Serrate RNA Effector Molecule (SRRT) Is Associated with Prostate Cancer Progression and Is a Predictor of Poor Prognosis in Lethal Prostate Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Tissue Microarray Construction
2.2. Immunohistochemistry
2.3. Pathological Analysis
2.4. TCGA PRAD Cohort and Data Analysis
2.5. Statistical Analysis
2.6. Software and Packages
3. Results
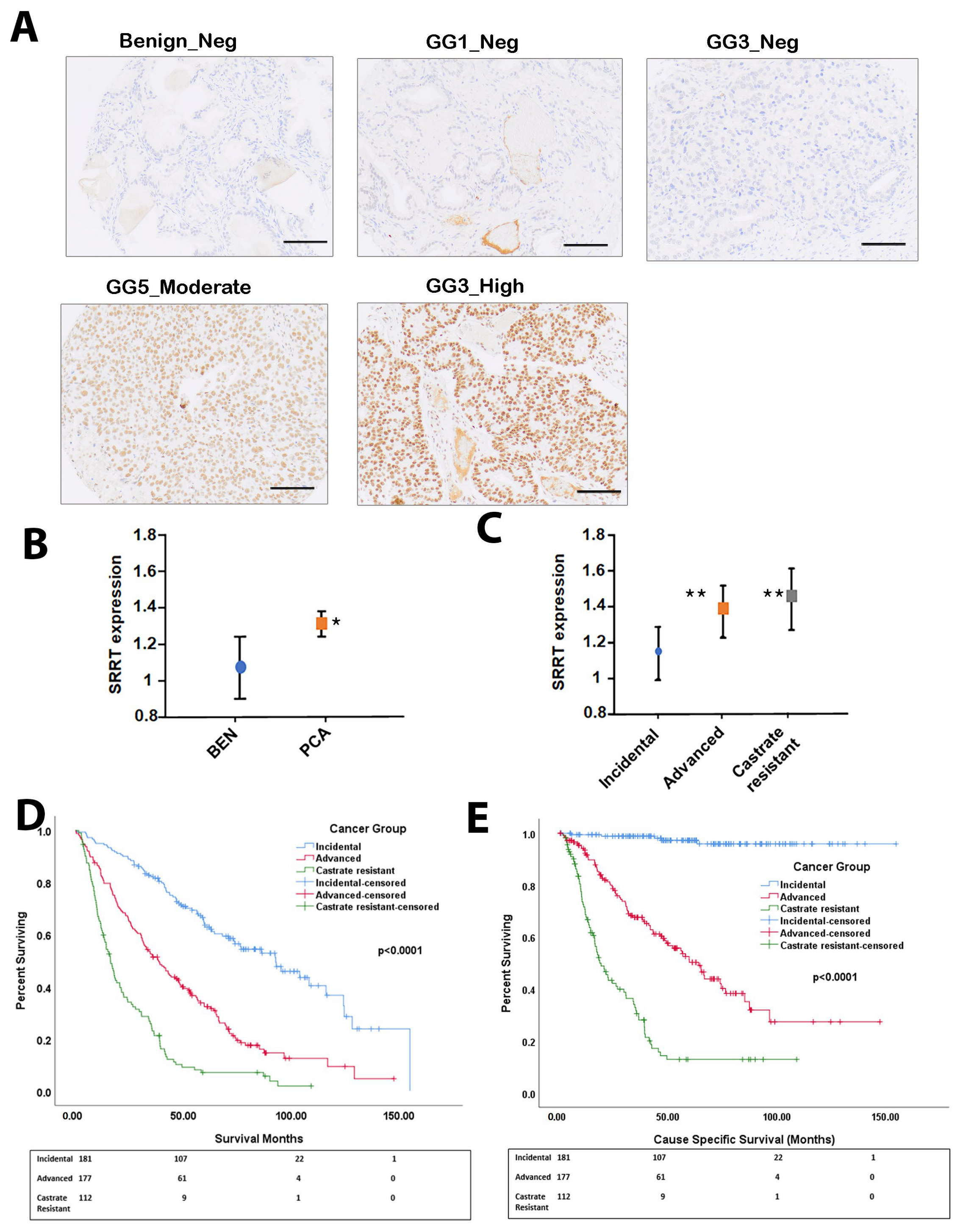
3.1. SRRT Expression in Prostate Cancer Cohort
3.2. SRRT Expression in Relation to Gleason Grade Grouping
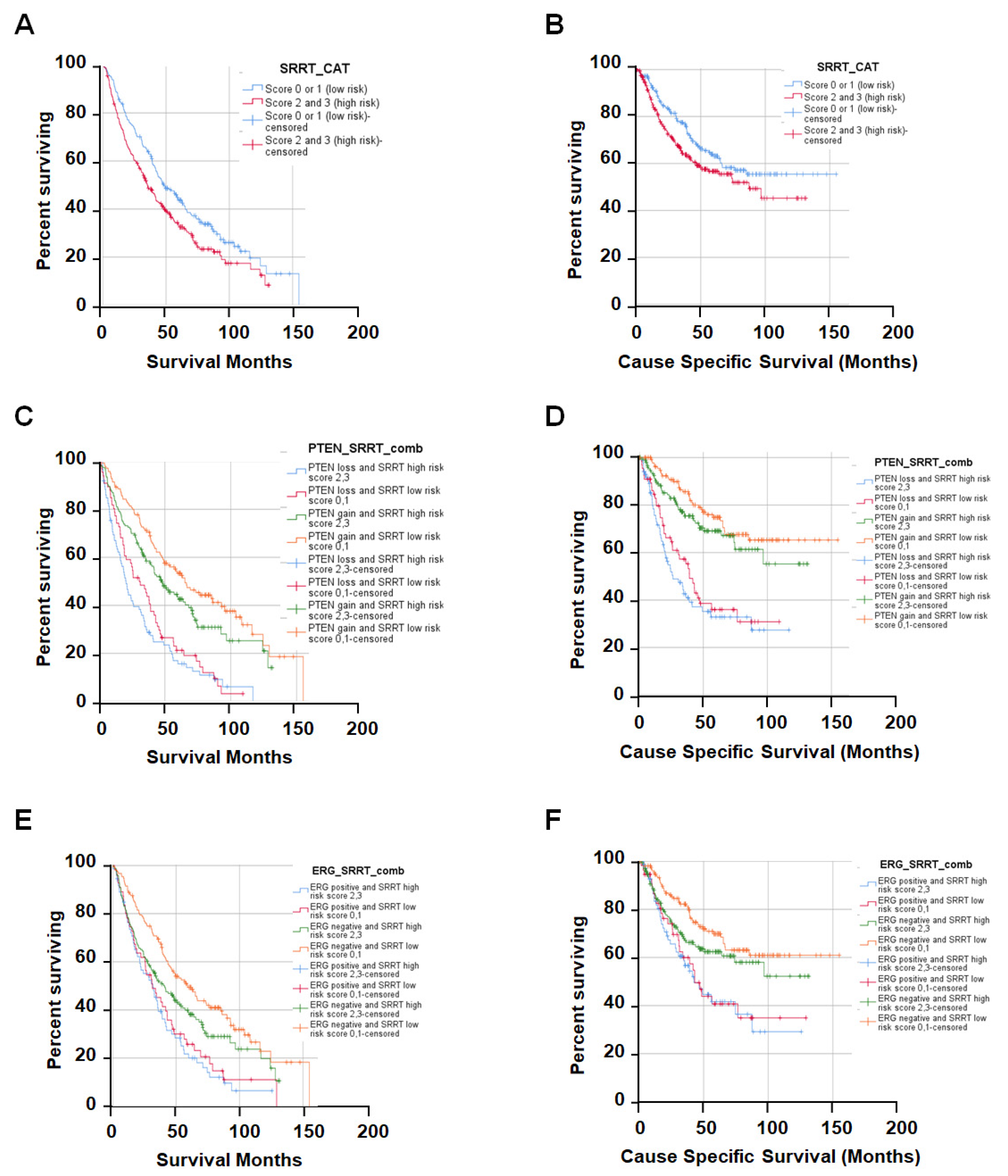
3.3. High SRRT Expression Associated with Overall Survival (OS) and Cause-Specific Survival (CSS) Related to Prostate Cancer Lethality
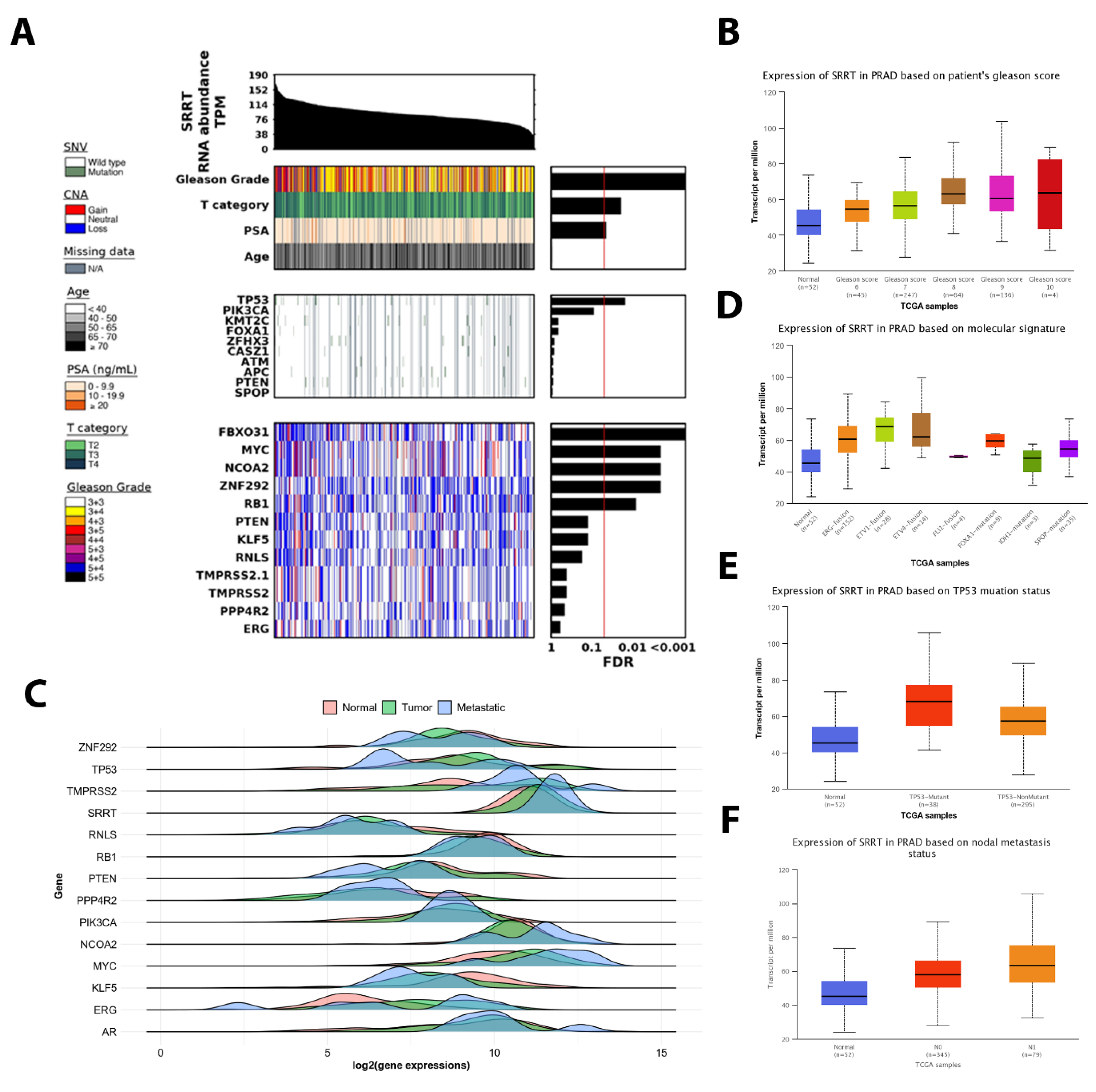
3.4. The Association between SRRT Expression with Age, T Category and PSA in TCGA PRAD
3.5. SRRT Expression Upregulated in Successively Higher Gleason Scores in TCGA PRAD
3.6. SRRT Expression Is Significantly Associated with Metastatic Disease and Oncogenic Driver Mutations in TCGA PRAD
3.7. TP53 Mutant Is Significantly Associated with High SRRT Expression in TCGA PRAD
3.8. The SRRT Association with Nodal Metastasis in TCGA PRAD
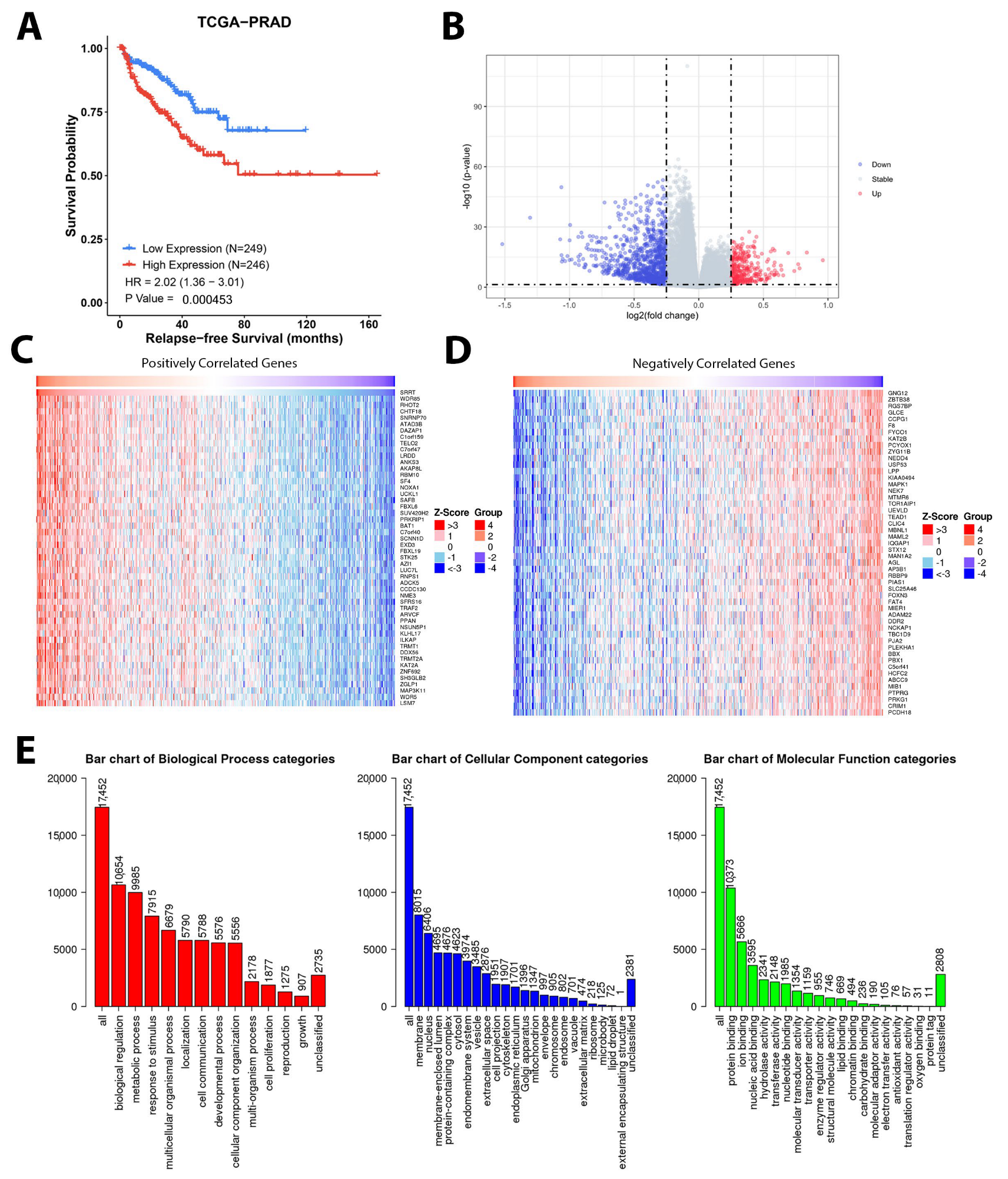
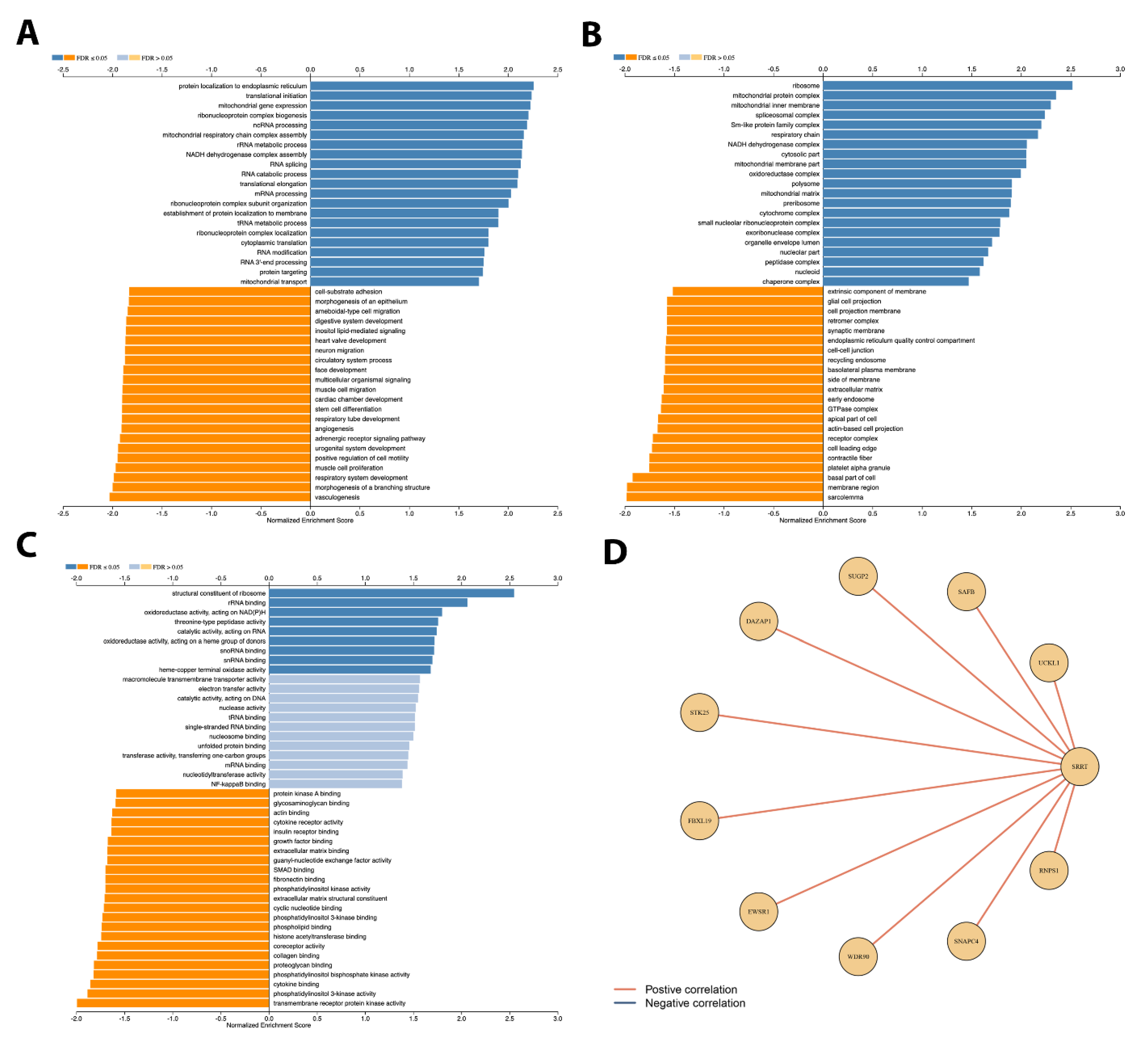
3.9. Gene Set Enrichment Analysis Depicted Potential Role of SRRT in PCa
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviations | Meaning |
| SRRT | ARS2/SRRT (Arsenite-resistance protein 2/Serrate RNA effector molecule) |
| PCa | Prostate cancer |
| TURP | Transurethral resection of the prostate |
| CSS | Cause-specific survival |
| OS | Overall survival |
| GG | Gleason Grade Group |
| Co-IP | Co-Immunoprecipitation |
| ERG | ETS-related gene |
| PTEN | Phosphatase tensin homologue |
| AR | Androgen receptor |
| CRPC | Castration-resistant prostate cancer |
| ADT | Androgen deprivation therapy |
| TMAs | Tissue microarrays |
| IHC | Immunohistochemistry |
| FISH | Fluorescence in situ hybridization |
| FCC | Fraction of Common Contacts |
| RSA | Relative solvent accessibility |
| KM | Kaplan–Meier |
| CI | Confidence intervals |
| HR | Hazard ratios |
| TAD | Transactivation domains |
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Chalmers, Z.R.; Burns, M.C.; Ebot, E.M.; Frampton, G.M.; Ross, J.S.; Hussain, M.H.A.; Abdulkadir, S.A. Early-onset metastatic and clinically advanced prostate cancer is a distinct clinical and molecular entity characterized by increased TMPRSS2-ERG fusions. Prostate Cancer Prostatic Dis. 2021, 24, 558–566. [Google Scholar] [CrossRef]
