The Effect of Induction Chemotherapy with VEGF Inhibition on Tumor Response in Synchronously Metastasized Potentially Resectable Colorectal Cancer
Abstract
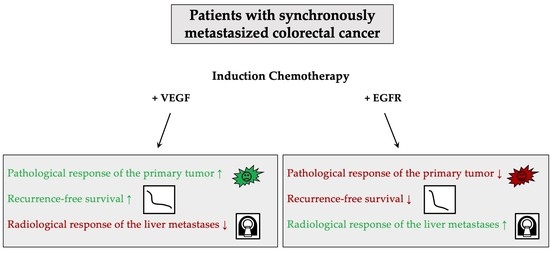
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Therapy
2.2. Pathological and Radiological Tumor Response Assessment
Ninety-Day Morbidity and Mortality
2.3. Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Patients
3.2. Radiological and Pathological Response
3.3. Ninety-Day Morbidity and Mortality
3.4. Survival Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Borner, M.M. Neoadjuvant chemotherapy for unresectable liver metastases of colorectal cancer--too good to be true? Ann. Oncol. 1999, 10, 623–626. [Google Scholar] [CrossRef]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef]
- Stintzing, S.; Modest, D.P.; Rossius, L.; Lerch, M.M.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.-E.; Heintges, T.; et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): A post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol. 2016, 17, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- Nordlinger, B.; Sorbye, H.; Glimelius, B.; Poston, G.J.; Schlag, P.M.; Rougier, P.; Bechstein, W.O.; Primrose, J.N.; Walpole, E.T.; Finch-Jones, M.; et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): Long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013, 14, 1208–1215. [Google Scholar] [CrossRef]
- Cremolini, C.; Loupakis, F.; Antoniotti, C.; Lupi, C.; Sensi, E.; Lonardi, S.; Mezi, S.; Tomasello, G.; Ronzoni, M.; Zaniboni, A.; et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: Updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015, 16, 1306–1315. [Google Scholar] [CrossRef]
- Primrose, J.; Falk, S.; Finch-Jones, M.; Valle, J.; O’Reilly, D.; Siriwardena, A.; Hornbuckle, J.; Peterson, M.; Rees, M.; Iveson, T.; et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: The New EPOC randomised controlled trial. Lancet Oncol. 2014, 15, 601–611. [Google Scholar] [CrossRef]
- Venook, A.P.; Niedzwiecki, D.; Lenz, H.-J.; Innocenti, F.; Fruth, B.; Meyerhardt, J.A.; Schrag, D.; Greene, C.; O’Neil, B.H.; Atkins, J.N.; et al. Effect of First-Line Chemotherapy Combined With Cetuximab or Bevacizumab on Overall Survival in Patients With KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA 2017, 317, 2392–2401. [Google Scholar] [CrossRef] [PubMed]
- Stremitzer, S.; Stift, J.; Singh, J.; Starlinger, P.; Gruenberger, B.; Tamandl, D.; Gruenberger, T. Histological response, pattern of tumor destruction and clinical outcome after neoadjuvant chemotherapy including bevacizumab or cetuximab in patients undergoing liver resection for colorectal liver metastases. Eur. J. Surg. Oncol. 2015, 41, 868–874. [Google Scholar] [CrossRef]
- Gruenberger, B.; Tamandl, D.; Schueller, J.; Scheithauer, W.; Zielinski, C.; Herbst, F.; Gruenberger, T. Bevacizumab, capecitabine, and oxaliplatin as neoadjuvant therapy for patients with potentially curable metastatic colorectal cancer. J. Clin. Oncol. 2008, 26, 1830–1835. [Google Scholar] [CrossRef] [PubMed]
- Loupakis, F.; Schirripa, M.; Caparello, C.; Funel, N.; Pollina, L.; Vasile, E.; Cremolini, C.; Salvatore, L.; Morvillo, M.; Antoniotti, C.; et al. Histopathologic evaluation of liver metastases from colorectal cancer in patients treated with FOLFOXIRI plus bevacizumab. Br. J. Cancer 2013, 108, 2549–2556. [Google Scholar] [CrossRef]
