Acute Myeloid Leukemia Stem Cells in Minimal/Measurable Residual Disease Detection
Abstract
Simple Summary
Abstract
1. Introduction
2. LSCs Drive AML Pathogenesis and Relapse
2.1. Stem Cell Origin for AML
2.2. LSC Heterogeneity in AML
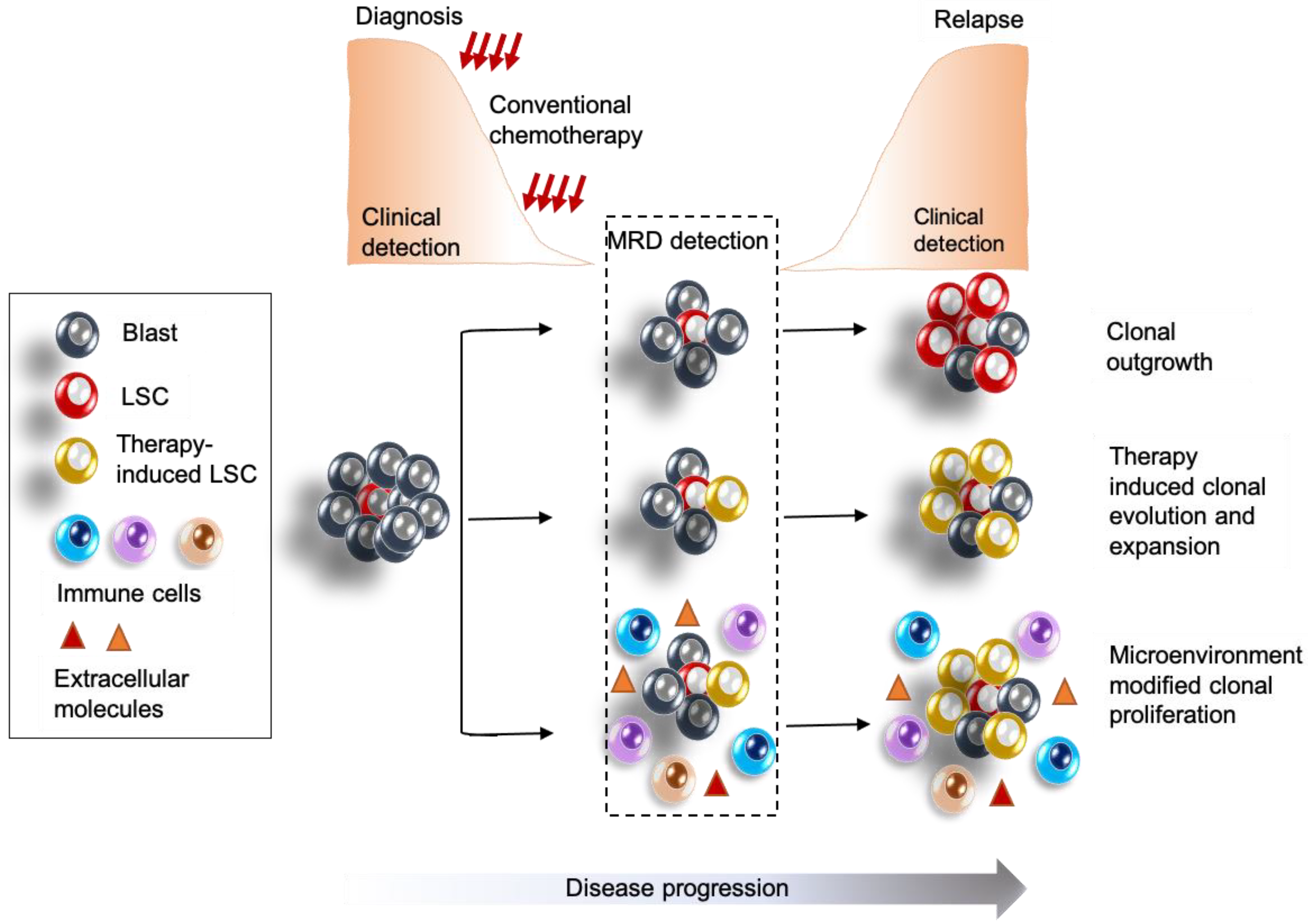
2.3. Contribution of LSCs to Relapse
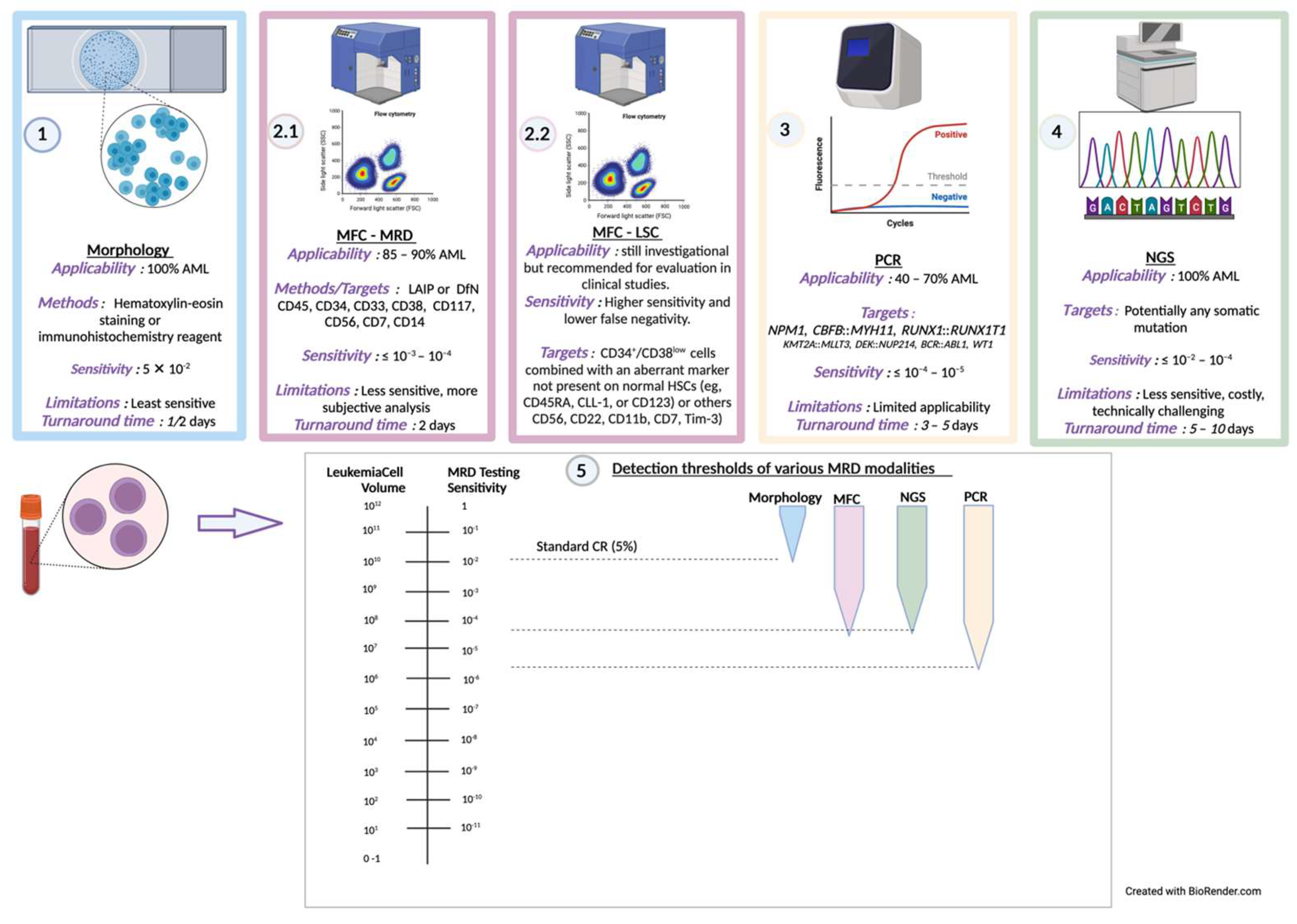
3. MRD in AML
3.1. Current Mechanisms and Considerations for MRD Detection
3.2. Prognostic Significance of MRD
3.3. Current State of MRD Guided Therapeutic Tailoring and Outcomes
4. LSC Detection as a Measure for Improving MRD Sensitivity
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Cancer Stat Facts: Leukemia—Acute Myeloid Leukemia (AML). Available online: https://seer.cancer.gov/statfacts/html/amyl.html (accessed on 6 March 2023).
