Midterm Results of High-Dose-Rate Intraoperative Brachytherapy in the Treatment of Soft Tissue Sarcomas
Abstract
Simple Summary
Abstract
1. Introduction
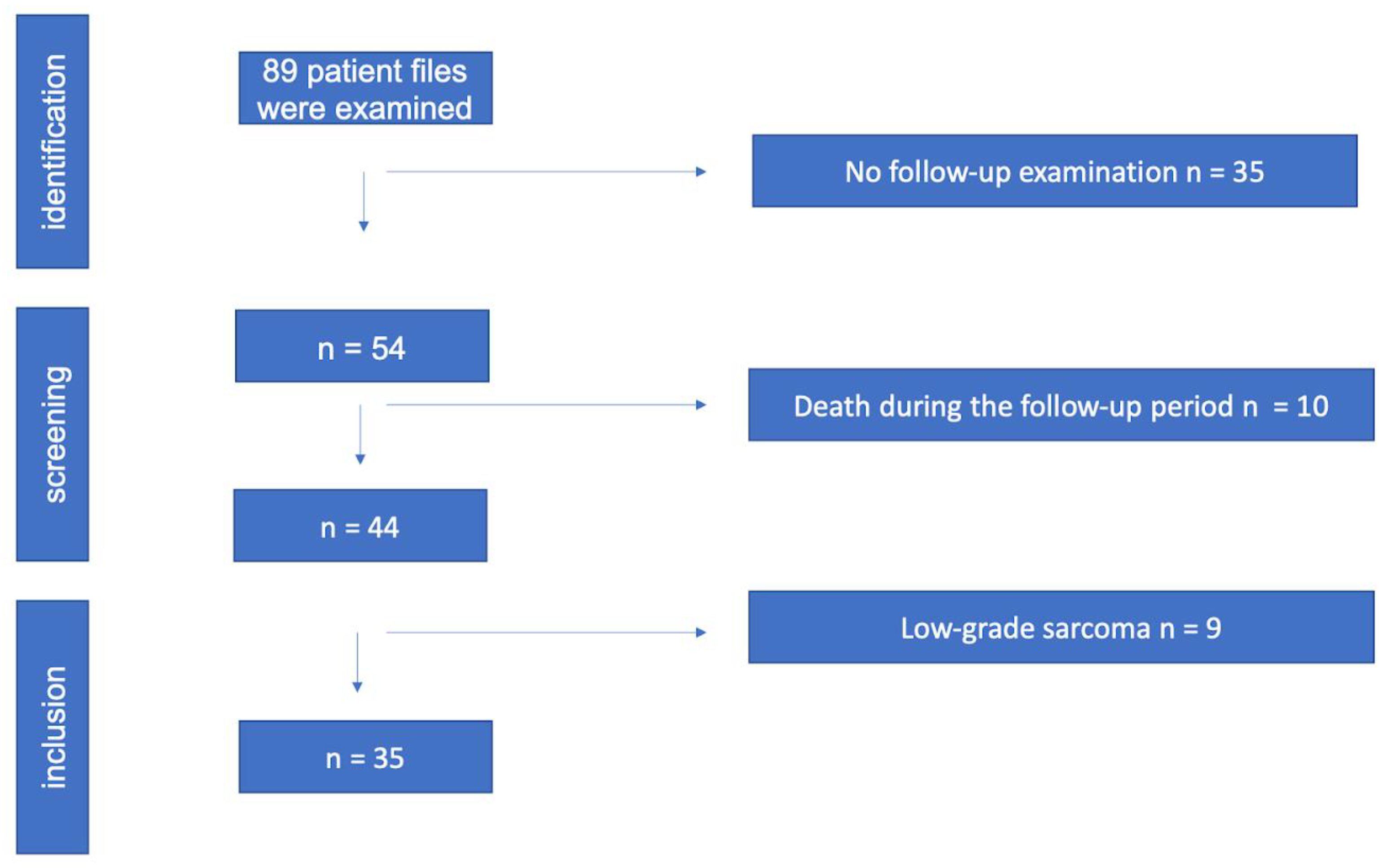
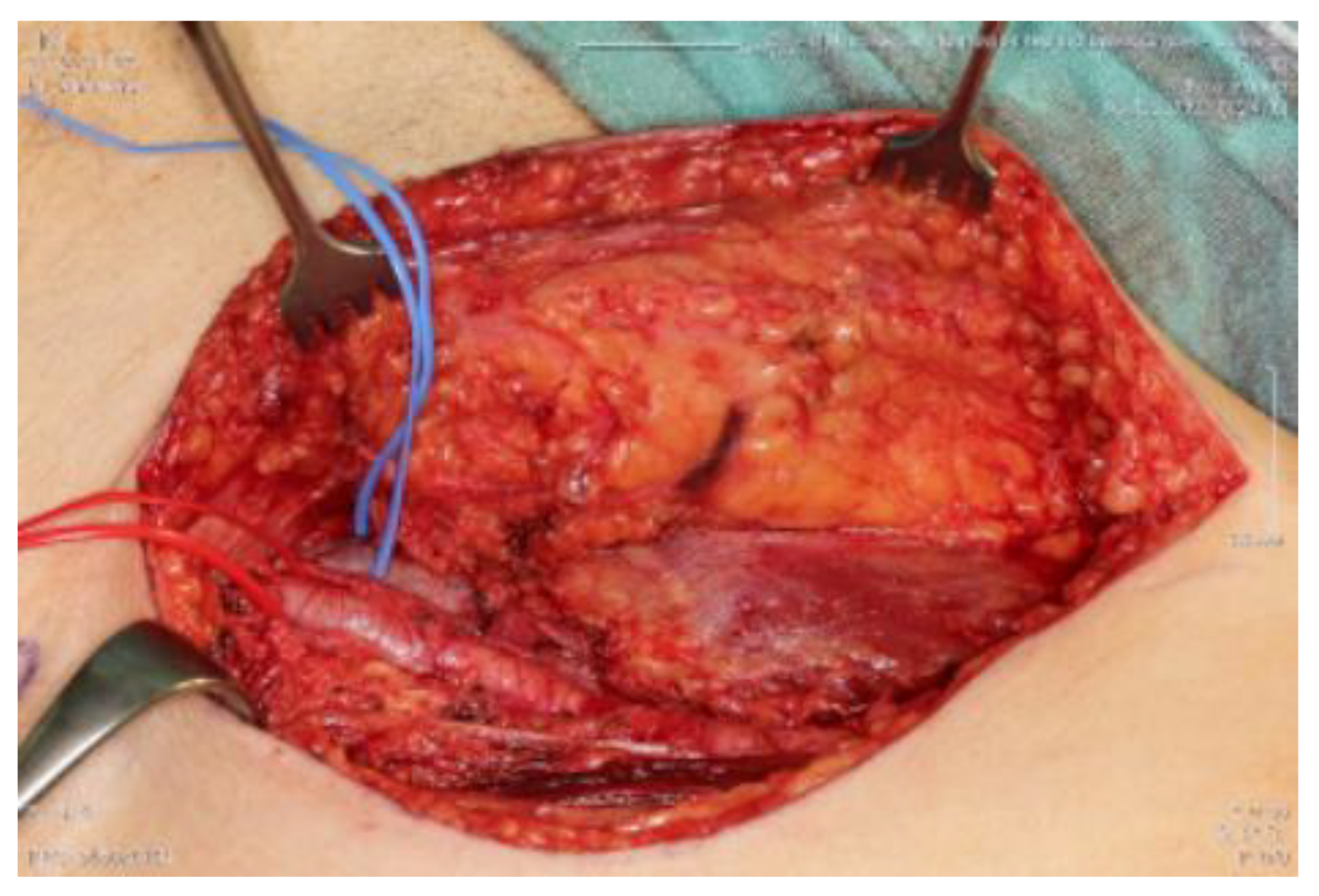
2. Material and Methods
3. Statistical Analysis
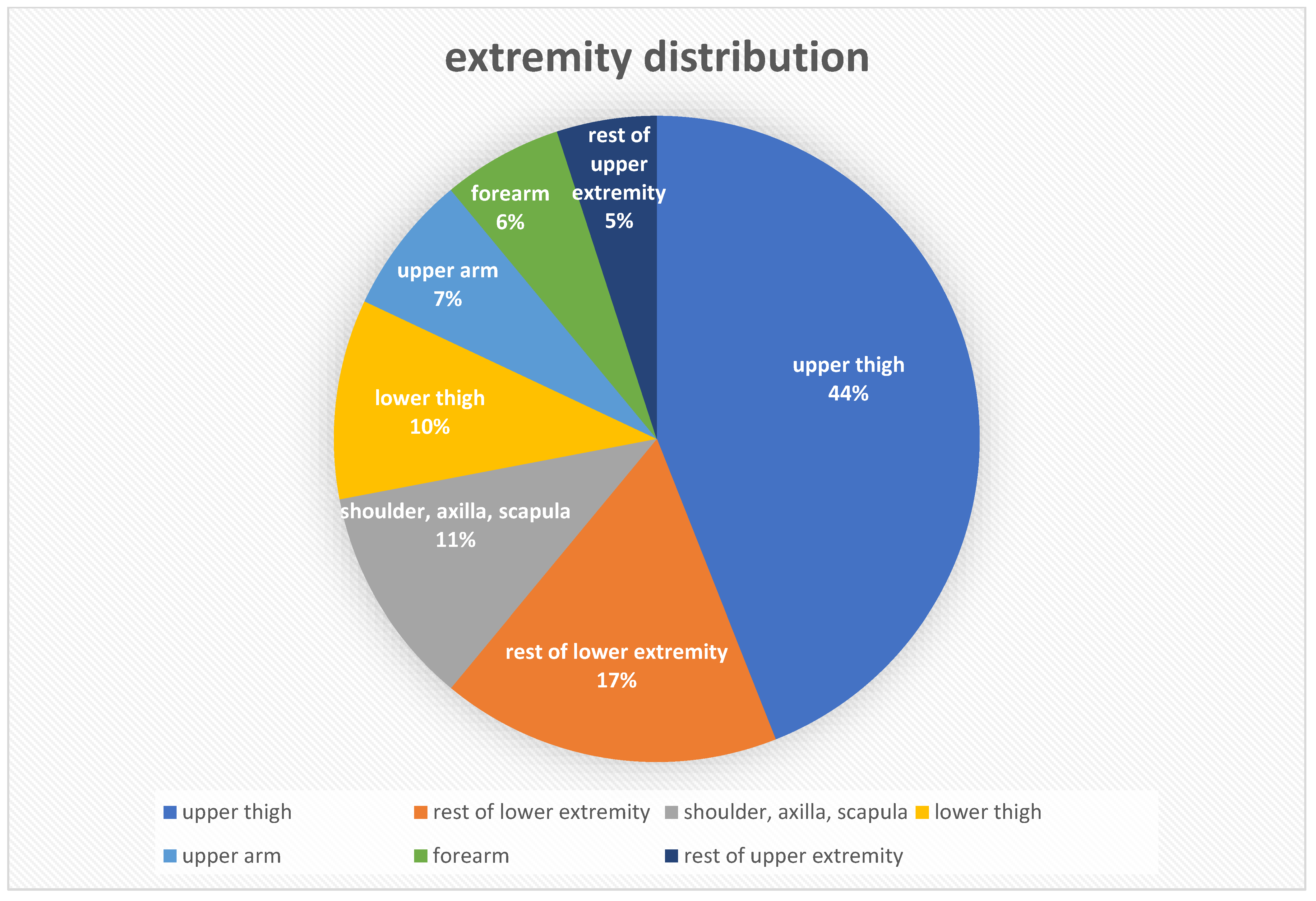
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Glossary
| LR | Local Recurrence |
| CT | Computertomography |
| MRI | Magnetic resonance imaging |
| STS | Soft tissue Sarcoma |
| LPS | Liposarcoma |
| LMS | Leiomyosarcoma |
| RT | Radiotherapy |
| GY | Gray |
| EBRT | External Beam Radiation Therapy, 2D, Protons |
| IO(HD)R-BT | Intraoperative (high-dose) Brachytherapy, 3D, Protons |
References
- Burningham, Z.; Hashibe, M.; Spector, L.; Schiffman, J.D. The Epidemiology of Sarcoma. Clin. Sarcoma Res. 2012, 2, 14. [Google Scholar] [CrossRef]
- SEER Cancer Statistics Review, 1975–2017. SEER. Available online: https://seer.cancer.gov/csr/1975_2017/index.html (accessed on 11 June 2022).
- Stiller, C.A.; Trama, A.; Serraino, D.; Rossi, S.; Navarro, C.; Chirlaque, M.D.; Casali, P.G.; RARECARE Working Group. Descriptive epidemiology of sarcomas in Europe: Report from the RARECARE project. Eur. J. Cancer 2013, 49, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Wibmer, C.; Leithner, A.; Zielonke, N.; Sperl, M.; Windhager, R. Increasing incidence rates of soft tissue sarcomas? A population-based epidemiologic study and literature review. Ann. Oncol. 2010, 21, 1106–1111. [Google Scholar] [CrossRef] [PubMed]
- Brodowicz, T.; Amann, G.; Leithner, A.; Sztankay, A.; Kainberger, F.; Eisterer, W.; Liegl-Atzwanger, B.; Rachbauer, F.; Rath, T.; Bergmann, M.; et al. Diagnose und Therapie von Weichteilsarkomen (interdisziplinär). Comprehensive Cancer Center Standard Operating Procedure; Version 3; 2017. Available online: https://www.ccc.ac.at/fileadmin/ccc/SOPs/CCC_SOP_Diagnose_und_Therapie_von_Weichteilsarkomen_interdisz_03_17.pdf (accessed on 11 June 2022).
- Rosenberg, S.A.; Tepper, J.; Glatstein, E.; Costa, J.; Baker, A.; Brennam, M.; Demoss, E.V.; Seipp, C.; Sindelar, W.F.; Sugarbaker, P.; et al. The treatment of soft-tissue sarcomas of the extremities: Prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann. Surg. 1982, 196, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Hoefkens, F.; Dehandschutter, C.; Somville, J.; Meijnders, P.; Van Gestel, D. Soft tissue sarcoma of the extremities: Pending questions on surgery and radiotherapy. Radiat. Oncol. 2016, 11, 136. [Google Scholar] [CrossRef]
