Benefit of Early Ruxolitinib Initiation Regardless of Fibrosis Grade in Patients with Primary Myelofibrosis: A Post Hoc Analysis of the Single-Arm Phase 3b JUMP Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Endpoints
2.3. Ethics
3. Results
3.1. Baseline Characteristics
3.2. Patient Disposition
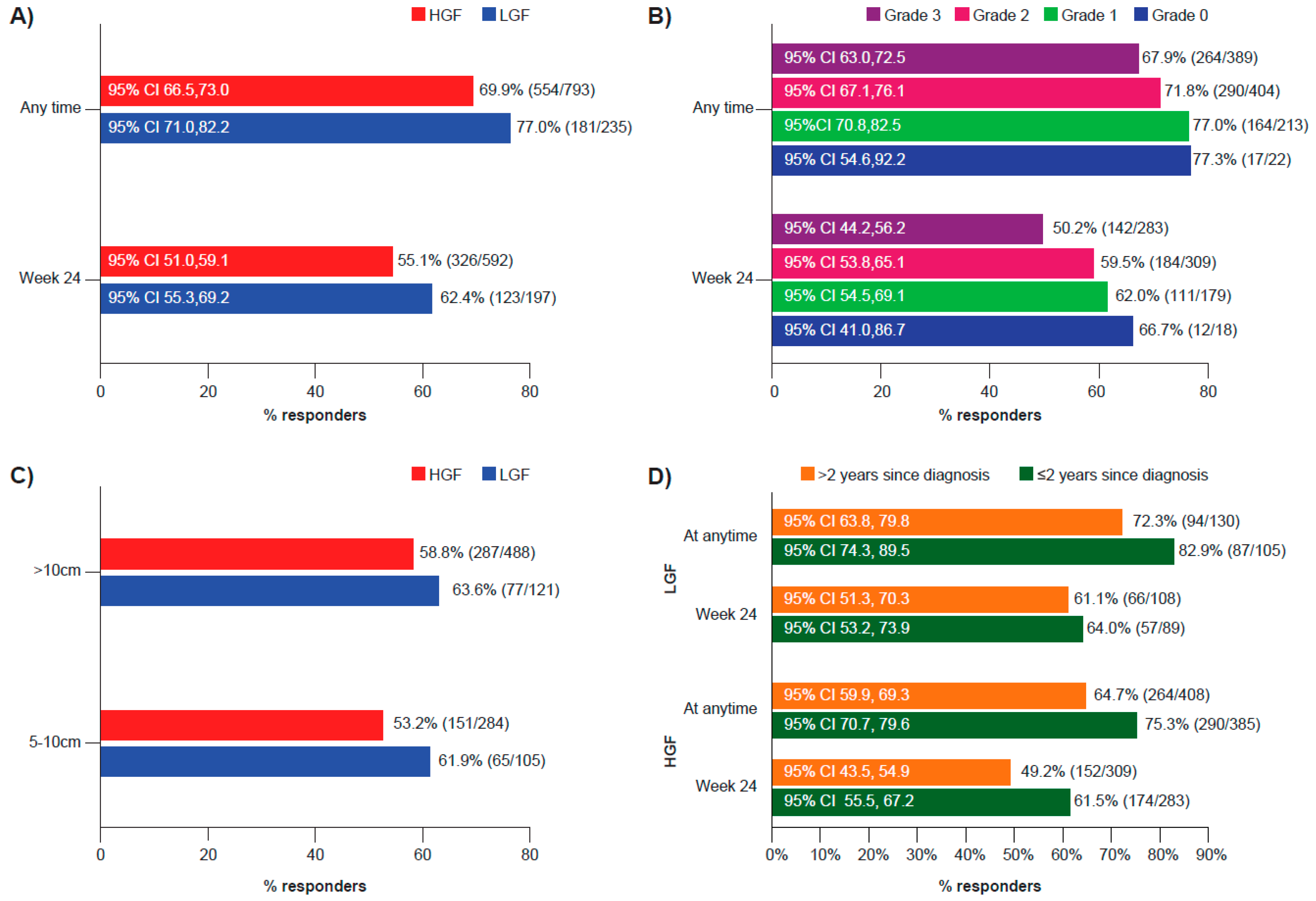
3.3. Spleen Response
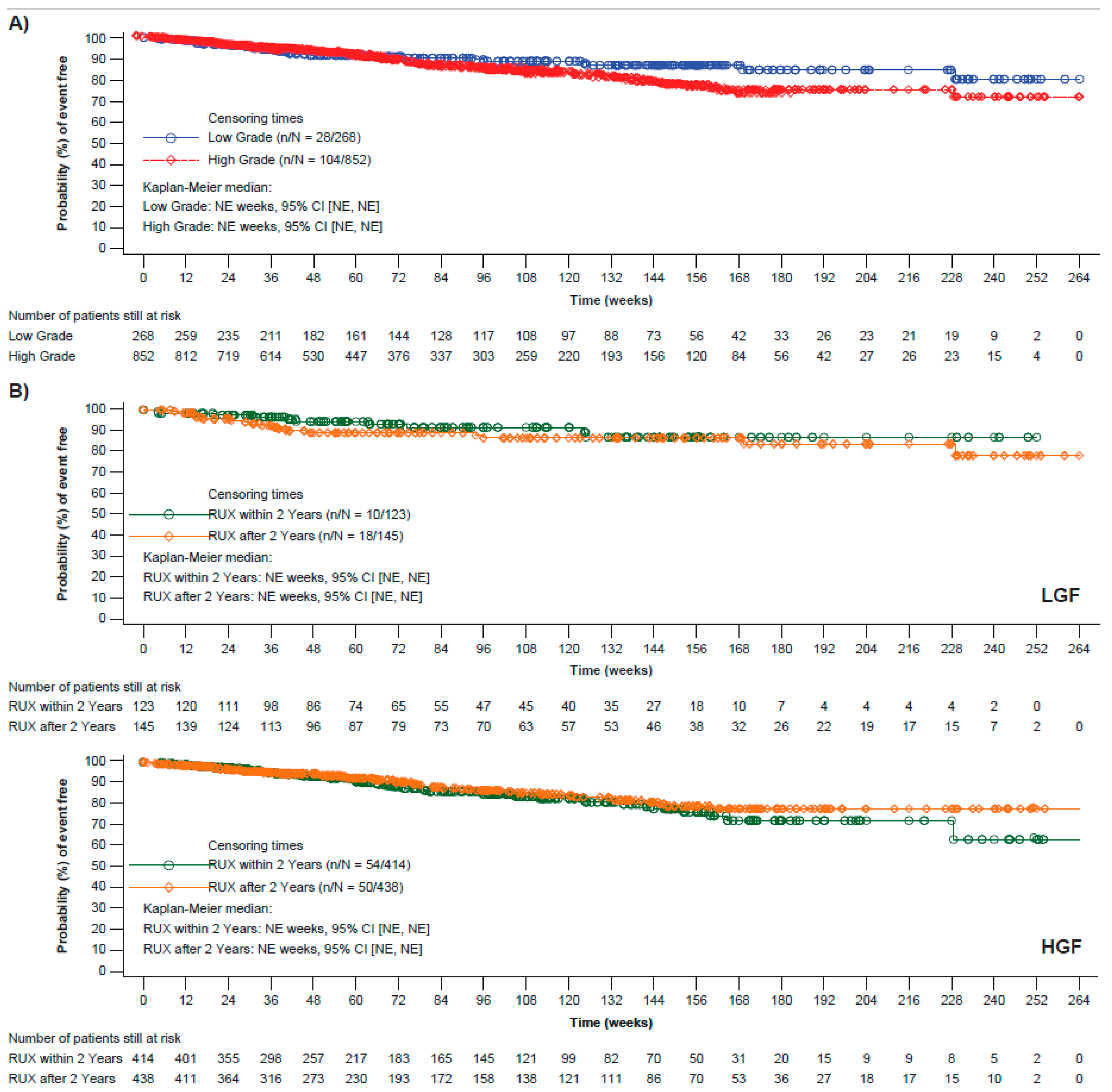
3.4. Progression-Free Survival
3.5. Overall Survival
3.6. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tefferi, A. Primary myelofibrosis: 2021 update on diagnosis, risk-stratification and management. Am. J. Hematol. 2021, 96, 145–162. [Google Scholar] [CrossRef]
- Garmezy, B.; Schaefer, J.K.; Mercer, J.; Talpaz, M. A provider’s guide to primary myelofibrosis: Pathophysiology, diagnosis, and management. Blood Rev. 2021, 45, 100691. [Google Scholar] [CrossRef] [PubMed]
- Passamonti, F.; Mora, B. Myelofibrosis. Blood 2022, 141, 1954–1970. [Google Scholar] [CrossRef]
- Titmarsh, G.J.; Duncombe, A.S.; McMullin, M.F.; O’Rorke, M.; Mesa, R.; De Vocht, F.; Horan, S.; Fritschi, L.; Clarke, M.; Anderson, L.A. How common are myeloproliferative neoplasms? A systematic review and meta-analysis. Am. J. Hematol. 2014, 89, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Lasho, T.L.; Jimma, T.; Finke, C.M.; Gangat, N.; Vaidya, R.; Begna, K.H.; Al-Kali, A.; Ketterling, R.P.; Hanson, C.A.; et al. One thousand patients with primary myelofibrosis: The mayo clinic experience. Mayo Clin. Proc. 2012, 87, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Agarwal, A.; Morrone, K.; Bartenstein, M.; Zhao, Z.J.; Verma, A.; Goel, S. Bone marrow fibrosis in primary myelofibrosis: Pathogenic mechanisms and the role of TGF-β. Stem Cell Investig. 2016, 3, 5. [Google Scholar] [CrossRef]
- Zahr, A.A.; Salama, M.E.; Carreau, N.; Tremblay, D.; Verstovsek, S.; Mesa, R.; Hoffman, R.; Mascarenhas, J. Bone marrow fibrosis in myelofibrosis: Pathogenesis, prognosis and targeted strategies. Haematologica 2016, 101, 660–671. [Google Scholar] [CrossRef]