- Leonard, J.P.; Martin, P.; Roboz, G.J. Practical Implications of the 2016 Revision of the World Health Organization Classification of Lymphoid and Myeloid Neoplasms and Acute Leukemia. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 2708–2715. [Google Scholar] [CrossRef] [PubMed]
- Adès, L.; Itzykson, R.; Fenaux, P. Myelodysplastic Syndromes. Lancet Lond. Engl. 2014, 383, 2239–2252. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Pardanani, A. Myeloproliferative Neoplasms: A Contemporary Review. JAMA Oncol. 2015, 1, 97–105. [Google Scholar] [CrossRef] [PubMed]
- McNerney, M.E.; Godley, L.A.; Le Beau, M.M. Therapy-Related Myeloid Neoplasms: When Genetics and Environment Collide. Nat. Rev. Cancer 2017, 17, 513–527. [Google Scholar] [CrossRef]
- Jaiswal, S.; Fontanillas, P.; Flannick, J.; Manning, A.; Grauman, P.V.; Mar, B.G.; Lindsley, R.C.; Mermel, C.H.; Burtt, N.; Chavez, A.; et al. Age-Related Clonal Hematopoiesis Associated with Adverse Outcomes. N. Engl. J. Med. 2014, 371, 2488–2498. [Google Scholar] [CrossRef]
- Genovese, G.; Kähler, A.K.; Handsaker, R.E.; Lindberg, J.; Rose, S.A.; Bakhoum, S.F.; Chambert, K.; Mick, E.; Neale, B.M.; Fromer, M.; et al. Clonal Hematopoiesis and Blood-Cancer Risk Inferred from Blood DNA Sequence. N. Engl. J. Med. 2014, 371, 2477–2487. [Google Scholar] [CrossRef]
- Bowman, R.L.; Busque, L.; Levine, R.L. Clonal Hematopoiesis and Evolution to Hematopoietic Malignancies. Cell Stem Cell 2018, 22, 157–170. [Google Scholar] [CrossRef]
- Jaiswal, S.; Ebert, B.L. Clonal Hematopoiesis in Human Aging and Disease. Science 2019, 366, eaan4673. [Google Scholar] [CrossRef]
- Lapidot, T.; Sirard, C.; Vormoor, J.; Murdoch, B.; Hoang, T.; Caceres-Cortes, J.; Minden, M.; Paterson, B.; Caligiuri, M.A.; Dick, J.E. A Cell Initiating Human Acute Myeloid Leukaemia after Transplantation into SCID Mice. Nature 1994, 367, 645–648. [Google Scholar] [CrossRef]
- Farge, T.; Saland, E.; De Toni, F.; Aroua, N.; Hosseini, M.; Perry, R.; Bosc, C.; Sugita, M.; Stuani, L.; Fraisse, M.; et al. Chemotherapy-Resistant Human Acute Myeloid Leukemia Cells Are Not Enriched for Leukemic Stem Cells but Require Oxidative Metabolism. Cancer Discov. 2017, 7, 716–735. [Google Scholar] [CrossRef]
- Marchand, T.; Pinho, S. Leukemic Stem Cells: From Leukemic Niche Biology to Treatment Opportunities. Front. Immunol. 2021, 12, 775128. [Google Scholar] [CrossRef]
- Pollyea, D.A.; Jordan, C.T. Therapeutic Targeting of Acute Myeloid Leukemia Stem Cells. Blood 2017, 129, 1627–1635. [Google Scholar] [CrossRef]
- Essers, M.A.G.; Trumpp, A. Targeting Leukemic Stem Cells by Breaking Their Dormancy. Mol. Oncol. 2010, 4, 443–450. [Google Scholar] [CrossRef]
- Ganzel, C.; Manola, J.; Douer, D.; Rowe, J.M.; Fernandez, H.F.; Paietta, E.M.; Litzow, M.R.; Lee, J.-W.; Luger, S.M.; Lazarus, H.M.; et al. Extramedullary Disease in Adult Acute Myeloid Leukemia Is Common but Lacks Independent Significance: Analysis of Patients in ECOG-ACRIN Cancer Research Group Trials, 1980–2008. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 3544–3553. [Google Scholar] [CrossRef]
- Grimwade, D.; Hills, R.K. Independent Prognostic Factors for AML Outcome. Hematology 2009, 2009, 385–395. [Google Scholar] [CrossRef]
- Grimwade, D.; Hills, R.K.; Moorman, A.V.; Walker, H.; Chatters, S.; Goldstone, A.H.; Wheatley, K.; Harrison, C.J.; Burnett, A.K.; on behalf of the National Cancer Research Institute Adult Leukaemia Working Group. Refinement of Cytogenetic Classification in Acute Myeloid Leukemia: Determination of Prognostic Significance of Rare Recurring Chromosomal Abnormalities among 5876 Younger Adult Patients Treated in the United Kingdom Medical Research Council Trials. Blood 2010, 116, 354–365. [Google Scholar] [CrossRef]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and Management of AML in Adults: 2022 Recommendations from an International Expert Panel on Behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef]
- Cornelissen, J.J.; Gratwohl, A.; Schlenk, R.F.; Sierra, J.; Bornhäuser, M.; Juliusson, G.; Råcil, Z.; Rowe, J.M.; Russell, N.; Mohty, M.; et al. The European LeukemiaNet AML Working Party Consensus Statement on Allogeneic HSCT for Patients with AML in Remission: An Integrated-Risk Adapted Approach. Nat. Rev. Clin. Oncol. 2012, 9, 579–590. [Google Scholar] [CrossRef]
- Koreth, J.; Schlenk, R.; Kopecky, K.J.; Honda, S.; Sierra, J.; Djulbegovic, B.J.; Wadleigh, M.; DeAngelo, D.J.; Stone, R.M.; Sakamaki, H.; et al. Allogeneic Stem Cell Transplantation for Acute Myeloid Leukemia in First Complete Remission: Systematic Review and Meta-Analysis of Prospective Clinical Trials. JAMA 2009, 301, 2349. [Google Scholar] [CrossRef]
- Burnett, A.K.; Goldstone, A.; Hills, R.K.; Milligan, D.; Prentice, A.; Yin, J.; Wheatley, K.; Hunter, A.; Russell, N. Curability of Patients With Acute Myeloid Leukemia Who Did Not Undergo Transplantation in First Remission. J. Clin. Oncol. 2013, 31, 1293–1301. [Google Scholar] [CrossRef]
- Stone, R.M.; Mandrekar, S.J.; Sanford, B.L.; Laumann, K.; Geyer, S.; Bloomfield, C.D.; Thiede, C.; Prior, T.W.; Döhner, K.; Marcucci, G.; et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N. Engl. J. Med. 2017, 377, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Altman, J.K.; Foran, J.M.; Pratz, K.W.; Trone, D.; Cortes, J.E.; Tallman, M.S. Phase 1 Study of Quizartinib in Combination with Induction and Consolidation Chemotherapy in Patients with Newly Diagnosed Acute Myeloid Leukemia. Am. J. Hematol. 2018, 93, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Komanduri, K.V.; Levine, R.L. Diagnosis and Therapy of Acute Myeloid Leukemia in the Era of Molecular Risk Stratification. Annu. Rev. Med. 2016, 67, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Hourigan, C.S.; Gale, R.P.; Gormley, N.J.; Ossenkoppele, G.J.; Walter, R.B. Measurable Residual Disease Testing in Acute Myeloid Leukaemia. Leukemia 2017, 31, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- Bisel, H.F. Criteria for the Evaluation of Response to Treatment in Acute Leukemia. Blood 1956, 11, 676–677. [Google Scholar]
