Clinical Outcomes of Cabozantinib in Patients Previously Treated with Atezolizumab/Bevacizumab for Advanced Hepatocellular Carcinoma—Importance of Good Liver Function and Good Performance Status
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Cabozantinib Treatment, Evaluation of Adverse Events and Changes in Liver Function
2.3. Evaluation of Antitumor Response
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
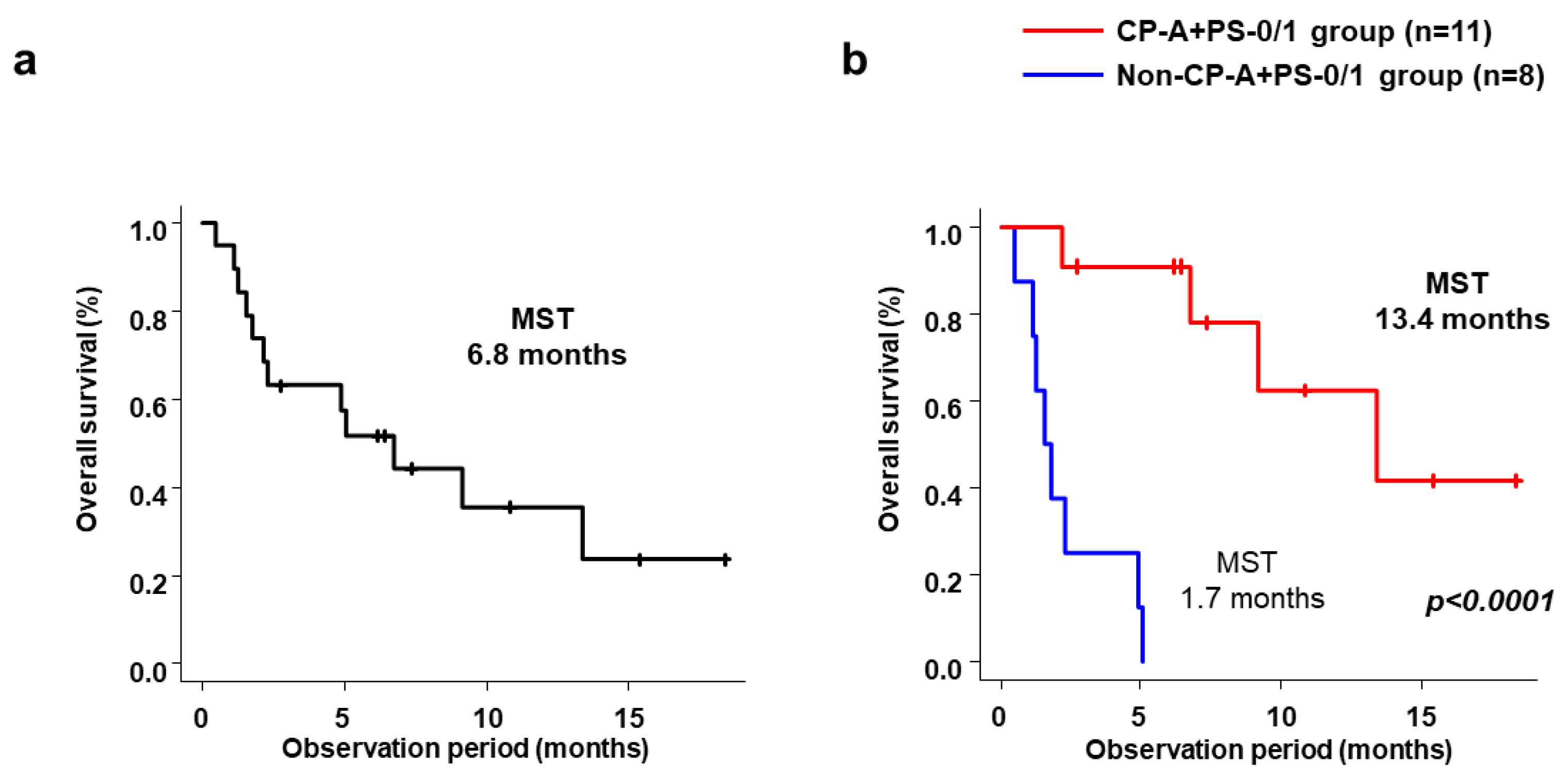
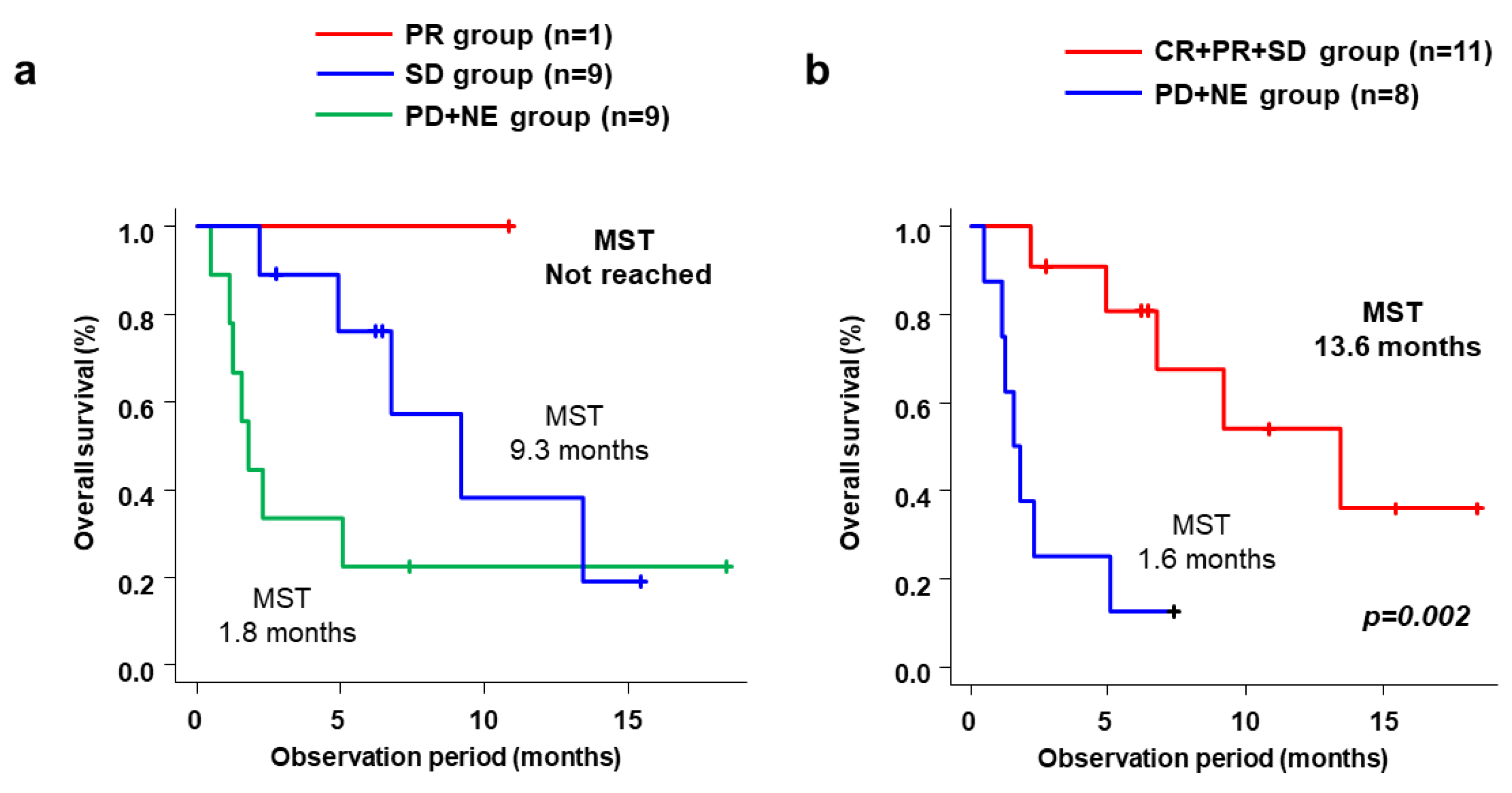
3.2. Efficacy
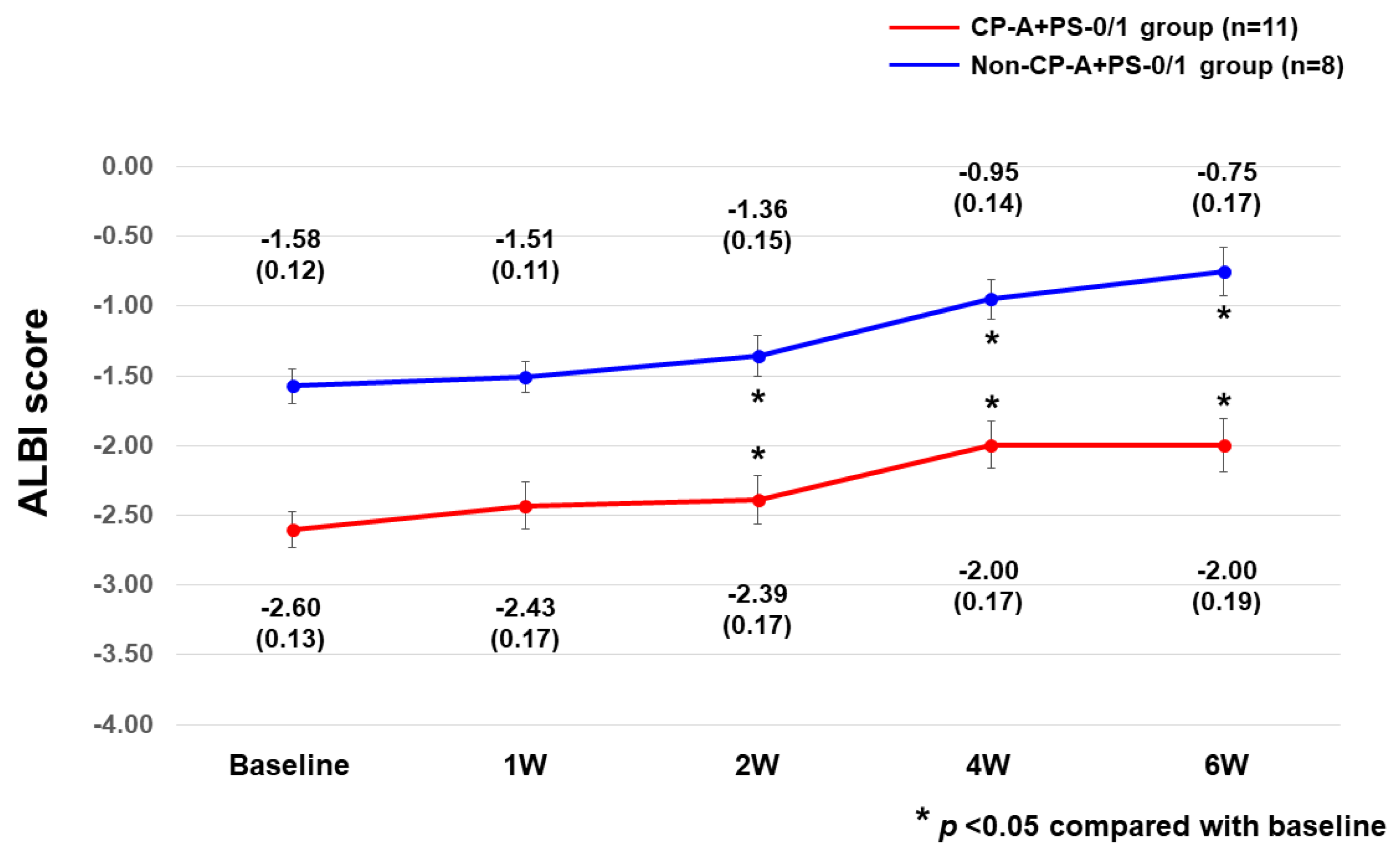
3.3. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Tada, T.; Hirooka, M.; Kariyama, K.; Tani, J.; Atsukawa, M.; Takaguchi, K.; Itobayashi, E.; Fukunishi, S.; et al. Atezolizumab plus bevacizumab treatment for unresectable hepatocellular carcinoma: Early clinical experience. Cancer Rep. 2022, 5, e1464. [Google Scholar] [CrossRef] [PubMed]
- Kuzuya, T.; Kawabe, N.; Hashimoto, S.; Miyahara, R.; Sawaki, A.; Nakano, T.; Nakaoka, K.; Tanaka, H.; Miyachi, Y.; Mii, A.; et al. Early Changes in Alpha-Fetoprotein Are a Useful Predictor of Efficacy of Atezolizumab plus Bevacizumab Treatment in Patients with Advanced Hepatocellular Carcinoma. Oncology 2022, 100, 12–21. [Google Scholar] [CrossRef]
- Kuzuya, T.; Kawabe, N.; Hashimoto, S.; Miyahara, R.; Nakano, T.; Nakaoka, K.; Tanaka, H.; Miyachi, Y.; Mii, A.; Tanahashi, Y.; et al. Initial Experience of Atezolizumab Plus Bevacizumab for Advanced Hepatocellular Carcinoma in Clinical Practice. Cancer Diagn. Progn. 2021, 1, 83–88. [Google Scholar] [CrossRef]
- Maesaka, K.; Sakamori, R.; Yamada, R.; Doi, A.; Tahata, Y.; Ohkawa, K.; Oshita, M.; Miyazaki, M.; Yakushijin, T.; Nozaki, Y.; et al. Pretreatment with antibiotics is associated with reduced therapeutic response to atezolizumab plus bevacizumab in patients with hepatocellular carcinoma. PLoS ONE 2023, 18, e0281459. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Kumada, T.; Hiraoka, A.; Kariyama, K.; Tani, J.; Hirooka, M.; Takaguchi, K.; Atsukawa, M.; Fukunishi, S.; Itobayashi, E.; et al. New prognostic system based on inflammation and liver function predicts prognosis in patients with advanced unresectable hepatocellular carcinoma treated with atezolizumab plus bevacizumab: A validation study. Cancer Med. 2022, 12, 6980–6993. [Google Scholar] [CrossRef]
