Carbon-Ion Radiotherapy Combined with Concurrent Chemotherapy for Locally Advanced Pancreatic Cancer: A Retrospective Case Series Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Eligibility
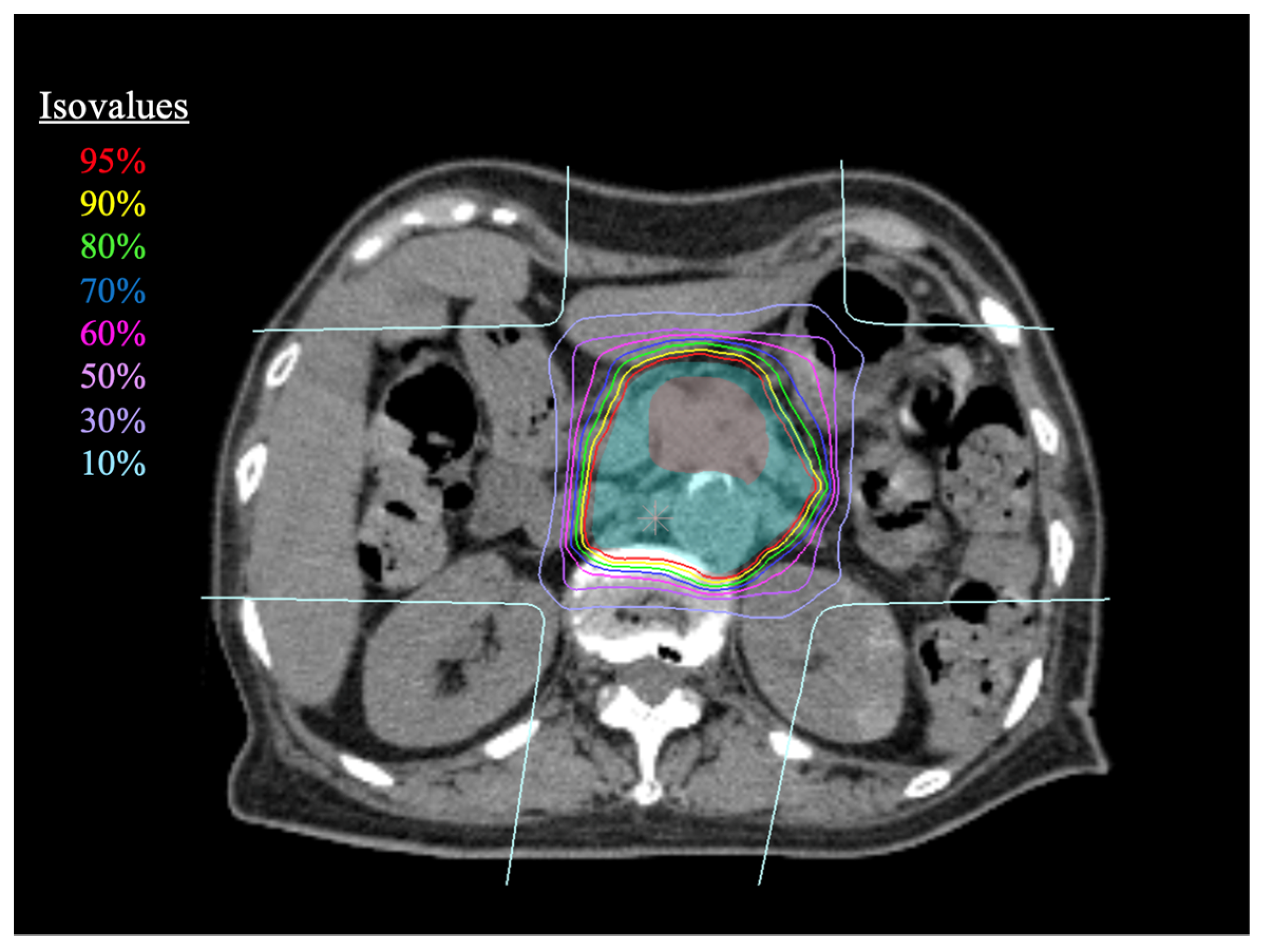
2.2. Carbon-Ion Radiotherapy
2.3. Treatment Planning
2.4. Chemotherapy
2.5. Evaluation and Follow-Up
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
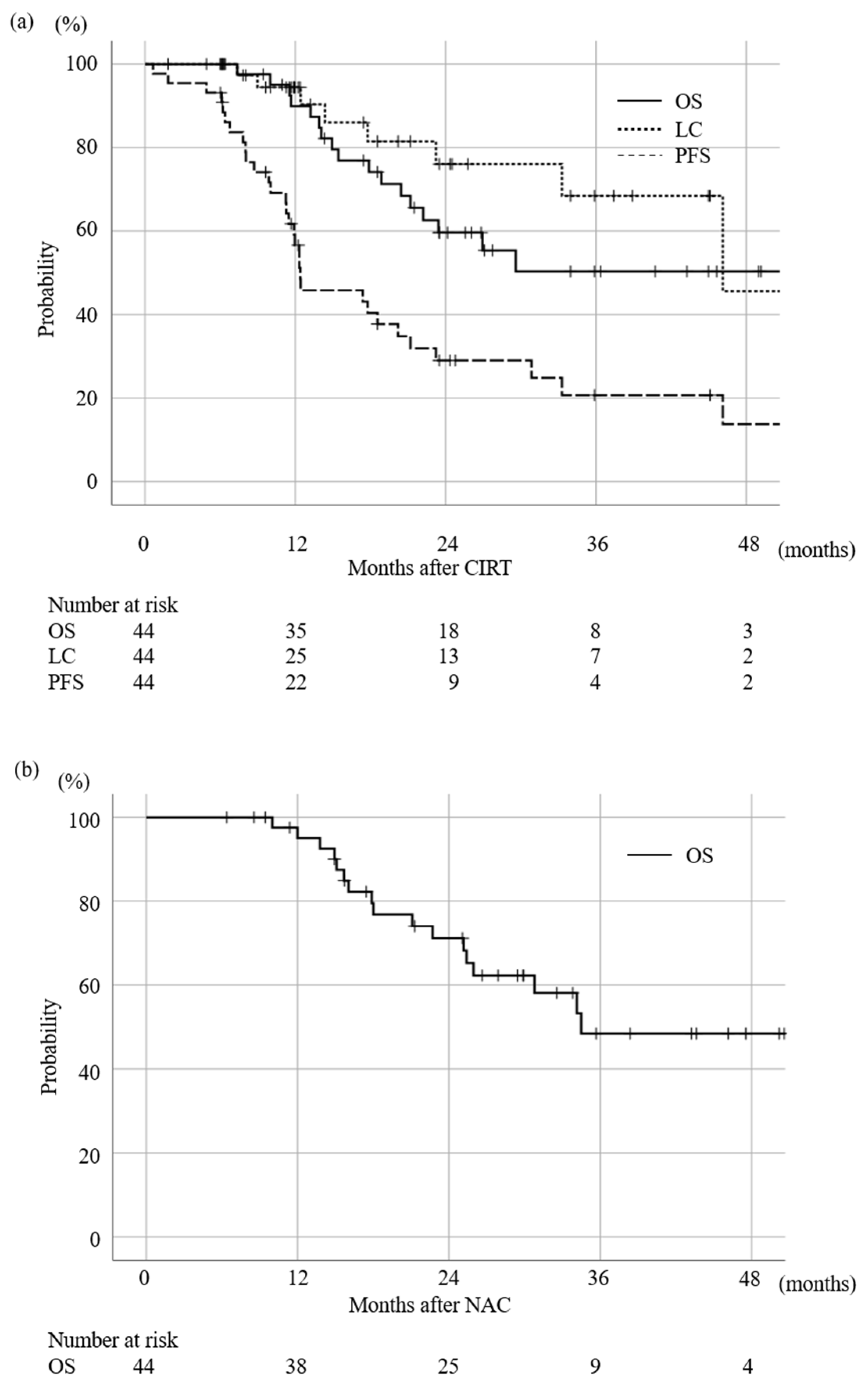
3.2. Survival and LC Rate
3.3. Toxicities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Sun, X.-J.; Jiang, T.-H.; Mao, A.-W. Combined radiochemotherapy in patients with locally advanced pancreatic cancer: A meta-analysis. World J. Gastroenterol. 2013, 19, 7461–7471. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.S.; Kim, T.H.; Woo, S.M.; Lee, W.J.; Lee, J.H.; Youn, S.H.; Han, S.S.; Park, S.J.; Kim, D.Y. Effectiveness and feasibility of concurrent chemoradiotherapy using simultaneous integrated boost-intensity modulated radiotherapy with and without induction chemotherapy for locally advanced pancreatic cancer. Radiat. Oncol. J. 2018, 36, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Nakamura, A.; Ashida, R.; Sakanaka, K.; Itasaka, S.; Shibuya, K.; Matsumoto, S.; Kanai, M.; Isoda, H.; Masui, T.; et al. Clinical evaluation of intensity-modulated radiotherapy for locally advanced pancreatic cancer. Radiat. Oncol. 2018, 13, 118. [Google Scholar] [CrossRef] [PubMed]
- Hammel, P.; Huguet, F.F.; Van Laethem, J.-L.; Goldstein, D.D.; Glimelius, B.; Artru, P.P.; Borbath, I.; Bouché, O.; Shannon, J.J.; André, T.; et al. Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients with Locally Advanced Pancreatic Cancer Controlled after 4 Months of Gemcitabine with or without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA 2016, 315, 1844–1853. [Google Scholar] [CrossRef]
