [CrossRef]
- González-Martínez, S.; Pérez-Mies, B.; Pizarro, D.; Caniego-Casas, T.; Cortés, J.; Palacios, J. Epithelial Mesenchymal Transition and Immune Response in Metaplastic Breast Carcinoma. Int. J. Mol. Sci. 2021, 22, 7398. [Google Scholar] [CrossRef]
- Gheldof, A.; Berx, G. Cadherins and Epithelial-to-Mesenchymal Transition. Prog. Mol. Biol. Transl. Sci. 2013, 116, 317–336. [Google Scholar] [CrossRef]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef]
- Yook, J.I.; Li, X.Y.; Ota, I.; Hu, C.; Kim, H.S.; Kim, N.H.; Cha, S.Y.; Ryu, J.K.; Choi, Y.J.; Kim, J.; et al. A Wnt-Axin2-GSK3beta cascade regulates Snail1 activity in breast cancer cells. Nat. Cell Biol. 2006, 8, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Kumar, U.; Hu, Y.; Masrour, N.; Castellanos-Uribe, M.; Harrod, A.; May, S.T.; Ali, S.; Speirs, V.; Coombes, R.C.; Yagüe, E. MicroRNA-495/TGF-beta/FOXC1 axis regulates multidrug resistance in metaplastic breast cancer cells. Biochem. Pharmacol. 2021, 192, 114692. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.J.; Thomas, D.; Emmons, A.; Giordano, T.J.; Kleer, C.G. Genetic changes of Wnt pathway genes are common events in metaplastic carcinomas of the breast. Clin. Cancer Res. 2008, 14, 4038–4044. [Google Scholar] [CrossRef] [PubMed]
- Nassar, A.; Sookhan, N.; Santisteban, M.; Bryant, S.C.; Boughey, J.C.; Giorgadze, T.; Degnim, A. Diagnostic utility of snail in metaplastic breast carcinoma. Diagn. Pathol. 2010, 5, 76. [Google Scholar] [CrossRef]
- Zhou, S.; Sun, X.; Yu, L.; Zhou, R.; Li, A.; Li, M.; Yang, W. Differential expression and clinical significance of epithelial-mesenchymal transition markers among different histological types of triple-negative breast cancer. J. Cancer 2018, 9, 604–613. [Google Scholar] [CrossRef]
- Alsuliman, A.; Colak, D.; Al-Harazi, O.; Fitwi, H.; Tulbah, A.; Al-Tweigeri, T.; Al-Alwan, M.; Ghebeh, H. Bidirectional crosstalk between PD-L1 expression and epithelial to mesenchymal transition: Significance in claudin-low breast cancer cells. Mol. Cancer 2015, 14, 149. [Google Scholar] [CrossRef]
- Joneja, U.; Vranic, S.; Swensen, J.; Feldman, R.; Chen, W.; Kimbrough, J.; Xiao, N.; Reddy, S.; Palazzo, J.; Gatalica, Z. Comprehensive profiling of metaplastic breast carcinomas reveals frequent overexpression of programmed death-ligand 1. J. Clin. Pathol. 2016, 70, 255–259. [Google Scholar] [CrossRef]
- Adams, S.; Loi, S.; Toppmeyer, D.; Cescon, D.W.; De Laurentiis, M.; Nanda, R.; Winer, E.P.; Mukai, H.; Tamura, K.; Armstrong, A.; et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: Cohort B of the phase II KEYNOTE-086 study. Ann. Oncol. 2019, 30, 405–411. [Google Scholar] [CrossRef]
- Adams, S. Dramatic response of metaplastic breast cancer to chemo-immunotherapy. NPJ Breast Cancer 2017, 3, 8. [Google Scholar] [CrossRef]
- Phase II Study of Pembrolizumab and Nab-Paclitaxel in HER-2 Negative Metastatic Breast Cancer. Available online: https://ClinicalTrials.gov/show/NCT02752685 (accessed on 10 May 2023).
- Nivolumab and Ipilimumab in Treating Patients with Rare Tumors. Available online: https://ClinicalTrials.gov/show/NCT02834013 (accessed on 10 May 2023).