- Guglielmelli, P.; Lasho, T.L.; Rotunno, G.; Mudireddy, M.; Mannarelli, C.; Nicolosi, M.; Pacilli, A.; Pardanani, A.; Rumi, E.; Rosti, V.; et al. MIPSS70: Mutation-Enhanced International Prognostic Score System for Transplantation-Age Patients with Primary Myelofibrosis. J. Clin. Oncol. 2018, 36, 310–318. [Google Scholar] [CrossRef]
- Abdel-Wahab, O.I.; Levine, R.L. Primary myelofibrosis: Update on definition, pathogenesis, and treatment. Annu. Rev. Med. 2009, 60, 233–245. [Google Scholar] [CrossRef]
- Harrison, C.N.; Vannucchi, A.M.; Kiladjian, J.J.; Al-Ali, H.K.; Gisslinger, H.; Knoops, L.; Cervantes, F.; Jones, M.M.; Sun, K.; McQuitty, M.; et al. Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs. best available therapy for myelofibrosis. Leukemia 2016, 30, 1701–1707. [Google Scholar] [CrossRef] [PubMed]
- Verstovsek, S.; Gotlib, J.; Mesa, R.A.; Vannucchi, A.M.; Kiladjian, J.J.; Cervantes, F.; Harrison, C.N.; Paquette, R.; Sun, W.; Naim, A.; et al. Long-term survival in patients treated with ruxolitinib for myelofibrosis: COMFORT-I and -II pooled analyses. J. Hematol. Oncol. 2017, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.; Kiladjian, J.J.; Al-Ali, H.K.; Gisslinger, H.; Waltzman, R.; Stalbovskaya, V.; McQuitty, M.; Hunter, D.S.; Levy, R.; Knoops, L.; et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N. Engl. J. Med. 2012, 366, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Mesa, R.A.; Gotlib, J.; Gupta, V.; Catalano, J.V.; Deininger, M.W.; Shields, A.L.; Miller, C.B.; Silver, R.T.; Talpaz, M.; Winton, E.F.; et al. Effect of ruxolitinib therapy on myelofibrosis-related symptoms and other patient-reported outcomes in COMFORT-I: A randomized, double-blind, placebo-controlled trial. J. Clin. Oncol. 2013, 31, 1285–1292. [Google Scholar] [CrossRef]
- Al-Ali, H.K.; Griesshammer, M.; Foltz, L.; Palumbo, G.A.; Martino, B.; Palandri, F.; Liberati, A.M.; le Coutre, P.; García-Hernández, C.; Zaritskey, A.; et al. Primary analysis of JUMP, a phase 3b, expanded-access study evaluating the safety and efficacy of ruxolitinib in patients with myelofibrosis, including those with low platelet counts. Br. J. Haematol. 2020, 189, 888–903. [Google Scholar] [CrossRef]