- Alarcon-Zendejas, A.P.; Scavuzzo, A.; Jimenez-Rios, M.A.; Alvarez-Gomez, R.M.; Montiel-Manriquez, R.; Castro-Hernandez, C.; Jimenez-Davila, M.A.; Perez-Montiel, D.; Gonzalez-Barrios, R.; Jimenez-Trejo, F.; et al. The promising role of new molecular biomarkers in prostate cancer: From coding and non-coding genes to artificial intelligence approaches. Prostate Cancer Prostatic Dis. 2022, 25, 431–443. [Google Scholar] [CrossRef]
- Walker, S.R.; Abdelsalam, R.; Ghosh, S.; Livingstone, J.; Palanisamy, N.; Boutros, P.C.; Yip, S.M.; Lees-Miller, S.P.; Bismar, T.A. Decreased ATM Protein Expression Is Substantiated with PTEN Loss in Defining Aggressive Phenotype of Prostate Cancer Associated with Lethal Disease. Eur. Urol. Open Sci. 2021, 29, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Arenas-Gallo, C.; Owiredu, J.; Weinstein, I.; Lewicki, P.; Basourakos, S.P.; Vince, R., Jr.; Al Hussein Al Awamlh, B.; Schumacher, F.R.; Spratt, D.E.; Barbieri, C.E.; et al. Race and prostate cancer: Genomic landscape. Nat. Rev. Urol. 2022, 19, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Khosh Kish, E.; Choudhry, M.; Gamallat, Y.; Buharideen, S.M.; Dhananjaya, D.; Bismar, T.A. The Expression of Proto-Oncogene ETS-Related Gene (ERG) Plays a Central Role in the Oncogenic Mechanism Involved in the Development and Progression of Prostate Cancer. Int. J. Mol. Sci. 2022, 23, 4772. [Google Scholar] [CrossRef] [PubMed]
- Turnham, D.J.; Bullock, N.; Dass, M.S.; Staffurth, J.N.; Pearson, H.B. The PTEN Conundrum: How to Target PTEN-Deficient Prostate Cancer. Cells 2020, 9, 2342. [Google Scholar] [CrossRef]
- Jamaspishvili, T.; Berman, D.M.; Ross, A.E.; Scher, H.I.; De Marzo, A.M.; Squire, J.A.; Lotan, T.L. Clinical implications of PTEN loss in prostate cancer. Nat. Rev. Urol. 2018, 15, 222–234. [Google Scholar] [CrossRef]
- Fragkoulis, C.; Glykas, I.; Dellis, A.; Ntoumas, K.; Papatsoris, A. Clinical impact of combined PTEN and ERG rearrangements in localized prostate cancer. Arch. Ital. Urol. Androl. 2021, 93, 84–85. [Google Scholar] [CrossRef]
- Jillson, L.K.; Yette, G.A.; Laajala, T.D.; Tilley, W.D.; Costello, J.C.; Cramer, S.D. Androgen Receptor Signaling in Prostate Cancer Genomic Subtypes. Cancers 2021, 13, 3272. [Google Scholar] [CrossRef]