- Kanai, T.; Endo, M.; Minohara, S.; Miyahara, N.; Koyama-Ito, H.; Tomura, H.; Matsufuji, N.; Futami, Y.; Fukumura, A.; Hiraoka, T.; et al. Biophysical characteristics of HIMAC clinical irradiation system for heavy-ion radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 1999, 44, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Suzuki, Y.; Ohno, T.; Kato, S.; Suzuki, M.; Morita, S.; Sato, S.; Oka, K.; Tsujii, H. Carbon Beam Therapy Overcomes the Radiation Resistance of Uterine Cervical Cancer Originating from Hypoxia. Clin. Cancer Res. 2006, 12, 2185–2190. [Google Scholar] [CrossRef]
- Cui, X.; Oonishi, K.; Tsujii, H.; Yasuda, T.; Matsumoto, Y.; Furusawa, Y.; Akashi, M.; Kamada, T.; Okayasu, R. Effects of Carbon Ion Beam on Putative Colon Cancer Stem Cells and Its Comparison with X-rays. Cancer Res. 2011, 71, 3676–3687. [Google Scholar] [CrossRef]
- Shinoto, M.; Yamada, S.; Terashima, K.; Yasuda, S.; Shioyama, Y.; Honda, H.; Kamada, T.; Tsujii, H.; Saisho, H.; Asano, T.; et al. Carbon Ion Radiation Therapy with Concurrent Gemcitabine for Patients with Locally Advanced Pancreatic Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 498–504. [Google Scholar] [CrossRef]
- Kawashiro, S.; Yamada, S.; Okamoto, M.; Ohno, T.; Nakano, T.; Shinoto, M.; Shioyama, Y.; Nemoto, K.; Isozaki, Y.; Tsuji, H.; et al. Multi-institutional Study of Carbon-ion Radiotherapy for Locally Advanced Pancreatic Cancer: Japan Carbon-ion Radiation Oncology Study Group (J-CROS) Study 1403 Pancreas. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 1212–1221. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; De La Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.; El-Maraghi, R.H.; Hammel, P.; Heinemann, V.; Kunzmann, V.; Sastre, J.; Scheithauer, W.; Siena, S.; Tabernero, J.; Teixeira, L.; et al. Nab-Paclitaxel Plus Gemcitabine for Metastatic Pancreatic Cancer: Long-Term Survival from a Phase III Trial. J. Natl. Cancer Inst. 2015, 107, dju413. [Google Scholar] [CrossRef] [PubMed]
- Fukahori, M.; Miwa, K.; Murotani, K.; Naito, Y.; Ushijima, T.; Sakaue, T.; Tanaka, T.; Nagasu, S.; Suga, H.; Kakuma, T.; et al. A phase II study of gemcitabine plus nab-paclitaxel as first-line therapy for locally advanced pancreatic cancer. Medicine 2021, 100, e26052. [Google Scholar] [CrossRef]
