Simple Summary
One of the main complications of cancer is delirium, especially in advanced stages. Our aim is to determine which delirium screening instrument is the most accurate in older people with cancer. A systematic review was performed on 13 different assessment tools, reporting an incidence of delirium ranging from 14.3% to 68.3%. The Delirium Observation Screening Scale (DOSS) showed the best metric properties, followed by the Nursing Delirium Screening Scale (NuDESC), Confusion Assessment Method (CAM) and Memorial Delirium Assessment Scale (MDAS). Screening tools for delirium are heterogeneous, and there is a need to analyze metric properties exclusively in the older population as knowing the tools with the greatest diagnostic accuracy will enable physicians and nurses to make the correct choice for early detection of delirium. In this way, the most appropriate measures could be implemented to avoid harmful consequences.
Abstract
Background: The increase in life expectancy worldwide has led to a larger population of older people, which in turn entails a rising prevalence of cancer. One of the main complications of cancer is delirium, especially in advanced stages. Objective: To determine which delirium screening instrument is the most accurate in older people with cancer. Methods: A systematic review was designed. A literature search was performed in MEDLINE, EBSCO and SCOPUS; additional records were identified by handsearching. Selection criteria were studies involving people with cancer and a mean sample age of 60 years or older, assessing delirium, and reporting the metric properties of the assessment instrument. Studies with post-surgical patients and substance abuse delirium were excluded. Results: From 2001 to 2021, 14 eligible studies evaluated 13 different assessment tools, reporting an incidence of delirium ranging from 14.3% to 68.3%. The Delirium Observation Screening Scale (DOSS) showed the best metric properties, followed by the Nursing Delirium Screening Scale (NuDESC), Confusion Assessment Method (CAM) and Memorial Delirium Assessment Scale (MDAS). Only two studies were considered to be at low risk of bias using the QUADAS-2 Tool. No study exclusively examined this population group. Conclusions: Screening tools for delirium are heterogeneous for older people with cancer, and there is a need to analyze metric properties exclusively in the older population. Registered on PROSPERO ID: CRD42022303530.
Keywords:
cancer; aged; delirium; assessment; accuracy; psychometric properties; incidence; prevalence 1. Introduction
The increase in life expectancy worldwide has led to a larger population of older people, which in turn entails a rising prevalence of cancer [1]. Indeed, oncological diseases are mostly diagnosed in people of advanced age and are currently the main cause of death in people over 65 years old [2].
One of the main complications of cancer is delirium [3], especially in advanced stages. Oncological disease encompasses metabolic abnormalities, metastatic disease, vascular disorders, paraneoplastic and autoimmune syndromes, and the promotion of inflammatory mediators, all of which are considered risk factors for delirium. Neurotoxicity related to cytostatic treatments, pain management, and other symptomatic treatments also increases the risk of delirium [4,5]. In addition, older people with cancer are more vulnerable due to changes derived from the aging process and a higher burden of comorbidities, such as diabetes, stroke, vascular diseases, dementia, frailty, and malnutrition, among others [6]. In older people, delirium can result in loss of function, increased cognitive impairment, loss of quality of life, and increased morbidity and mortality. In addition, the longer the duration and severity, the worse the consequences [7].
The prevalence of delirium ranges from 18% to 33% in general medical or oncology wards and 42% to 58% in palliative care units. In older people with cancer, reported prevalence ranges from 22% to 57% [8]; however, data show that up to 84.2% of delirium cases remain undiagnosed [8,9]. Physicians diagnose delirium based on criteria in the Diagnostic and Statistical Manual of Mental Disorders (DSM-V) [10] or the International Classification of Diseases (ICD-10), but different screening tools have been validated [11] for use by other healthcare professionals, including nurses and psychologists [12]. Specifically analyzing the available assessment instruments in older people with cancer would enable a more precise estimate of the true prevalence of delirium in this population [8]. There are more than 30 validated tools for screening, diagnosis or assessment of the severity of delirium [11], including everything from brief screening tools for settings such as intensive care or the emergency department, where the assessment needs to be performed in 2–3 min [13], to other, more comprehensive instruments that take 20–30 min [14] for settings such as inpatient wards or long-term care. Most instruments require short training by experienced psychiatrists, doctors, or nurses [11]. Therefore, depending on the setting, type of patient, assessment time, or team, the validated tool with the highest diagnostic accuracy would be chosen.
In addition to instruments validated in adults, there are some specific instruments for the pediatric population, but there is no distinction in the validation of the scales in younger versus older adults [11].