- Rossman, T.G.; Wang, Z. Expression cloning for arsenite-resistance resulted in isolation of tumor-suppressor fau cDNA: Possible involvement of the ubiquitin system in arsenic carcinogenesis. Carcinogenesis 1999, 20, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Andreu-Agullo, C.; Maurin, T.; Thompson, C.B.; Lai, E.C. Ars2 maintains neural stem-cell identity through direct transcriptional activation of Sox2. Nature 2011, 481, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Kainov, Y.A.; Makeyev, E.V. A transcriptome-wide antitermination mechanism sustaining identity of embryonic stem cells. Nat. Commun. 2020, 11, 361. [Google Scholar] [CrossRef]
- Ke, X.X.; Pang, Y.; Chen, K.; Zhang, D.; Wang, F.; Zhu, S.; Mao, J.; Hu, X.; Zhang, G.; Cui, H. Knockdown of arsenic resistance protein 2 inhibits human glioblastoma cell proliferation through the MAPK/ERK pathway. Oncol. Rep. 2018, 40, 3313–3322. [Google Scholar] [CrossRef] [PubMed]
- Gruber, J.J.; Zatechka, D.S.; Sabin, L.R.; Yong, J.; Lum, J.J.; Kong, M.; Zong, W.X.; Zhang, Z.; Lau, C.K.; Rawlings, J.; et al. Ars2 links the nuclear cap-binding complex to RNA interference and cell proliferation. Cell 2009, 138, 328–339. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, X.; Li, Y.; Zhang, H.; Fu, R.; Liu, Y.; Hu, J.; Deng, Q.; Luo, Q.; Zhang, D.; et al. Ars2 promotes cell proliferation and tumorigenicity in glioblastoma through regulating miR-6798-3p. Sci. Rep. 2018, 8, 15602. [Google Scholar] [CrossRef]
- Hu, X.; Xu, S.; Chen, Y.; Gao, Z.; Li, Y.; Hu, J.; Huang, X.; Zhang, Y.; Jiang, X.; Li, L.; et al. Depletion of Ars2 inhibits cell proliferation and leukemogenesis in acute myeloid leukemia by modulating the miR-6734-3p/p27 axis. Leukemia 2019, 33, 1090–1101. [Google Scholar] [CrossRef]
- Elahi, S.; Egan, S.M.; Holling, G.A.; Kandefer, R.L.; Nemeth, M.J.; Olejniczak, S.H. The RNA binding protein Ars2 supports hematopoiesis at multiple levels. Exp. Hematol. 2018, 64, 45–58.e9. [Google Scholar] [CrossRef]
- Voinnet, O. Fly antiviral RNA silencing and miRNA biogenesis claim ARS2. Cell Host Microbe 2009, 6, 99–101. [Google Scholar] [CrossRef]
- Lykke-Andersen, S.; Rouviere, J.O.; Jensen, T.H. ARS2/SRRT: At the nexus of RNA polymerase II transcription, transcript maturation and quality control. Biochem. Soc. Trans. 2021, 49, 1325–1336. [Google Scholar] [CrossRef]