- Barosi, G.; Mesa, R.A.; Thiele, J.; Cervantes, F.; Campbell, P.J.; Verstovsek, S.; Dupriez, B.; Levine, R.L.; Passamonti, F.; Gotlib, J.; et al. Proposed criteria for the diagnosis of post-polycythemia vera and post-essential thrombocythemia myelofibrosis: A consensus statement from the International Working Group for Myelofibrosis Research and Treatment. Leukemia 2008, 22, 437–438. [Google Scholar] [CrossRef]
- Vardiman, J.W.; Thiele, J.; Arber, D.A.; Brunning, R.D.; Borowitz, M.J.; Porwit, A.; Harris, N.L.; Le Beau, M.M.; Hellström-Lindberg, E.; Tefferi, A.; et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: Rationale and important changes. Blood 2009, 114, 937–951. [Google Scholar] [CrossRef]
- Tefferi, A.; Cervantes, F.; Mesa, R.; Passamonti, F.; Verstovsek, S.; Vannucchi, A.M.; Gotlib, J.; Dupriez, B.; Pardanani, A.; Harrison, C.; et al. Revised response criteria for myelofibrosis: International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) and European LeukemiaNet (ELN) consensus report. Blood 2013, 122, 1395–1398. [Google Scholar] [CrossRef]
- Tefferi, A.; Barosi, G.; Mesa, R.A.; Cervantes, F.; Deeg, H.J.; Reilly, J.T.; Verstovsek, S.; Dupriez, B.; Silver, R.T.; Odenike, O.; et al. International Working Group (IWG) consensus criteria for treatment response in myelofibrosis with myeloid metaplasia, for the IWG for Myelofibrosis Research and Treatment (IWG-MRT). Blood 2006, 108, 1497–1503. [Google Scholar] [CrossRef]
- Cervantes, F.; Dupriez, B.; Pereira, A.; Passamonti, F.; Reilly, J.T.; Morra, E.; Vannucchi, A.M.; Mesa, R.A.; Demory, J.L.; Barosi, G.; et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood 2009, 113, 2895–2901. [Google Scholar] [CrossRef]
- Gangat, N.; Caramazza, D.; Vaidya, R.; George, G.; Begna, K.; Schwager, S.; Van Dyke, D.; Hanson, C.; Wu, W.; Pardanani, A.; et al. DIPSS plus: A refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J. Clin. Oncol. 2011, 29, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, J.; Chatain, N.; Sofias, A.M.; Lammers, T.; Koschmieder, S. Progression of Myeloproliferative Neoplasms (MPN): Diagnostic and Therapeutic Perspectives. Cells 2021, 10, 3551. [Google Scholar] [CrossRef] [PubMed]