There have been reviews of screening instruments for delirium in hospitalized older people [15], in adults with cancer [8], and in palliative care [16,17,18]. Nevertheless, to our knowledge, there are no published systematic reviews of delirium screening instruments in older adults with cancer. The primary aim of this review was to determine which instrument has the best metric properties for detecting delirium in older people with cancer, and the secondary aim was to evaluate the incidence and prevalence of delirium in this population.
2. Materials and Methods
This review followed the methods laid out in the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. The study protocol is registered in PROSPERO (ID: CRD42022303530).
2.1. Literature Search
The research question was defined in the PICO format (population/intervention/comparison/outcomes), with the following search strategy:
(((((Delirium OR (Deliri*) OR (Confusion*) AND ((Diagnosis) OR (Comprehensive Geriatric Assessment) OR (Method) OR (Diagnostic) OR (diagnostical) OR (Diagnosis) OR (Assessment) OR (Assessing) OR (Scale) OR (Test) OR (testing) OR (Tests) OR (Screen) OR (screening) OR (Measurement) OR (Measurements) OR (validation) OR (tool) OR (instrument) OR (Delirium Detection)) AND ((Aged) OR (Frail Elderly) OR (Older) OR (Elder*) OR (Geriatric patient) OR (Geriatric*)) AND ((Cancer) OR (Oncol*)))
A literature search was conducted in the electronic databases MEDLINE, Scopus, and EBSCO, over the period from database inception to 20 December 2021. Each database was searched using the terms shown as a single search term or in combination using Medical Subjects Headings (MeSH) with the Boolean operators AND/OR.
To identify possible additional articles, reference lists of all relevant articles were manually cross-referenced. The search for unpublished studies included an electronic search of trial records: current controlled trials (www.controlled-trials.com, accessed on 20 December 2021), the National Institute of Clinical Health Databases (clinicaltrials.gov, accessed on 20 December 2021), Mednar, as well as a review of the grey literature and Google Search.
2.2. Inclusion and Exclusion Criteria
In order to answer our research questions, we applied the following criteria to select records for inclusion in the review: (1) full-text articles published in English or Spanish; (2) peer-reviewed original studies with experimental (randomized controlled trials), observational, and cross-sectional designs; (3) studies assessing delirium in older people (mean age ≥ 60 years) with oncological diseases; (4) inclusion of a description in the Methods section of the instrument used to detect delirium; and (5) reporting of the metric properties of the assessment tool(s). Studies in animals, case reports, qualitative studies, letters to the editor, abstracts from conferences, books and doctoral theses were excluded.
2.3. Data Collection and Analysis
A web-based system was used to manage the screening process and remove any duplicate citations. Thereafter, two members of the review team (PP-R and AP-G) independently screened the titles and abstracts against our selection criteria and retrieved all relevant full-text reports. Any discrepancies between review authors were resolved by consensus with a third member of the review team (FMM-A).
The same review authors Independently extracted the following data from each article: date of publication, study design, country, number and characteristics of the older participants (age and sex, type of cancer and setting), description of incidence or prevalence of delirium, assessment scale, gold standard, and validity and reliability of the instrument in the sample. In case of disagreement between the two reviewers regarding the manuscripts and the data extracted from them, the other author (FMM-A) acted as adjudicator.
2.4. Assessment of Risk of Bias in the Selected Studies
The included studies were assessed using the QUADAS-2 tool, developed by the Cochrane Review Group to evaluate the quality of diagnostic accuracy studies [19]. Studies were rated as being at low, unclear, or high risk of bias according to applicability concerns in the following four domains: (1) patient selection; (2) index test(s); (3) reference standard; and (4) flow and timing (“high” ratings indicate a high risk of bias and thus lower methodological quality). Studies were considered to be of acceptable methodological quality if they had an unclear risk of bias or applicability concern in one or two of the four domains. Studies with a high risk of bias or applicability concern in any of the domains were not considered to be of satisfactory methodological quality.
The judgments were made independently by two review authors (AP-G and FMM-A), and any discrepancies were resolved through consensus in consultation with a third review author (PP-R).
3. Results
3.1. Characteristics of Studies
The literature search yielded a total of 10,373 articles and 4 additional records through other sources. After the study selection process, 14 articles were included in the analysis. The full study selection process is presented in a PRISMA flow chart (Figure 1).
