Biosynthetic Mesh Reconstruction after Abdominoperineal Resection for Low Rectal Cancer: Cross Relation of Surgical Healing and Oncological Outcomes: A Multicentric Observational Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
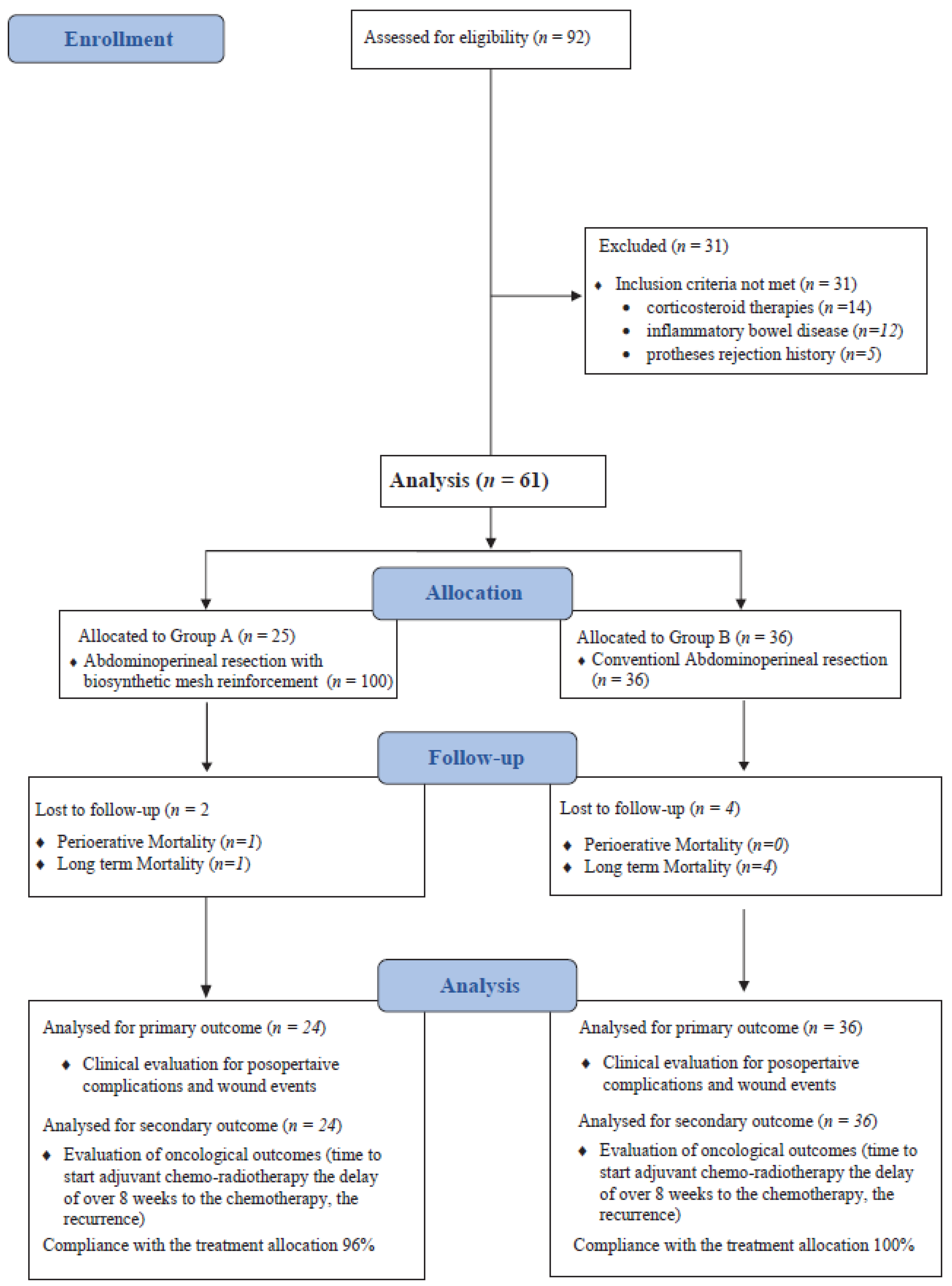
2.2. Study Setting and Study Population
2.3. Surgical Technique
2.3.1. Conventional Abdominoperineal Resection
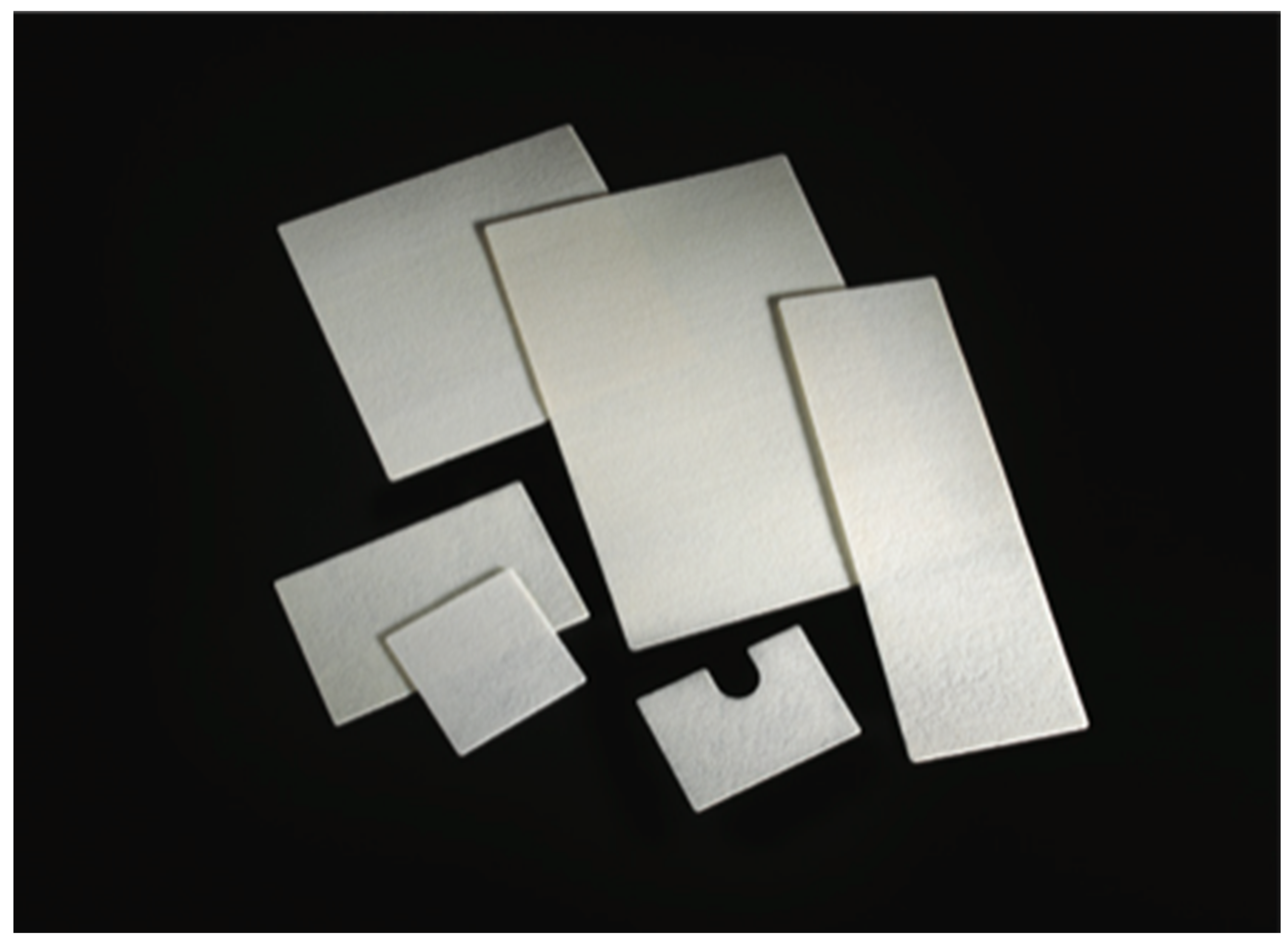
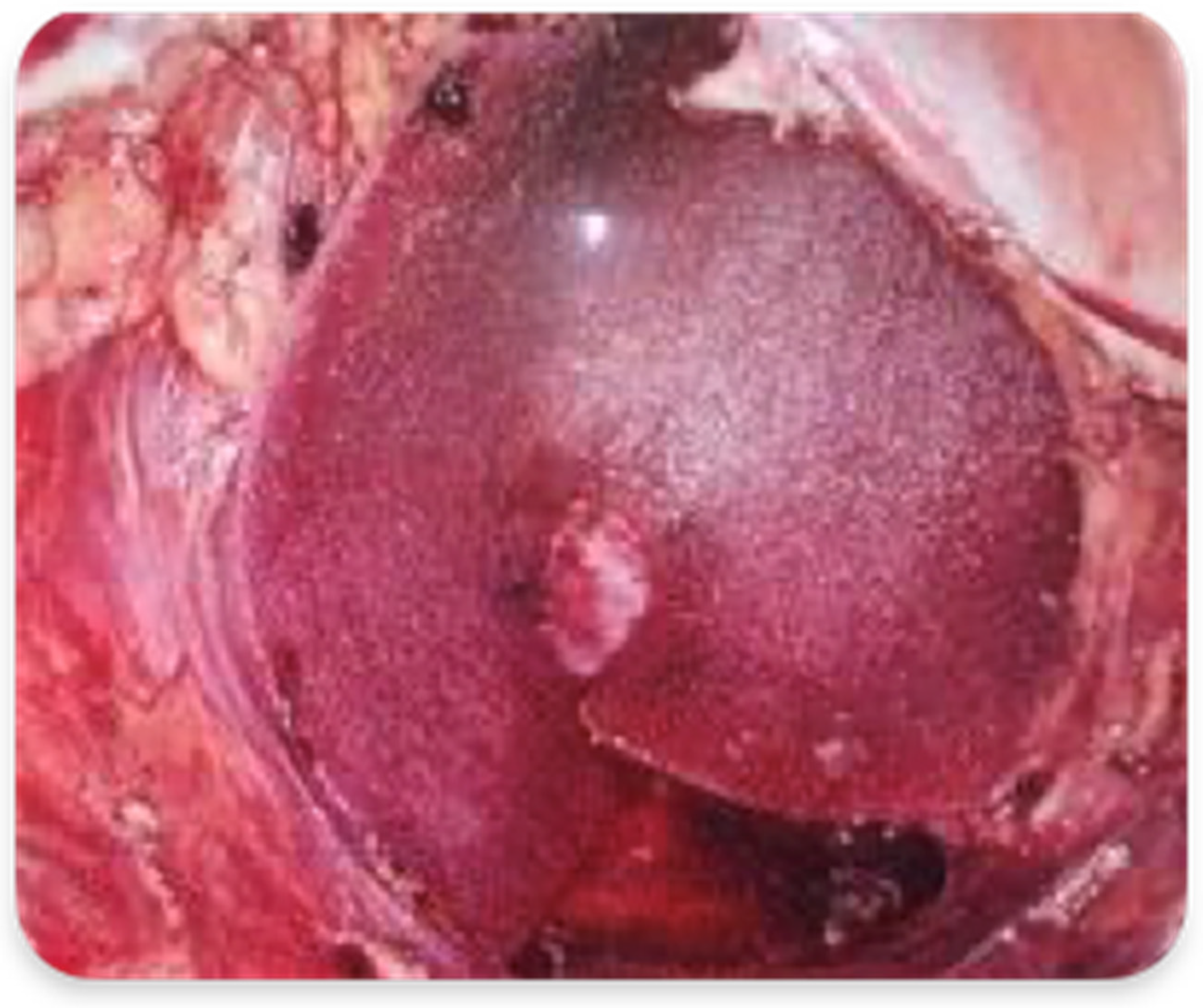
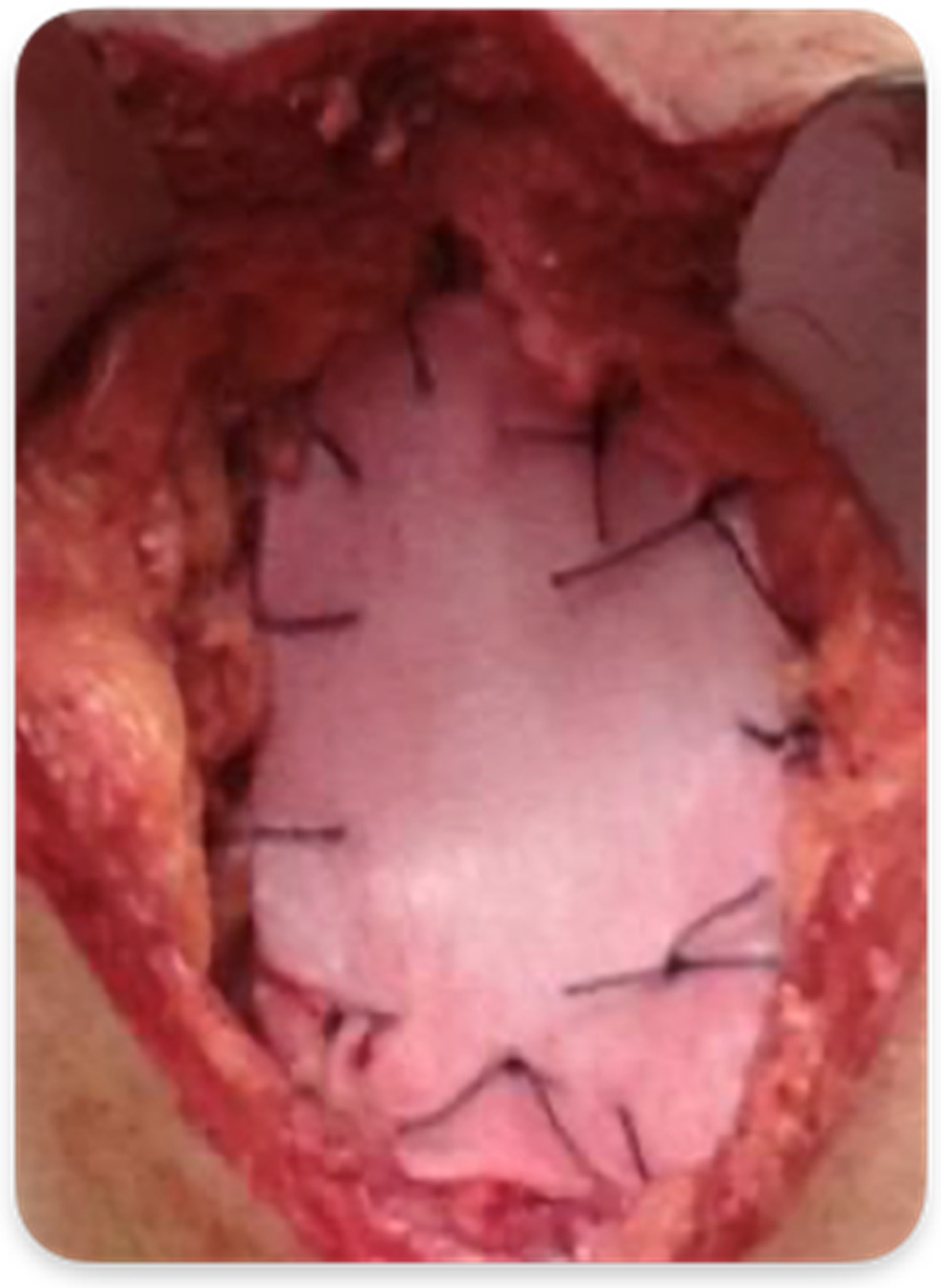
2.3.2. Abdominoperineal Resection with Mesh Reinforcement of the Perineal Plane
2.4. Outcome Measures
2.5. Study Outcomes
2.6. Statistical Analysis
3. Results
3.1. Primary Outcome
3.2. Secondary Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef]
- Torre, L.A.; Bray, F.; Siegel RLFerlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Wibe, A.; Syse, A.; Andersen, E.; Tretli, S.; Myrvold, H.E.; Søreide, O.; Norwegian Rectal Cancer Group. Oncological outcomes after total mesorectal excision for cure for cancer of the lower rectum: Anterior vs. abdominoperineal resection. Dis. Colon Rectum 2004, 47, 48–58. [Google Scholar] [CrossRef]
- Atallah, S.; Albert, M.; DeBeche-Adams, T.; Nassif, G.; Polavarapu, H.; Larach, S. Transanal minimally invasive surgery for total mesorectal excision (TAMIS-TME): A stepwise description of the surgical technique with video demonstration. Tech. Coloproctol. 2013, 17, 321–325. [Google Scholar] [CrossRef]
- Garcia-Henriquez, N.; Galante, D.J.; Monson, J.R.T. Selection and Outcomes in Abdominoperineal Resection. Front. Oncol. 2020, 10, 1339. [Google Scholar] [CrossRef]
- Butt, H.Z.; Salem, M.K.; Vijaynagar, B.; Chaudhri, S.; Singh, B. Perineal reconstruction after extra-levator abdominoperineal excision (eLAPE): A systematic review. Int. J. Color. Dis. 2013, 28, 1459–1468. [Google Scholar] [CrossRef]
- Kamrava, A.; Mahmoud, N.N. Prevention and management of nonhealing perineal wounds. Clin. Colon Rectal Surg. 2013, 26, 106–111. [Google Scholar]