- Matsumoto, I.; Kamei, K.; Omae, K.; Suzuki, S.; Matsuoka, H.; Mizuno, N.; Ozaka, M.; Ueno, H.; Kobayashi, S.; Uesugi, K.; et al. FOLFIRINOX for locally advanced pancreatic cancer: Results and prognostic factors of subset analysis from a nation-wide multicenter observational study in Japan. Pancreatology 2019, 19, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Brada, L.J.H.; Walma, M.S.; Daamen, L.A.; van Roessel, S.; Dam, R.M.; Hingh, I.H.; Liem, M.L.S.; Meijer, V.E.; Patijn, G.A.; Festen, S.; et al. Predicting overall survival and resection in patients with locally advanced pancreatic cancer treated with FOLFIRINOX: Development and internal validation of two nomograms. J. Surg. Oncol. 2021, 124, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Suker, M.; Beumer, B.R.; Sadot, E.; Marthey, L.; Faris, J.E.; Mellon, E.A.; El-Rayes, B.F.; Wang-Gillam, A.; Lacy, J.; Hosein, P.J.; et al. FOLFIRINOX for locally advanced pancreatic cancer: A systematic review and patient-level meta-analysis. Lancet Oncol. 2016, 17, 801–810. [Google Scholar] [CrossRef]
- Inaniwa, T.; Kanematsu, N.; Matsufuji, N.; Kanai, T.; Shirai, T.; Noda, K.; Tsuji, H.; Kamada, T.; Tsujii, H. Reformulation of a clinical-dose system for carbon-ion radiotherapy treatment planning at the National Institute of Radiological Sciences, Japan. Phys. Med. Biol. 2015, 60, 3271–3286. [Google Scholar] [CrossRef]
- Maisey, N.R.; Norman, A.R.; Hill, A.; Massey, A.; Oates, J.; Cunningham, D. CA19-9 as a prognostic factor in inoperable pancreatic cancer: The implication for clinical trials. Br. J. Cancer 2005, 93, 740–743. [Google Scholar] [CrossRef]
- Zhao, B.; Zhao, B.; Chen, F. Diagnostic value of serum carbohydrate antigen 19-9 in pancreatic cancer: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2022, 34, 891–904. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, P.; Wang, Z.; Huang, X.; Wu, C.; Li, M.; Wang, L.; Tian, B. Adjusting CA19-9 values with clinical stage and bilirubin to better predict survival of resectable pancreatic cancer patients: 5-year-follow-up of a single center. Front. Oncol. 2022, 12, 3939. [Google Scholar] [CrossRef]