- Hatanaka, T.; Yata, Y.; Naganuma, A.; Kakizaki, S. Treatment Strategy for Intermediate-Stage Hepatocellular Carcinoma: Transarterial Chemoembolization, Systemic Therapy, and Conversion Therapy. Cancers 2023, 15, 1798. [Google Scholar] [CrossRef]
- Vogel, A.; Martinelli, E.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.; Meyer, T.; Nault, J.-C.; Neumann, U.; Ricke, J.; et al. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann. Oncol. 2021, 32, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.-L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.-Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.-W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation Barcelona Clinic Liver Cancer (BCLC) staging system: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.H.; Huang, Y.H.; Lin, S.M.; Hsu, C. AXL and MET in Hepatocellular Carcinoma: A Systematic Literature Review. Liver Cancer 2022, 11, 94–112. [Google Scholar] [CrossRef]
- Pinato, D.J.; Brown, M.W.; Trousil, S.; Aboagye, E.O.; Beaumont, J.; Zhang, H.; Coley, H.M.; Mauri, F.A.; Sharma, R. Integrated analysis of multiple receptor tyrosine kinases identifies Axl as a therapeutic target and mediator of resistance to sorafenib in hepatocellular carcinoma. Br. J. Cancer 2019, 120, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Chen, W.; Ren, M.; Wang, J.; Zhang, H.; Deng, D.Y.; Zhang, L.; Shang, C.; Chen, Y. Cabozantinib Suppresses Tumor Growth and Metastasis in Hepatocellular Carcinoma by a Dual Blockade of VEGFR2 and MET. Clin. Cancer Res. 2014, 20, 2959–2970. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Jiang, S.; Li, J.; Chen, H.; Zhang, X. Activation of the HGF/c-MET axis promotes lenvatinib resistance in hepatocellular carcinoma cells with high c-MET expression. Med. Oncol. 2020, 37, 24. [Google Scholar] [CrossRef]
- Balan, M.; Teran, E.M.Y.; Waaga-Gasser, A.M.; Gasser, M.; Choueiri, T.K.; Freeman, G.; Pal, S. Novel Roles of c-Met in the Survival of Renal Cancer Cells through the Regulation of HO-1 and PD-L1 Expression. J. Biol. Chem. 2015, 290, 8110–8120. [Google Scholar] [CrossRef]
- Kwilas, A.R.; Ardiani, A.; Donahue, R.N.; Aftab, D.T.; Hodge, J.W. Dual effects of a targeted small-molecule inhibitor (cabozantinib) on immune-mediated killing of tumor cells and immune tumor microenvironment permissiveness when combined with a cancer vaccine. J. Transl. Med. 2014, 12, 294. [Google Scholar] [CrossRef]