- Moertel, C.G.; Fleming, T.R.; Macdonald, J.S.; Haller, D.G.; Laurie, J.A.; Goodman, P.J.; Ungerleider, J.S.; Emerson, W.A.; Tormey, D.C.; Glick, J.H.; et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N. Engl. J. Med. 1990, 322, 352–358. [Google Scholar] [CrossRef]
- Laurie, J.A.; Moertel, C.G.; Fleming, T.R.; Wieand, H.S.; Leigh, J.E.; Rubin, J.; McCormack, G.W.; Gerstner, J.B.; Krook, J.E.; Malliard, J.; et al. Surgical adjuvant therapy of large-bowel carcinoma: An evaluation of levamisole and the combination of levamisole and fluorouracil. The North Central Cancer Treatment Group and the Mayo Clinic. J. Clin. Oncol. 1989, 7, 1447–1456. [Google Scholar] [CrossRef]
- Merkow, R.P.; Bentrem, D.J.; Mulcahy, M.F.; Chung, J.W.; Abbott, D.E.; Kmiecik, T.E.; Stewart, A.K.; Winchester, D.P.; Ko, C.Y.; Bilimoria, K.Y. Effect of postoperative complications on adjuvant chemotherapy use for stage III colon cancer. Ann. Surg. 2013, 258, 847–853. [Google Scholar] [CrossRef]
- Hendren, S.; Birkmeyer, J.D.; Yin, H.; Banerjee, M.; Sonnenday, C.; Morris, A.M. Surgical complications are associated with omission of chemotherapy for stage III colorectal cancer. Dis. Colon Rectum 2010, 53, 1587–1593. [Google Scholar] [CrossRef]
- Lefevre, J.H.; Parc, Y.; Kerne´is, S.; Shields, C.; Touboul, E.; Chaouat, M.; Tiret, E. Abdomino-perineal resection for anal cancer, impact of a vertical rectus abdominis myocutaneus flap on survival, recurrence, morbidity, and wound healing. Ann. Surg. 2009, 250, 707–711. [Google Scholar] [CrossRef]
- Arnold, P.G.; Lovich, S.F.; Pairolero, P.C. Muscle flaps in irradiated wounds: An account of 100 consecutive cases. Plast. Reconstr. Surg. 1994, 93, 324–327. [Google Scholar] [CrossRef]
- Chessin, D.B.; Hartley, J.; Cohen, A.M.; Mazumdar, M.; Cordeiro, P.; Disa, J.; Mehrara, B.; Minsky, B.D.; Paty, P.; Weiser, M.; et al. Rectus flap reconstruction decreases perineal wound complications after chemoradiation and surgery: A cohort study. Ann. Surg. Oncol. 2005, 12, 104–110. [Google Scholar] [CrossRef]
