A Retrospective Multi-Institutional Cohort Analysis of Clinical Characteristics and Outcomes in Dedifferentiated Chondrosarcoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Patient Population
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murphey, M.D.; Walker, E.A.; Wilson, A.J.; Kransdorf, M.J.; Temple, H.T.; Gannon, F.H. From the Archives of the AFIP: Imaging of Primary Chondrosarcoma: Radiologic-Pathologic Correlation. Radiographics 2003, 23, 1245–1278. [Google Scholar] [CrossRef] [PubMed]
- Cranmer, L.D.; Chau, B.; Mantilla, J.G.; Loggers, E.T.; Pollack, S.M.; Kim, T.S.; Kim, E.Y.; Kane, G.M.; Thompson, M.J.; Harwood, J.L.; et al. Is Chemotherapy Associated with Improved Overall Survival in Patients with Dedifferentiated Chondrosarcoma? A SEER Database Analysis. Clin. Orthop. Relat. Res. 2022, 480, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Daly, P.J.; Sim, F.H.; Wold, L.E. Dedifferentiated Chondrosarcoma of Bone. Orthopedics 1989, 12, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, D.C.; Beabout, J.W. Dedifferentiation of Low-Grade Chondrosarcomas. Cancer 1971, 28, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Grimer, R.J.; Gosheger, G.; Taminiau, A.; Biau, D.; Matejovsky, Z.; Kollender, Y.; San-Julian, M.; Gherlinzoni, F.; Ferrari, C. Dedifferentiated Chondrosarcoma: Prognostic Factors and Outcome from a European Group. Eur. J. Cancer 2007, 43, 2060–2065. [Google Scholar] [CrossRef] [PubMed]
- Biermann, J.S.; Chow, W.; Reed, D.R.; Lucas, D.; Adkins, D.R.; Agulnik, M.; Benjamin, R.S.; Brigman, B.; Budd, G.T.; Curry, W.T.; et al. NCCN Guidelines Insights: Bone Cancer, Version 2.2017. J. Natl. Compr. Canc. Netw. 2017, 15, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Casali, P.G.; Bielack, S.; Abecassis, N.; Aro, H.T.; Bauer, S.; Biagini, R.; Bonvalot, S.; Boukovinas, I.; Bovee, J.V.M.G.; Brennan, B.; et al. Bone Sarcomas: ESMO–PaedCan–EURACAN Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2018, 29, iv79–iv95. [Google Scholar] [CrossRef] [PubMed]