- Storandt, M.H.; Gile, J.J.; Palmer, M.E.; Zemla, T.J.; Ahn, D.H.; Bekaii-Saab, T.S.; Jin, Z.; Tran, N.H.; Mahipal, A. Cabozantinib Following Immunotherapy in Patients with Advanced Hepatocellular Carcinoma. Cancers 2022, 14, 5173. [Google Scholar] [CrossRef]
- Tomonari, T.; Tani, J.; Ogawa, C.; Deguchi, A.; Senoh, T.; Moriya, A.; Shibata, H.; Fukuno, H.; Tanaka, H.; Tanaka, T.; et al. Multicenter retrospective study of initial treatment outcome and feasibility of initiating dose reduction of cabozantinib in unresectable hepatocellular carcinoma. Hepatol. Res. 2023, 53, 172–178. [Google Scholar] [CrossRef]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of Liver Function in Patients With Hepatocellular Carcinoma: A New Evidence-Based Approach—The ALBI Grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the Freely Available Easy-to-Use Software ‘EZR’ for Medical Statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Meyer, T.; Cheng, A.-L.; Rimassa, L.; Sen, S.; Milwee, S.; Kelley, R.K.; Abou-Alfa, G.K. Safety and efficacy of cabozantinib for patients with advanced hepatocellular carcinoma who advanced to Child–Pugh B liver function at study week 8: A retrospective analysis of the CELESTIAL randomised controlled trial. BMC Cancer 2022, 22, 377. [Google Scholar] [CrossRef]
- Rimassa, L.; Kelley, R.K.; Meyer, T.; Ryoo, B.-Y.; Merle, P.; Park, J.-W.; Blanc, J.-F.; Lim, H.Y.; Tran, A.; Chan, Y.-W.; et al. Outcomes Based on Plasma Biomarkers for the Phase 3 CELESTIAL Trial of Cabozantinib versus Placebo in Advanced Hepatocellular Carcinoma. Liver Cancer 2021, 11, 38–47. [Google Scholar] [CrossRef]
- Kelley, R.K.; Miksad, R.; Cicin, I.; Chen, Y.; Klümpen, H.-J.; Kim, S.; Lin, Z.-Z.; Youkstetter, J.; Hazra, S.; Sen, S.; et al. Efficacy and safety of cabozantinib for patients with advanced hepatocellular carcinoma based on albumin-bilirubin grade. Br. J. Cancer 2022, 126, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.K.; Ryoo, B.-Y.; Merle, P.; Park, J.-W.; Bolondi, L.; Chan, S.L.; Lim, H.Y.; Baron, A.D.; Parnis, F.; Knox, J.; et al. Second-line cabozantinib after sorafenib treatment for advanced hepatocellular carcinoma: A subgroup analysis of the phase 3 CELESTIAL trial. ESMO Open 2020, 5, e000714. [Google Scholar] [CrossRef]