- Biagi, J.J.; Raphael, M.J.; Mackillop, W.J.; Kong, W.; King, W.D.; Booth, C.M. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: A systematic review and meta-analysis. JAMA 2011, 305, 2335–2342. [Google Scholar] [CrossRef]
- Kolehmainen, M.; Suominen, S.; Tukiainen, E. Pelvic, perineal and genital reconstructions. Scand. J. Surg. 2013, 102, 25–31. [Google Scholar] [CrossRef]
- Terranova, O.; Sandei, F.; Rebuffat, C.; Maruotti, R.; Pezzuoli, G. Management of the perineal wound after rectal excision for neoplastic disease: A controlled clinical trial. Dis. Colon Rectum 1979, 22, 228–233. [Google Scholar] [CrossRef]
- Moreno-Sanz, C.; Manzanera-Díaz, M.; Clerveus, M.; Cortina-Oliva, F.J.; de Pedro-Conal, J.; Picazo-Yeste, J. Pelvic reconstruction after abdominoperineal resection of the rectum. Cir. Esp. 2011, 89, 77–81. [Google Scholar] [CrossRef]
- Mughal, M.; Baker, R.; Muneer, A.; Mosahebi, A. Reconstruction of perineal defects. Ann. R. Coll. Surg. Engl. 2013, 95, 539–544. [Google Scholar] [CrossRef]
- Ozturk, E.O.H.; Yilmazlar, T. The use of vacuum assisted closure therapy in the management of Fournier’s gangrene. Am. J. Surg. 2009, 197, 660–665. [Google Scholar] [CrossRef]
- Boulanger, L.; Boukerrrou, M.; Collinet, P.; Fruchard, A.; Courcol, R.J.; Cosson, M. Bacteriological analysis of meshes removed for complications after surgical management of urinary incontinence or pelvic organ prolapse. Int. Urogynecology J. 2008, 19, 827–831. [Google Scholar] [CrossRef]
- Bluebond-Langner, R.; Keifa, E.S.; Mithani, S.; Bochicchio, G.V.; Scalea, T.; Rodriguez, E.D. Recurrent abdominal laxity following interpositional human acellular dermal matrix. Ann. Plast. Surg. 2008, 60, 76–80. [Google Scholar] [CrossRef]
- Candage, R.; Jones, K.; Luchette, F.A.; Sinacore, J.M.; Vandevender, D.; Reed, R.L., 2nd. Use of human acellular dermal matrix for hernia repair: Friend or foe? Surgery 2008, 144, 703–711. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef]