- Gruenberger, T.; Bridgewater, J.; Chau, I.; García Alfonso, P.; Rivoire, M.; Mudan, S.; Lasserre, S.; Hermann, F.; Waterkamp, D.; Adam, R. Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: The OLIVIA multinational randomised phase II trial. Ann. Oncol. 2015, 26, 702–708. [Google Scholar] [CrossRef]
- Cremolini, C.; Antoniotti, C.; Stein, A.; Bendell, J.; Gruenberger, T.; Rossini, D.; Masi, G.; Ongaro, E.; Hurwitz, H.; Falcone, A.; et al. Individual Patient Data Meta-Analysis of FOLFOXIRI Plus Bevacizumab Versus Doublets Plus Bevacizumab as Initial Therapy of Unresectable Metastatic Colorectal Cancer. J. Clin. Oncol. 2020, 38, 3314–3324. [Google Scholar] [CrossRef]
- Morton, D.; Seymour, M.; Magill, L.; Handley, K.; Glasbey, J.; Glimelius, B.; Palmer, A.; Seligmann, J.; Laurberg, S.; Murakami, K.; et al. Preoperative Chemotherapy for Operable Colon Cancer: Mature Results of an International Randomized Controlled Trial. J. Clin. Oncol. 2023, 41, 1541–1552. [Google Scholar] [CrossRef]
- Karoui, M.; Rullier, A.; Luciani, A.; Bonnetain, F.; Auriault, M.-L.; Sarran, A.; Monges, G.; Trillaud, H.; Le Malicot, K.; Leroy, K.; et al. Neoadjuvant FOLFOX 4 versus FOLFOX 4 with Cetuximab versus immediate surgery for high-risk stage II and III colon cancers: A multicentre randomised controlled phase II trial--the PRODIGE 22--ECKINOXE trial. BMC Cancer 2015, 15, 511. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Becker, K. Tumor regression grading of gastrointestinal cancers after neoadjuvant therapy. Virchows Arch. 2018, 472, 175–186. [Google Scholar] [CrossRef]
- Song, C.; Chung, J.-H.; Kang, S.-B.; Kim, D.-W.; Oh, H.-K.; Lee, H.S.; Kim, J.W.; Lee, K.-W.; Kim, J.H.; Kim, J.-S. Impact of Tumor Regression Grade as a Major Prognostic Factor in Locally Advanced Rectal Cancer after Neoadjuvant Chemoradiotherapy: A Proposal for a Modified Staging System. Cancers 2018, 10, 319. [Google Scholar] [CrossRef] [PubMed]
- Rödel, C.; Martus, P.; Papadoupolos, T.; Füzesi, L.; Klimpfinger, M.; Fietkau, R.; Liersch, T.; Hohenberger, W.; Raab, R.; Sauer, R.; et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J. Clin. Oncol. 2005, 23, 8688–8696. [Google Scholar] [CrossRef] [PubMed]
- Kanemitsu, Y.; Shitara, K.; Mizusawa, J.; Hamaguchi, T.; Shida, D.; Komori, K.; Ikeda, S.; Ojima, H.; Ike, H.; Shiomi, A.; et al. Primary Tumor Resection Plus Chemotherapy Versus Chemotherapy Alone for Colorectal Cancer Patients with Asymptomatic, Synchronous Unresectable Metastases (JCOG1007; iPACS): A Randomized Clinical Trial. J. Clin. Oncol. 2021, 39, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Van der Kruijssen, D.E.W.; Elias, S.G.; Vink, G.R.; van Rooijen, K.L.; ’t Lam-Boer, J.; Mol, L.; Punt, C.J.A.; de Wilt, J.H.W.; Koopman, M.; CAIRO4 Working Group. Sixty-Day Mortality of Patients With Metastatic Colorectal Cancer Randomized to Systemic Treatment vs Primary Tumor Resection Followed by Systemic Treatment: The CAIRO4 Phase 3 Randomized Clinical Trial. JAMA Surg. 2021, 156, 1093. [Google Scholar] [CrossRef] [PubMed]