- Grimwade, D.; Freeman, S.D. Defining Minimal Residual Disease in Acute Myeloid Leukemia: Which Platforms Are Ready for “Prime Time”? Blood 2014, 124, 3345–3355. [Google Scholar] [CrossRef]
- Hogge, D.E.; Shannon, K.M.; Kalousek, D.K.; Schonberg, S.; Schaffner, V.; Zoger, S.; Eaves, C.J.; Eaves, A.C. Juvenile Monosomy 7 Syndrome: Evidence That the Disease Originates in a Pluripotent Hemopoietic Stem Cell. Leuk. Res. 1987, 11, 705–709. [Google Scholar] [CrossRef]
- Tamura, S.; Kanamaru, A.; Takemoto, Y.; Kakishita, E.; Nagai, K. Clonal Evolutions during Long-Term Cultures of Bone Marrow from de Novo Acute Myeloid Leukaemia with Trilineage Myelodysplasia and with Myelodysplastic Remission Marrow. Br. J. Haematol. 1993, 84, 219–226. [Google Scholar] [CrossRef]
- Sun, G.X.; Wormsley, S.; Sparkes, R.S.; Naeim, F.; Gale, R.P. Where Does Transformation Occur in Acute Leukemia? Leuk. Res. 1991, 15, 1183–1189. [Google Scholar] [CrossRef]
- Will, B.; Zhou, L.; Vogler, T.O.; Ben-Neriah, S.; Schinke, C.; Tamari, R.; Yu, Y.; Bhagat, T.D.; Bhattacharyya, S.; Barreyro, L.; et al. Stem and Progenitor Cells in Myelodysplastic Syndromes Show Aberrant Stage-Specific Expansion and Harbor Genetic and Epigenetic Alterations. Blood 2012, 120, 2076–2086. [Google Scholar] [CrossRef]
- Engel, H.; Drach, J.; Keyhani, A.; Jiang, S.; Van, N.T.; Kimmel, M.; Sanchez-Williams, G.; Goodacre, A.; Andreeff, M. Quantitation of Minimal Residual Disease in Acute Myelogenous Leukemia and Myelodysplastic Syndromes in Complete Remission by Molecular Cytogenetics of Progenitor Cells. Leukemia 1999, 13, 568–577. [Google Scholar] [CrossRef]
- Will, B.; Vogler, T.O.; Narayanagari, S.; Bartholdy, B.; Todorova, T.I.; da Silva Ferreira, M.; Chen, J.; Yu, Y.; Mayer, J.; Barreyro, L.; et al. Minimal PU.1 Reduction Induces a Preleukemic State and Promotes Development of Acute Myeloid Leukemia. Nat. Med. 2015, 21, 1172–1181. [Google Scholar] [CrossRef]
- Kuo, Y.-H.; Landrette, S.F.; Heilman, S.A.; Perrat, P.N.; Garrett, L.; Liu, P.P.; Le Beau, M.M.; Kogan, S.C.; Castilla, L.H. Cbf Beta-SMMHC Induces Distinct Abnormal Myeloid Progenitors Able to Develop Acute Myeloid Leukemia. Cancer Cell 2006, 9, 57–68. [Google Scholar] [CrossRef]
- Somervaille, T.C.P.; Cleary, M.L. Identification and Characterization of Leukemia Stem Cells in Murine MLL-AF9 Acute Myeloid Leukemia. Cancer Cell 2006, 10, 257–268. [Google Scholar] [CrossRef]
- Bereshchenko, O.; Mancini, E.; Moore, S.; Bilbao, D.; Månsson, R.; Luc, S.; Grover, A.; Jacobsen, S.E.W.; Bryder, D.; Nerlov, C. Hematopoietic Stem Cell Expansion Precedes the Generation of Committed Myeloid Leukemia-Initiating Cells in C/EBPalpha Mutant AML. Cancer Cell 2009, 16, 390–400. [Google Scholar] [CrossRef]
- Gentles, A.J.; Plevritis, S.K.; Majeti, R.; Alizadeh, A.A. Association of a Leukemic Stem Cell Gene Expression Signature with Clinical Outcomes in Acute Myeloid Leukemia. JAMA 2010, 304, 2706–2715. [Google Scholar] [CrossRef]
- Eppert, K.; Takenaka, K.; Lechman, E.R.; Waldron, L.; Nilsson, B.; van Galen, P.; Metzeler, K.H.; Poeppl, A.; Ling, V.; Beyene, J.; et al. Stem Cell Gene Expression Programs Influence Clinical Outcome in Human Leukemia. Nat. Med. 2011, 17, 1086–1093. [Google Scholar] [CrossRef]
- Majeti, R.; Becker, M.W.; Tian, Q.; Lee, T.-L.M.; Yan, X.; Liu, R.; Chiang, J.-H.; Hood, L.; Clarke, M.F.; Weissman, I.L. Dysregulated Gene Expression Networks in Human Acute Myelogenous Leukemia Stem Cells. Proc. Natl. Acad. Sci. USA 2009, 106, 3396–3401. [Google Scholar] [CrossRef]
- Barreyro, L.; Will, B.; Bartholdy, B.; Zhou, L.; Todorova, T.I.; Stanley, R.F.; Ben-Neriah, S.; Montagna, C.; Parekh, S.; Pellagatti, A.; et al. Overexpression of IL-1 Receptor Accessory Protein in Stem and Progenitor Cells and Outcome Correlation in AML and MDS. Blood 2012, 120, 1290–1298. [Google Scholar] [CrossRef]
- Jan, M.; Snyder, T.M.; Corces-Zimmerman, M.R.; Vyas, P.; Weissman, I.L.; Quake, S.R.; Majeti, R. Clonal Evolution of Preleukemic Hematopoietic Stem Cells Precedes Human Acute Myeloid Leukemia. Sci. Transl. Med. 2012, 4, 149ra118. [Google Scholar] [CrossRef]
- Corces-Zimmerman, M.R.; Hong, W.-J.; Weissman, I.L.; Medeiros, B.C.; Majeti, R. Preleukemic Mutations in Human Acute Myeloid Leukemia Affect Epigenetic Regulators and Persist in Remission. Proc. Natl. Acad. Sci. USA 2014, 111, 2548–2553. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, D.; Dick, J.E. Human Acute Myeloid Leukemia Is Organized as a Hierarchy That Originates from a Primitive Hematopoietic Cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, H.J.; Blair, A.; Zapf, R.W. Characterization of a Hierarchy in Human Acute Myeloid Leukemia Progenitor Cells. Blood 1996, 87, 4754–4761. [Google Scholar] [CrossRef]
- Hope, K.J.; Jin, L.; Dick, J.E. Acute Myeloid Leukemia Originates from a Hierarchy of Leukemic Stem Cell Classes That Differ in Self-Renewal Capacity. Nat. Immunol. 2004, 5, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Martelli, M.P.; Pettirossi, V.; Thiede, C.; Bonifacio, E.; Mezzasoma, F.; Cecchini, D.; Pacini, R.; Tabarrini, A.; Ciurnelli, R.; Gionfriddo, I.; et al. CD34+ Cells from AML with Mutated NPM1 Harbor Cytoplasmic Mutated Nucleophosmin and Generate Leukemia in Immunocompromised Mice. Blood 2010, 116, 3907–3922. [Google Scholar] [CrossRef]
- Taussig, D.C.; Vargaftig, J.; Miraki-Moud, F.; Griessinger, E.; Sharrock, K.; Luke, T.; Lillington, D.; Oakervee, H.; Cavenagh, J.; Agrawal, S.G.; et al. Leukemia-Initiating Cells from Some Acute Myeloid Leukemia Patients with Mutated Nucleophosmin Reside in the CD34(−) Fraction. Blood 2010, 115, 1976–1984. [Google Scholar] [CrossRef]