- He, Q.; Huang, Y.; Cai, L.; Zhang, S.; Zhang, C. Expression and prognostic value of Ars2 in hepatocellular carcinoma. Int. J. Clin. Oncol. 2014, 19, 880–888. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Cai, L.; Shuai, L.; Li, D.; Wang, C.; Liu, Y.; Li, X.; Li, Z.; Wang, S. Ars2 is overexpressed in human cholangiocarcinomas and its depletion increases PTEN and PDCD4 by decreasing microRNA-21. Mol. Carcinog. 2013, 52, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Bismar, T.A.; Yoshimoto, M.; Duan, Q.; Liu, S.; Sircar, K.; Squire, J.A. Interactions and relationships of PTEN, ERG, SPINK1 and AR in castration-resistant prostate cancer. Histopathology 2012, 60, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.C.; Begin, L.R.; Palanisamy, N.; Donnelly, B.; Bismar, T.A. SPINK1 expression in relation to PTEN and ERG in matched primary and lymph node metastatic prostate cancer: Implications for biomarker development. Urol. Oncol. 2016, 34, 235.e1–235.e10. [Google Scholar] [CrossRef]
- Yemelyanova, A.; Vang, R.; Kshirsagar, M.; Lu, D.; Marks, M.A.; Shih Ie, M.; Kurman, R.J. Immunohistochemical staining patterns of p53 can serve as a surrogate marker for TP53 mutations in ovarian carcinoma: An immunohistochemical and nucleotide sequencing analysis. Mod. Pathol. 2011, 24, 1248–1253. [Google Scholar] [CrossRef]
- Guedes, L.B.; Almutairi, F.; Haffner, M.C.; Rajoria, G.; Liu, Z.; Klimek, S.; Zoino, R.; Yousefi, K.; Sharma, R.; De Marzo, A.M.; et al. Analytic, Preanalytic, and Clinical Validation of p53 IHC for Detection of TP53 Missense Mutation in Prostate Cancer. Clin. Cancer Res. 2017, 23, 4693–4703. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research, N. The Molecular Taxonomy of Primary Prostate Cancer. Cell 2015, 163, 1011–1025. [Google Scholar] [CrossRef]
- Ellrott, K.; Bailey, M.H.; Saksena, G.; Covington, K.R.; Kandoth, C.; Stewart, C.; Hess, J.; Ma, S.; Chiotti, K.E.; McLellan, M.; et al. Scalable Open Science Approach for Mutation Calling of Tumor Exomes Using Multiple Genomic Pipelines. Cell Syst. 2018, 6, 271–281.e7. [Google Scholar] [CrossRef]
- Vasaikar, S.V.; Straub, P.; Wang, J.; Zhang, B. LinkedOmics: Analyzing multi-omics data within and across 32 cancer types. Nucleic Acids Res. 2018, 46, D956–D963. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Karthikeyan, S.K.; Korla, P.K.; Patel, H.; Shovon, A.R.; Athar, M.; Netto, G.J.; Qin, Z.S.; Kumar, S.; Manne, U.; et al. UALCAN: An update to the integrated cancer data analysis platform. Neoplasia 2022, 25, 18–27. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.; Varambally, S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef] [PubMed]