- Fong, Y.; Fortner, J.; Sun, R.L.; Brennan, M.F.; Blumgart, L.H. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: Analysis of 1001 consecutive cases. Ann. Surg. 1999, 230, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.S.; Vauthey, J.-N.; Boonsirikamchai, P.; Maru, D.M.; Kopetz, S.; Palavecino, M.; Curley, S.A.; Abdalla, E.K.; Kaur, H.; Charnsangavej, C.; et al. Association of computed tomography morphological criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA 2009, 302, 2338–2344. [Google Scholar] [CrossRef]
- Poultsides, G.A.; Servais, E.L.; Saltz, L.B.; Patil, S.; Kemeny, N.E.; Guillem, J.G.; Weiser, M.; Temple, L.K.F.; Wong, W.D.; Paty, P.B. Outcome of primary tumor in patients with synchronous stage IV colorectal cancer receiving combination chemotherapy without surgery as initial treatment. J. Clin. Oncol. 2009, 27, 3379–3384. [Google Scholar] [CrossRef]
- Foxtrot Collaborative Group, null Feasibility of preoperative chemotherapy for locally advanced, operable colon cancer: The pilot phase of a randomised controlled trial. Lancet Oncol. 2012, 13, 1152–1160. [CrossRef]
- Ribero, D.; Wang, H.; Donadon, M.; Zorzi, D.; Thomas, M.B.; Eng, C.; Chang, D.Z.; Curley, S.A.; Abdalla, E.K.; Ellis, L.M.; et al. Bevacizumab improves pathologic response and protects against hepatic injury in patients treated with oxaliplatin-based chemotherapy for colorectal liver metastases. Cancer 2007, 110, 2761–2767. [Google Scholar] [CrossRef]
- Kishi, Y.; Zorzi, D.; Contreras, C.M.; Maru, D.M.; Kopetz, S.; Ribero, D.; Motta, M.; Ravarino, N.; Risio, M.; Curley, S.A.; et al. Extended preoperative chemotherapy does not improve pathologic response and increases postoperative liver insufficiency after hepatic resection for colorectal liver metastases. Ann. Surg. Oncol. 2010, 17, 2870–2876. [Google Scholar] [CrossRef] [PubMed]
- Granieri, S.; Cotsoglou, C.; Bonomi, A.; Salvatore, L.; Filippi, R.; Nigro, O.; Gelsomino, F.; Zurlo, I.V.; Depetris, I.; Giampieri, R.; et al. Conversion Strategy in Left-Sided RAS/BRAF Wild-Type Metastatic Colorectal Cancer Patients with Unresectable Liver-Limited Disease: A Multicenter Cohort Study. Cancers 2022, 14, 5513. [Google Scholar] [CrossRef]
- Pramanik, A.; Xu, Z.; Shamsuddin, S.H.; Khaled, Y.S.; Ingram, N.; Maisey, T.; Tomlinson, D.; Coletta, P.L.; Jayne, D.; Hughes, T.A.; et al. Affimer Tagged Cubosomes: Targeting of Carcinoembryonic Antigen Expressing Colorectal Cancer Cells Using In Vitro and In Vivo Models. ACS Appl. Mater. Interfaces 2022, 14, 11078–11091. [Google Scholar] [CrossRef] [PubMed]
- Rossini, D.; Boccaccino, A.; Carullo, M.; Antoniotti, C.; Dima, G.; Ciracì, P.; Marmorino, F.; Moretto, R.; Masi, G.; Cremolini, C. Primary tumour side as a driver for treatment choice in RAS wild-type metastatic colorectal cancer patients: A systematic review and pooled analysis of randomised trials. Eur. J. Cancer 2023, 184, 106–116. [Google Scholar] [CrossRef] [PubMed]


| TRG | Tumor Regression |
|---|---|
| 0 | No regression |
| 1 | <25% of tumor mass |
| 2 | 25–50% of tumor mass |
| 3 | >50% of tumor mass |
| 4 | Complete regression |
| Patient Characteristics, n | 60 | |
|---|---|---|
| Age, years, median (range) | 62.0 (42–88) | |
| Sex, n (%) | Male Female | 41 (68.3) |
| 19 (31.7) | ||
| Liver resection, n (%) | Major Minor | 32 (53.3) 28 (46.7) |
| Primary tumor, n (%) | Rectum Left colon and sigmoid colon Right colon | 25 (41.7) 24 (40.0) 11 (18.3) |
| Surgical treatment, n (%) | Liver first Primum first Synchronous surgery | 34 (56.7) |