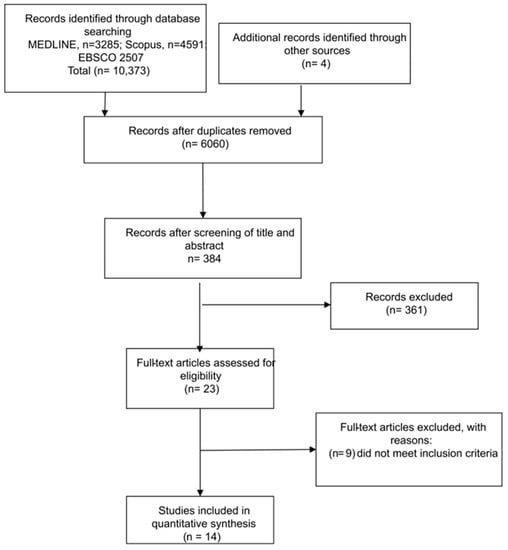
Figure 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) workflow for literature search.
In all included studies, any patient aged 18 years or older was eligible; none of the articles used old age as an inclusion criterion, although the mean age of all samples was over 60 years, ranging from 60.9 years [20] to 76 years [21].
Half the studies were European [20,21,22,23,24,25,26], three took place in Asia, two in Canada, one in Australia and one in the USA. The studies included participants with any type of advanced cancer who were recruited in hospital oncology services or palliative care units. The incidence/prevalence of delirium in the included studies ranged from 14.3% [20] to 68.3% [27]. Fifty percent (n = 7) of the studies had a higher proportion of men than women, but some did not indicate data regarding sex (Table 1).
The metric properties of 13 assessment instruments were analyzed: the most commonly used was the Memorial Delirium Assessment Scale (MDAS) [27,28] and its versions in Spanish [21], Thai [29], Korean [30] and Italian [24]. Grassi also analyzed the Italian versions of the Delirium Rating Scale (DRS-98) and Mini Mental State Examination (MMSE).
Two studies assessed the Nursing Delirium Screening Scale (Nu–DESC) [28,31], and two the Delirium Observation Screening Scale (DOSS) [22,25].
The least commonly used instruments were the Confusion Assessment Method (CAM) [26,32], the Single Question in Delirium (SQiD) [32], and its Spanish translation [20]. The rest of the instruments were analyzed only in single studies (Table 2). The professionals in charge of using the assessment instruments were mainly nursing staff, although there are also studies where physicians, caregivers, psychologists, palliative and clinical staff were the evaluators (Table 2).

Table 2.
Screening instruments, gold standard and analyzed metric properties of included studies.

Table 1.
Characteristics of included studies.
Table 1.
Characteristics of included studies.
| Study | Country | Type of Cancer | Setting | n | Mean Age (SD or Range)/% Men | Prevalence (%) |
| Barahona et al., 2018 [21] | Spain | All, advanced cancer | Hospice and general hospital | 60 | 76 (69–83)/48 | 41.8 |
| De la Cruz et al., 2015 [31] | USA | All, advanced cancer | Home, receiving hospice care | 78 | 69 (49–91)/55 | 44, MDAS 42, Nu–DESC Nurse 24, Nu–DESC Caregiver evening 15, Nu–DESC Caregiver night |
| Detroyer et al., 2014 [22] | Belgium | All, palliative care | PCU and MOW | 48 | 72 (67.25–78)/62.5 | 22.9 |
| Gaudreau et al., 2005 [28] | Canada | All, palliative care | Hemato–oncology/Internal medicine hospital unit | 59 | 61 (15–92)/NA | 35.59 |
| Grandahl et al., 2016 [23] | Danish | All, oncology inpatient | MOW | 81 | 68.5 (7.8)/42 | 33 |
| Grassi et al., 2001 [24] | Italy | All, derived from psychiatrist consultation | PCU and MOW | 105 | 67.7 (13.2)/52.4 | 62.8 |
| Hamamo et al., 2015 [33] | Japan | All, palliative care | PCU and MOW | 2343 | 69.1 (12.8) | 19.9 |
| Kang et al., 2018 [30] | Korea | All, advanced cancer | PCU | 123 | 66.92 (12.09)/42.28 | 23.52 |
| Klankluang et al., 2019 [29] | Thailand | All | PCU | 194 | 63.9 (13.3)/51.5 | 51 (8.1 hyperactive; 38.4 hypoactive; 53.5 mixed) |
| Lawlor et al., 2000 [27] | Canada | All, advanced cancer | PCU | 104 | 64.4 (10)/NA | 68.3 |
| Nefjees et al., 2019 [25] | Netherlands | All, advanced cancer | MOW | 187 | 64 (12)/66.3 | 50.26 |
| Ryan et al., 2009 [26] | Ireland | All, advanced cancer | PCU | 52 | 69.19 (36–93)/46.15 | 29.41 |
| Sancho–Espinosa et al., 2018 [20] | Spain | Patients with solid tumors | MOW | 42 | 60.9 (1.9)/71.4 | 14.3 |
| Sands et al., 2021 [32] | Australia | All | MOW | 73 | 68 (60.5–78)/42 | 38 (14.8 hyperactive; 59.3 hypoactive; 22.2 mixed) |
MDAS: Memorial Delirium Assessment Scale; MOW: medical oncology ward; Nu–DESC: Nursing Delirium Screening Scale; PCU: palliative care unit.