- Goardon, N.; Marchi, E.; Atzberger, A.; Quek, L.; Schuh, A.; Soneji, S.; Woll, P.; Mead, A.; Alford, K.A.; Rout, R.; et al. Coexistence of LMPP-like and GMP-like Leukemia Stem Cells in Acute Myeloid Leukemia. Cancer Cell 2011, 19, 138–152. [Google Scholar] [CrossRef]
- Quek, L.; Otto, G.W.; Garnett, C.; Lhermitte, L.; Karamitros, D.; Stoilova, B.; Lau, I.-J.; Doondeea, J.; Usukhbayar, B.; Kennedy, A.; et al. Genetically Distinct Leukemic Stem Cells in Human CD34− Acute Myeloid Leukemia Are Arrested at a Hemopoietic Precursor-like Stage. J. Exp. Med. 2016, 213, 1513–1535. [Google Scholar] [CrossRef]
- Terpstra, W.; Ploemacher, R.; Prins, A.; Van Lom, K.; Pouwels, K.; Wognum, A.; Wagemaker, G.; Lowenberg, B.; Wielenga, J. Fluorouracil Selectively Spares Acute Myeloid Leukemia Cells with Long- Term Growth Abilities in Immunodeficient Mice and in Culture. Blood 1996, 88, 1944–1950. [Google Scholar] [CrossRef]
- Costello, R.T.; Mallet, F.; Gaugler, B.; Sainty, D.; Arnoulet, C.; Gastaut, J.A.; Olive, D. Human Acute Myeloid Leukemia CD34+/CD38− Progenitor Cells Have Decreased Sensitivity to Chemotherapy and Fas-Induced Apoptosis, Reduced Immunogenicity, and Impaired Dendritic Cell Transformation Capacities. Cancer Res. 2000, 60, 4403–4411. [Google Scholar]
- Ishikawa, F.; Yoshida, S.; Saito, Y.; Hijikata, A.; Kitamura, H.; Tanaka, S.; Nakamura, R.; Tanaka, T.; Tomiyama, H.; Saito, N.; et al. Chemotherapy-Resistant Human AML Stem Cells Home to and Engraft within the Bone-Marrow Endosteal Region. Nat. Biotechnol. 2007, 25, 1315–1321. [Google Scholar] [CrossRef]
- Ding, L.; Ley, T.J.; Larson, D.E.; Miller, C.A.; Koboldt, D.C.; Welch, J.S.; Ritchey, J.K.; Young, M.A.; Lamprecht, T.; McLellan, M.D.; et al. Clonal Evolution in Relapsed Acute Myeloid Leukaemia Revealed by Whole-Genome Sequencing. Nature 2012, 481, 506–510. [Google Scholar] [CrossRef]
- Hourigan, C.S.; Dillon, L.W.; Gui, G.; Logan, B.R.; Fei, M.; Ghannam, J.; Li, Y.; Licon, A.; Alyea, E.P.; Bashey, A.; et al. Impact of Conditioning Intensity of Allogeneic Transplantation for Acute Myeloid Leukemia with Genomic Evidence of Residual Disease. J. Clin. Oncol. 2020, 38, 1273–1283. [Google Scholar] [CrossRef]
- Höllein, A.; Jeromin, S.; Meggendorfer, M.; Fasan, A.; Nadarajah, N.; Kern, W.; Haferlach, C.; Haferlach, T. Minimal Residual Disease (MRD) Monitoring and Mutational Landscape in AML with RUNX1-RUNX1T1: A Study on 134 Patients. Leukemia 2018, 32, 2270–2274. [Google Scholar] [CrossRef]
- Jan, M.; Majeti, R. Clonal Evolution of Acute Leukemia Genomes. Oncogene 2013, 32, 135–140. [Google Scholar] [CrossRef]
- Shlush, L.I.; Mitchell, A.; Heisler, L.; Abelson, S.; Ng, S.W.K.; Trotman-Grant, A.; Medeiros, J.J.F.; Rao-Bhatia, A.; Jaciw-Zurakowsky, I.; Marke, R.; et al. Tracing the Origins of Relapse in Acute Myeloid Leukaemia to Stem Cells. Nature 2017, 547, 104–108. [Google Scholar] [CrossRef]
- Walter, R.B.; Ofran, Y.; Wierzbowska, A.; Ravandi, F.; Hourigan, C.S.; Ngai, L.L.; Venditti, A.; Buccisano, F.; Ossenkoppele, G.J.; Roboz, G.J. Measurable Residual Disease as a Biomarker in Acute Myeloid Leukemia: Theoretical and Practical Considerations. Leukemia 2021, 35, 1529–1538. [Google Scholar] [CrossRef]
- Short, N.J.; Zhou, S.; Fu, C.; Berry, D.A.; Walter, R.B.; Freeman, S.D.; Hourigan, C.S.; Huang, X.; Nogueras Gonzalez, G.; Hwang, H.; et al. Association of Measurable Residual Disease with Survival Outcomes in Patients with Acute Myeloid Leukemia: A Systematic Review and Meta-Analysis. JAMA Oncol. 2020, 6, 1890. [Google Scholar] [CrossRef]
- Araki, D.; Wood, B.L.; Othus, M.; Radich, J.P.; Halpern, A.B.; Zhou, Y.; Mielcarek, M.; Estey, E.H.; Appelbaum, F.R.; Walter, R.B. Allogeneic Hematopoietic Cell Transplantation for Acute Myeloid Leukemia: Time to Move toward a Minimal Residual Disease–Based Definition of Complete Remission? J. Clin. Oncol. 2016, 34, 329–336. [Google Scholar] [CrossRef]
- Rau, R.E.; Dai, Y.; Devidas, M.; Rabin, K.R.; Zweidler-McKay, P.; Angiolillo, A.; Schore, R.J.; Burke, M.J.; Salzer, W.L.; Heerema, N.A.; et al. Prognostic Impact of Minimal Residual Disease at the End of Consolidation in NCI Standard-Risk B-Lymphoblastic Leukemia: A Report from the Children’s Oncology Group. Pediatr. Blood Cancer 2021, 68, e28929. [Google Scholar] [CrossRef]
- Buccisano, F.; Hourigan, C.S.; Walter, R.B. The Prognostic Significance of Measurable (“Minimal”) Residual Disease in Acute Myeloid Leukemia. Curr. Hematol. Malig. Rep. 2017, 12, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Blachly, J.S.; Walter, R.B.; Hourigan, C.S. The Present and Future of Measurable Residual Disease Testing in Acute Myeloid Leukemia. Haematologica 2022, 107, 2810–2822. [Google Scholar] [CrossRef] [PubMed]
- De Haas, V.; Ismaila, N.; Advani, A.; Arber, D.A.; Dabney, R.S.; Patel-Donelly, D.; Kitlas, E.; Pieters, R.; Pui, C.-H.; Sweet, K.; et al. Initial Diagnostic Work-Up of Acute Leukemia: ASCO Clinical Practice Guideline Endorsement of the College of American Pathologists and American Society of Hematology Guideline. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Patkar, N.; Kakirde, C.; Shaikh, A.F.; Salve, R.; Bhanshe, P.; Chatterjee, G.; Rajpal, S.; Joshi, S.; Chaudhary, S.; Kodgule, R.; et al. Clinical Impact of Panel-Based Error-Corrected next Generation Sequencing versus Flow Cytometry to Detect Measurable Residual Disease (MRD) in Acute Myeloid Leukemia (AML). Leukemia 2021, 35, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Jongen-Lavrencic, M.; Grob, T.; Hanekamp, D.; Kavelaars, F.G.; Al Hinai, A.; Zeilemaker, A.; Erpelinck-Verschueren, C.A.J.; Gradowska, P.L.; Meijer, R.; Cloos, J.; et al. Molecular Minimal Residual Disease in Acute Myeloid Leukemia. N. Engl. J. Med. 2018, 378, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Daga, S.; Rosenberger, A.; Kashofer, K.; Heitzer, E.; Quehenberger, F.; Halbwedl, I.; Graf, R.; Krisper, N.; Prietl, B.; Höfler, G.; et al. Sensitive and Broadly Applicable Residual Disease Detection in Acute Myeloid Leukemia Using Flow Cytometry-Based Leukemic Cell Enrichment Followed by Mutational Profiling. Am. J. Hematol. 2020, 95, 1148–1157. [Google Scholar] [CrossRef]