- P’ng, C.; Green, J.; Chong, L.C.; Waggott, D.; Prokopec, S.D.; Shamsi, M.; Nguyen, F.; Mak, D.Y.F.; Lam, F.; Albuquerque, M.A.; et al. BPG: Seamless, automated and interactive visualization of scientific data. BMC Bioinform. 2019, 20, 42. [Google Scholar] [CrossRef]
- Cantley, L.C.; Neel, B.G. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc. Natl. Acad. Sci. USA 1999, 96, 4240–4245. [Google Scholar] [CrossRef] [PubMed]
- Lahdensuo, K.; Erickson, A.; Saarinen, I.; Seikkula, H.; Lundin, J.; Lundin, M.; Nordling, S.; Butzow, A.; Vasarainen, H.; Bostrom, P.J.; et al. Loss of PTEN expression in ERG-negative prostate cancer predicts secondary therapies and leads to shorter disease-specific survival time after radical prostatectomy. Mod. Pathol. 2016, 29, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Guo, H.; Zhu, Y.; Xia, Y.; Cui, J.; Shi, K.; Fan, Y.; Shi, B.; Chen, S. TP53 alterations of hormone-naive prostate cancer in the Chinese population. Prostate Cancer Prostatic Dis. 2021, 24, 482–491. [Google Scholar] [CrossRef]
- Maxwell, K.N.; Cheng, H.H.; Powers, J.; Gulati, R.; Ledet, E.M.; Morrison, C.; Le, A.; Hausler, R.; Stopfer, J.; Hyman, S.; et al. Inherited TP53 Variants and Risk of Prostate Cancer. Eur. Urol. 2022, 81, 243–250. [Google Scholar] [CrossRef]
- Yamada, Y.; Beltran, H. Clinical and Biological Features of Neuroendocrine Prostate Cancer. Curr. Oncol. Rep. 2021, 23, 15. [Google Scholar] [CrossRef]
- Karlsson, Q.; Brook, M.N.; Dadaev, T.; Wakerell, S.; Saunders, E.J.; Muir, K.; Neal, D.E.; Giles, G.G.; MacInnis, R.J.; Thibodeau, S.N.; et al. Rare Germline Variants in ATM Predispose to Prostate Cancer: A PRACTICAL Consortium Study. Eur. Urol. Oncol. 2021, 4, 570–579. [Google Scholar] [CrossRef]
| Variables | Frequency (%) |
|---|---|
| ISUP Grade Group (Gleason Score) | |
| Grade Group 1 | 144 (29.0) |
| Grade Group 2 (3 + 4) | 48 (9.7) |
| Grade Group 3 (4 + 3) | 33 (6.7) |
| Grade Group 4 (8) | 32 (6.5) |
| Grade Group 5 (9,10) | 205 (41.3) |
| Missing | 34 (6.9) |
| Deceased | |
| Yes | 318 (64.1) |
| No | 163 (32.9) |
| Missing | 15 (3.0) |
| Prostate-Cancer-Specific Survival | |
| Yes | 164 (33.1) |
| No | 317 (63.9) |