The gold standard was mainly the DSM diagnostic criteria in its different versions and the CAM scale, as well as the MDAS and DSR-98, with the responsible professionals including physicians, nurses, psychiatrists or researchers (Table 2).
All instruments were assessed for sensitivity and specificity. Four studies did not report other measures, such as PPV and negative predictive value [23,26,27,28]. Validity, reliability, and other data such as area under the receiver operating curve (AUC) are also reported in Table 2. The instrument with the best diagnostic metric properties was the DOSS, assessed by Neefjes et al. [25] as having sensitivity, specificity and negative predictive values over 99% and PPV values of 95%. The instruments yielding the lowest sensitivity were the Nu-DESC scale, for use by caregivers [31], and the SQiD and CAM, for use by clinical staff [32].
Analyses of the Spanish, Thai, Korean and Italian versions of the MDAS found the highest sensitivity and specificity with different cutoffs: ≥7 points in studies by [21,27,28], ≥9 points in studies by Kang et al. and Klankluang et al. [29,30], and ≥13 points in the study by Grassi et al. [24] (Table 2).
3.2. Risk of Bias
After analyzing methodological quality with QUADAS-2, only two studies were deemed to be at low risk of bias on all domains and of low concern in terms of applicability [23,25]; one presented the instrument with the highest sensitivity and specificity [25].
Eight studies (57.14%) had excellent or acceptable methodological quality, while the other six (42.86%) had shortcomings due to a lack of generalizability to all older people with cancer. The sample selected by Grassi et al. [24] was previously screened via psychiatric consultation. Klankluang et al. [29] excluded patients with dementia, mental retardation, coma, or communication problems, while Ryan et al. [26] excluded patients who were terminally ill, unconscious or had communication problems, and repeated the assessment if participants had disorganized thinking or behavioral problems. Sancho-Espinosa et al. [20] included only solid tumors, not hematological disease, and the SQiD question was not validated in Spanish. Hamano et al. [33] analyzed only one item from a validated instrument, and Lawlor et al. [27] presented data from assessments in the same patients at different time points and carried out by different staff (Table 3 and Figure 2).

Table 3.
Risk of bias and applicability concerns of included diagnostic test accuracy studies, according to the QUADAS-2 tool.
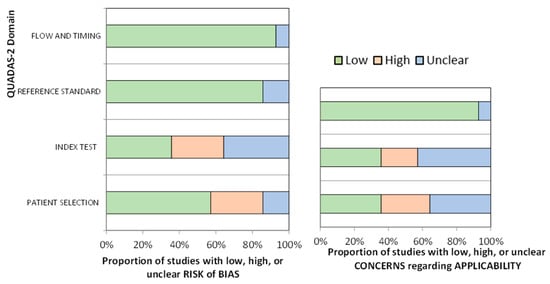
Figure 2.
Risk of bias and applicability concerns of included studies, according to QUADAS-2.
4. Discussion
In addition to being a neuropsychiatric syndrome, delirium can derive from the oncological pathophysiological process itself and is a possible side effect of the different cytostatic, radiotherapeutic and surgical treatments or interventions for symptom management. The prevalence of delirium increases in elderly oncological patients, in whom additional factors related to the aging process, such as advanced age and comorbidity, are also at play. Early detection of delirium enables prompt, appropriate treatment, and the literature suggests that the earlier treatment is administered, the less severe the consequences [34]. The most effective treatment is based on multi-component (pharmacological and non-pharmacological) interventions including re-orientation, appropriate lighting and noise levels, massages, and early mobility. This helps to reduce its duration and severity and, in turn, fatal consequences [34]. Early detection enables prompt treatment. This systematic review aimed to determine which delirium assessment tool has the best metric properties in older people with cancer, as well as to determine the prevalence of this syndrome in that population. The instrument with the highest sensitivity, specificity, positive predictive value, and negative predictive value was the DOSS.
4.1. Incidence
The incidence of delirium in the included studies ranged from 14% to 68%. Reviews estimate an incidence in older cancer patients of 22% to 57%, and more specifically in older patients in palliative care units, of up to 30% [8]. This wide range may be due to heterogeneous inclusion criteria or study settings. The lowest rate in our included studies was in older people with solid tumors in an inpatient oncology service [20], while the highest rates were after psychiatric consultation [24] and in patients with any type of advanced tumor [27]. The end-of-life process and the presence of psychiatric disease or disorders increase the risk even more and could explain the high incidence.