- Italiano, A.; Mir, O.; Cioffi, A.; Palmerini, E.; Piperno-Neumann, S.; Perrin, C.; Chaigneau, L.; Penel, N.; Duffaud, F.; Kurtz, J.E.; et al. Advanced Chondrosarcomas: Role of Chemotherapy and Survival. Ann. Oncol. 2013, 24, 2916–2922. [Google Scholar] [CrossRef] [PubMed]
- Dickey, I.D.; Rose, P.S.; Fuchs, B.; Wold, L.E.; Okuno, S.H.; Sim, F.H.; Scully, S.P. Dedifferentiated Chondrosarcoma: The Role of Chemotherapy with Updated Outcomes. J. Bone Jt. Surg. Am. Vol. 2004, 86, 2412–2418. [Google Scholar] [CrossRef]
- Strotman, P.K.; Reif, T.J.; Kliethermes, S.A.; Sandhu, J.K.; Nystrom, L.M. Dedifferentiated Chondrosarcoma: A Survival Analysis of 159 Cases from the SEER Database (2001–2011). J. Surg. Oncol. 2017, 116, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Van Maldegem, A.; Conley, A.P.; Rutkowski, P.; Patel, S.R.; Lugowska, I.; Desar, I.M.E.; Bovée, J.V.M.G.; Gelderblom, H. Outcome of First-Line Systemic Treatment for Unresectable Conventional, Dedifferentiated, Mesenchymal, and Clear Cell Chondrosarcoma. Oncologist 2019, 24, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, S.; Sun, T.; Lin, P.P.; Deavers, M.; Harun, N.; Lewis, V.O. Does Ifosfamide Therapy Improve Survival of Patients with Dedifferentiated Chondrosarcoma? Clin. Orthop. Relat. Res. 2014, 472, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Gusho, C.A.; Lee, L.; Zavras, A.; Seikel, Z.; Miller, I.; Colman, M.W.; Gitelis, S.; Blank, A.T. Dedifferentiated Chondrosarcoma: A Case Series and Review of the Literature. Orthop. Rev. 2022, 14, 35488. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.P.; Chebib, I.; Bredella, M.A.; Berner, E.A.; Taylor-Black, Q.; Choy, E.; Cote, G.M.; Chen, Y.; MacDonald, S.M.; Schwab, J.H.; et al. Progonostic Significance of Percentage and Size of Dedifferentiation in Dedifferentiated Chondrosarcoma. Mod. Pathol. 2023, 36, 100069. [Google Scholar] [CrossRef] [PubMed]