- Pasquali, S.; Palassini, E.; Stacchiotti, S.; Casali, P.G.; Gronchi, A. Neoadjuvant treatment: A novel standard? Curr. Opin. Oncol. 2017, 29, 253–259. [Google Scholar] [CrossRef]
- von Mehren, M.; Kane, J.M.; Agulnik, M.; Bui, M.M.; Carr-Ascher, J.; Choy, E.; Connelly, M.; Dry, S.; Ganjoo, K.N.; Gonzalez, R.J.; et al. Soft Tissue Sarcoma, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 815–833. [Google Scholar] [CrossRef]
- Neugebauer, J.; Blum, P.; Keiler, A.; Süß, M.; Neubauer, M.; Moser, L.; Dammerer, D. Brachytherapy in the Treatment of Soft-Tissue Sarcomas of the Extremities—A Current Concept and Systematic Review of the Literature. Cancers 2023, 15, 1133. [Google Scholar] [CrossRef]
- Ballo, M.T.; Lee, A.K. Current results of brachytherapy for soft tissue sarcoma. Curr. Opin. Oncol. 2003, 15, 313–318. [Google Scholar] [CrossRef]
- Pisters, P.W.; Harrison, L.B.; Leung, D.H.Y.; Woodruff, J.M.; Casper, E.S.; Brennan, M.F. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J. Clin. Oncol. 1996, 14, 859–868. [Google Scholar] [CrossRef]
- Dammerer, D.; VAN Beeck, A.; Wabro, K.; Blockhuys, K.; Michielsen, J. Intraoperative Brachytherapy Combined With Adjuvant Radiotherapy Shows Equal Results in the Treatment of Leiomyosarcoma on the Extremities Compared to Adjuvant Radiotherapy Only—A Multicentric Retrospective Analysis. Anticancer. Res. 2022, 42, 4485–4492. [Google Scholar] [CrossRef]
- Haas, R.L.; DeLaney, T.F.; O’sullivan, B.; Keus, R.B.; Le Pechoux, C.; Olmi, P.; Poulsen, J.-P.; Seddon, B.; Wang, D. Radiotherapy for Management of Extremity Soft Tissue Sarcomas: Why, When, and Where? Int. J. Radiat. Oncol. 2012, 84, 572–580. [Google Scholar] [CrossRef]
- DeLaney, T.F.; Kepka, L.; Goldberg, S.I.; Hornicek, F.J.; Gebhardt, M.C.; Yoon, S.S.; Springfield, D.S.; Raskin, K.A.; Harmon, D.C.; Kirsch, D.G.; et al. Radiation Therapy for Control of Soft-Tissue Sarcomas Resected With Positive Margins. Int. J. Radiat. Oncol. 2007, 67, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Rachbauer, F.; Sununu, T.; Bach, C.; Nogler, M.; Krismer, M.; Sztankay, A.; Eichberger, P.; Schiestl, B.; Lukas, P.; Kreczy, A. High-dose-rate intraoperative brachytherapy (IOHDR) using flab technique in the treatment of soft tissue sarcomas. Strahlenther. Onkol. 2003, 179, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Spanier, S. A System for the Surgical Staging of Musculoskeletal Sarcoma. Clin. Orthop. 1980, 15, 106–120. [Google Scholar]