| Missing | 15 (3.0) |
| Cancer Subgroup | |
| Incidental | 185 (37.3) |
| Advanced | 186 (37.5) |
| Castrate-resistant | 125 (25.2) |
| SRRT Score (by Cancer Subgroup) | |
| Score 0/1 | 237 (47.8) |
| Incidental | 100 (42.2) |
| Advanced | 86 (36.3) |
| Castrate-resistant | 51 (21.5) |
| Score 2/3 | 259 (52.2) |
| Incidental | 85 (32.8) |
| Advanced | 100 (38.6) |
| Castrate-resistant | 74 (28.6) |
| TP53 and SRRT Combined | |
| TP53 scores 0, 2, 3 and SRRT score 2/3 | 54 (10.9) |
| TP53 scores 0, 2, 3 and SRRT score 0/1 | 30 (6.0) |
| TP53 score 1 and SRRT score 2/3 | 200 (40.3) |
| TP53 score 1 and SRRT score 0/1 | 176 (35.5) |
| Missing | 36 (7.3) |
| PTEN and SRRT Combined | |
| PTEN loss and SRRT score 2/3 | 93 (18.8) |
| PTEN loss and SRRT score 0/1 | 70 (14.1) |
| PTEN-intact and SRRT score 2/3 | 164 (33.1) |
| PTEN-intact and SRRT score 0/1 | 156 (31.5) |
| Missing | 12 (2.4) |
| ERG and SRRT Combined | |
| ERG-positive and SRRT score 2/3 | 77 (15.5) |
| ERG-positive and SRRT score 0/1 | 57 (11.5) |
| ERG-negative and SRRT score 2/3 | 180 (36.3) |
| ERG-negative and SRRT score 0/1 | 170 (34.3) |
| Missing | 12 (2.4) |
| ATM and SRRT Combined | |
| ATM score 0/1 and SRRT score 2/3 | 93 (18.8) |
| ATM score 0/1 and SRRT score 0/1 | 70 (14.1) |
| ATM score 2/3 and SRRT score 2/3 | 74 (14.9) |
| ATM score 2/3 and SRRT score 0/1 | 48 (9.7) |
| Missing | 211 (42.5) |
| Variables | SRRT Low Risk (Scores 0, 1) | SRRT High Risk (Scores 2, 3) | p-Value |
|---|---|---|---|
| Gleason Score | |||
| <=6 | 75 (33.3) | 69 (29.1) | 0.497 |
| 3 + 4 | 18 (8.0) | 15 (6.3) | |
| 4 + 3 | 23 (10.2) | 25 (10.5) | |
| 8 | 18 (8.0) | 14 (5.9) | |
| 9–10 | 91 (40.4) | 114 (48.1) | |
| PTEN Intensity | |||
| Score 0 | 70 (31.0) | 93 (36.2) | 0.227 |
| Score >0 | 156 (69.0) | 164 (63.8) | |
| TP53 | |||
| Score 1 | 176 (85.4) | 200 (78.7) | 0.065 |
| Scores 0, 2, 3 | 30 (14.6) | 54 (21.3) | |
| ATM Score | |||
| Score 2/3 | 48 (40.7) | 74 (44.3) | 0.541 |
| Score 0/1 | 70 (59.3) | 93 (55.7) | |
| ERG Dual Intensity | |||
| Negative | 170 (74.9) | 180 (70.0) | 0.234 |
| Positive | 57 (25.1) | 77 (30.0) |
| Variables | Overall Survival HR (95% CI) | p-Value | Cause-Specific Survival HR (95% CI) | p-Value |
|---|---|---|---|---|
| PTEN (Intact scores 1, 2, or 3) | ||||
| Loss score 0 | 2.35 (1.88–2.95) | <0.0001 | 3.20 (2.35–4.36) | <0.0001 |
| ERG (Negative) | ||||