- Owens, W.D.; Felts, J.A.; Spitznagel, E.L., Jr. ASA physical status classifications: A study of consistency of ratings. Anesthesiology 1978, 49, 239–243. [Google Scholar] [CrossRef]
- Brauker, J.H.; Carr-Brendel, V.E.; Martinson, L.A.; Crudele, J.; Johnston, W.D.; Johnson, R.C. Neovascularization of synthetic membranes directed by membrane microarchitecture. J. Biomed. Mater. Res. 1995, 29, 1517–1524. [Google Scholar] [CrossRef]
- Sharkawy, A.A.; Klitzman, B.; Truskey, G.A.; Reichert, W.M. Engineering the tissue which encapsulates subcutaneous implants. I. Diffusion properties. J. Biomed. Mater. Res. 1997, 37, 401–412. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Longoria, M.; Welbourne, S.; Sabio, A.; Wilson, S.E. Glycolide copolymer staple-line reinforcement reduces staple site bleeding during laparoscopic gastric bypass: A prospective randomized trial. Arch. Surg. 2005, 140, 773–778. [Google Scholar] [CrossRef]
- de la Portilla, F.; Zbar, A.P.; Rada, R.; Vega, J.; Cisneros, N.; Maldonado, V.H.; Utrera, A.; Espinosa, E. Bioabsorbable staple-line reinforcement to reduce staple-line bleeding in the transection of mesenteric vessels during laparoscopic colorectal resection: A pilot study. Tech. Coloproctol. 2006, 10, 335–338. [Google Scholar] [CrossRef]
- Onyekwelu, I.; Yakkanti, R.; Protzer, L.; Pinkston, C.M.; Tucker, C.; Seligson, D. Surgical wound classification and surgical site infections in the orthopaedic patient. JAAOS Glob. Res. Rev. 2017, 1, e022. [Google Scholar] [CrossRef]
- National Institutes of Health. Adjuvant Therapy for Patients with Colon and Rectum Cancer: NIH Consensus Statement; National Institutes of Health: Bethesda, MY, USA, 1990; Volume 8, pp. 1–25. [Google Scholar]
- Vaesen Bentein, H.; De Roeck, L.; Pirenne, Y.; Vissers, G.; Tondu, T.; Thiessen, F.; Willemsen, P. Perineal bowel evisceration after extralevator abdominoperineal excision and vertical rectus abdominis myocutaneous flap closure. Acta Chir. Belg. 2022, 12, 1–6. [Google Scholar] [CrossRef]