- Coindre, J.-M. Grading of Soft Tissue Sarcomas: Review and Update. Arch. Pathol. Lab. Med. 2006, 130, 1448–1453. [Google Scholar] [CrossRef] [PubMed]
- Hermanek, P.; Wittekind, C. The Pathologist and the Residual Tumor (R) Classification. Pathol.-Res. Pr. 1994, 190, 115–123. [Google Scholar] [CrossRef]
- Gronchi, A.; Miah, A.B.; Dei Tos, A.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; et al. Soft tissue and visceral sarcomas: ESMO–EURACAN–GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up☆. Ann. Oncol. 2021, 32, 1348–1365. [Google Scholar] [CrossRef]
- Sindelar, W.F.; Kinsella, T.J.; Chen, P.W.; Delaney, T.F.; Tepper, J.E.; Rosenberg, S.A.; Glatstein, E. Intraoperative Radiotherapy in Retroperitoneal Sarcomas: Final Results of a Prospective, Randomized, Clinical Trial. Arch. Surg. 1993, 128, 402–410. [Google Scholar] [CrossRef]
- Alektiar, K.M.; Hu, K.; Anderson, L.; Brennan, M.F.; Harrison, L.B. High-dose-rate intraoperative radiation therapy (HDR-IORT) for retroperitoneal sarcomas. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 157–163. [Google Scholar] [CrossRef]
- Gieschen, H.L.; Spiro, I.J.; Suit, H.D.; Ott, M.J.; Rattner, D.W.; Ancukiewicz, M.; Willett, C.G. Long-term results of intraoperative electron beam radiotherapy for primary and recurrent retroperitoneal soft tissue sarcoma. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Petersen, I.A.; Haddock, M.G.; Donohue, J.H.; Nagorney, D.M.; Grill, J.P.; Sargent, D.J.; Gunderson, L.L. Use of intraoperative electron beam radiotherapy in the management of retroperitoneal soft tissue sarcomas. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 469–475. [Google Scholar] [CrossRef]
- Bobin, J.; Al-Lawati, T.; Granero, L.; Adham, M.; Romestaing, P.; Chapet, O.; Issac, S.; Gerard, J. Surgical management of retroperitoneal sarcomas associated with external and intraoperative electron beam radiotherapy. Eur. J. Surg. Oncol. (EJSO) 2003, 29, 676–681. [Google Scholar] [CrossRef]