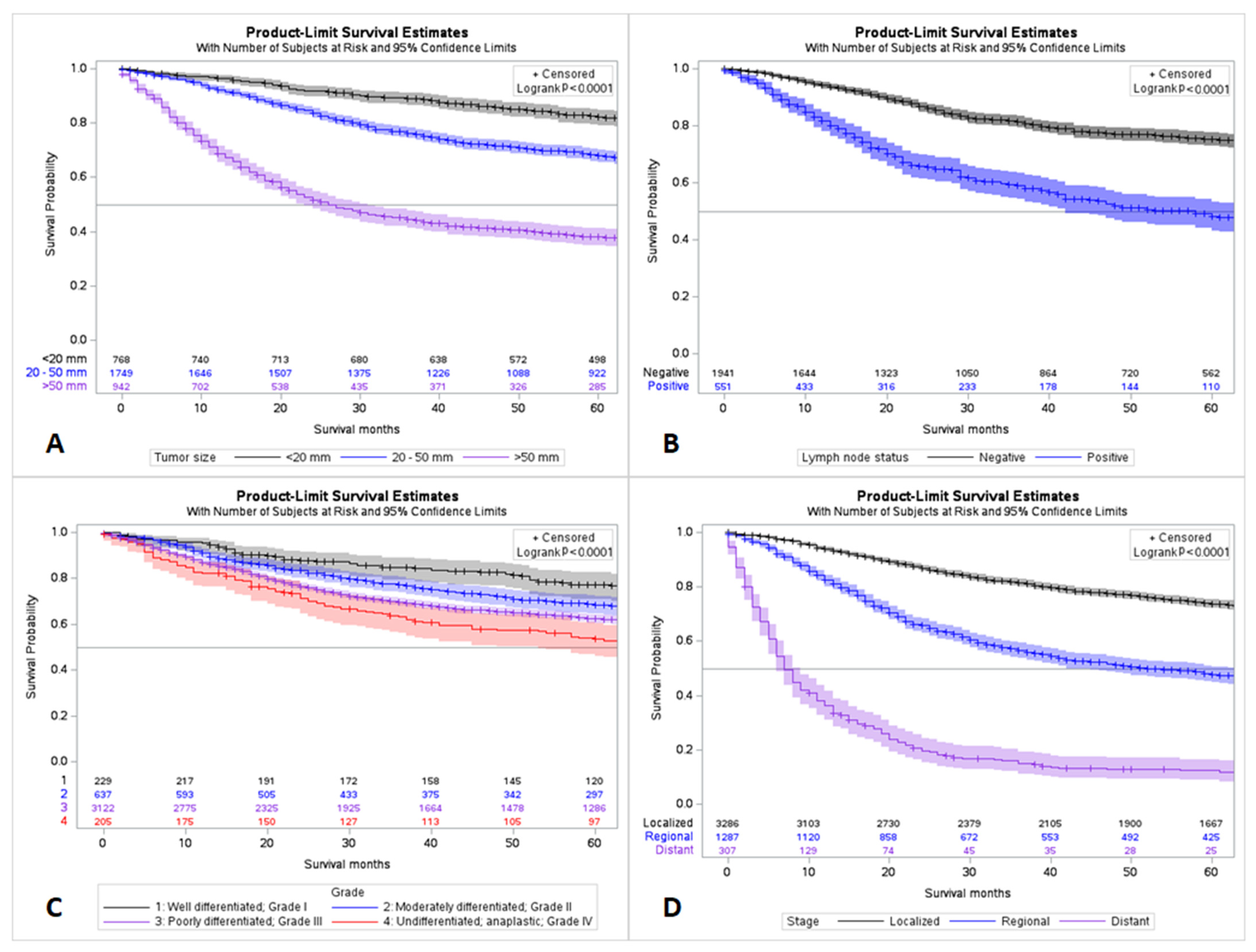
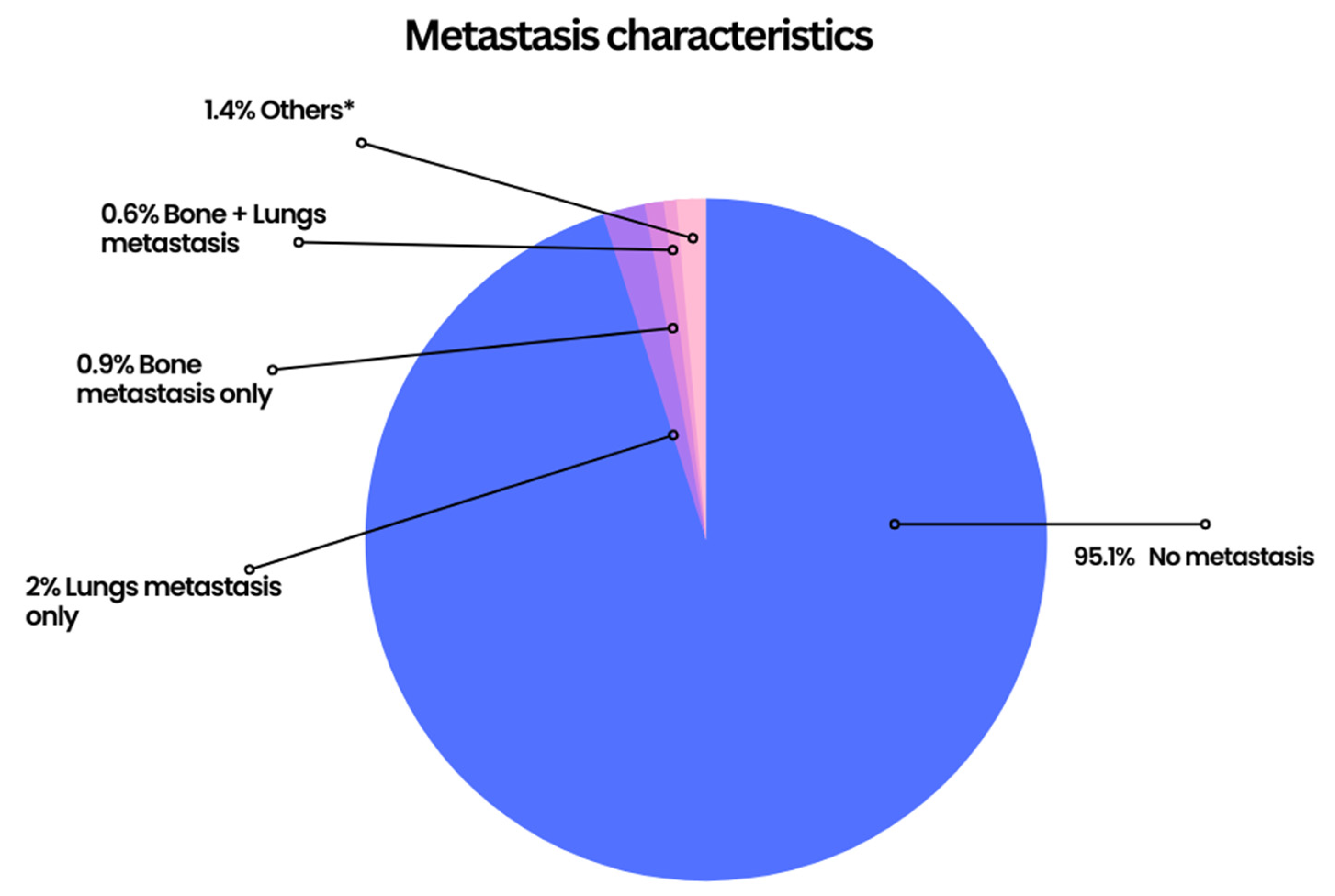
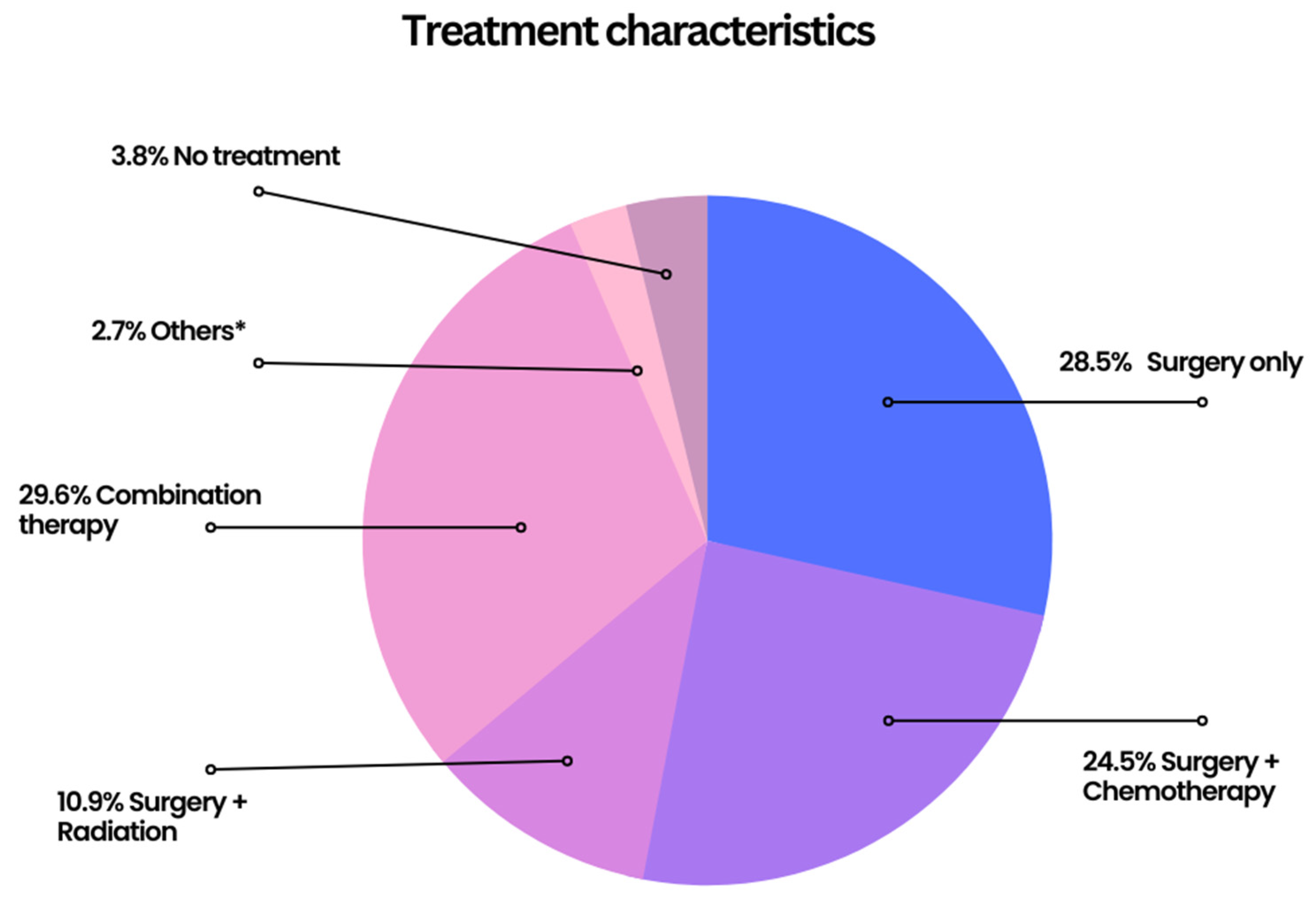
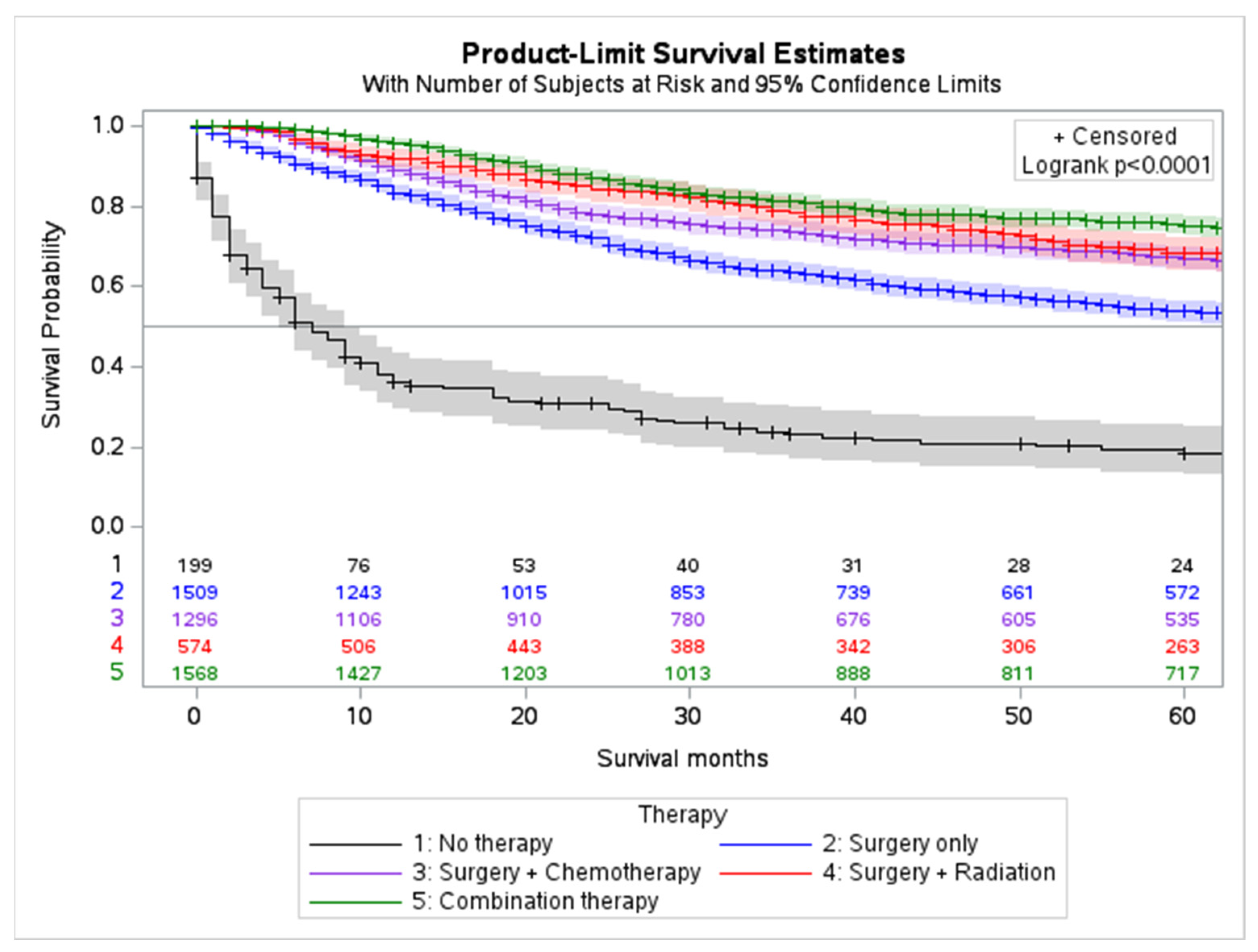
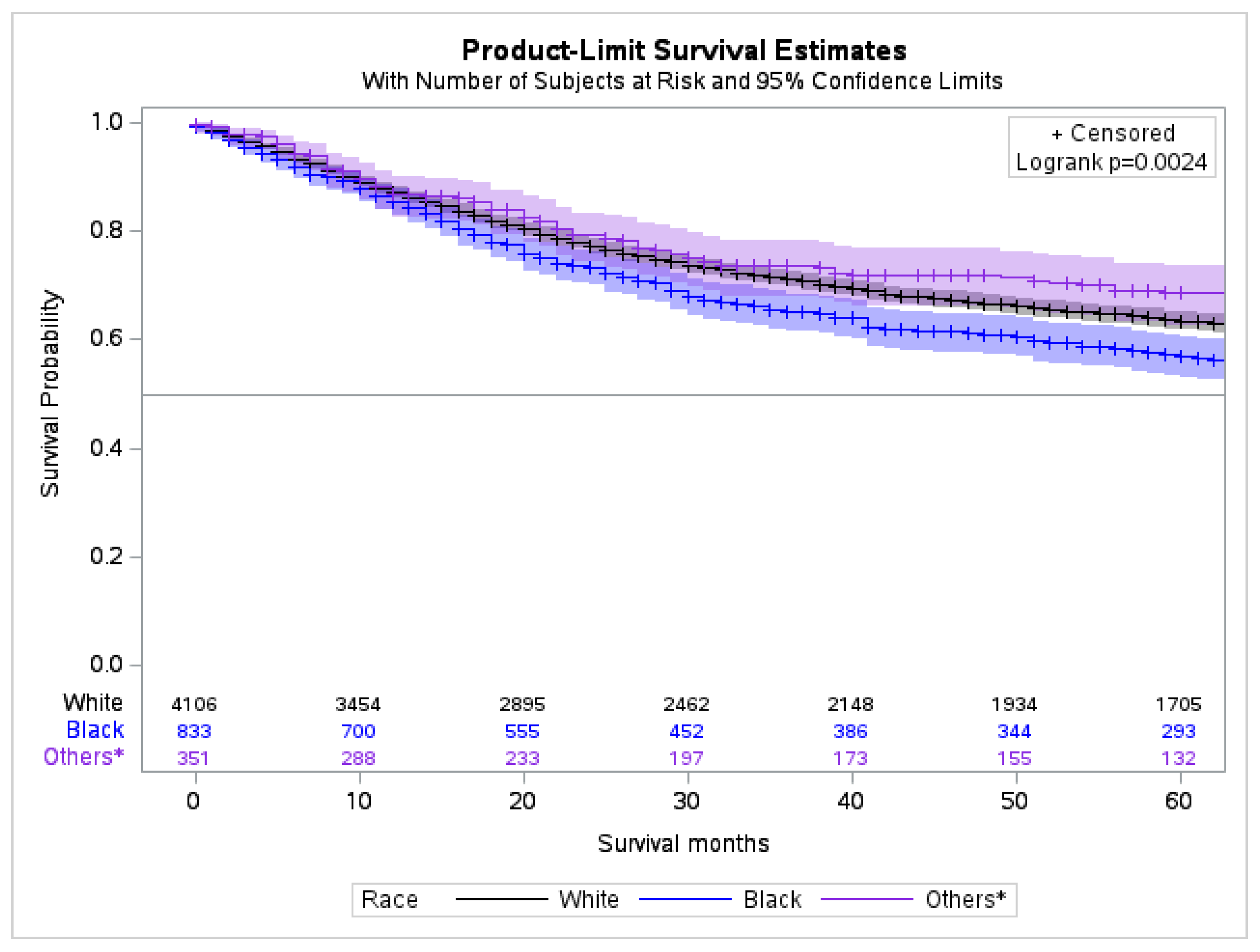
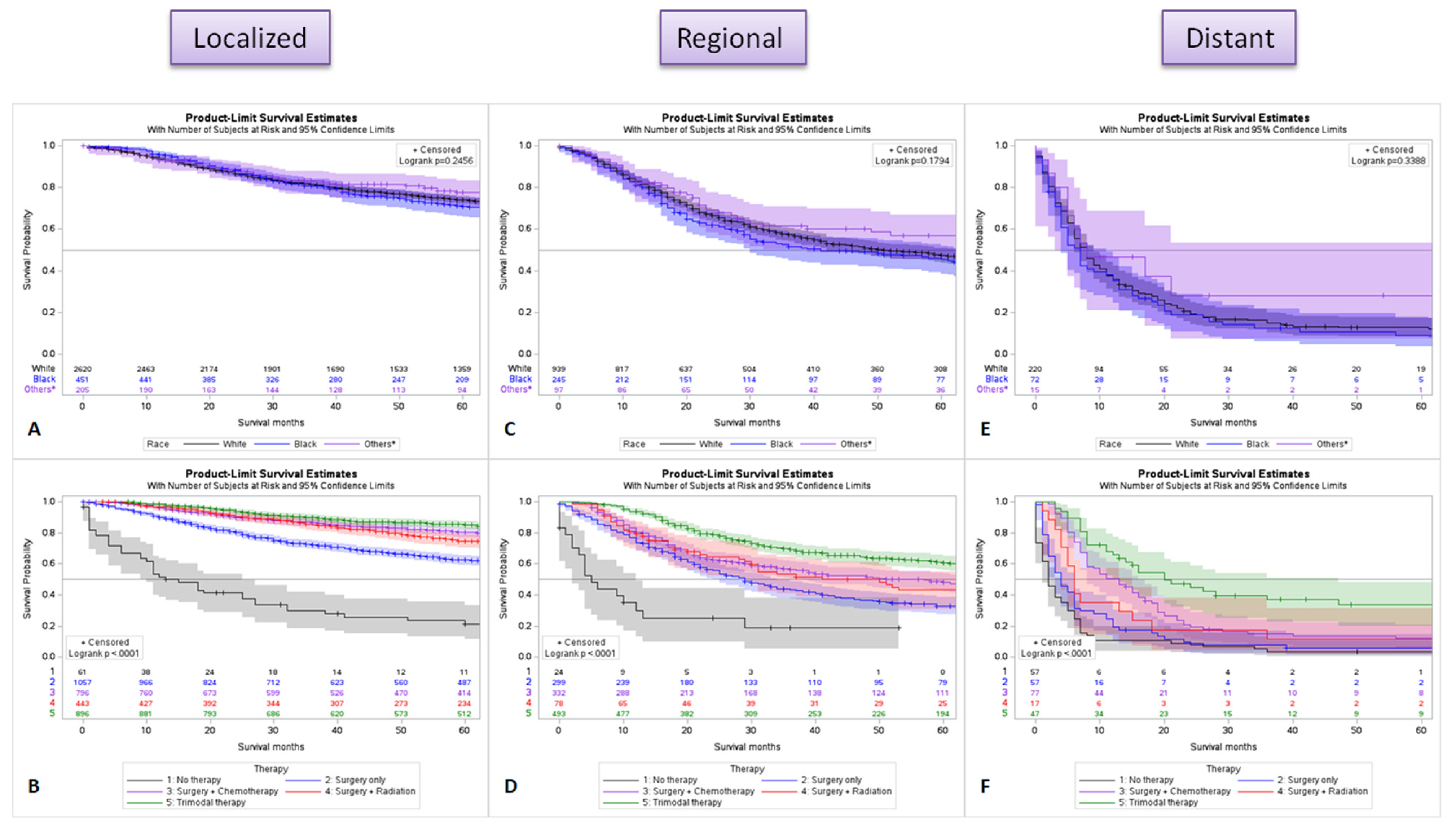
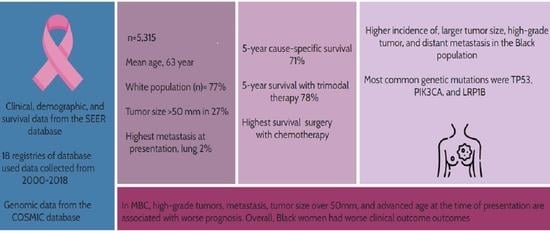
| Variable (n = 5315) | Frequency (%) | ||
|---|---|---|---|
| Age | 18–29 | 46 (0.9%) | |
| 30–39 | 256 (4.8%) | ||
| 40–49 | 690 (13.0%) | ||
| 50–59 | 1152 (21.7%) | ||
| 60–69 | 1243 (23.4%) | ||
| 70–79 | 1088 (20.5%) | ||
| ≥80 | 840 (15.8%) | ||
| Race | Unknown | 25 (0.5%) | |
| White | 4106 (77.3%) | ||
| Black | 833 (15.7%) | ||
| Asian or Pacific Islander | 324 (6.1%) | ||