- Mesa, R.; Miller, C.B.; Thyne, M.; Mangan, J.; Goldberger, S.; Fazal, S.; Ma, X.; Wilson, W.; Paranagama, D.C.; Dubinski, D.G.; et al. Myeloproliferative neoplasms (MPNs) have a significant impact on patients’ overall health and productivity: The MPN Landmark survey. BMC Cancer 2016, 16, 167. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.N.; Koschmieder, S.; Foltz, L.; Guglielmelli, P.; Flindt, T.; Koehler, M.; Mathias, J.; Komatsu, N.; Boothroyd, R.N.; Spierer, A.; et al. The impact of myeloproliferative neoplasms (MPNs) on patient quality of life and productivity: Results from the international MPN Landmark survey. Ann. Hematol. 2017, 96, 1653–1665. [Google Scholar] [CrossRef] [PubMed]
- Barosi, G.; Rosti, V.; Bonetti, E.; Campanelli, R.; Carolei, A.; Catarsi, P.; Isgrò, A.M.; Lupo, L.; Massa, M.; Poletto, V.; et al. Evidence that prefibrotic myelofibrosis is aligned along a clinical and biological continuum featuring primary myelofibrosis. PLoS ONE 2012, 7, e35631. [Google Scholar] [CrossRef]
- Kvasnicka, H.M.; Thiele, J.; Bueso-Ramos, C.E.; Sun, W.; Cortes, J.; Kantarjian, H.M.; Verstovsek, S. Long-term effects of ruxolitinib versus best available therapy on bone marrow fibrosis in patients with myelofibrosis. J. Hematol. Oncol. 2018, 11, 42. [Google Scholar] [CrossRef]
- Gupta, V.; Griesshammer, M.; Martino, B.; Foltz, L.; Tavares, R.; Al-Ali, H.K.; Giraldo, P.; Guglielmelli, P.; Lomaia, E.; Bouard, C.; et al. Analysis of predictors of response to ruxolitinib in patients with myelofibrosis in the phase 3b expanded-access JUMP study. Leuk. Lymphoma 2021, 62, 918–926. [Google Scholar] [CrossRef]
- Vannucchi, A.M.; Kantarjian, H.M.; Kiladjian, J.J.; Gotlib, J.; Cervantes, F.; Mesa, R.A.; Sarlis, N.J.; Peng, W.; Sandor, V.; Gopalakrishna, P.; et al. A pooled analysis of overall survival in COMFORT-I and COMFORT-II, 2 randomized phase III trials of ruxolitinib for the treatment of myelofibrosis. Haematologica 2015, 100, 1139–1145. [Google Scholar] [CrossRef]
- Verstovsek, S.; Kiladjian, J.J.; Vannucchi, A.M.; Mesa, R.A.; Scherber, R.; Hamer-Maansson, J.E.; Harrison, C.N. Does Early Intervention in Myelofibrosis Impact Outcomes? a Pooled Analysis of the Comfort I and II Studies. Blood 2021, 138, 1505. [Google Scholar] [CrossRef]