- Fox, E.; Patel, S.; Wathen, J.K.; Schuetze, S.; Chawla, S.; Harmon, D.; Reinke, D.; Chugh, R.; Benjamin, R.S.; Helman, L.J. Phase II Study of Sequential Gemcitabine Followed by Docetaxel for Recurrent Ewing Sarcoma, Osteosarcoma, or Unresectable or Locally Recurrent Chondrosarcoma: Results of Sarcoma Alliance for Research Through Collaboration Study 003. Oncologist 2012, 17, e321–e329. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Burgess, M.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; Priebat, D.A.; et al. Pembrolizumab in Advanced Soft-Tissue Sarcoma and Bone Sarcoma(SARC028): A Multicentre, Two-Cohort, Single-Arm, Open-Label, Phase 2 Trial. Lancet Oncol. 2017, 18, 1493–1501. [Google Scholar] [CrossRef]
| Patient Characteristics | All Patients (n = 74) |
|---|---|
| Median age in years (range) | 63 (23–87) |
| Sex | |
| Male | 45 (61%) |
| Female | 29 (39%) |
| Race | |
| White | 51 (69%) |
| Black | 3 (4%) |
| Asian | 2 (2.7%) |
| Native Hawaiian/Pacific Islander | 1 (1.3%) |
| Unknown | 17 (23%) |
| Sarcoma Center | |
| Vanderbilt University Medical Center | 27 |
| Stanford University | 17 |
| Washington University Siteman Cancer Center | 12 |
| Fred Hutchinson Cancer Center | 12 |
| Royal Marsden Hospital | 6 |
| Disease Characteristics | |
| Median tumor size in centimeters (range) | 11 (2–34) |
| Tumor grade | |
| High | 66 (96%) |
| Low | 3 (4%) |
| Primary site | |
| Extremity | 54 (73%) |
| Abdomen/pelvis | 10 (13.5%) |
| Chest | 6 (8.1%) |
| Head/neck | 2 (2.7%) |
| Other | 2 (2.7%) |
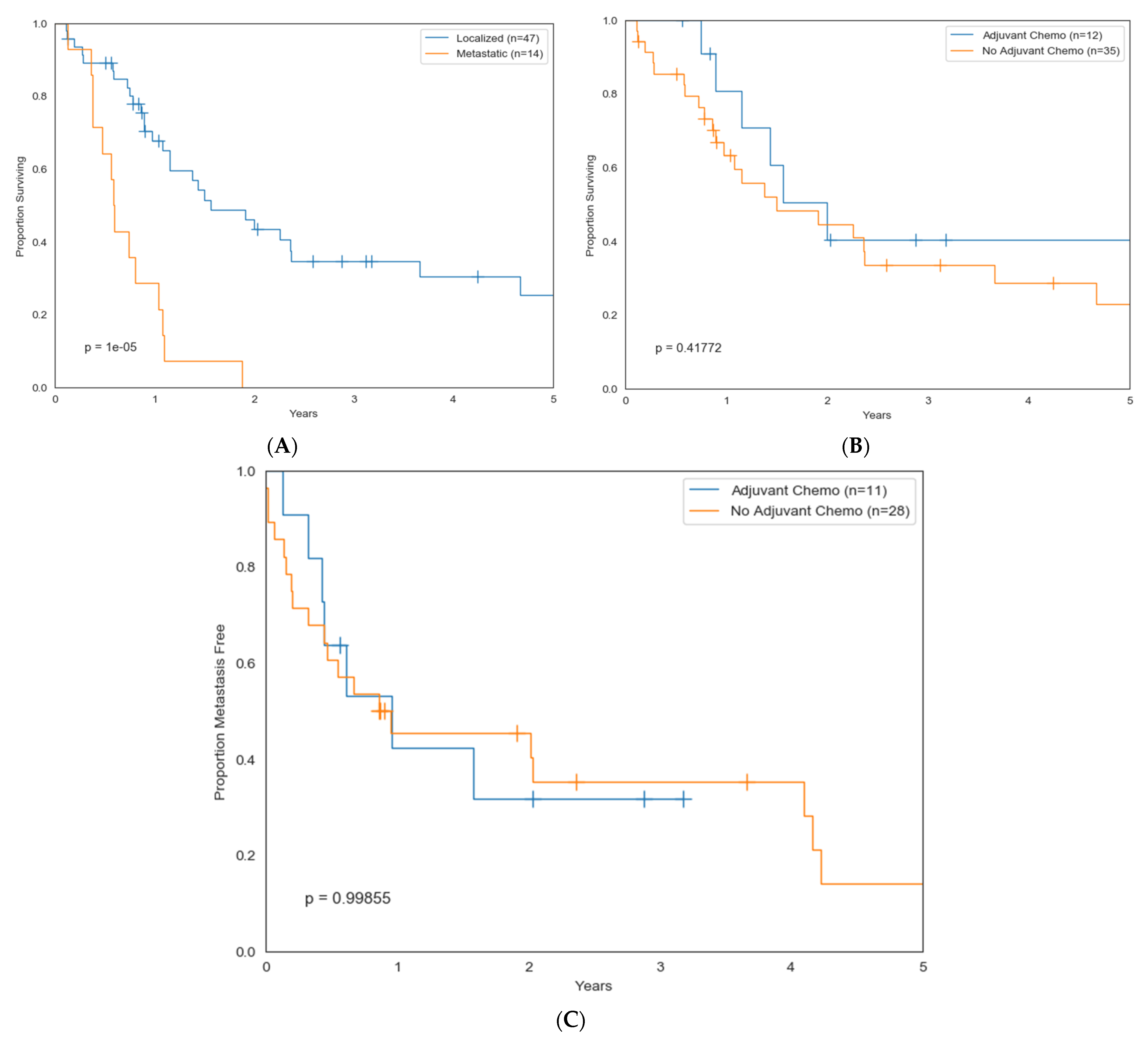
| Extent of disease at diagnosis | |
| Localized | 57 (77%) |
| Metastatic | 17 (23%) |