| American Indian or Alaska Native | 27 (0.5%) | ||
| Grade (n = 5315) | Frequency (%) | ||
| Unknown | 1122 (21.1%) | ||
| Known | 4193 (78.9%) | ||
| Grade where known (n = 4193) | |||
| Well differentiated—Grade I | 229 (5.5%) | ||
| Moderately differentiated—Grade II | 637 (15.2%) | ||
| Poorly differentiated—Grade III | 3122 (74.4%) | ||
| Undifferentiated/Anaplastic—Grade IV | 205 (4.9%) | ||
| Receptor status (n = 5315) | Frequency (%) | ||
| Triple negative | 1978 (37.2%) | ||
| HR+/HER2− | 696 (13.1%) | ||
| HR−/HER2+ | 107 (2%) | ||
| HR+/HER2+ | 60 (1.1%) | ||
| Borderline/Unknown | 2474 (46.5%) | ||
| Variable (n = 5315) | Frequency (%) | ||
| Stage | Unknown | 435 (8.2%) | |
| Known | 4880 (91.8%) | ||
| Stage where known (n = 4880) | |||
| Localized | 3286 (67.3%) | ||
| Regional | 1287 (26.3%) | ||
| Distant | 307 (6.3%) | ||
| Size | Unknown | 1856 (34.9%) | |
| Known | 3459 (65.0%) | ||
| Size where known (n = 3459) | |||
| <20 mm | 768 (22.2%) | ||
| 20–50 mm | 1749 (50.6%) | ||
| >50 mm | 942 (27.2%) | ||
| Laterality | Left—origin of primary | 2708 (51.0%) | |
| Right—origin of primary | 2597 (48.9%) | ||
| Bilateral—single primary | 4 (0.1%) | ||
| Only one side—side unspecified | 1 (0.09%) | ||
| Paired site—but no information concerning laterality | 5 (0.1%) | ||
| Variables | Race | |
|---|---|---|
| White (n = 4106) | Black (n = 833) | |
| Grade III | 2359 (73.0%) | 542 (80.5%) |