- Palandri, F.; Palumbo, G.A.; Bonifacio, M.; Tiribelli, M.; Benevolo, G.; Martino, B.; Abruzzese, E.; D’Adda, M.; Polverelli, N.; Bergamaschi, M.; et al. Baseline factors associated with response to ruxolitinib: An independent study on 408 patients with myelofibrosis. Oncotarget 2017, 8, 79073–79086. [Google Scholar] [CrossRef]
- Passamonti, F.; Gupta, V.; Martino, B.; Foltz, L.; Zaritskey, A.; Al-Ali, H.K.; Tavares, R.; Maffioli, M.; Raanani, P.; Giraldo, P.; et al. Comparing the safety and efficacy of ruxolitinib in patients with Dynamic International Prognostic Scoring System low-, intermediate-1-, intermediate-2-, and high-risk myelofibrosis in JUMP, a Phase 3b, expanded-access study. Hematol. Oncol. 2021, 39, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Palandri, F.; Palumbo, G.A.; Abruzzese, E.; Iurlo, A.; Polverelli, N.; Elli, E.; Bonifacio, M.; Bergamaschi, M.; Martino, B.; Tiribelli, M.; et al. Impact of 2016 WHO diagnosis of early and overt primary myelofibrosis on presentation and outcome of 232 patients treated with ruxolitinib. Hematol. Oncol. 2019, 37, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.A. Fedratinib: First Approval. Drugs 2019, 79, 1719–1725. [Google Scholar] [CrossRef]
- Bose, P.; Verstovsek, S. JAK Inhibition for the Treatment of Myelofibrosis: Limitations and Future Perspectives. Hemasphere 2020, 4, e424. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, J. Pacritinib for the treatment of patients with myelofibrosis and thrombocytopenia. Expert Rev. Hematol. 2022, 15, 671–684. [Google Scholar] [CrossRef]
- Chifotides, H.T.; Bose, P.; Verstovsek, S. Momelotinib: An emerging treatment for myelofibrosis patients with anemia. J. Hematol. Oncol. 2022, 15, 7. [Google Scholar] [CrossRef]
- Moyo, T.K.; Kishtagari, A.; Villaume, M.; McMahon, B.; Mohan, S.R.; Stopczynski, T.; Chen, S.C.; Fan, R.; Huo, Y.; Moon, H.; et al. PI3K Inhibition Restores and Amplifies Response to Ruxolitinib in patients with Myelofibrosis. Clin. Cancer Res. 2023, CCR-22. [Google Scholar] [CrossRef]