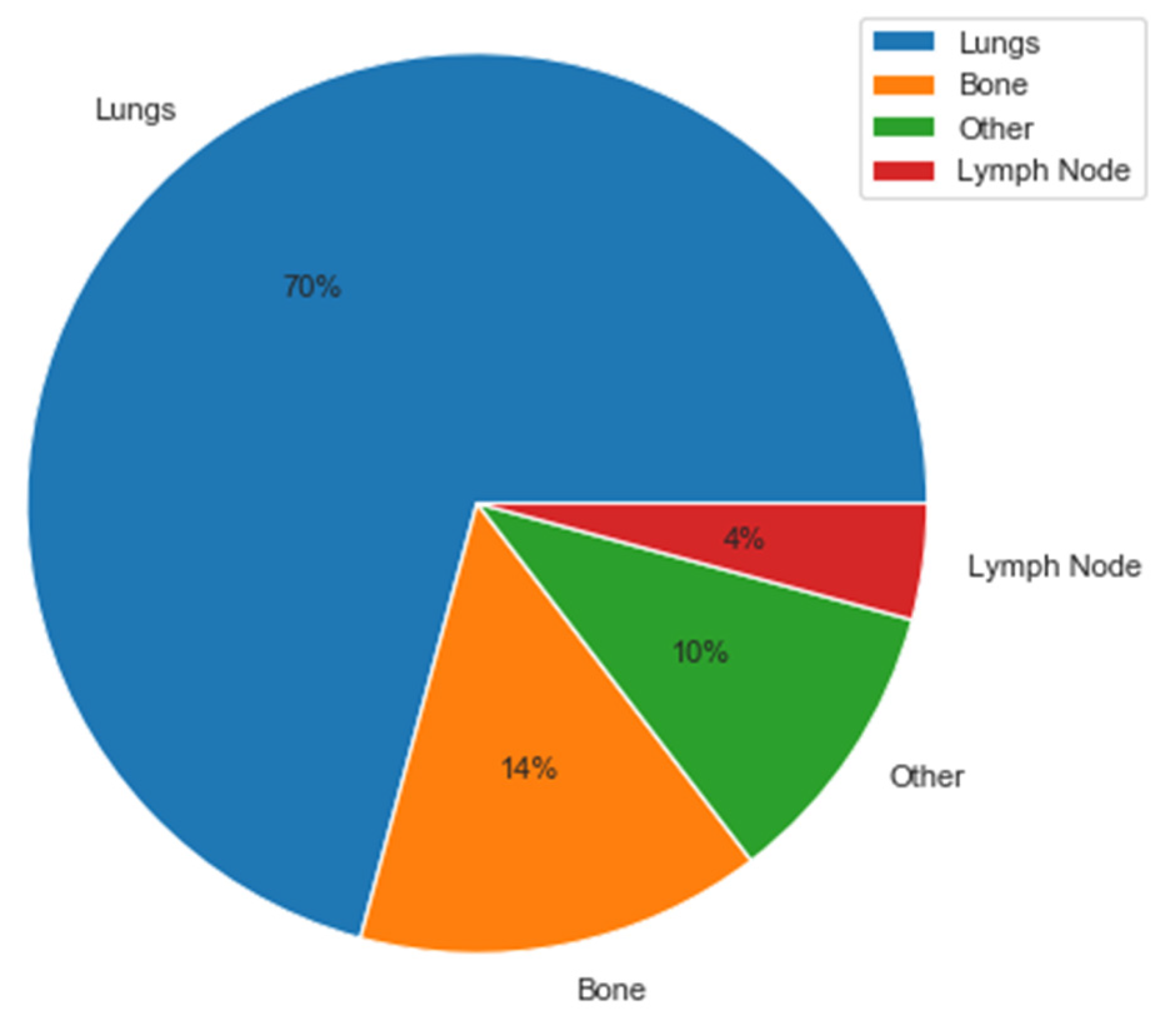
| Sites of metastatic disease | n = 42 |
| Lung | 38 (90%) |
| Bone | 8 (20%) |
| Lymph node | 3 (7%) |
| Other | 8 (20%) |
| Mutation Status | |
| IDH1 | 5 (6.8%) |
| IDH2 | 2 (2.7%) |
| Other | 2 (2.7%) |
| Unknown | 65 (88%) |
| Treatment Plan | All DDCS (n = 74) | Localized DDCS (n = 57) | Metastatic DDCS (n = 17) |
|---|---|---|---|
| Initial surgical resection | |||
| Yes | 66 (89%) | 56 (98%) | 10 (59%) |
| No | 8 (11%) | 1 (2%) | 7 (41%) |
| Radiation | |||
| Yes | 20 (27%) | 15 (26%) | 5 (29%) |
| No/unknown | 54 (73%) | 42 (74%) | 12 (71%) |
| Chemotherapy | |||
| Yes | 26 (35%) | 13 (23%) | 13 (76%) |
| No/unknown | 48 (65%) | 44 (77%) | 4 (24%) |
| Variable | Hazard Ratio | 95% CI | p-Value |
|---|---|---|---|
| Age > 60 years | 1.2 | 0.68–2.1 | 0.53 |
| Sex (Male) | 1.35 | 0.76–2.4 | 0.3 |
| Tumor Size (cm) | 1.01 | 0.95–1.07 | 0.74 |
| Location (Extremity) | 1.37 | 0.7–2.7 | 0.36 |
| Metastasis at Dx | 4.7 | 2.3–9.3 | 0.00001 |
| Systemic Treatment Regimen | Number of Patients | Median Weeks of Treatment (Range) | Best Response to Systemic Therapy |
|---|---|---|---|
| Neo/adjuvant * | 13 | ||
| Doxorubicin/cisplatin | 6 | 10.5 (3–16) | SD |
| Doxorubicin | 4 | 11.5 (6–18) | NA |
| Methotrexate/doxorubicin/cisplatin | 3 | 8 (4–16) | SD |
| Doxorubicin/methotrexate | 1 | 8 | NA |
| Doxorubicin/carboplatin | 1 | 20 | NA |
| Doxorubicin/ifosfamide | 1 | 18 | NA |
| Gemcitabine/docetaxel | 1 | 18 | NA |
| Imatinib | 1 | 2 | NR |
| Metastatic * | 30 | ||
| Chemotherapy | |||
| Doxorubicin/cisplatin | 12 | 7.5 (2–23) | PR |
| Gemcitabine/docetaxel | 10 | 6 (0.5–22) | SD |
| Doxorubicin | 4 | 10.6 (6–18) | SD |
| Doxorubicin/ifosfamide | 3 | 9 (9–18) | PR |
| Methotrexate/doxorubicin/cisplatin | 3 | 9 (8–10) | PD |
| Ifosfamide/etoposide | 2 | 6 | PD |
| Doxorubicin/olaratumab | 1 | 19 | SD |
| Doxorubicin/carboplatin | 1 | 12 | PD |