| Distant metastases | 220 (5.8%) | 72 (9.4%) |
| Tumor size > 50 mm | 689 (25.8%) | 193 (34.8%) |
| Positive lymph nodes | 398 (21.1%) | 102 (25.6%) |
| Bone metastasis | 41 (1.8%) | 15 (3.1%) |
| Brain metastasis | 14 (0.6%) | 2 (0.4%) |
| Liver metastasis | 21 (0.9%) | 8 (1.6%) |
| Lung metastasis | 74 (3.2%) | 25 (5.1%) |
| Triple negative | 1531 (37.3%) | 315 (37.8%) |
| HR+/HER2- | 512 (12.5%) | 108 (13%) |
| HR-/HER2+ | 76 (1.9%) | 22 (2.6%) |
| HR+/HER2+ | 41 (1%) | 12 (1.4%) |
| Borderline/Unknown | 1946 (47.4%) | 376 (45.1%) |
| Variables | Multivariate Analysis; Hazard Ratio (p-Value) | |
|---|---|---|
| Age | >60 | 1.958 (0.001) |
| Grade | Undifferentiated/Anaplastic—Grade IV | 3.692 (0.002) |
| Stage | Distant | 2.613 (0.012) |
| Tumor size | >50 mm | 3.275 (0.001) |
| Brain metastasis | Yes | 29.266 (0.001) |
| Trial Number | Study Title | Study Type | Intervention | Primary Outcome | Status |
|---|---|---|---|---|---|
| NCT02834013 [52] | DART: Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors | Phase II, multicenter, Open-label | Arm 1: Nivolumab IV on days 1, 15 and 29 + Ipilimumab IV on day 1 for up to 17 42-day cyclesArm 2: Ipilimumab IV on days 1, 15, 29 for up to 17 42-day cycles | Overall response rate | Active |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, A.; Khan, J.; Yasinzai, A.Q.K.; Tracy, K.; Nguyen, T.; Tareen, B.; Garcia, A.A.; Heneidi, S.; Segura, S.E. Metaplastic