- Pollyea, D.A.; Bixby, D.; Perl, A.; Bhatt, V.R.; Altman, J.K.; Appelbaum, F.R.; de Lima, M.; Fathi, A.T.; Foran, J.M.; Gojo, I.; et al. NCCN Guidelines Insights: Acute Myeloid Leukemia, Version 2. J. Natl. Compr. Cancer Netw. JNCCN 2021, 19, 16–27. [Google Scholar] [CrossRef]
- Bernasconi, P.; Borsani, O. Eradication of Measurable Residual Disease in AML: A Challenging Clinical Goal. Cancers 2021, 13, 3170. [Google Scholar] [CrossRef]
- Li, S.-Q.; Xu, L.-P.; Wang, Y.; Zhang, X.-H.; Chen, H.; Chen, Y.-H.; Wang, F.-R.; Han, W.; Sun, Y.-Q.; Yan, C.-H.; et al. An LSC-Based MRD Assay to Complement the Traditional MFC Method for Prediction of AML Relapse: A Prospective Study. Blood 2022, 140, 516–520. [Google Scholar] [CrossRef]
- Ngai, L.L.; Kelder, A.; Janssen, J.J.W.M.; Ossenkoppele, G.J.; Cloos, J. MRD Tailored Therapy in AML: What We Have Learned So Far. Front. Oncol. 2021, 10, 603636. [Google Scholar] [CrossRef]
- Panuzzo, C.; Jovanovski, A.; Ali, M.S.; Cilloni, D.; Pergolizzi, B. Revealing the Mysteries of Acute Myeloid Leukemia: From Quantitative PCR through Next-Generation Sequencing and Systemic Metabolomic Profiling. J. Clin. Med. 2022, 11, 483. [Google Scholar] [CrossRef]
- Onecha, E.; Rapado, I.; Luz Morales, M.; Carreño-Tarragona, G.; Martinez-Sanchez, P.; Gutierrez, X.; Sáchez Pina, J.M.; Linares, M.; Gallardo, M.; Martinez-López, J.; et al. Monitoring of Clonal Evolution of Acute Myeloid Leukemia Identifies the Leukemia Subtype, Clinical Outcome and Potential New Drug Targets for Post-Remission Strategies or Relapse. Haematologica 2021, 106, 2325–2333. [Google Scholar] [CrossRef]
- Duncavage, E.J.; Schroeder, M.C.; O’Laughlin, M.; Wilson, R.; MacMillan, S.; Bohannon, A.; Kruchowski, S.; Garza, J.; Du, F.; Hughes, A.E.O.; et al. Genome Sequencing as an Alternative to Cytogenetic Analysis in Myeloid Cancers. N. Engl. J. Med. 2021, 384, 924–935. [Google Scholar] [CrossRef]
- Short, N.J.; Ravandi, F. How Close Are We to Incorporating Measurable Residual Disease into Clinical Practice for Acute Myeloid Leukemia? Haematologica 2019, 104, 1532–1541. [Google Scholar] [CrossRef]
- Juul-Dam, K.L.; Ommen, H.B.; Nyvold, C.G.; Walter, C.; Vålerhaugen, H.; Kairisto, V.; Abrahamsson, J.; Alm, S.J.; Jahnukainen, K.; Lausen, B.; et al. Measurable Residual Disease Assessment by QPCR in Peripheral Blood Is an Informative Tool for Disease Surveillance in Childhood Acute Myeloid Leukaemia. Br. J. Haematol. 2020, 190, 198–208. [Google Scholar] [CrossRef]
- Godwin, C.D.; Zhou, Y.; Othus, M.; Asmuth, M.M.; Shaw, C.M.; Gardner, K.M.; Wood, B.L.; Walter, R.B.; Estey, E.H. Acute Myeloid Leukemia Measurable Residual Disease Detection by Flow Cytometry in Peripheral Blood vs. Bone Marrow. Blood 2021, 137, 569–572. [Google Scholar] [CrossRef]
- Freeman, S.D.; Hills, R.K.; Virgo, P.; Khan, N.; Couzens, S.; Dillon, R.; Gilkes, A.; Upton, L.; Nielsen, O.J.; Cavenagh, J.D.; et al. Measurable Residual Disease at Induction Redefines Partial Response in Acute Myeloid Leukemia and Stratifies Outcomes in Patients at Standard Risk Without NPM1 Mutations. J. Clin. Oncol. 2018, 36, 1486–1497. [Google Scholar] [CrossRef]
- Pratz, K.W.; Jonas, B.A.; Pullarkat, V.; Recher, C.; Schuh, A.C.; Thirman, M.J.; Garcia, J.S.; DiNardo, C.D.; Vorobyev, V.; Fracchiolla, N.S.; et al. Measurable Residual Disease Response and Prognosis in Treatment-Naïve Acute Myeloid Leukemia with Venetoclax and Azacitidine. J. Clin. Oncol. 2022, 40, 855–865. [Google Scholar] [CrossRef]
- Yin, J.A.L.; O’Brien, M.A.; Hills, R.K.; Daly, S.B.; Wheatley, K.; Burnett, A.K. Minimal Residual Disease Monitoring by Quantitative RT-PCR in Core Binding Factor AML Allows Risk Stratification and Predicts Relapse: Results of the United Kingdom MRC AML-15 Trial. Blood 2012, 120, 2826–2835. [Google Scholar] [CrossRef]
- Rücker, F.G.; Agrawal, M.; Corbacioglu, A.; Weber, D.; Kapp-Schwoerer, S.; Gaidzik, V.I.; Jahn, N.; Schroeder, T.; Wattad, M.; Lübbert, M.; et al. Measurable Residual Disease Monitoring in Acute Myeloid Leukemia with t(8;21)(Q22;Q22.1): Results from the AML Study Group. Blood 2019, 134, 1608–1618. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Tang, J.-L.; Tien, F.-M.; Kuo, Y.-Y.; Wu, D.-C.; Lin, C.-C.; Tseng, M.-H.; Peng, Y.-L.; Hou, M.-F.; Chuang, Y.-K.; et al. Clinical Implications of Sequential MRD Monitoring by NGS at 2 Time Points after Chemotherapy in Patients with AML. Blood Adv. 2021, 5, 2456–2466. [Google Scholar] [CrossRef] [PubMed]
- Puckrin, R.; Atenafu, E.G.; Claudio, J.O.; Chan, S.; Gupta, V.; Maze, D.; McNamara, C.; Murphy, T.; Shuh, A.C.; Yee, K.; et al. Measurable Residual Disease Monitoring Provides Insufficient Lead-Time to Prevent Morphologic Relapse in the Majority of Patients with Core-Binding Factor Acute Myeloid Leukemia. Haematologica 2021, 106, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Hourigan, C.S.; Karp, J.E. Minimal residual disease in acute myeloid leukaemia. Nat. Rev. Clin. Oncol. 2013, 10, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Ivey, A.; Hills, R.K.; Simpson, M.A.; Jovanovic, J.V.; Gilkes, A.; Grech, A.; Patel, Y.; Bhudia, N.; Farah, H.; Mason, J.; et al. Assessment of Minimal Residual Disease in Standard-Risk AML. N. Engl. J. Med. 2016, 374, 422–433. [Google Scholar] [CrossRef]