- Kelley, R.K.; Meyer, T.; Rimassa, L.; Merle, P.; Park, J.-W.; Yau, T.; Chan, S.L.; Blanc, J.-F.; Tam, V.C.; Tran, A.; et al. Serum Alpha-fetoprotein Levels and Clinical Outcomes in the Phase III CELESTIAL Study of Cabozantinib versus Placebo in Patients with Advanced Hepatocellular Carcinoma. Clin. Cancer Res. 2020, 26, 4795–4804. [Google Scholar] [CrossRef]
- Kudo, M.; Tsuchiya, K.; Kato, N.; Hagihara, A.; Numata, K.; Aikata, H.; Inaba, Y.; Kondo, S.; Motomura, K.; Furuse, J.; et al. Cabozantinib in Japanese patients with advanced hepatocellular carcinoma: A phase 2 multicenter study. J. Gastroenterol. 2021, 56, 181–190. [Google Scholar] [CrossRef]
- Kato, N.; Kudo, M.; Tsuchiya, K.; Hagihara, A.; Numata, K.; Aikata, H.; Inaba, Y.; Kondo, S.; Motomura, K.; Okano, N.; et al. Cabozantinib in Japanese patients with advanced hepatocellular carcinoma: Final results of a multicenter phase II study. Hepatol. Res. 2023, 53, 409–416. [Google Scholar] [CrossRef]
- Finkelmeier, F.; Scheiner, B.; Leyh, C.; Best, J.; Fründt, T.W.; Czauderna, C.; Beutel, A.; Bettinger, D.; Weiß, J.; Meischl, T.; et al. Cabozantinib in Advanced Hepatocellular Carcinoma: Efficacy and Safety Data from an International Multicenter Real-Life Cohort. Liver Cancer 2021, 10, 360–369. [Google Scholar] [CrossRef]
- Adhoute, X.; De Matharel, M.; Mineur, L.; Pénaranda, G.; Ouizeman, D.; Toullec, C.; Tran, A.; Castellani, P.; Rollet, A.; Oules, V.; et al. Second-line therapy for advanced hepatocellular carcinoma with regorafenib or cabozantinib: Multicenter French clinical experience in real-life after matching. World J. Gastrointest. Oncol. 2022, 14, 1510–1527. [Google Scholar] [CrossRef]
- Tovoli, F.; Dadduzio, V.; De Lorenzo, S.; Rimassa, L.; Masi, G.; Iavarone, M.; Marra, F.; Garajova, I.; Brizzi, M.P.; Daniele, B.; et al. Real-Life Clinical Data of Cabozantinib for Unresectable Hepatocellular Carcinoma. Liver Cancer 2021, 10, 370–379. [Google Scholar] [CrossRef]
- Kudo, M.; Ikeda, M.; Takayama, T.; Numata, K.; Izumi, N.; Furuse, J.; Okusaka, T.; Kadoya, M.; Yamashita, S.; Ito, Y.; et al. Safety and efficacy of sorafenib in Japanese patients with hepatocellular carcinoma in clinical practice: A subgroup analysis of GIDEON. J. Gastroenterol. 2016, 51, 1150–1160. [Google Scholar] [CrossRef]