- Yacoub, A.; Borate, U.; Rampal, R.; Ali, H.; Wang, R.; Gerds, A.; Hobbs, G.; Kremyanskaya, M.; Winton, E.; O’Connell, C.; et al. Add-on parsaclisib (a PI3K-delta inhibitor) in patients with myelofibrosis and suboptimal response to ruxolitinib: Interim analysis from a phase 2 study. HemaSphere 2021, 5, 512. [Google Scholar]
- Mascarenhas, J.; Kremyanskaya, M.; Patriarca, A.; Palandri, F.; Devos, T.; Passamonti, F.; Rampal, R.K.; Mead, A.J.; Hobbs, G.; Scandura, J.M.; et al. MANIFEST: Pelabresib in Combination with Ruxolitinib for Janus Kinase Inhibitor Treatment-Naïve Myelofibrosis. J. Clin. Oncol. 2023, JCO-22. [Google Scholar] [CrossRef]
- Pemmaraju, N.; Garcia, J.S.; Potluri, J.; Harb, J.G.; Sun, Y.; Jung, P.; Qin, Q.Q.; Tantravahi, S.K.; Verstovsek, S.; Harrison, C. Addition of navitoclax to ongoing ruxolitinib treatment in patients with myelofibrosis (REFINE): A post-hoc analysis of molecular biomarkers in a phase 2 study. Lancet Haematol. 2022, 9, e434–e444. [Google Scholar] [CrossRef]
- El Chaer, F.; McCloskey, J.; Rein, L.A.M.; Brown, R.A.; Green, S.D.; Pu, J.J.; Shirane, S.; Shimoda, K.; Ichii, M.; Yuda, J.; et al. Preliminary Data from the Phase I/II Study of TP-3654, a Selective Oral PIM1 Kinase Inhibitor, in Patients with Myelofibrosis Previously Treated with or Ineligible for JAK Inhibitor Therapy. Blood 2022, 140, 594–595. [Google Scholar] [CrossRef]
| LGF N = 268 | HGF N = 852 | |
|---|---|---|
| Median age (range), years | 66.0 (26.0–88.0) | 67.0 (18.0–89.0) |
| ≥65 years, n (%) | 143 (53.4) | 536 (62.9) |
| Male, n (%) | 164 (61.2) | 503 (59.0) |
| Mean time since initial diagnosis (SD), months | 49.1 (54.9) | 47.9 (58.4) |
| Mean time since last biopsy (SD), months | 35.5 (45.7) | 26.1 (36.0) |
| Dynamic IPSS risk group at study entry, n (%) | ||
| Low risk | 14 (5.2) | 14 (1.6) |
| Intermediate risk 1 | 112 (41.8) | 290 (34.0) |
| Intermediate risk 2 | 71 (26.5) | 337 (39.6) |
| High risk | 15 (5.6) | 87 (10.2) |
| Missing | 56 (20.9) | 124 (14.6) |
| Hemoglobin level < 100 g/L, n (%) | 87 (32.5) | 395 (46.4) |
| Platelets < 100 × 109/L, n (%) | 11 (4.1) | 73 (8.6) |
| Peripheral blasts ≥ 1%, n (%) | 65 (24.3) | 279 (32.7) |
| Palpable spleen, n (%) | 239 (89.2) | 807 (94.7) |
| Mean palpable spleen length below costal margin (SD), cm | 11.3 (7.1) | 12.9 (7.2) |