- Fingerhut, A.; Hay, J.M.; Delalande, J.P.; Paquet, J.C. The French Association for Surgical Research Passive vs closed suction drainage after wound closure following abdominoperineal rectal excision for carcinoma. A multicenter, controlled trial. Dis. Colon Rectum 1995, 38, 926–932. [Google Scholar] [CrossRef]
- Raba, G.; Chamula, W. Management of selected complications following urogynecological surgeries with the use of synthetic prostheses-own observations. Ginekol. Pol. 2008, 79, 550–554. [Google Scholar]
- Falagas Me Velakoulis, S.; Iavazzo, C.; Athanasiou, S. Mesh-related infections after pelvic organ prolapse repair surgery. Eur. J. Obstet. Gynecol. Reprod. Biol. 2007, 134, 147–156. [Google Scholar] [CrossRef]
- Irvin, T.T.; Coligher, J.C. A controlled clinical trial of three different methods of perineal wound management following excision of the rectum. Br. J. Surg. 1975, 62, 287–291. [Google Scholar] [CrossRef]
- Brusciano, L.; Gambardella, C.; Tolone, S.; Del Genio, G.; Terracciano, G.; Gualtieri, G.; Schiano di Visconte, M.; Docimo, L. An imaginary cuboid: Chest, abdomen, vertebral column and perineum, different parts of the same whole in the harmonic functioning of the pelvic floor. Tech. Coloproctol. 2019, 23, 603–605. [Google Scholar] [CrossRef]
- Gambardella, C.; Brusciano, L.; Del Genio, G.; Tolone, S.; Terracciano, G.; Gualtieri, G.; Lucido, F.S.; Docimo, L. Predictive parameters to identify incontinent patients amenable for rehabilitation treatment: The muscular synergies evaluation. Arq. De Gastroenterol. 2019, 56, 452–453. [Google Scholar] [CrossRef]
- Musters, G.D.; Bemelman, W.A.; Bosker, R.J.; Burger, J.W.; van Duijvendijk, P.; van Etten, B.; van Geloven, A.A.; de Graaf, E.J.; Hoff, C.; de Korte, N.; et al. Randomized controlled multicentre study comparing biological mesh closure of the pelvic floor with primary perineal wound closure after extralevator abdominoperineal resection for rectal cancer (BIOPEX-study). BMC Surg. 2014, 14, 58. [Google Scholar] [CrossRef]