Previous studies such as Watt et al. [17] found higher rates in palliative care units, although the studies analyzed also include patients with diagnoses other than cancer. Van Velthuijsen et al. [15] also found higher rates in older people hospitalized in general medicine wards. The heterogeneity of included studies in terms of selection criteria, setting, screening instruments and comorbidity make any comparison difficult, although the higher the presence of risk factors, the higher the figures.
4.2. Assessment Tools
The 14 included studies evaluated 13 assessment tools. The DOSS showed the best predictive capacity in the study by Neefjes et al. [25], although Detroyer et al. [22] reported lower sensitivity, specificity, and positive and negative predictive values using the same cutoff (≥3 points) and the same assessor (nurses). The study sample or setting may interfere with this difference, and it may also be possible to analyze the gold standard used in each case.
The most widely used instrument was the MDAS, although it was not the one with the best metric properties. Despite being the most widely used in several languages, it is difficult to compare results, as the analyses used different cutoffs. Indeed, the reported predictive capacity varied widely, with the sensitivity of the MDAS ranging from 68% with a cutoff of ≥13 [24] to 97% with a cutoff of ≥7 [27]. Other studies used cutoffs ≥9 [29,30]. In validation of this scale, Breitbart et al. [35] proposed a cutoff of ≥13, citing a sensitivity of 70.59% and specificity of 93.75%.
The Nu-DESC is another widely used scale, validated in different languages in palliative care, yielding a sensitivity of 85.7% [15,36,37]. However, the use of different cutoffs modifies its sensitivity [31].
Despite being the most widely used instrument worldwide, only two studies analyzed the CAM scale [38]: Ryan et al. [26] obtained very good metric properties in palliative care units, while Sands [32] reported unacceptable sensitivity in an inpatient oncology service. Grassi et al. [24] analyzed two cutoffs in the Italian version of the DRS, with moderate results. This scale is primarily used for assessing delirium severity, with no explicit cutoff point for initial validation [39].
The tools with the lowest predictive capacity were the CDT, the Minicog and the DST, although combining them increased their sensitivity [23]. The SQID also showed unacceptable sensitivity rates in its validation study [40] and its (unvalidated) Spanish translation [20].
In addition to participants’ sociodemographic characteristics, the setting and the type or stage of cancer may also have influenced the results. The wide use of different instruments with different cutoff points, the construct itself, or the assessor (ranging from caregivers to clinical staff, nurses or physicians with or without previous education or training) are other factors that may affect accuracy. For example, de la Cruz et al. [31] obtained different incidence rates depending on the instrument used, the people in charge of carrying it out, and the time of the assessment, obtaining lower prevalence and lower sensitivity when caregivers performed the assessments instead of nurses, or when these took place at night instead of the evening.
Finally, few studies are at low risk of bias in older people with cancer. There is a need for studies that analyze the incidence and prevalence of delirium as well as all the factors that could influence its onset, to favor early identification and better outcomes.
4.3. Strengths and Limitations
This systematic review is the first to compare the diagnostic accuracy of delirium screening tools in older people with cancer. The use of the DSM or ICD for diagnosis is limited to physicians, while the use of validated assessment instruments allows the rest of the healthcare team to screen for delirium in this vulnerable population.
Previous studies have concluded that there is a need for rapid, useful tools for detecting delirium [9]. Given the existence of multiple delirium screening tools, understanding the diagnostic accuracy of each in the target population or setting of implementation allows for an informed choice.
The main limitation of the review evidence resides in the lack of studies exclusively in older people. In addition, the studies were heterogeneous in terms of population, setting, cancer types, and stages, precluding a robust comparison of the results. Furthermore, some studies analyzed modified tools or different cutoff points and used different gold standards or assessors, further complicating matters. In addition, we reviewed a limited number of databases and only studies published in English or Spanish were included.
4.4. Implications for Practice
The results of this systematic review raise several points to consider in future research into delirium in older people with oncological disease:
- Studies should include only older people with oncological disease in order to determine the specific predictive capacity of test(s) in this population and to analyze the results by age group.
- Comorbidities, hospital unit, and type of cancer should be analyzed in addition to possible risk factors derived from cytostatic treatment, radiotherapy, or other healthcare interventions.
- Only validated scales, such as the DOSS, CAM or Nu-DESC, should be used whenever possible, respecting the validated cutoff points.
- Whenever possible, the metric properties of the instrument should be measured in the population analyzed.
- Details of the assessment should be described, including the time when it is performed, the person carrying it out, and their previous training or experience in the use of the instrument.