Breast Carcinoma in U.S. Population: Racial Disparities, Survival Benefit of Adjuvant Chemoradiation and Future Personalized Treatment with Genomic Landscape. Cancers 2023, 15, 2954. https://doi.org/10.3390/cancers15112954
Ullah A, Khan J, Yasinzai AQK, Tracy K, Nguyen T, Tareen B, Garcia AA, Heneidi S, Segura SE. Metaplastic Breast Carcinoma in U.S. Population: Racial Disparities, Survival Benefit of Adjuvant Chemoradiation and Future Personalized Treatment with Genomic Landscape. Cancers. 2023; 15(11):2954. https://doi.org/10.3390/cancers15112954
Chicago/Turabian StyleUllah, Asad, Jaffar Khan, Abdul Qahar Khan Yasinzai, Katharine Tracy, Tena Nguyen, Bisma Tareen, Andrea Agualimpia Garcia, Saleh Heneidi, and Sheila E. Segura. 2023. "Metaplastic Breast Carcinoma in U.S. Population: Racial Disparities, Survival Benefit of Adjuvant Chemoradiation and Future Personalized Treatment with Genomic Landscape" Cancers 15, no. 11: 2954. https://doi.org/10.3390/cancers15112954
APA StyleUllah, A., Khan, J., Yasinzai, A. Q. K., Tracy, K., Nguyen, T., Tareen, B., Garcia, A. A., Heneidi, S., & Segura, S. E. (2023). Metaplastic Breast Carcinoma in U.S. Population: Racial Disparities, Survival Benefit of Adjuvant Chemoradiation and Future Personalized Treatment with Genomic Landscape. Cancers, 15(11), 2954. https://doi.org/10.3390/cancers15112954