- Krempien, R.; Roeder, F.; Oertel, S.; Weitz, J.; Hensley, F.W.; Timke, C.; Funk, A.; Lindel, K.; Harms, W.; Buchler, M.W.; et al. Intraoperative electron-beam therapy for primary and recurrent retroperitoneal soft-tissue sarcoma. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 773–779. [Google Scholar] [CrossRef]
- Ballo, M.T.; Zagars, G.K.; Pollock, R.; Benjamin, R.S.; Feig, B.W.; Cormier, J.N.; Hunt, K.K.; Patel, S.R.; Trent, J.C.; Beddar, S.; et al. Retroperitoneal soft tissue sarcoma: An analysis of radiation and surgical treatment. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 158–163. [Google Scholar] [CrossRef]
- Dziewirski, W.; Rutkowski, P.; Nowecki, Z.I.; Sałamacha, M.; Morysiński, T.; Kulik, A.; Kawczyńska, M.; Kasprowicz, A.; Łyczek, J.; Ruka, W. Surgery Combined With Intraoperative Brachytherapy in the Treatment of Retroperitoneal Sarcomas. Ann. Surg. Oncol. 2006, 13, 245–252. [Google Scholar] [CrossRef]
- Sweeting, R.S.; Deal, A.M.; Llaguna, O.H.; Bednarski, B.K.; Meyers, M.O.; Yeh, J.J.; Calvo, B.F.; Tepper, J.E.; Kim, H.J. Intraoperative electron radiation therapy as an important treatment modality in retroperitoneal sarcoma. J. Surg. Res. 2013, 185, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Roeder, F.; Ulrich, A.; Habl, G.; Uhl, M.; Saleh-Ebrahimi, L.; Huber, P.E.; Schulz-Ertner, D.; Nikoghosyan, A.V.; Alldinger, I.; Krempien, R.; et al. Clinical Phase I/II trial to Investigate Preoperative Dose-Escalated Intensity-Modulated Radiation Therapy (IMRT) and Intraoperative Radiation Therapy (IORT) in patients with retroperitoneal soft tissue sarcoma: Interim analysis. BMC Cancer 2014, 14, 617. [Google Scholar] [CrossRef]
- Stucky, C.-C.H.; Wasif, N.; Ashman, J.B.; Pockaj, B.A.; Gunderson, L.L.; Gray, R.J. Excellent local control with preoperative radiation therapy, surgical resection, and intra-operative electron radiation therapy for retroperitoneal sarcoma. J. Surg. Oncol. 2014, 109, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Gronchi, A.; De Paoli, A.; Dani, C.; Merlo, D.F.; Quagliuolo, V.; Grignani, G.; Bertola, G.; Navarria, P.; Sangalli, C.; Buonadonna, A.; et al. Preoperative chemo-radiation therapy for localised retroperitoneal sarcoma: A phase I–II study from the Italian Sarcoma Group. Eur. J. Cancer 2013, 50, 784–792. [Google Scholar] [CrossRef]
- Roeder, F.; Lehner, B.; Schmitt, T.; Kasper, B.; Egerer, G.; Sedlaczek, O.; Grüllich, C.; Mechtersheimer, G.; Wuchter, P.; Hens-ley, F.W.; et al. Excellent local control with IOERT and postoperative EBRT in high grade extremity sarcoma: Results from a subgroup analysis of a prospective trial. BMC Cancer 2014, 14, 350. [Google Scholar] [CrossRef] [PubMed]