- Studies should be designed to minimize the risk of bias in order to enable extrapolation of the data to the entire older population with oncological disease.
5. Conclusions
This is the first review to analyze screening instruments for delirium in older people with cancer. The tool with the best diagnostic accuracy is the DOSS, with a cutoff of ≥3 points. Prevalence of delirium ranges from 14% to 68% in settings including inpatient oncology services, palliative care units, and home care. Future studies should exclusively include older people and standardize the use of assessment tools.
Author Contributions
Conceptualization P.P.-R.; methodology, P.P.-R. and F.M.M.-A.; data curation, P.P.-R., A.P.-G. and F.M.M.-A.; writing—original draft preparation, P.P.-R., A.P.-G. and F.M.M.-A.; writing—review and editing, P.P.-R. and F.M.M.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mattiuzzi, C.; Lippi, G. Worldwide Disease Epidemiology in the Older Persons. Eur. Geriatr. Med. 2020, 11, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Word Health Organization. Mortality Rate in Older People—Top 20 Causes (Global and Regions). Available online: https://platform.who.int/data/maternal-newborn-child-adolescent-ageing/static-visualizations/mca (accessed on 27 June 2022).
- Lawlor, P.G.; Bush, S.H. Delirium in Patients with Cancer: Assessment, Impact, Mechanisms and Management. Nat. Rev. Clin. Oncol. 2015, 12, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Bush, S.H.; Lawlor, P.G.; Ryan, K.; Centeno, C.; Lucchesi, M.; Kanji, S.; Siddiqi, N.; Morandi, A.; Davis, D.H.J.; Laurent, M.; et al. Delirium in Adult Cancer Patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29, iv143–iv165. [Google Scholar] [CrossRef] [PubMed]
- Nolan, C.; DeAngelis, L.M. The Confused Oncologic Patient: A Rational Clinical Approach. Curr. Opin. Neurol. 2016, 29, 789–796. [Google Scholar] [CrossRef]
- Meehan, A.M.; Kassab, L.; Qin, H. Cancer and Older Adult Patient Care. Hosp. Pract. 2020, 48, 17–25. [Google Scholar] [CrossRef]
- Oh, E.S.; Fong, T.G.; Hshieh, T.T.; Inouye, S.K. Delirium in Older Persons: Advances in Diagnosis and Treatment. JAMA 2017, 318, 1161–1174. [Google Scholar] [CrossRef]
- Sands, M.B.; Wee, I.; Agar, M.; Vardy, J.L. The Detection of Delirium in Admitted Oncology Patients: A Scoping Review. Eur. Geriatr. Med. 2022, 13, 33–51. [Google Scholar] [CrossRef]
- Boettger, S.; Zipser, C.M.; Bode, L.; Spiller, T.; Deuel, J.; Osterhoff, G.; Ernst, J.; Petry, H.; Volbracht, J.; von Känel, R. The Prevalence Rates and Adversities of Delirium: Too Common and Disadvantageous. Palliat. Support. Care 2021, 19, 161–169. [Google Scholar] [CrossRef]
- Carter, M.J. Diagnostic and Statistical Manual of Mental Disorders; Sagamore Publishing LLC: Urbana, IL, USA, 2014; Volume 48, pp. 275–277. [Google Scholar]
- Network for Investigation of Delirium: Unifying Scientists (NIDUS). 2018. Delirium Measurement Info Cards. Available online: https://deliriumnetwork.org/measurement/delirium-info-cards/ (accessed on 9 April 2023).