| Gemcitabine | 1 | 1 | PD |
| Ifosfamide | 1 | 15 | PD |
| Small-molecule inhibitors (SMI) | |||
| Pazopanib/regorafenib/sorafenib | 6 | 11 (6–20) | SD |
| Everolimus/temsirolimus | 2 | 16 (12–20) | SD |
| IDH inhibitor | 1 | NR | NR |
| Immune checkpoint inhibitors (ICI) | |||
| Ipilimumab/nivolumab | 4 | 11 (1–24) | SD |
| Pembrolizumab | 5 | 21 (12–48) | PR |
| Atezolizumab | 1 | 12 | PD |
| SMI + ICI | |||
| Pazopanib + pembrolizumab | 1 | 8 | PD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bui, N.; Dietz, H.; Farag, S.; Hirbe, A.C.; Wagner, M.J.; Van Tine, B.A.; Ganjoo, K.; Jones, R.L.; Keedy, V.L.; Davis, E.J. A Retrospective Multi-Institutional Cohort Analysis of Clinical Characteristics and Outcomes in Dedifferentiated Chondrosarcoma. Cancers 2023, 15, 2617. https://doi.org/10.3390/cancers15092617
Bui N, Dietz H, Farag S, Hirbe AC, Wagner MJ, Van Tine BA, Ganjoo K, Jones RL, Keedy VL, Davis EJ. A Retrospective Multi-Institutional Cohort Analysis of Clinical Characteristics and Outcomes in Dedifferentiated Chondrosarcoma. Cancers. 2023; 15(9):2617. https://doi.org/10.3390/cancers15092617
Chicago/Turabian StyleBui, Nam, Hilary Dietz, Sheima Farag, Angela C. Hirbe, Michael J. Wagner, Brian A. Van Tine, Kristen Ganjoo, Robin L. Jones, Vicki L. Keedy, and Elizabeth J. Davis. 2023. "A Retrospective Multi-Institutional Cohort Analysis of Clinical Characteristics and Outcomes in Dedifferentiated Chondrosarcoma" Cancers 15, no. 9: 2617. https://doi.org/10.3390/cancers15092617
APA StyleBui, N., Dietz, H., Farag, S., Hirbe, A. C., Wagner, M. J., Van Tine, B. A., Ganjoo, K., Jones, R. L., Keedy, V. L., & Davis, E. J. (2023). A Retrospective Multi-Institutional Cohort Analysis of Clinical Characteristics and Outcomes in Dedifferentiated Chondrosarcoma. Cancers, 15(9), 2617. https://doi.org/10.3390/cancers15092617