- Hollebecque, A.; Cattan, S.; Romano, O.; Sergent, G.; Mourad, A.; Louvet, A.; Dharancy, S.; Boleslawski, E.; Truant, S.; Pruvot, F.-R.; et al. Safety and efficacy of sorafenib in hepatocellular carcinoma: The impact of the Child-Pugh score. Aliment. Pharmacol. Ther. 2011, 34, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Ueshima, K.; Nishida, N.; Hagiwara, S.; Aoki, T.; Minami, T.; Chishina, H.; Takita, M.; Minami, Y.; Ida, H.; Takenaka, M.; et al. Impact of Baseline ALBI Grade on the Outcomes of Hepatocellular Carcinoma Patients Treated with Lenvatinib: A Multicenter Study. Cancers 2019, 11, 952. [Google Scholar] [CrossRef] [PubMed]
- Kariyama, K.; Hiraoka, A.; Kumada, T.; Yasuda, S.; Toyoda, H.; Tsuji, K.; Hatanaka, T.; Kakizaki, S.; Naganuma, A.; Tada, T.; et al. Chronological change in serum albumin as a prognostic factor in patients with hepatocellular carcinoma treated with lenvatinib: Proposal of albumin simplified grading based on the modified albumin–bilirubin score (ALBS grade). J. Gastroenterol. 2022, 57, 581–586. [Google Scholar] [CrossRef]
- Hiraoka, A.; Tanizawa, Y.; Huang, Y.-J.; Cai, Z.; Sakaguchi, S. Association of Albumin-Bilirubin Grade and Sequential Treatment with Standard Systemic Therapies for Advanced Hepatocellular Carcinoma: A Retrospective Cohort Study Using a Japanese Administrative Database. Drugs-Real World Outcomes 2021, 8, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Muto, H.; Kuzuya, T.; Ito, T.; Ishizu, Y.; Honda, T.; Ishikawa, T.; Ishigami, M.; Fujishiro, M. Transient deterioration of albumin-bilirubin scores in early post-dose period of molecular targeted therapies in advanced hepatocellular carcinoma with 50% or higher liver occupation: A STROBE-compliant retrospective observational study. Medicine 2021, 100, e26820. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Kurosaki, M.; Sakamoto, A.; Marusawa, H.; Kojima, Y.; Hasebe, C.; Arai, H.; Joko, K.; Kondo, M.; Tsuji, K.; et al. The Real-World Data in Japanese Patients with Unresectable Hepatocellular Carcinoma Treated with Lenvatinib from a Nationwide Multicenter Study. Cancers 2021, 13, 2608. [Google Scholar] [CrossRef]
- Kuzuya, T.; Ishigami, M.; Ishizu, Y.; Honda, T.; Hayashi, K.; Katano, Y.; Hirooka, Y.; Ishikawa, T.; Nakano, I.; Goto, H. Early Clinical Response after 2 Weeks of Sorafenib Therapy Predicts Outcomes and Anti-Tumor Response in Patients with Advanced Hepatocellular Carcinoma. PLoS ONE 2015, 10, e0138776. [Google Scholar] [CrossRef]