- Chemotherapy, Irradiation, and Surgery for Function-Preserving Therapy of Primary Extremity Soft Tissue Sarcomas—Edmonson—2002—Cancer—Wiley Online Library. Available online: https://acsjournals.onlinelibrary.wiley.com/doi/full/10.1002/cncr.10259?sid=nlm%3Apubmed (accessed on 11 June 2022).
- Azinovic, I.; Monge, R.M.; Aristu, J.J.; Salgado, E.; Villafranca, E.; Hidalgo, O.F.; Amillo, S.; Julian, M.S.; Villas, C.; Aramendía, J.M.; et al. Intraoperative radiotherapy electron boost followed by moderate doses of external beam radiotherapy in resected soft-tissue sarcoma of the extremities. Radiother. Oncol. 2003, 67, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Oertel, S.; Niethammer, A.G.; Krempien, R.; Roeder, F.; Eble, M.J.; Baer, C.; Huber, P.E.; Kulozik, A.; Waag, K.-L.; Treiber, M.; et al. Combination of external-beam radiotherapy with intraoperative electron-beam therapy is effective in incompletely resected pediatric malignancies. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Call, J.A.; Stafford, S.L.; Petersen, I.A.; Haddock, M.G. Use of Intraoperative Radiotherapy for Upper-extremity Soft-tissue Sarcomas: Analysis of Disease Outcomes and Toxicity. Am. J. Clin. Oncol. 2014, 37, 81–85. [Google Scholar] [CrossRef]
- Calvo, F.A.; Sole, C.V.; Polo, A.; Cambeiro, M.; Montero, A.; Álvarez, A.; Cuervo, M.; Julian, M.S.; Martínez-Monge, R. Limb-sparing management with surgical resection, external-beam and intraoperative electron-beam radiation therapy boost for patients with primary soft tissue sarcoma of the extremity. Strahlenther. Onkol. 2014, 190, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Roeder, F.; Lehner, B.; Saleh-Ebrahimi, L.; Hensley, F.W.; Ulrich, A.; Alldinger, I.; Mechtersheimer, G.; Huber, P.E.; Krempien, R.; Bischof, M.; et al. Intraoperative electron radiation therapy combined with external beam radiation therapy and limb sparing surgery in extremity soft tissue sarcoma: A retrospective single center analysis of 183 cases. Radiother. Oncol. 2015, 119, 22–29. [Google Scholar] [CrossRef]
- Niewald, M.; Fleckenstein, J.; Licht, N.; Bleuzen, C.; Ruebe, C. Intraoperative radiotherapy (IORT) combined with external beam radiotherapy (EBRT) for soft-tissue sarcomas—A retrospective evaluation of the Homburg experience in the years 1995–2007. Radiat. Oncol. 2009, 4, 32. [Google Scholar] [CrossRef]