- Hartlapp, I.; Valta-Seufzer, D.; Siveke, J.; Algül, H.; Goekkurt, E.; Siegler, G.; Martens, U.; Waldschmidt, D.; Pelzer, U.; Fuchs, M.; et al. Prognostic and predictive value of CA 19-9 in locally advanced pancreatic cancer treated with multiagent induction chemotherapy: Results from a prospective, multicenter phase II trial (NEOLAP-AIO-PAK-0113). ESMO Open 2022, 7, 100552. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Shiba, S.; Okazaki, S.; Miyasaka, Y.; Shibuya, K.; Kiyohara, H.; Ohno, T. Feasibility and Safety of Repeated Carbon Ion Radiotherapy for Locally Advanced Unresectable Pancreatic Cancer. Cancers 2021, 13, 665. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, Y.; Yamada, S.; Isozaki, Y.; Takiyama, H.; Shinoto, M.; Kawashiro, S.; Bhattacharyya, T.; Nemoto, K.; Tsuji, H. Efficacy and feasibility of re-irradiation using carbon ions for pancreatic cancer that recurs after carbon-ion radiotherapy. Clin. Transl. Radiat. Oncol. 2021, 26, 24–29. [Google Scholar] [CrossRef]
- Kawashiro, S.; Yamada, S.; Isozaki, Y.; Nemoto, K.; Tsuji, H.; Kamada, T. Carbon-ion radiotherapy for locoregional recurrence after primary surgery for pancreatic cancer. Radiother. Oncol. 2018, 129, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Molinelli, S.; Vai, A.; Russo, S.; Loap, P.; Meschini, G.; Paganelli, C.; Barcellini, A.; Vitolo, V.; Orlandi, E.; Ciocca, M. The Role of Multiple Anatomical Scenarios in Plan Optimization for Carbon Ion Radiotherapy of Pancreatic Cancer: Inter-Fraction Robustness in CIRT for Pancreatic Cancer. Radiother. Oncol. 2022, 176, 1–8. [Google Scholar] [CrossRef]
| Structure | Constraints |
|---|---|
| Stomach, Duodenum | Max dose < 45 Gy; volume receive more than 30 Gy < 10 cm3 |
| Spinal cord | Max dose < 30 Gy |
| Liver | Volume received more than 20 Gy < 35% |