- Kuzuya, T.; Ishigami, M.; Ito, T.; Ishizu, Y.; Honda, T.; Ishikawa, T.; Fujishiro, M. Favorable radiological antitumor response at 2 weeks after starting lenvatinib for patients with advanced hepatocellular carcinoma. Hepatol. Res. 2020, 50, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.; Holland, J.; Ramies, D.; Mamelok, R.; Benrimoh, N.; Ciric, S.; Marbury, T.; Preston, R.A.; Heuman, D.M.; Gavis, E.; et al. Effect of Renal and Hepatic Impairment on the Pharmacokinetics of Cabozantinib. J. Clin. Pharmacol. 2016, 56, 1130–1140. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, A.; Kumada, T.; Atsukawa, M.; Hirooka, M.; Tsuji, K.; Ishikawa, T.; Takaguchi, K.; Kariyama, K.; Itobayashi, E.; Tajiri, K.; et al. Prognostic factor of lenvatinib for unresectable hepatocellular carcinoma in real-world conditions—Multicenter analysis. Cancer Med. 2019, 8, 3719–3728. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Moriguchi, M.; Iwai, K.; Tsuchiya, S.; Seko, Y.; Takahashi, A.; Kobayashi, K.; Ogasawara, S.; Watanabe, S.; Morimoto, N.; et al. Real-world outcomes of molecular targeted agents for patients with hepatocellular carcinoma over 80 years old. Hepatol. Res. 2022, 52, 859–871. [Google Scholar] [CrossRef]
- Nishikawa, H.; Osaki, Y.; Endo, M.; Takeda, H.; Tsuchiya, K.; Joko, K.; Ogawa, C.; Taniguchi, H.; Orito, E.; Uchida, Y.; et al. Comparison of standard-dose and half-dose sorafenib therapy on clinical outcome in patients with unresectable hepatocellular carcinoma in field practice: A propensity score matching analysis. Int. J. Oncol. 2014, 45, 2295–2302. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.K.; Dao, T.V. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022, 1, EVIDoa2100070. [Google Scholar] [CrossRef]


| Characteristics | n = 19 |
|---|---|
| Median age (years, range) | 67 (39–79) |
| Sex (male/female) | 15/4 |
| Etiology (HBV/HCV/non-viral) | 6/4/9 |
| Child–Pugh score (5/6/7/8/9) | 8/4/3/2/2 |
| ECOG-PS (0/1/2) | 10/6/3 |
| Both Child–Pugh class A and ECOG-PS score 0/1 (+/−) | 11/8 |
| BCLC stage (B/C) | 4/15 |
| HCC size (<50 mm/≥50 mm) | 11/8 |
| Number of HCCs (<4/≥4) | 1/18 |
| Portal vein tumor thrombosis (−/+) | 12/7 |
| Extrahepatic spread (−/+) | 8/11 |
| Median serum AFP level (ng/mL, range) | 885 (2.1–625,505) |
| History of hepatic resection (+/−) | 10/9 |
| History of radiofrequency ablation (+/−) | 3/16 |
| History of trans arterial chemoembolization (+/−) | 17/2 |
| History of radiation therapy (+/−) | 4/15 |
| History of atezolizumab plus bevacizumab (+/−) | 19/0 |