- Platzbecker, U.; Middeke, J.M.; Sockel, K.; Herbst, R.; Wolf, D.; Baldus, C.D.; Oelschlägel, U.; Mütherig, A.; Fransecky, L.; Noppeney, R.; et al. Measurable Residual Disease-Guided Treatment with Azacitidine to Prevent Haematological Relapse in Patients with Myelodysplastic Syndrome and Acute Myeloid Leukaemia (RELAZA2): An Open-Label, Multicentre, Phase 2 Trial. Lancet Oncol. 2018, 19, 1668–1679. [Google Scholar] [CrossRef] [PubMed]
- Short, N.J.; Rafei, H.; Daver, N.; Hwang, H.; Ning, J.; Jorgensen, J.L.; Kadia, T.M.; DiNardo, C.D.; Wang, S.A.; Jabbour, E.; et al. Prognostic Impact of Complete Remission with MRD Negativity in Patients with Relapsed or Refractory AML. Blood Adv. 2020, 4, 6117–6126. [Google Scholar] [CrossRef] [PubMed]
- Simoes, C.; Paiva, B.; Martínez-Cuadrón, D.; Bergua, J.-M.; Vives, S.; Algarra, L.; Tormo, M.; Martinez, P.; Serrano, J.; Herrera, P.; et al. Measurable Residual Disease in Elderly Acute Myeloid Leukemia: Results from the PETHEMA-FLUGAZA Phase 3 Clinical Trial. Blood Adv. 2021, 5, 760–770. [Google Scholar] [CrossRef]
- Maiti, A.; DiNardo, C.D.; Wang, S.A.; Jorgensen, J.; Kadia, T.M.; Daver, N.G.; Short, N.J.; Yilmaz, M.; Pemmaraju, N.; Borthakur, G.; et al. Prognostic Value of Measurable Residual Disease after Venetoclax and Decitabine in Acute Myeloid Leukemia. Blood Adv. 2021, 5, 1876–1883. [Google Scholar] [CrossRef]
- Roboz, G.J.; Ravandi, F.; Wei, A.H.; Dombret, H.; Thol, F.; Voso, M.T.; Schuh, A.C.; Porkka, K.; La Torre, I.; Skikne, B.; et al. Oral Azacitidine Prolongs Survival of Patients with AML in Remission Independently of Measurable Residual Disease Status. Blood 2022, 139, 2145–2155. [Google Scholar] [CrossRef]
- Balsat, M.; Renneville, A.; Thomas, X.; De Botton, S.; Caillot, D.; Marceau, A.; Lemasle, E.; Marolleau, J.-P.; Nibourel, O.; Berthon, C.; et al. Postinduction Minimal Residual Disease Predicts Outcome and Benefit from Allogeneic Stem Cell Transplantation in Acute Myeloid Leukemia with NPM1 Mutation: A Study by the Acute Leukemia French Association Group. J. Clin. Oncol. 2017, 35, 185–193. [Google Scholar] [CrossRef]
- Sockel, K.; Wermke, M.; Radke, J.; Kiani, A.; Schaich, M.; Bornhauser, M.; Ehninger, G.; Thiede, C.; Platzbecker, U. Minimal Residual Disease-Directed Preemptive Treatment with Azacitidine in Patients with NPM1-Mutant Acute Myeloid Leukemia and Molecular Relapse. Haematologica 2011, 96, 1568–1570. [Google Scholar] [CrossRef]
- Platzbecker, U.; Wermke, M.; Radke, J.; Oelschlaegel, U.; Seltmann, F.; Kiani, A.; Klut, I.-M.; Knoth, H.; Röllig, C.; Schetelig, J.; et al. Azacitidine for Treatment of Imminent Relapse in MDS or AML Patients after Allogeneic HSCT: Results of the RELAZA Trial. Leukemia 2012, 26, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Kapp-Schwoerer, S.; Weber, D.; Corbacioglu, A.; Gaidzik, V.I.; Paschka, P.; Krönke, J.; Theis, F.; Rücker, F.G.; Teleanu, M.-V.; Panina, E.; et al. Impact of Gemtuzumab Ozogamicin on MRD and Relapse Risk in Patients with NPM1-Mutated AML: Results from the AMLSG 09-09 Trial. Blood 2020, 136, 3041–3050. [Google Scholar] [CrossRef] [PubMed]
- Tiong, I.S.; Dillon, R.; Ivey, A.; Teh, T.-C.; Nguyen, P.; Cummings, N.; Taussig, D.C.; Latif, A.-L.; Potter, N.E.; Runglall, M.; et al. Venetoclax Induces Rapid Elimination of NPM1 Mutant Measurable Residual Disease in Combination with Low-Intensity Chemotherapy in Acute Myeloid Leukaemia. Br. J. Haematol. 2021, 192, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Tiong, I.S.; Quaglieri, A.; MacRaild, S.; Loghavi, S.; Brown, F.C.; Thijssen, R.; Pomilio, G.; Ivey, A.; Salmon, J.M.; et al. Molecular Patterns of Response and Treatment Failure after Frontline Venetoclax Combinations in Older Patients with AML. Blood 2020, 135, 791–803. [Google Scholar] [CrossRef]
- Burnett, A.K.; Russell, N.H.; Hills, R.K.; Knapper, S.; Freeman, S.; Huntly, B.; Clark, R.E.; Thomas, I.F.; Kjeldsen, L.; McMullin, M.F.; et al. Defining the Optimal Total Number of Chemotherapy Courses in Younger Patients with Acute Myeloid Leukemia: A Comparison of Three versus Four Courses. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 890–901. [Google Scholar] [CrossRef]
- Dillon, R.; Hills, R.; Freeman, S.; Potter, N.; Jovanovic, J.; Ivey, A.; Kanda, A.S.; Runglall, M.; Foot, N.; Valganon, M.; et al. Molecular MRD Status and Outcome after Transplantation in NPM1-Mutated AML. Blood 2020, 135, 680–688. [Google Scholar] [CrossRef]
- Morsink, L.M.; Sandmaier, B.M.; Othus, M.; Palmieri, R.; Granot, N.; Bezerra, E.D.; Wood, B.L.; Mielcarek, M.; Schoch, G.; Davis, C.; et al. Conditioning Intensity, Pre-Transplant Flow Cytometric Measurable Residual Disease, and Outcome in Adults with Acute Myeloid Leukemia Undergoing Allogeneic Hematopoietic Cell Transplantation. Cancers 2020, 12, 2339. [Google Scholar] [CrossRef]
- Morsink, L.M.; Bezerra, E.D.; Othus, M.; Wood, B.L.; Fang, M.; Sandmaier, B.M.; Mielcarek, M.B.; Deeg, H.J.; Schoch, G.; Appelbaum, F.R.; et al. Comparative Analysis of Total Body Irradiation (TBI)-Based and Non-TBI-Based Myeloablative Conditioning for Acute Myeloid Leukemia in Remission with or without Measurable Residual Disease. Leukemia 2020, 34, 1701–1705. [Google Scholar] [CrossRef]
- Walter, R.B.; Gyurkocza, B.; Storer, B.E.; Godwin, C.D.; Pagel, J.M.; Buckley, S.A.; Sorror, M.L.; Wood, B.L.; Storb, R.; Appelbaum, F.R.; et al. Comparison of Minimal Residual Disease as Outcome Predictor for AML Patients in First Complete Remission Undergoing Myeloablative or Nonmyeloablative Allogeneic Hematopoietic Cell Transplantation. Leukemia 2015, 29, 137–144. [Google Scholar] [CrossRef]
- Walter, R.B.; Buckley, S.A.; Pagel, J.M.; Wood, B.L.; Storer, B.E.; Sandmaier, B.M.; Fang, M.; Gyurkocza, B.; Delaney, C.; Radich, J.P.; et al. Significance of Minimal Residual Disease before Myeloablative Allogeneic Hematopoietic Cell Transplantation for AML in First and Second Complete Remission. Blood 2013, 122, 1813–1821. [Google Scholar] [CrossRef]