- Rutkowski, P.; Ługowska, I. Follow-up in soft tissue sarcomas. Memo-Mag. Eur. Med. Oncol. 2014, 7, 92–96. [Google Scholar] [CrossRef]
- von Konow, A.; Ghanei, I.; Styring, E.; von Steyern, F.V. Late Local Recurrence and Metastasis in Soft Tissue Sarcoma of the Extremities and Trunk Wall: Better Outcome After Treatment of Late Events Compared with Early. Ann. Surg. Oncol. 2021, 28, 7891–7902. [Google Scholar] [CrossRef]
- Huber, F.T.; Stepan, R.; Zimmermann, F.; Fink, U.; Molls, M.; Siewert, J.R. Locally advanced rectal cancer: Resection and intraoperative radiotherapy using the flab method combined with preoperative or postoperative radiochemotherapy. Dis. Colon Rectum 1996, 39, 774–779. [Google Scholar] [CrossRef]
- Haase, G.M.; Meagher, D.P.; McNeely, L.K.; Daniel, W.E.; Poole, M.A.; Blake, M.; Odom, L.F. Electron beam intraoperative radiation therapy for pediatric neoplasms. Cancer 1994, 74, 740–747. [Google Scholar] [CrossRef]
- Fu, S.; Lu, J.J.; Zhang, Q.; Yang, Z.; Peng, L.; Xiong, F. Intraoperative Radiotherapy Combined With Adjuvant Chemoradiotherapy for Locally Advanced Gastric Adenocarcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 1488–1494. [Google Scholar] [CrossRef]
- Zagars, G.K.; Ballo, M.; Pisters, P.W.; Pollock, R.E.; Patel, S.R.; Benjamin, R.S. Preoperative vs. postoperative radiation therapy for soft tissue sarcoma: A retrospective comparative evaluation of disease outcome. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 482–488. [Google Scholar] [CrossRef]
- Grimer, R.J. Size Matters for Sarcomas! Ann. R. Coll. Surg. Engl. 2006, 88, 519–524. [Google Scholar] [CrossRef]
- Gibbs, J.F.; Huang, P.P.; Lee, R.J.; McGrath, B.; Brooks, J.; McKinley, B.; Driscoll, D.; Kraybill, W.G. Malignant Fibrous Histiocytoma: An Institutional Reviews. Cancer Investig. 2001, 19, 23–27. [Google Scholar] [CrossRef]
- Lehnert, T.; Schwarzbach, M.; Willeke, F.; Treiber, M.; Hinz, U.; Wannenmacher, M.M.; Herfarth, C. Intraoperative radiotherapy for primary and locally recurrent soft tissue sarcoma: Morbidity and long-term prognosis. Eur. J. Surg. Oncol. 