- Jensen, K.K.; Rashid, L.; Pilsgaard, B.; Møller, P.; Wille-Jørgensen, P. Pelvic floor reconstruction with a biological mesh after extralevator abdominoperineal excision leads to few perineal hernias and acceptable wound complication rates with minor movement limitations: Single-centre experience including clinical examination and interview. Colorectal Dis. 2014, 16, 192–197. [Google Scholar]
- Cui, J.; Xiang, J.; Huang, M.J.; Huang, Y.H.; Wang, J.P. Application of GORE-TEX Dual Mesh fixing into peritoneum in sigmoid-colostomy to prevent peristomal hernia. Zhonghua Wei Chang. Wai Ke Za Zhi 2009, 12, 480–482. [Google Scholar]
- Tao, Y.; Han, J.G.; Wang, Z.J. Comparison of perineal morbidity between biologic mesh reconstruction and primary closure following extralevator abdominoperineal excision: A systematic review and meta-analysis. Int. J. Color. Dis. 2021, 36, 893–902. [Google Scholar] [CrossRef]
- Althumairi, A.A.; Canner, J.K.; Ahuja, N.; Sacks, J.M.; Safar, B.; Efron, J.E. Time to Chemotherapy After Abdominoperineal Resection: Comparison Between Primary Closure and Perineal Flap Reconstruction. World J. Surg. 2016, 40, 225–230. [Google Scholar] [CrossRef]

| Group A 25 Patients | Group B 36 Patients | p | |
|---|---|---|---|
| Gender (Male/Female) | 13/12 (52%/48%) | 21/15 (58.3%/41.7%) | 0.624 |
| Age (Years) ° | 69.3 (36–85) | 67.6 (43–78) | 0.160 |
| BMI | 28.2 (22–35.7) | 29.3 (24–38) | 0.304 |
| ASA I-II/III-IV | 18/7 (72%/28%) | 19/17 (52.7%/47.3%) | 0.130 |
| Hypertension | 16 (64%) | 26 (72.2%) | 0.495 |
| Diabetes | 9 (36%) | 14 (38.9%) | 0.818 |
| Chronic obstructive pulmonary disease | 4 (16%) | 7 19.4%) | 0.730 |
| Cerebrovascular Disease | 0 | 1 (2.7%) | 0.391 |
| Smoking | 9 (36%) | 15 (41.6%) | 0.655 |
| Chronic kidney disease | 1 (4%) | 2 (5.5%) | 0.800 |
| Heart ischemic attack | 2 (8%) | 2 (5.5%) | 0.704 |
| Preoperative symptoms | |||
| 21 (84%) 11 (44%) 18 (72%) 3 (12%) | 29 (80.5%) 15 (41.6%) 28 (77%) 5 (13.8%) | 0.730 0.856 0.606 0.829 |