| Kidney | Volume received more than 20 Gy < 50% of each kidney |
| Patient Characteristics | Value | |
|---|---|---|
| Age (years) | median (range) | 68 (44–79) |
| Sex | male/female | 30:14 |
| Tumor location | head/body | 17:27 |
| Tumor to GI tract distance (mm) | median (range) | 3 (0–24) |
| N stage | N0:N1 | 36:8 |
| Neoadjuvant CT | multi agent/single/none | 35:2:7 |
| NAC regimen | GnP/FFX/GEM/GEM + TGO/TGO | 29:5:1:1:1 |
| NAC duration (days) | median (range) | 88 (31–343) |
| Concurrent CT | GEM:TGO | 26:18 |
| Adjuvant CT | multi agent/single | 27:17 |
| AC regimen | GnP/FFX/GEM/GEM + TGO/TGO | 23:3:7:1:10 |
| Factor | Category | Number | 95% CI of mOS (Month) | p-Value |
|---|---|---|---|---|
| Age | ≥70 <70 | 16 28 | 17.5–32.2 40.1–60.5 | 0.038 |
| Sex | Male Female | 30 14 | 30.4–43.3 20.0–47.9 | 0.069 |
| Tumor location | Head Body | 17 27 | 19.8–33.4 36.6–58.1 | 0.169 |
| CA19-9 at CIRT | ≥150 (ng/mL) <150 (ng/mL) | 11 33 | 12.7–24.0 39.6–59.1 | 0.001 |
| Concurrent CT | GEM TGO | 26 18 | 29.3–43.4 25.8–51.1 | 0.436 |
| Neoadjuvant CT | Single agent Multiple agents None | 2 35 7 | 23.4–23.4 29.7–41.0 6.0–55.9 | 0.406 |
| NAC duration | ≥100 days <100 days None | 16 21 7 | 24.3–42.5 28.4–42.8 6.0–55.9 | 0.449 |
| Adjuvant CT | Single agent Multiple agents | 17 27 | 21.6–46.4 32.1–45.0 | 0.077 |
| Tumor to GI distance | ≥3 mm <3 mm | 28 16 | 30.1–51.8 26.6–41.9 | 0.555 |
| Factor | Category | Number | 95% CI of mLC (Month) | p-Value |
|---|---|---|---|---|
| Age | ≥70 y.o <70 y.o | 16 28 | 38.4–56.2 33.1–64.9 | 0.390 |
| Tumor location | Head Body | 17 27 | NIA * 35.4–63.5 | 0.122 |
| CA19-9 at CIRT | ≥150 (U/mL) <150 (U/mL) | 11 33 | 28.3–49.9 40.3–67.4 | 0.551 |
| Concurrent CT | GEM TGO | 26 18 | 36.5–52.2 33.7–69.5 | 0.857 |
| Tumor to GI distance | ≥3 mm <3 mm | 28 16 | 34.5–66.8 31.8–54.1 | 0.878 |
| Tumor diameter | ≥30 mm <30 mm | 33 11 | 48.8–70.7 26.3–54.7 | 0.603 |
| GTV D95 | ≥52.8 Gy <52.8 Gy | 30 14 | 56.3–74.1 16.3–46.1 | 0.015 |