| 2 (3.3) | ||
| 24 (40.0) | ||
| Induction chemotherapy with antibody prior to primary resection and resection of the liver metastases, n (%) | Chemotherapy + VEGF antibody therapy Chemotherapy + EGFR antibody therapy | 37 (61.7) |
| 23 (38.3) | ||
| Tumor recurrence, n (%) | Yes No | 42 (70.0) |
| 18 (30.0) | ||
| Location of tumor recurrence, n (%) | Intrahepatic Extrahepatic Intra- and extrahepatic | 19 (31.7) 9 (15.0) 14 (23.3) |
| Ninety-day morbidity and mortality, Dindo et al. [24], n (%) | Dindo 0 Dindo I Dindo II Dindo IIIa Dindo IIIb Dindo IVa Dindo IVb Dindo V | 45 (75.0) |
| 2 (3.3) | ||
| 6 (10.0) | ||
| 3 (5.0) | ||
| 1 (1.7) | ||
| 1 (1.7) | ||
| 0 (0) | ||
| 3 (5.0) |
| Therapy | n = 60 | % | Rödel Score, Median, 95% CI |
|---|---|---|---|
| Chemotherapy + VEGF antibody therapy | 37 | 61.6 | 3 (2–4) |
| Chemotherapy + EGFR antibody therapy | 23 | 38.3 | 1 (0–2) |
| p-value | 0.005 |
| Therapy | n | % | Rödel Score, Median, 95% CI |
|---|---|---|---|
| Chemotherapy + VEGF antibody therapy in patients with RAS mt | 22 | 50.0 | 3 (2–4) |
| Chemotherapy + EGFR antibody therapy in patients with RAS wt | 22 | 50.0 | 1 (0–2) |
| p-value | 0.011 | ||
| Chemotherapy + VEGF antibody therapy in patients with RAS wt | 13 | 37.1 | 3 (1–4) |
| Chemotherapy + EGFR antibody therapy in patients with RAS wt | 22 | 62.9 | 1 (0–2) |
| p-value | 0.010 |
| Antibody | ORR % | CR n, % | PR n, % | SD n, % | PD n, % |
|---|---|---|---|---|---|
| VEGF | 78.9 | 0 (0.0) | 30 (78.9) | 6 (15.8) | 2 (5.2) |
| EGFR | 90.9 | 0 (0.0) | 20 (90.9) | 2 (10.0) | 0 (0.0) |
| Antibody | RFS Median, 95% CI | OS Median, 95% CI |
|---|---|---|
| VEGF | 12.33 (10.39–14.26) | 38.07 (27.29–48.85) |
| EGFR | 8.40 (5.85–10.95) | 27.53 (15.98–39.07) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thonhauser, R.; Poglitsch, M.; Jonas, J.P.; Dong, Y.; Tschögl, M.; Gramberger, M.; Salem, M.; Santol, J.; Brandl, I.; Klimpfinger, M.; et al. The Effect of Induction Chemotherapy with VEGF Inhibition on Tumor Response in Synchronously Metastasized Potentially Resectable Colorectal Cancer. Cancers 2023, 15, 2900. https://doi.org/10.3390/cancers15112900
Thonhauser R, Poglitsch M, Jonas JP, Dong Y, Tschögl M, Gramberger M, Salem M, Santol J, Brandl I, Klimpfinger M, et al. The Effect of Induction Chemotherapy with VEGF Inhibition on Tumor Response in Synchronously Metastasized Potentially Resectable Colorectal Cancer. Cancers. 2023; 15(11):2900. https://doi.org/10.3390/cancers15112900
Chicago/Turabian StyleThonhauser, Rebecca, Marcus Poglitsch, Jan Philipp Jonas, Yawen Dong, Madita Tschögl, Mariel Gramberger, Mohamed Salem, Jonas Santol, Irmgard Brandl, Martin Klimpfinger, and et al. 2023. "The Effect of Induction Chemotherapy with VEGF Inhibition on Tumor Response in Synchronously Metastasized Potentially Resectable Colorectal Cancer" Cancers 15, no. 11: 2900. https://doi.org/10.3390/cancers15112900
APA StyleThonhauser, R., Poglitsch, M., Jonas, J. P., Dong, Y., Tschögl, M., Gramberger, M., Salem, M., Santol, J., Brandl, I., Klimpfinger, M., Vierziger, C., & Gruenberger, T. (2023). The Effect of Induction Chemotherapy with VEGF Inhibition on Tumor Response in Synchronously Metastasized Potentially Resectable Colorectal Cancer. Cancers, 15(11), 2900. https://doi.org/10.3390/cancers15112900