2000, 26 (Suppl. A), S21–S24. [Google Scholar]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P.; et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Guzik, G.; Łyczek, J.; Kowalik, Ł. Surgical resection with adjuvant brachytherapy in soft tissue sarcoma of the extremity—A case report. J. Contemp. Brachytherapy 2012, 4, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Monge, R.; Julián, M.S.; Amillo, S.; Cambeiro, M.; Arbea, L.; Valero, J.; González-Cao, M.; Martín-Algarra, S. Perioperative high-dose-rate brachytherapy in soft tissue sarcomas of the extremity and superficial trunk in adults: Initial results of a pilot study. Brachytherapy 2005, 4, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Sarria, G.R.; Petrova, V.; Wenz, F.; Abo-Madyan, Y.; Sperk, E.; Giordano, F.A. Intraoperative radiotherapy with low energy x-rays for primary and recurrent soft-tissue sarcomas. Radiat. Oncol. 2020, 15, 110. [Google Scholar] [CrossRef] [PubMed]
- Schneider, F.; Clausen, S.; Thölking, J.; Wenz, F.; Abo-Madyan, Y. A novel approach for superficial intraoperative radiotherapy (IORT) using a 50 kV X-ray source: A technical and case report. J. Appl. Clin. Med. Phys. 2014, 15, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Abarca, T.; Gao, Y.; Monga, V.; Tanas, M.; Milhem, M.; Miller, B.J. Improved survival for extremity soft tissue sarcoma treated in high-volume facilities. J. Surg. Oncol. 2018, 117, 1479–1486. [Google Scholar] [CrossRef] [PubMed]

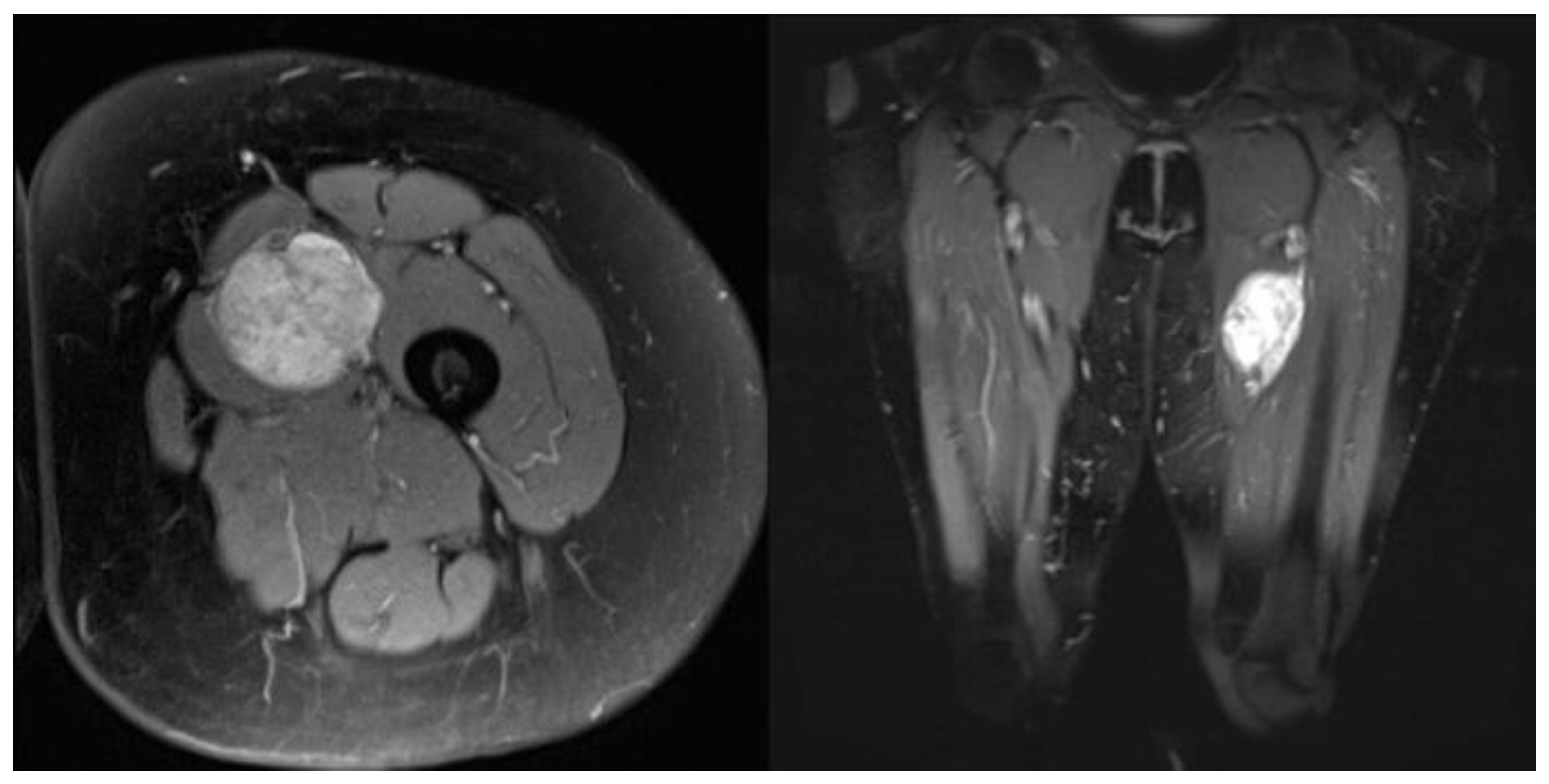
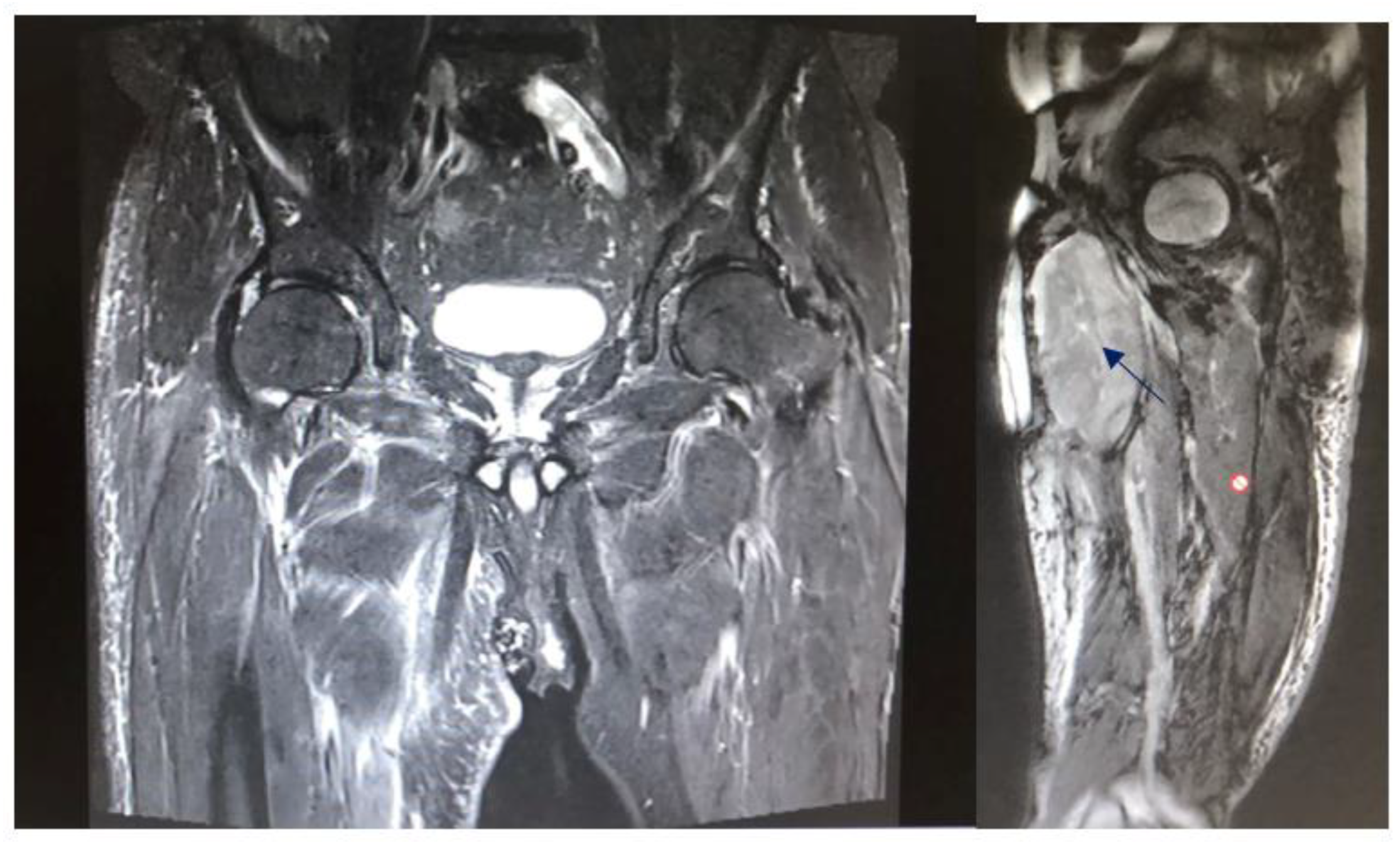

| Complication | Number (%) |
|---|---|
| Radiodermatitis | 20 (57) |
| Stage 1 | 15 (43) |
| Stage 2 | 4 (11) |
| Stage 3 | 1 (3) |
| Surgical Debridement | 2 (6) |
| Surgical Revision | 4 (11) |
| Splitskin Coverage | 4 (11) |
| Status | Description |
|---|---|
| R0 | No residual Tumor |
| R1 | Residual tumour microscopically detectable/resection along the pseudocapsule |
| R2 | Residual tumour macroscopically visible |
| Curative tumour resection = R0 in the absence or after resection of metastases | |
| Types of Sarcoma | n = 35 (%) |
|---|---|
| Liposarcoma | 11 (31) |
| Pleomorphic undifferentiated sarcoma | 9 (26) |
| Myxofibrosarcoma | 6 (17) |
| extraskeletal myxoid chondrosarcoma | 2 (6) |
| Myofibrosarcoma | 2 (6) |
| Synovial sarcoma | 2 (6) |
| Rhabdomyosarcoma (pleomorphic) | 1 (3) |
| Leiomyosarcoma | 1 (3) |
| Malignant peripheral nerve sheath tumour | 1 (3) |
| Resection Status | Number of LR (%) | No LR (%) | Total |
|---|---|---|---|