| Positive | 1.74 (1.37–2.19) | <0.0001 | 1.95 (1.42–2.67) | <0.0001 |
| GS (<=6) | ||||
| GS 3 + 4 | 2.30 (1.41–3.73) | 0.001 | 13.36 (4.11–43.38) | <0.0001 |
| GS 4 + 3 | 1.68 (1.08–2.63) | 0.022 | 5.00 (1.41–17.71) | 0.013 |
| GS 8 | 4.25 (2.63–6.85) | <0.0001 | 31.42 (10.37–95.21) | <0.0001 |
| GS 9, 10 | 4.93 (3.64–6.68) | <0.0001 | 45.05 (16.60–122.29) | <0.0001 |
| SRRT-low-risk scores 0, 1 | ||||
| SRRT-high-risk scores 2, 3 | 1.36 (1.09–1.70) | 0.006 | 1.36 (1.00–1.85) | 0.052 |
| Combination PTEN and SRRT (PTEN scores 1, 2, 3 and SRRT scores 0, 1) | ||||
| PTEN score 0 and SRRT scores 0, 1 | 3.02 (2.20–4.15) | <0.0001 | 3.99 (2.57–6.19) | <0.0001 |
| PTEN score 0 and SRRT scores 2, 3 | 2.49 (1.76–3.49) | <0.0001 | 3.27 (2.06–5.20) | <0.0001 |
| PTEN scores 1, 2, 3 and SRRT scores 2, 3 | 1.38 (1.02–1.85) | 0.036 | 1.35 (0.86–2.11) | 0.186 |
| Combination PTEN and SRRT (PTEN scores 1, 2, 3 and SRRT scores 0, 1) * | ||||
| PTEN score 0 and SRRT scores 0, 1 | 1.82 (1.29–2.55) | 0.001 | 1.71 (1.08–2.71) | 0.022 |
| PTEN score 0 and SRRT scores 2, 3 | 1.52 (1.06–2.18) | 0.023 | 1.46 (0.90–2.36) | 0.122 |
| PTEN scores 1, 2, 3 and SRRT scores 2, 3 | 1.26 (0.93–1.71) | 0.136 | 1.14 (0.72- -1.81) | 0.578 |
| Combination ERG and SRRT (ERG loss and SRRT scores 0, 1) | ||||
| ERG gain and SRRT scores 0, 1 | 2.15 (1.55–2.98) | <0.0001 | 2.35 (1.51–3.66) | <0.0001 |
| ERG gain and SRRT scores 2, 3 | 1.94 (1.36–2.76) | <0.0001 | 2.21 (1.37–3.56) | 0.001 |
| ERG loss and SRRT scores 2, 3 | 1.42 (1.08–1.87) | 0.013 | 1.42 (0.96–2.10) | 0.08 |
| Combination ERG and SRRT (ERG loss and SRRT scores 0, 1) * | ||||
| ERG gain and SRRT scores 0, 1 | 1.35 (0.96–1.91) | 0.085 | 1.13 (0.71–1.82) | 0.607 |
| ERG gain and SRRT scores 2, 3 | 1.22 (0.84–1.77) | 0.061 | 1.12 (0.69–1.83) | 0.647 |
| ERG loss and SRRT scores 0, 1 | 1.31 (0.98–1.73) | 0.065 | 1.26 (0.84–1.89) | 0.27 |
| Combination TP53 and SRRT (TP53 score 1 and SRRT scores 0, 1) | ||||
| TP53 scores 0, 2, 3 and SRRT scores 2, 3 | 4.13 (2.91–5.86) | <0.0001 | 5.79 (3.69–9.06) | <0.0001 |
| TP53 scores 0, 2, 3 and SRRT scores 0, 1 | 2.57 (1.63–4.05) | <0.0001 | 4.34 (2.51–7.51) | <0.0001 |
| TP53 score 1 and SRRT scores 2, 3 | 1.24 (0.95–1.61) | 0.113 | 1.18 (0.79–1.75) | 0.415 |
| Combination TP53 and SRRT (TP53 score 1 and SRRT scores 0, 1) * | ||||
| TP53 scores 0, 2, 3 and SRRT scores 2, 3 | 2.27 (1.55–3.31) | <0.0001 | 2.25 (1.40–3.61) | 0.001 |