- Jentzsch, M.; Bischof, L.; Backhaus, D.; Brauer, D.; Schulz, J.; Franke, G.-N.; Vucinic, V.; Niederwieser, D.; Platzbecker, U.; Schwind, S. Impact of MRD Status in Patients with AML Undergoing Allogeneic Stem Cell Transplantation in the First vs. the Second Remission. Blood Adv. 2022, 6, 4570–4580. [Google Scholar] [CrossRef]
- Gilleece, M.H.; Shimoni, A.; Labopin, M.; Robinson, S.; Beelen, D.; Socié, G.; Unal, A.; Ganser, A.; Vitek, A.; Sengeloev, H.; et al. Measurable Residual Disease Status and Outcome of Transplant in Acute Myeloid Leukemia in Second Complete Remission: A Study by the Acute Leukemia Working Party of the EBMT. Blood Cancer J. 2021, 11, 88. [Google Scholar] [CrossRef]
- Ambinder, A.J.; Levis, M. Potential Targeting of FLT3 Acute Myeloid Leukemia. Haematologica 2021, 106, 671–681. [Google Scholar] [CrossRef]
- Cortes, J.E.; Khaled, S.; Martinelli, G.; Perl, A.E.; Ganguly, S.; Russell, N.; Krämer, A.; Dombret, H.; Hogge, D.; Jonas, B.A.; et al. Quizartinib versus Salvage Chemotherapy in Relapsed or Refractory FLT3-ITD Acute Myeloid Leukaemia (QuANTUM-R): A Multicentre, Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet Oncol. 2019, 20, 984–997. [Google Scholar] [CrossRef]
- Perl, A.E.; Martinelli, G.; Cortes, J.E.; Neubauer, A.; Berman, E.; Paolini, S.; Montesinos, P.; Baer, M.R.; Larson, R.A.; Ustun, C.; et al. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3-Mutated AML. N. Engl. J. Med. 2019, 381, 1728–1740. [Google Scholar] [CrossRef]
- Burchert, A.; Bug, G.; Fritz, L.V.; Finke, J.; Stelljes, M.; Röllig, C.; Wollmer, E.; Wäsch, R.; Bornhäuser, M.; Berg, T.; et al. Sorafenib Maintenance after Allogeneic Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia with FLT3-Internal Tandem Duplication Mutation (SORMAIN). J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 2993–3002. [Google Scholar] [CrossRef]
- Bataller, A.; Oñate, G.; Diaz-Beyá, M.; Guijarro, F.; Garrido, A.; Vives, S.; Tormo, M.; Arnan, M.; Salamero, O.; Sampol, A.; et al. Acute Myeloid Leukemia with NPM1 Mutation and Favorable European LeukemiaNet Category: Outcome after Preemptive Intervention Based on Measurable Residual Disease. Br. J. Haematol. 2020, 191, 52–61. [Google Scholar] [CrossRef]
- Venditti, A.; Piciocchi, A.; Candoni, A.; Melillo, L.; Calafiore, V.; Cairoli, R.; De Fabritiis, P.; Storti, G.; Salutari, P.; Lanza, F.; et al. GIMEMA AML1310 Trial of Risk-Adapted, MRD-Directed Therapy for Young Adults with Newly Diagnosed Acute Myeloid Leukemia. Blood 2019, 134, 935–945. [Google Scholar] [CrossRef]
- Venditti, A.; Gale, R.P.; Buccisano, F.; Ossenkoppele, G. Should Persons with Acute Myeloid Leukemia (AML) in 1st Histological Complete Remission Who Are Measurable Residual Disease (MRD) Test Positive Receive an Allotransplant? Leukemia 2020, 34, 963–965. [Google Scholar] [CrossRef]
- Zhu, H.-H.; Zhang, X.-H.; Qin, Y.-Z.; Liu, D.-H.; Jiang, H.; Chen, H.; Jiang, Q.; Xu, L.-P.; Lu, J.; Han, W.; et al. MRD-Directed Risk Stratification Treatment May Improve Outcomes of t(8;21) AML in the First Complete Remission: Results from the AML05 Multicenter Trial. Blood 2013, 121, 4056–4062. [Google Scholar] [CrossRef]
- Jaramillo, S.; Krisam, J.; Le Cornet, L.; Kratzmann, M.; Baumann, L.; Sauer, T.; Crysandt, M.; Rank, A.; Behringer, D.; Teichmann, L.; et al. Rationale and Design of the 2 by 2 Factorial Design GnG-Trial: A Randomized Phase-III Study to Compare Two Schedules of Gemtuzumab Ozogamicin as Adjunct to Intensive Induction Therapy and to Compare Double-Blinded Intensive Postremission Therapy with or without Glasdegib in Older Patients with Newly Diagnosed AML. Trials 2021, 22, 765. [Google Scholar] [CrossRef] [PubMed]
- Schlenk, P.D.R.F. Randomized Phase-III Study to Compare Two Schedules of Gemtuzumab Ozogamicin as Adjunct to Intensive Induction Therapy and to Compare Intensive Postremission Therapy Double Blinded with or without Glasdegib in Older Patients with Newly Diagnosed AML. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04093505 (accessed on 11 May 2023).
- National Cancer Institute (NCI). Blockade of PD-1 Added to Standard Therapy to Target Measurable Residual Disease in Acute Myeloid Leukemia 2 (BLAST MRD AML-2): A Randomized Phase 2 Study of the Venetoclax, Azacitadine, and Pembrolizumab (VAP) Versus Venetoclax and Azacitadine as First Line Therapy in Older Patients with Acute Myeloid Leukemia (AML) Who Are Ineligible or Who Refuse Intensive Chemotherapy. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT04284787 (accessed on 11 May 2023).
- National Cancer Institute (NCI). Blockade of PD-1 Added to Standard Therapy to Target Measurable Residual Disease in Acute Myeloid Leukemia 1 (BLAST MRD AML-1): A Randomized Phase 2 Study of the Anti-PD-1 Antibody Pembrolizumab in Combination with Conventional Intensive Chemotherapy as Frontline Therapy in Patients with Acute Myeloid Leukemia. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT04214249 (accessed on 11 May 2023).
- Atallah, E.L. A Phase II Study of the Efficacy and Pharmacogenomics of Cladribine-Based Salvage Chemotherapy in Patients with Relapse/Refractory and Secondary Acute Myeloid Leukemia (AML) and High Risk Myelodysplastic Syndrome (MDS). 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT03150004 (accessed on 11 May 2023).
- Cytovia, Inc. Open-Label, Multicenter, Effects of Remission Maintenance Therapy with Ceplene®, Given in Conjunction with Low-Dose Interleukin-2, on Immune Response and Minimal Residual Disease in Adult Patients with AML in First Complete Remission. 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT01347996 (accessed on 11 May 2023).