| History of lenvatinib (+/−) | 19/0 |
| History of ramucirumab (+/−) | 10/9 |
| History of sorafenib (+/−) | 1/18 |
| History of regorafenib (+/−) | 1/18 |
| Cabozantinib treatment line (3rd-/4th-/5th/6th) | 9/7/2/1 |
| Cabozantinib initial dose (60/40/20 mg/day) | 3/12/4 |
| Median duration from initial diagnosis of HCC (months) | 22.5 (7.9–120.6) |
| Median observation period (months) | 5.1 (0.5–18.7) |
| RECIST | All Patients (n = 19) | CP-A+PS-0/1 Group (n = 11) | Non-CP-A+PS-0/1 Group (n = 8) | p Value * | |
|---|---|---|---|---|---|
| Cabozantinib alone | CR/PR/SD/PD/NE, n | 0/1/9/2/7 | 0/1/8/1/1 | 0/0/1/1/6 | |
| ORR | 5.3% | 9.1% | 0% | 1.000 | |
| DCR | 52.6% | 81.1% | 12.5% | 0.006 | |
| Cabozantinib + additional treatment | CR/PR/SD/PD/NE, n | 2/0/9/1/7 | 2/0/8/0/1 | 0/0/1/1/6 | |
| ORR | 10.5% | 18.2% | 0% | 0.485 | |
| DCR | 57.9% | 90.9% | 12.5% | 0.001 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Factors | HR (95%CI) | p Value | HR (95%CI) | p Value |
| Age (≥67 years) | 0.313 (0.099–0.989) | 0.048 | 0.493 (0.144–1.689) | 0.260 |
| Sex (male) | 0.539 (0.164–1.769) | 0.308 | ||
| Etiology (HBV or HCV) | 0.950 (0.354–3.030) | 0.918 | ||
| Child–Pugh A and ECOG-PS 0/1 (+) | 0.184 (0.054–0.633) | 0.007 | 0.136 (0.035–0.530) | 0.031 |
| HCC number (<4) | 0.420 (0.051–3.443) | 0.419 | ||
| HCC size (≥5 cm) | 0.874 (0.310–2.463) | 0.800 | ||
| Vp (−) | 0.761 (0.258–2.244) | 0.620 | ||
| EHS (+) | 0.504 (0.174–1.458) | 0.206 | ||
| AFP level (<400 ng/mL) | 0.406 (0.134–1.235) | 0.112 | ||
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Factors | HR (95%CI) | p Value | HR (95%CI) | p Value |
| Age (≥67 years) | 0.634 (0.200–2.013) | 0.440 | ||
| Sex (female) | 0.901 (0.241–3.368) | 0.877 | ||
| Etiology (HBV or HCV) | 0.527 (0.167–1.669) | 0.276 | ||
| Child–Pugh A and ECOG-PS 0/1 (+) | 0.039 (0.005–0.324) | 0.003 | 0.044 (0.005–0.401) | 0.005 |
| HCC number (≥4) | 0.976 (0.127–7.806) | 0.981 | ||
| HCC size (<5 cm) | 0.524 (0.155–1.770) | 0.298 | ||
| Vp (−) | 0.296 (0.078–1.118) | 0.072 | 0.096 (0.014–0.656) | 0.017 |
| EHS (−) | 0.226 (0.048–.053) | 0.058 | 0.055 (0.005–0.584) | 0.016 |
| AFP level (<400 ng/mL) | 0.417 (0.123–1.413) | 0.160 | ||
| All Patients (n = 19) | CP-A+PS-0/1 Group (n = 11) | Non-CP-A+PS-0/1 Group (n = 8) | ||||
|---|---|---|---|---|---|---|
| Any Grade n, (%) | Grade ≥3 n, (%) | Any Grade n, (%) | Grade ≥3 n, (%) | Any Grade n, (%) | Grade ≥3 n, (%) | |