| R0 | 1 (6) | 16 (94) | 17 |
| R1 | 2 (13) | 13 (87) | 15 |
| R2 | 2 (100) | - | 2 |
| Lost to follow-up | 1 (100) | - | 1 |
| Types of Sarcoma | |||
| Liposarcoma | 4 (36) | 7 (64) | 11 |
| UPS | 1 (11) | 8 (89) | 9 |
| Myxofibrosarcoma | 1 (17) | 5 (83) | 6 |
| Other entities | - | 9 (100) | 9 |
| Localization | |||
| Extremities | 3 (11) | 25 (89) | 28 |
| Retroperitoneum | 3 (60) | 2 (40) | 5 |
| Superficial trunk | - | 2 (100) | 2 |
| Size | |||
| <5 cm | 2 (18) | 9 (82) | 11 |
| >5 cm | 4 (17) | 20 (83) | 24 |
| Grading | |||
| 2 | 1 (8) | 12 (92) | 13 |
| 3 | 5 (23) | 17 (77) | 22 |
| Staging | |||
| T1 N0 M0 | 2 (18) | 9 (82) | 11 |
| T2 N0 M0 | 4 (27) | 11 (73) | 15 |
| Other class | - | 9 (100) | 9 |
| Additional therapy | |||
| Adjuvant RT | 5 (15) | 28 (85) | 33 |
| Chemotherapy | 1 (11) | 8 (89) | 9 |
| TX | Primary tumours can not be assessed |
| T0 | No evidence of primary tumour |
| T1 | ≤5 cm in greatest spread |
| T2 | 5–10 cm |
| T3 | 10–15 cm |
| T4 | >15 cm |
| N0 | No regional lymph node metastases |
| N1 | regional lymph node metastases |
| M0 | No metastases |
| M1 | Metastases |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dammerer, D.; Neugebauer, J.; Braito, M.; Wagner, M.; Neubauer, M.; Moser, L.; Süß, M.; Liebensteiner, M.; Putzer, D. Midterm Results of High-Dose-Rate Intraoperative Brachytherapy in the Treatment of Soft Tissue Sarcomas. Cancers 2023, 15, 2854. https://doi.org/10.3390/cancers15102854
Dammerer D, Neugebauer J, Braito M, Wagner M, Neubauer M, Moser L, Süß M, Liebensteiner M, Putzer D. Midterm Results of High-Dose-Rate Intraoperative Brachytherapy in the Treatment of Soft Tissue Sarcomas. Cancers. 2023; 15(10):2854. https://doi.org/10.3390/cancers15102854
Chicago/Turabian StyleDammerer, Dietmar, Johannes Neugebauer, Matthias Braito, Moritz Wagner, Markus Neubauer, Lukas Moser, Markus Süß, Michael Liebensteiner, and David Putzer. 2023. "Midterm Results of High-Dose-Rate Intraoperative Brachytherapy in the Treatment of Soft Tissue Sarcomas" Cancers 15, no. 10: 2854. https://doi.org/10.3390/cancers15102854
APA StyleDammerer, D., Neugebauer, J., Braito, M., Wagner, M., Neubauer, M., Moser, L., Süß, M., Liebensteiner, M., & Putzer, D. (2023). Midterm Results of High-Dose-Rate Intraoperative Brachytherapy in the Treatment of Soft Tissue Sarcomas. Cancers, 15(10), 2854. https://doi.org/10.3390/cancers15102854