| Tumour Stage | |||
| 0 7 (28%) 16 (64%) 2 (8%) | 0 14 (38.9%) 18 (50%) 4 (11.1%) | - 0.378 0.279 0.688 |
| Tumour dimension (Centimetres) | 6.3 (3–9) | 5 (2–7) | 0.347 |
| Height from the anal margin (Centimetres) | 2.2 (0–3) | 2.5 (0–3) | 0.734 |
| Neo-adjuvant CHT-RT | 19 (76%) | 29 (80.5%) | 0.512 |
| Group A 25 Patients | Group B 36 Patients | p | |
|---|---|---|---|
| Operative time (min) ° | 235 (160–330) | 195 (140–295) | 0.125 |
| Intraoperative blood loss (mL) ° | 275 (140–730) | 230 (110–650) | 0.423 |
| Intraoperative complications | 0 | 1 (Bleeding) | 0.391 |
| Hospitalization (days) ° | 6 (4–12) | 8 (6–18) | 0.572 |
| Perioperative mortality | 1 (4%) | 0 | 0.226 |
| Until Discharge | Until 30 Days | After 30 Days | ||||
|---|---|---|---|---|---|---|
| Group A | Group B | Group A | Group B | Group A | Group B | |
| Uneventful postoperative course | >95% | >99% | >85% | >60% | >95% | >95% |
| Readmission | - | - | <1% | <1% | <1% | <1% |
| Reoperation | <1% | <1% | <1% | <1% | <1% | <1% |
| Clavien–Dindo Grade I-II | <1% | <1% | <15% | <40% | <1% | <1% |
| Clavien–Dindo Grade >III | <4% | <1% | <1% | <1% | <90% | <85% |
| Mortality | 1 | 0 | 0 | 0 | 2 | 4 |
| Bowel leak | 0 | 0 | 0 | 0 | 0 | 0 |
| Small bowel obstruction/internal hernia | 0 | 0 | 0 | 0 | 0 | 0 |
| Bleeding | 0 | 0 | 0 | 0 | 0 | 0 |
| Wound infection | 0 | 0 | 2 | 11 | 0 | 0 |
| Perineal wound dehiscence | 0 | 0 | 1 | 9 | 0 | 0 |
| Group A 24 Patients | Group B 36 Patients | p | |
|---|---|---|---|
| Hematoma | 4 (16.6%) | 5 (13.8%) | 0.767 |
| Seroma | 7 (29.1%) | 6 (16.6%) | 0.147 |
| Superficial incisional infections | 2 (8.3%) | 8 (22.2%) | 0.157 |
| Deep incisional infections | 0 | 3 (8.3%) | 0.146 |
| Perineal wound dehiscence | 1 (4.2%) | 9 (25%) | 0.033 * |
| Time needed for wound healing (Days) ° | 16 (12–26) | 24 (18–32) | 0.015 * |
| Group A 24 Patients | Group B 36 Patients | p | |
|---|---|---|---|
| Time to start adjuvant chemotherapy (days) ° | 26 (15–40) | 70 (30–120) | 0.003 * |