- Heuser, M.; Freeman, S.D.; Ossenkoppele, G.J.; Buccisano, F.; Hourigan, C.S.; Ngai, L.L.; Tettero, J.M.; Bachas, C.; Baer, C.; Béné, M.-C.; et al. 2021 Update on MRD in Acute Myeloid Leukemia: A Consensus Document from the European LeukemiaNet MRD Working Party. Blood 2021, 138, 2753–2767. [Google Scholar] [CrossRef] [PubMed]
- Zeijlemaker, W.; Gratama, J.W.; Schuurhuis, G.J. Tumor Heterogeneity Makes AML a “Moving Target” for Detection of Residual Disease. Cytom. B Clin. Cytom. 2014, 86, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Baer, M.R.; Stewart, C.C.; Dodge, R.K.; Leget, G.; Sulé, N.; Mrózek, K.; Schiffer, C.A.; Powell, B.L.; Kolitz, J.E.; Moore, J.O.; et al. High Frequency of Immunophenotype Changes in Acute Myeloid Leukemia at Relapse: Implications for Residual Disease Detection (Cancer and Leukemia Group B Study 8361). Blood 2001, 97, 3574–3580. [Google Scholar] [CrossRef] [PubMed]
- Zeijlemaker, W.; Kelder, A.; Oussoren-Brockhoff, Y.J.M.; Scholten, W.J.; Snel, A.N.; Veldhuizen, D.; Cloos, J.; Ossenkoppele, G.J.; Schuurhuis, G.J. A Simple One-Tube Assay for Immunophenotypical Quantification of Leukemic Stem Cells in Acute Myeloid Leukemia. Leukemia 2016, 30, 439–446. [Google Scholar] [CrossRef]
- Kamel, A.M.; Elsharkawy, N.M.; Kandeel, E.Z.; Hanafi, M.; Samra, M.; Osman, R.A. Leukemia Stem Cell Frequency at Diagnosis Correlates with Measurable/Minimal Residual Disease and Impacts Survival in Adult Acute Myeloid Leukemia. Front. Oncol. 2022, 12, 867684. [Google Scholar] [CrossRef]
- Thomas, D.; Majeti, R. Biology and Relevance of Human Acute Myeloid Leukemia Stem Cells. Blood 2017, 129, 1577–1585. [Google Scholar] [CrossRef]
- Saygin, C.; Hu, E.; Zhang, P.; Sher, S.; Lozanski, A.; Doong, T.-J.; Nicolet, D.; Orwick, S.; Labanowska, J.; Skinner, J.N.; et al. Genomic Analysis of Cellular Hierarchy in Acute Myeloid Leukemia Using Ultrasensitive LC-FACSeq. Leukemia 2021, 35, 3406–3420. [Google Scholar] [CrossRef]
- Schuurhuis, G.J.; Heuser, M.; Freeman, S.; Béné, M.-C.; Buccisano, F.; Cloos, J.; Grimwade, D.; Haferlach, T.; Hills, R.K.; Hourigan, C.S.; et al. Minimal/Measurable Residual Disease in AML: A Consensus Document from the European LeukemiaNet MRD Working Party. Blood 2018, 131, 1275–1291. [Google Scholar] [CrossRef]
- Li, S.; Xu, L.-P.; Wang, Y.; Zhang, X.; Chen, H.; Chen, Y.-H.; Wang, F.; Han, W.; Sun, Y.; Yan, C.; et al. Superiority of Leukemic Stem Cell-Based Minimal Residual Disease Assay to Traditional Multiparameter Flow Cytometry-Based Method for Relapse Prediction in AML Patients: A Prospective Study with Randomized Training and Validation Sets. Blood 2021, 138, 517. [Google Scholar] [CrossRef]
- Srinivasan, K.; Bhaskar, A.; Alexandre, J.; Winters, A.; Zhang, E.; Akker, Y.; Liu, C.; Arbini, A.; Chattopadhyay, P.; Park, C. Novel Multi-Parameter Flow Cytometry Approaches and Data Analysis Tools for the Evaluation and Detection of Leukemia Stem Cells; Nature Publishing Group: London, UK, 2019; Volume 99. [Google Scholar]
- Canali, A.; Vergnolle, I.; Bertoli, S.; Largeaud, L.; Nicolau, M.-L.; Rieu, J.-B.; Tavitian, S.; Huguet, F.; Picard, M.; Bories, P.; et al. Prognostic Impact of Unsupervised Early Assessment of Bulk and Leukemic Stem Cell Measurable Residual Disease in Acute Myeloid Leukemia. Clin. Cancer Res. 2023, 29, 134–142. [Google Scholar] [CrossRef]
- Nakamura, S.; Yokoyama, K.; Shimizu, E.; Yusa, N.; Kondoh, K.; Ogawa, M.; Takei, T.; Kobayashi, A.; Ito, M.; Isobe, M.; et al. Prognostic Impact of Circulating Tumor DNA Status Post–Allogeneic Hematopoietic Stem Cell Transplantation in AML and MDS. Blood 2019, 133, 2682–2695. [Google Scholar] [CrossRef]
- Ho, T.-C.; LaMere, M.; Stevens, B.M.; Ashton, J.M.; Myers, J.R.; O’Dwyer, K.M.; Liesveld, J.L.; Mendler, J.H.; Guzman, M.; Morrissette, J.D.; et al. Evolution of Acute Myelogenous Leukemia Stem Cell Properties after Treatment and Progression. Blood 2016, 128, 1671–1678. [Google Scholar] [CrossRef]
- Lasry, A.; Nadorp, B.; Fornerod, M.; Nicolet, D.; Wu, H.; Walker, C.J.; Sun, Z.; Witkowski, M.T.; Tikhonova, A.N.; Guillamot-Ruano, M.; et al. An Inflammatory State Remodels the Immune Microenvironment and Improves Risk Stratification in Acute Myeloid Leukemia. Nat. Cancer 2023, 4, 27–42. [Google Scholar] [CrossRef]
| Name of Trial | Description |
|---|---|
| NCT04168502 [113] | Currently ongoing phase 3 study examining MRD levels in AML patients treated with Gemtuzumab in combination with standard chemotherapy. |
| NCT04093505 [114] | Investigating the role of Glasdegib and Gemtuzumab Ozogamicin (GO) as maintenance therapy post-transplant and as adjunct to consolidation therapy, respectively, to gain evidence of anti-leukemic activity of GO and Glasdegib in older patients with newly diagnosed AML. |
| NCT04284787 [115] | Currently recruiting unfit AML patients for a phase 2 study for anti-PD 1 pembrolizumab in combination with azacitidine and venetoclax. |
| NCT04214249 [116] | Currently recruiting AML patients for a phase 2 study for blockade of PD-1 Pembrolizumab in combination with intensive chemotherapy (cytarabine and idarubicin or daunorubicin) as frontline therapy. |
| NCT03150004 [117] | Currently recruiting patients with R/R and secondary AML to test the efficacy and pharmacogenomics of salvage CLAG-M (cladribine, cytarabine, mitoxantrone, G-CSF) chemotherapy. |
| NCT01347996 [118] | Evaluating the effects of remission maintenance therapy using Ceplene/IL-2 in adult patients with AML in CR1 on specific immune system cells (T and NK cells) and prospectively defining markers of immune response that are known to reflect T and NK cell ability to combat AML. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srinivasan Rajsri, K.; Roy, N.; Chakraborty, S. Acute Myeloid Leukemia Stem Cells in Minimal/Measurable Residual Disease Detection. Cancers 2023, 15, 2866. https://doi.org/10.3390/cancers15102866
Srinivasan Rajsri K, Roy N, Chakraborty S. Acute Myeloid Leukemia Stem Cells in Minimal/Measurable Residual Disease Detection. Cancers. 2023; 15(10):2866. https://doi.org/10.3390/cancers15102866
Chicago/Turabian StyleSrinivasan Rajsri, Kritika, Nainita Roy, and Sohini Chakraborty. 2023. "Acute Myeloid Leukemia Stem Cells in Minimal/Measurable Residual Disease Detection" Cancers 15, no. 10: 2866. https://doi.org/10.3390/cancers15102866
APA StyleSrinivasan Rajsri, K., Roy, N., & Chakraborty, S. (2023). Acute Myeloid Leukemia Stem Cells in Minimal/Measurable Residual Disease Detection. Cancers, 15(10), 2866. https://doi.org/10.3390/cancers15102866