- Pérez-Ros, P.; Martínez-Arnau, F.M. Delirium Assessment in Older People in Emergency Departments. A Literature Review. Diseases 2019, 7, 14. [Google Scholar] [CrossRef]
- Bellelli, G.; Morandi, A.; Davis, D.H.J.; Mazzola, P.; Turco, R.; Gentile, S.; Ryan, T.; Cash, H.; Guerini, F.; Torpilliesi, T.; et al. Validation of the 4AT, a New Instrument for Rapid Delirium Screening: A Study in 234 Hospitalised Older People. Age Ageing 2014, 43, 496–502. [Google Scholar] [CrossRef]
- O’Sullivan, R.; Meagher, D.; Leonard, M.; Watne, L.O.; Hall, R.J.; Maclullich, A.M.J.; Trzepacz, P.; Adamis, D. A Comparison of the Revised Delirium Rating Scale (DRS-R98) and the Memorial Delirium Assessment Scale (MDAS) in a Palliative Care Cohort with DSM-IV Delirium. Palliat. Support. Care 2015, 13, 937–944. [Google Scholar] [CrossRef]
- Van Velthuijsen, E.L.; Zwakhalen, S.M.G.; Warnier, R.M.J.; Mulder, W.J.; Verhey, F.R.J.; Kempen, G.I.J.M. Psychometric Properties and Feasibility of Instruments for the Detection of Delirium in Older Hospitalized Patients: A Systematic Review. Int. J. Geriatr. Psychiatry 2016, 31, 974–989. [Google Scholar] [CrossRef]
- Bramati, P.; Bruera, E. Delirium in Palliative Care. Cancers 2021, 13, 5893. [Google Scholar] [CrossRef]
- Watt, C.L.; Scott, M.; Webber, C.; Sikora, L.; Bush, S.H.; Kabir, M.; Boland, J.W.; Woodhouse, R.; Sands, M.B.; Lawlor, P.G. Delirium Screening Tools Validated in the Context of Palliative Care: A Systematic Review. Palliat. Med. 2021, 35, 683–696. [Google Scholar] [CrossRef]
- Yang, E.J.; Hahm, B.-J.; Shim, E.-J. Screening and Assessment Tools for Measuring Delirium in Patients with Cancer in Hospice and Palliative Care: A Systematic Review. J. Hosp. Palliat. Care 2021, 24, 214–225. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M.; QUADAS-2 Group. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Sancho-Espinosa, I.; Calvo-Espinós, C.; Arasanz Esteban, H.; Lacalle Emboroujo, A.; de Gaona Lana, E. Value of a Single-Question Screening Tool for Delirium Screening by a Nurse Team in Cancer Patients at an Oncology Service. Med. Paliativa 2018, 25, 214–221. [Google Scholar] [CrossRef]
- Barahona, E.; Pinhao, R.; Galindo, V.; Noguera, A. The Diagnostic Sensitivity of the Memorial Delirium Assessment Scale-Spanish Version. J. Pain Symptom Manag. 2018, 55, 968–972. [Google Scholar] [CrossRef]
- Detroyer, E.; Clement, P.M.; Baeten, N.; Pennemans, M.; Decruyenaere, M.; Vandenberghe, J.; Menten, J.; Joosten, E.; Milisen, K. Detection of Delirium in Palliative Care Unit Patients: A Prospective Descriptive Study of the Delirium Observation Screening Scale Administered by Bedside Nurses. Palliat. Med. 2014, 28, 79–86. [Google Scholar] [CrossRef]
- Grandahl, M.G.; Nielsen, S.E.; Koerner, E.A.; Schultz, H.H.; Arnfred, S.M. Prevalence of Delirium among Patients at a Cancer Ward: Clinical Risk Factors and Prediction by Bedside Cognitive Tests. Nord. J. Psychiatry 2016, 70, 413–417. [Google Scholar] [CrossRef]
- Grassi, L.; Caraceni, A.; Beltrami, E.; Borreani, C.; Zamorani, M.; Maltoni, M.; Monti, M.; Luzzani, M.; Mercadante, S.; De Conno, F. Assessing Delirium in Cancer Patients: The Italian Versions of the Delirium Rating Scale and the Memorial Delirium Assessment Scale. J. Pain Symptom Manag. 2001, 21, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Neefjes, E.C.W.; Van Der Vorst, M.J.D.L.; Boddaert, M.S.A.; Verdegaal, B.A.T.T.; Beeker, A.; Teunissen, S.C.C.; Beekman, A.T.F.; Zuurmond, W.W.A.; Berkhof, J.; Verheul, H.M.W. Accuracy of the Delirium Observational Screening Scale (DOS) as a Screening Tool for Delirium in Patients with Advanced Cancer. BMC Cancer 2019, 19, 160. [Google Scholar] [CrossRef] [PubMed]