| Anorexia | 10 (52.6) | 1 (5.2) | 5 (45.5) | 0 | 5 (62.5) | 1 (12.5) |
| Proteinuria | 8 (42.1) | 6 (31.6) | 6 (54.5) | 5 (45.5) | 2 (25.0) | 1 (12.5) |
| General fatigue | 8 (42.1) | 2 (10.5) | 4 (36.4) | 0 | 4 (50.0) | 2 (25.0) |
| Hand-foot syndrome | 7 (36.8) | 1 (5.3) | 6 (54.5) | 1 (9.1) | 1 (12.5) | 0 |
| Diarrhea | 6 (31.6) | 1 (5.3) | 5 (45.5) | 0 | 1 (12.5) | 1 (12.5) |
| Hypothyroidism | 6 (31.6) | 0 | 3 (27.3) | 0 | 3 (37.5) | 0 |
| Bleeding | 3 (15.8) | 1 (5.3) | 1 (9.1) | 0 | 2 (25.0) | 1 (12.5) |
| Hypertension | 3 (15.8) | 0 | 2 (18.2) | 0 | 1 (12.5) | 0 |
| Fever | 3 (15.8) | 0 | 2 (18.2) | 0 | 1 (12.5) | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuzuya, T.; Kawabe, N.; Ariga, M.; Ohno, E.; Funasaka, K.; Nagasaka, M.; Nakagawa, Y.; Miyahara, R.; Shibata, T.; Takahara, T.; et al. Clinical Outcomes of Cabozantinib in Patients Previously Treated with Atezolizumab/Bevacizumab for Advanced Hepatocellular Carcinoma—Importance of Good Liver Function and Good Performance Status. Cancers 2023, 15, 2952. https://doi.org/10.3390/cancers15112952
Kuzuya T, Kawabe N, Ariga M, Ohno E, Funasaka K, Nagasaka M, Nakagawa Y, Miyahara R, Shibata T, Takahara T, et al. Clinical Outcomes of Cabozantinib in Patients Previously Treated with Atezolizumab/Bevacizumab for Advanced Hepatocellular Carcinoma—Importance of Good Liver Function and Good Performance Status. Cancers. 2023; 15(11):2952. https://doi.org/10.3390/cancers15112952
Chicago/Turabian StyleKuzuya, Teiji, Naoto Kawabe, Mizuki Ariga, Eizaburo Ohno, Kohei Funasaka, Mitsuo Nagasaka, Yoshihito Nakagawa, Ryoji Miyahara, Tomoyuki Shibata, Takeshi Takahara, and et al. 2023. "Clinical Outcomes of Cabozantinib in Patients Previously Treated with Atezolizumab/Bevacizumab for Advanced Hepatocellular Carcinoma—Importance of Good Liver Function and Good Performance Status" Cancers 15, no. 11: 2952. https://doi.org/10.3390/cancers15112952
APA StyleKuzuya, T., Kawabe, N., Ariga, M., Ohno, E., Funasaka, K., Nagasaka, M., Nakagawa, Y., Miyahara, R., Shibata, T., Takahara, T., Kato, Y., & Hirooka, Y. (2023). Clinical Outcomes of Cabozantinib in Patients Previously Treated with Atezolizumab/Bevacizumab for Advanced Hepatocellular Carcinoma—Importance of Good Liver Function and Good Performance Status. Cancers, 15(11), 2952. https://doi.org/10.3390/cancers15112952