| Delay in chemotherapy (more than 8 weeks) | 4 (16.6%) | 16 (44.4%) | 0.025 * |
| Mortality | 2 (8.3%) | 4 (11.1%) | 0.725 |
| Long-term recurrence | 4 (16.6%) | 12 (33.3%) | 0.152 |
| Follow-up (months) ° | 30 (28–32) | 31 (27–33) | 0.645 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gambardella, C.; Mongardini, F.M.; Karpathiotakis, M.; Lucido, F.S.; Pizza, F.; Tolone, S.; Parisi, S.; Nesta, G.; Brusciano, L.; Gambardella, A.; et al. Biosynthetic Mesh Reconstruction after Abdominoperineal Resection for Low Rectal Cancer: Cross Relation of Surgical Healing and Oncological Outcomes: A Multicentric Observational Study. Cancers 2023, 15, 2725. https://doi.org/10.3390/cancers15102725
Gambardella C, Mongardini FM, Karpathiotakis M, Lucido FS, Pizza F, Tolone S, Parisi S, Nesta G, Brusciano L, Gambardella A, et al. Biosynthetic Mesh Reconstruction after Abdominoperineal Resection for Low Rectal Cancer: Cross Relation of Surgical Healing and Oncological Outcomes: A Multicentric Observational Study. Cancers. 2023; 15(10):2725. https://doi.org/10.3390/cancers15102725
Chicago/Turabian StyleGambardella, Claudio, Federico Maria Mongardini, Menelaos Karpathiotakis, Francesco Saverio Lucido, Francesco Pizza, Salvatore Tolone, Simona Parisi, Giusiana Nesta, Luigi Brusciano, Antonio Gambardella, and et al. 2023. "Biosynthetic Mesh Reconstruction after Abdominoperineal Resection for Low Rectal Cancer: Cross Relation of Surgical Healing and Oncological Outcomes: A Multicentric Observational Study" Cancers 15, no. 10: 2725. https://doi.org/10.3390/cancers15102725
APA StyleGambardella, C., Mongardini, F. M., Karpathiotakis, M., Lucido, F. S., Pizza, F., Tolone, S., Parisi, S., Nesta, G., Brusciano, L., Gambardella, A., Docimo, L., & Mongardini, M. (2023). Biosynthetic Mesh Reconstruction after Abdominoperineal Resection for Low Rectal Cancer: Cross Relation of Surgical Healing and Oncological Outcomes: A Multicentric Observational Study. Cancers, 15(10), 2725. https://doi.org/10.3390/cancers15102725