| Adjuvant CT | Single agent Multiple agents | 17 27 | 31.9–66.8 36.5–45.9 | 0.079 |
| Acute Toxicity | |||||
|---|---|---|---|---|---|
| Adverse event | Grade 0–1 | Grade 2 | Grade 3 | Grade 4–5 | |
| Upper GI tract | 40 | 4 | 0 | 0 | |
| Lower GI tract | 42 | 2 | 0 | 0 | |
| Biliary tract | 41 | 3 | 0 | 0 | |
| Dermatitis | 44 | 0 | 0 | 0 | |
| Nausea | 40 | 4 | 0 | 0 | |
| GEM | Leukopenia | 6 | 11 | 9 | 0 |
| Thrombocytopenia | 18 | 2 | 6 | 0 | |
| TGO | Leukopenia | 14 | 3 | 1 | 0 |
| Thrombocytopenia | 18 | 0 | 0 | 0 | |
| Late toxicity | |||||
| Adverse event | Grade 0–1 | Grade 2 | Grade 3 | Grade 4–5 | |
| Upper GI tract | 41 | 2 | 1 | 0 | |
| Lower GI tract | 41 | 3 | 0 | 0 | |
| Biliary tract | 39 | 2 | 3 | 0 | |
| Dermatitis | 44 | 0 | 0 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okamoto, M.; Shiba, S.; Kobayashi, D.; Miyasaka, Y.; Okazaki, S.; Shibuya, K.; Ohno, T. Carbon-Ion Radiotherapy Combined with Concurrent Chemotherapy for Locally Advanced Pancreatic Cancer: A Retrospective Case Series Analysis. Cancers 2023, 15, 2857. https://doi.org/10.3390/cancers15102857
Okamoto M, Shiba S, Kobayashi D, Miyasaka Y, Okazaki S, Shibuya K, Ohno T. Carbon-Ion Radiotherapy Combined with Concurrent Chemotherapy for Locally Advanced Pancreatic Cancer: A Retrospective Case Series Analysis. Cancers. 2023; 15(10):2857. https://doi.org/10.3390/cancers15102857
Chicago/Turabian StyleOkamoto, Masahiko, Shintaro Shiba, Daijiro Kobayashi, Yuhei Miyasaka, Shohei Okazaki, Kei Shibuya, and Tatsuya Ohno. 2023. "Carbon-Ion Radiotherapy Combined with Concurrent Chemotherapy for Locally Advanced Pancreatic Cancer: A Retrospective Case Series Analysis" Cancers 15, no. 10: 2857. https://doi.org/10.3390/cancers15102857
APA StyleOkamoto, M., Shiba, S., Kobayashi, D., Miyasaka, Y., Okazaki, S., Shibuya, K., & Ohno, T. (2023). Carbon-Ion Radiotherapy Combined with Concurrent Chemotherapy for Locally Advanced Pancreatic Cancer: A Retrospective Case Series Analysis. Cancers, 15(10), 2857. https://doi.org/10.3390/cancers15102857