| TP53 scores 0, 2, 3 and SRRT scores 0, 1 | 1.34 (0.83–2.17) | 0.227 | 1.58 (0.90–2.79) | 0.112 |
| TP53 score 1 and SRRT scores 2, 3 | 1.12 (0.86–1.47) | 0.405 | 0.97 (0.64–1.46) | 0.874 |
| Combination ATM and SRRT (ATM scores 2, 3 and SRRT scores 0, 1) | ||||
| ATM scores 0, 1 and SRRT scores 2, 3 | 1.70 (1.05–2.74) | 0.03 | 2.02 (1.06–3.86) | 0.033 |
| ATM scores 0, 1 and SRRT scores 0, 1 | 1.57 (0.95–2.58) | 0.076 | 1.64 (0.83–3.26) | 0.155 |
| ATM scores 2, 3 and SRRT scores 2, 3 | 1.14 (0.68–1.91) | 0.627 | 0.75 (0.34–1.65) | 0.478 |
| Combination ATM and SRRT (ATM scores 2, 3 and SRRT scores 0, 1) * | ||||
| ATM scores 0, 1 and SRRT scores 2, 3 | 1.51 (0.91–2.51) | 0.11 | 1.40 (0.70–2.81) | 0.342 |
| ATM scores 0, 1 and SRRT scores 0, 1 | 1.25 (0.74–2.13) | 0.406 | 0.94 (0.45–1.97) | 0.875 |
| ATM scores 2, 3 and SRRT scores 2, 3 | 1.13 (0.65–1.96) | 0.672 | 0.56 (0.34–1.35) | 0.198 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamallat, Y.; Choudhry, M.; Li, Q.; Rokne, J.G.; Alhajj, R.; Abdelsalam, R.; Ghosh, S.; Arbet, J.; Boutros, P.C.; Bismar, T.A. Serrate RNA Effector Molecule (SRRT) Is Associated with Prostate Cancer Progression and Is a Predictor of Poor Prognosis in Lethal Prostate Cancer. Cancers 2023, 15, 2867. https://doi.org/10.3390/cancers15102867
Gamallat Y, Choudhry M, Li Q, Rokne JG, Alhajj R, Abdelsalam R, Ghosh S, Arbet J, Boutros PC, Bismar TA. Serrate RNA Effector Molecule (SRRT) Is Associated with Prostate Cancer Progression and Is a Predictor of Poor Prognosis in Lethal Prostate Cancer. Cancers. 2023; 15(10):2867. https://doi.org/10.3390/cancers15102867
Chicago/Turabian StyleGamallat, Yaser, Muhammad Choudhry, Qiaowang Li, Jon George Rokne, Reda Alhajj, Ramy Abdelsalam, Sunita Ghosh, Jaron Arbet, Paul C. Boutros, and Tarek A. Bismar. 2023. "Serrate RNA Effector Molecule (SRRT) Is Associated with Prostate Cancer Progression and Is a Predictor of Poor Prognosis in Lethal Prostate Cancer" Cancers 15, no. 10: 2867. https://doi.org/10.3390/cancers15102867
APA StyleGamallat, Y., Choudhry, M., Li, Q., Rokne, J. G., Alhajj, R., Abdelsalam, R., Ghosh, S., Arbet, J., Boutros, P. C., & Bismar, T. A. (2023). Serrate RNA Effector Molecule (SRRT) Is Associated with Prostate Cancer Progression and Is a Predictor of Poor Prognosis in Lethal Prostate Cancer. Cancers, 15(10), 2867. https://doi.org/10.3390/cancers15102867