| Spleen length, n (%) | ||
| <5 cm | 34 (12.7) | 65 (7.6) |
| 5–10 cm | 105 (39.2) | 284 (33.3) |
| >10 cm | 121 (45.1) | 488 (57.3) |
| Missing | 8 (3.0) | 15 (1.8) |
| Prior transfusions, n (%) | 60 (22.4) | 261 (30.6) |
| Mean FACIT–Fatigue total score (SD) a | 34.5 (10.8) | 32.8 (12.0) |
| Mean FACT–Lymphoma total score (SD) a | 115.4 (22.0) | 114.9 (23.9) |
| LGF | HGF | |||
|---|---|---|---|---|
| n (%) | n (%) | |||
| N = 268 | N = 852 | |||
| All grades | Grade 3/4 | All grades | Grade 3/4 | |
| Total | 205 (76.5) | 105 (39.2) | 632 (74.2) | 410 (48.1) |
| Anemia | 171 (63.8) | 90 (33.6) | 489 (57.4) | 334 (39.2) |
| Thrombocytopenia | 112 (41.8) | 36 (13.4) | 397 (46.6) | 164 (19.2) |
| Infection | 1 (0.4) | 1 (0.4) | 4 (0.5) | 1 (0.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palandri, F.; Al-Ali, H.K.; Guglielmelli, P.; Zuurman, M.W.; Sarkar, R.; Gupta, V. Benefit of Early Ruxolitinib Initiation Regardless of Fibrosis Grade in Patients with Primary Myelofibrosis: A Post Hoc Analysis of the Single-Arm Phase 3b JUMP Study. Cancers 2023, 15, 2859. https://doi.org/10.3390/cancers15102859
Palandri F, Al-Ali HK, Guglielmelli P, Zuurman MW, Sarkar R, Gupta V. Benefit of Early Ruxolitinib Initiation Regardless of Fibrosis Grade in Patients with Primary Myelofibrosis: A Post Hoc Analysis of the Single-Arm Phase 3b JUMP Study. Cancers. 2023; 15(10):2859. https://doi.org/10.3390/cancers15102859
Chicago/Turabian StylePalandri, Francesca, Haifa Kathrin Al-Ali, Paola Guglielmelli, Mike W. Zuurman, Rajendra Sarkar, and Vikas Gupta. 2023. "Benefit of Early Ruxolitinib Initiation Regardless of Fibrosis Grade in Patients with Primary Myelofibrosis: A Post Hoc Analysis of the Single-Arm Phase 3b JUMP Study" Cancers 15, no. 10: 2859. https://doi.org/10.3390/cancers15102859
APA StylePalandri, F., Al-Ali, H. K., Guglielmelli, P., Zuurman, M. W., Sarkar, R., & Gupta, V. (2023). Benefit of Early Ruxolitinib Initiation Regardless of Fibrosis Grade in Patients with Primary Myelofibrosis: A Post Hoc Analysis of the Single-Arm Phase 3b JUMP Study. Cancers, 15(10), 2859. https://doi.org/10.3390/cancers15102859