- Ryan, K.; Leonard, M.; Guerin, S.; Donnelly, S.; Conroy, M.; Meagher, D. Validation of the Confusion Assessment Method in the Palliative Care Setting. Palliat. Med. 2009, 23, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, P.G.; Nekolaichuk, C.; Gagnon, B.; Mancini, I.L.; Pereira, J.L.; Bruera, E.D. Clinical Utility, Factor Analysis, and Further Validation of the Memorial Delirium Assessment Scale in Patients with Advanced Cancer: Assessing Delirium in Advanced Cancer. Cancer 2000, 88, 2859–2867. [Google Scholar] [CrossRef] [PubMed]
- Gaudreau, J.-D.; Gagnon, P.; Harel, F.; Tremblay, A.; Roy, M.-A. Fast, Systematic, and Continuous Delirium Assessment in Hospitalized Patients: The Nursing Delirium Screening Scale. J. Pain Symptom Manag. 2005, 29, 368–375. [Google Scholar] [CrossRef]
- Klankluang, W.; Pukrittayakamee, P.; Atsariyasing, W.; Siriussawakul, A.; Chanthong, P.; Tongsai, S.; Tayjasanant, S. Validity and Reliability of the Memorial Delirium Assessment Scale-Thai Version (MDAS-T) for Assessment of Delirium in Palliative Care Patients. Oncologist 2020, 25, e335–e340. [Google Scholar] [CrossRef]
- Kang, B.; Kim, Y.J.; Suh, S.W.; Son, K.-L.; Ahn, G.S.; Park, H.Y. Delirium and Its Consequences in the Specialized Palliative Care Unit: Validation of the Korean Version of Memorial Delirium Assessment Scale. Psycho-Oncology 2019, 28, 160–166. [Google Scholar] [CrossRef]
- De la Cruz, M.; Noguera, A.; San Miguel-Arregui, M.T.; Williams, J.; Chisholm, G.; Bruera, E. Delirium, Agitation, and Symptom Distress within the Final Seven Days of Life among Cancer Patients Receiving Hospice Care. Palliat. Support. Care 2015, 13, 211–216. [Google Scholar] [CrossRef]
- Sands, M.B.; Sharma, S.; Carpenter, L.; Hartshorn, A.; Lee, J.T.; Lujic, S.; Congdon, M.E.; Buchanan, A.M.; Agar, M.; Vardy, J.L. “SQiD, the Single Question in Delirium; Can a Single Question Help Clinicians to Detect Delirium in Hospitalised Cancer Patients?” Running Heading Single Question in Delirium” (Bcan-D-20-01665). BMC Cancer 2021, 21, 75. [Google Scholar] [CrossRef]
- Hamano, J.; Morita, T.; Ozawa, T.; Shishido, H.; Kawahara, M.; Aoki, S.; Demizu, A.; Goshima, M.; Goto, K.; Gyoda, Y.; et al. Validation of the Simplified Palliative Prognostic Index Using a Single Item from the Communication Capacity Scale. J. Pain Symptom Manag. 2015, 50, 542–547.e4. [Google Scholar] [CrossRef]
- León-Salas, B.; Trujillo-Martín, M.M.; Martínez del Castillo, L.P.; García-García, J.; Pérez-Ros, P.; Rivas-Ruiz, F.; Serrano-Aguilar, P. Multicomponent Interventions for the Prevention of Delirium in Hospitalized Older People: A Meta-Analysis. J. Am. Geriatr. Soc. 2020, 68, 2947–2954. [Google Scholar] [CrossRef]
- Breitbart, W.; Rosenfeld, B.; Roth, A.; Smith, M.J.; Cohen, K.; Passik, S. The Memorial Delirium Assessment Scale. J. Pain Symptom Manag. 1997, 13, 128–137. [Google Scholar] [CrossRef]
- Gélinas, C.; Bérubé, M.; Chevrier, A.; Pun, B.T.; Ely, E.W.; Skrobik, Y.; Barr, J. Delirium Assessment Tools for Use in Critically Ill Adults: A Psychometric Analysis and Systematic Review. Crit. Care Nurse 2018, 38, 38–49. [Google Scholar] [CrossRef]
- Gnatta, J.R.; Cavassana, T.M.; Nascimento, A.S.d.; Poveda, V.d.B. Instruments to Identify Delirium in Patients Recovering from Anesthesia: A Scoping Review. J. Perianesth. Nurs. 2022, 37, 961–965.e7. [Google Scholar] [CrossRef]
- Inouye, S.K.; van Dyck, C.H.; Alessi, C.A.; Balkin, S.; Siegal, A.P.; Horwitz, R.I. Clarifying Confusion: The Confusion Assessment Method. A New Method for Detection of Delirium. Ann. Intern. Med. 1990, 113, 941–948. [Google Scholar] [CrossRef]
- Trzepacz, P.T.; Mittal, D.; Torres, R.; Kanary, K.; Norton, J.; Jimerson, N. Validation of the Delirium Rating Scale-Revised-98: Comparison with the Delirium Rating Scale and the Cognitive Test for Delirium. J. Neuropsychiatry Clin. Neurosci. 2001, 13, 229–242. [Google Scholar] [CrossRef]
- Sands, M.B.; Dantoc, B.P.; Hartshorn, A.; Ryan, C.J.; Lujic, S. Single Question in Delirium (SQiD): Testing Its Efficacy against Psychiatrist Interview, the Confusion Assessment Method and the Memorial Delirium Assessment Scale. Palliat. Med. 2010, 24, 561–565. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).