Delayed Reconstruction after Major Head and Neck Cancer Resection: An Interdisciplinary Feasibility Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Surgical Procedure
2.3. Surgical Outcome Parameters
2.4. Functional Outcome Parameters
2.5. Data Analysis
3. Results
3.1. Study Population
3.2. Surgical Procedure
3.3. Surgical Outcome
3.4. Functional Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gabrysz-Forget, F.; Tabet, P.; Rahal, A.; Bissada, E.; Christopoulos, A.; Ayad, T. Free versus pedicled flaps for reconstruction of head and neck cancer defects: A systematic review. J. Otolaryngol. Head Neck Surg. 2019, 48, 13. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J. Microvascular reconstruction in the head and neck. Mayo Clin. Proc. 1986, 61, 451–458. [Google Scholar] [CrossRef]
- Wang, W.; Ong, A.; Vincent, A.G.; Shokri, T.; Scott, B.; Ducic, Y. Flap Failure and Salvage in Head and Neck Reconstruction. Semin. Plast. Surg. 2020, 34, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Godina, M. Early microsurgical reconstruction of complex trauma of the extremities. Plast. Reconstr. Surg. 1986, 78, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Lee, Z.H.; Stranix, J.T.; Rifkin, W.J.; Daar, D.A.; Anzai, L.; Ceradini, D.J.; Thanik, V.; Saadeh, P.B.; Levine, J.P. Timing of Microsurgical Reconstruction in Lower Extremity Trauma: An Update of the Godina Paradigm. Plast. Reconstr. Surg. 2019, 144, 759–767. [Google Scholar] [CrossRef]
- Starnes-Roubaud, M.J.; Peric, M.; Chowdry, F.; Nguyen, J.T.; Schooler, W.; Sherman, R.; Carey, J.N. Microsurgical Lower Extremity Reconstruction in the Subacute Period: A Safe Alternative. Plast. Reconstr. Surg. Glob. Open 2015, 3, e449. [Google Scholar] [CrossRef]
- Pfister, D.G.; Spencer, S.; Adelstein, D.; Adkins, D.; Anzai, Y.; Brizel, D.M.; Bruce, J.Y.; Busse, P.M.; Caudell, J.J.; Cmelak, A.J.; et al. Head and Neck Cancers, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 873–898. [Google Scholar] [CrossRef]
- Mak, P.H.; Campbell, R.C.; Irwin, M.G. The ASA Physical Status Classification: Inter-observer consistency. American Society of Anesthesiologists. Anaesth. Intensive Care 2002, 30, 633–640. [Google Scholar] [CrossRef]
- UICC. TNM Klassifikation Maligner Tumoren; Wiley-VCH: Weinheim, Germany, 2017; Volume 8. [Google Scholar]
- Grassner, L.; Marhold, F.; Yousif, M.; Grillhosl, A.; Ungersboeck, K.; Schulz, J.; Strowitzki, M. Experiences with a temporary synthetic skin substitute after decompressive craniectomy: A retrospective two-center analysis. Acta Neurochir. 2019, 161, 493–499. [Google Scholar] [CrossRef]
- Chorath, K.; Go, B.; Shinn, J.R.; Mady, L.J.; Poonia, S.; Newman, J.; Cannady, S.; Revenaugh, P.C.; Moreira, A.; Rajasekaran, K. Enhanced recovery after surgery for head and neck free flap reconstruction: A systematic review and meta-analysis. Oral Oncol. 2021, 113, 105117. [Google Scholar] [CrossRef]
- Abraham, M.; Badhey, A.; Hu, S.; Kadakia, S.; Rasamny, J.K.; Moscatello, A.; Ducic, Y. Thromboprophylaxis in Head and Neck Microvascular Reconstruction. Craniomaxillofac. Trauma Reconstr. 2018, 11, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Van Gijn, D.R.; D’Souza, J.; King, W.; Bater, M. Free Flap Head and Neck Reconstruction with an Emphasis on Postoperative Care. Facial Plast. Surg. 2018, 34, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Kamolz, L.P.; Giovanoli, P.; Haslik, W.; Koller, R.; Frey, M. Continuous free-flap monitoring with tissue-oxygen measurements: Three-year experience. J. Reconstr. Microsurg. 2002, 18, 487–491; discussion 492–493. [Google Scholar] [CrossRef] [PubMed]
- Szakmany, T.; Dodd, M.; Dempsey, G.A.; Lowe, D.; Brown, J.S.; Vaughan, E.D.; Rogers, S.N. The influence of allogenic blood transfusion in patients having free-flap primary surgery for oral and oropharyngeal squamous cell carcinoma. Br. J. Cancer 2006, 94, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Dejaco, D.; Riedl, D.; Gasser, S.; Schartinger, V.H.; Innerhofer, V.; Gottfried, T.; Steinbichler, T.B.; Riechelmann, F.; Moschen, R.; Galvan, O.; et al. A Tool for Rapid Assessment of Functional Outcomes in Patients with Head and Neck Cancer. Cancers 2021, 13, 5529. [Google Scholar] [CrossRef]
- Schemper, M.; Smith, T.L. A note on quantifying follow-up in studies of failure time. Control. Clin. Trials 1996, 17, 343–346. [Google Scholar] [CrossRef]
- Jacobs, J.P.; Mavroudis, C.; Jacobs, M.L.; Maruszewski, B.; Tchervenkov, C.I.; Lacour-Gayet, F.G.; Clarke, D.R.; Yeh, T., Jr.; Walters, H.L., 3rd; Kurosawa, H.; et al. What is operative mortality? Defining death in a surgical registry database: A report of the STS Congenital Database Taskforce and the Joint EACTS-STS Congenital Database Committee. Ann. Thorac. Surg. 2006, 81, 1937–1941. [Google Scholar] [CrossRef]
- Brady, J.S.; Desai, S.V.; Crippen, M.M.; Eloy, J.A.; Gubenko, Y.; Baredes, S.; Park, R.C.W. Association of Anesthesia Duration With Complications After Microvascular Reconstruction of the Head and Neck. JAMA Facial Plast. Surg. 2018, 20, 188–195. [Google Scholar] [CrossRef]
- Bulbul, M.G.; Tarabichi, O.; Sethi, R.K.; Parikh, A.S.; Varvares, M.A. Does Clearance of Positive Margins Improve Local Control in Oral Cavity Cancer? A Meta-analysis. Otolaryngol. Head Neck Surg. 2019, 161, 235–244. [Google Scholar] [CrossRef]
- Heimbach, D.M.; Warden, G.D.; Luterman, A.; Jordan, M.H.; Ozobia, N.; Ryan, C.M.; Voigt, D.W.; Hickerson, W.L.; Saffle, J.R.; DeClement, F.A.; et al. Multicenter postapproval clinical trial of Integra dermal regeneration template for burn treatment. J. Burn Care Rehabil. 2003, 24, 42–48. [Google Scholar] [CrossRef]
- Maus, J.C.; Hemal, K.; Khan, M.; Calder, B.W.; Marks, M.W.; Defranzo, A.J.; Pestana, I.A. Dermal Regeneration Template and Staged Skin Grafting for Extirpative Scalp Wound Reconstruction: A 14-Year Experience. Otolaryngol. Head Neck Surg. 2021, 165, 275–281. [Google Scholar] [CrossRef]
- Seth, A.K.; Ratanshi, I.; Dayan, J.H.; Disa, J.J.; Mehrara, B.J. Nasal Reconstruction Using the Integra Dermal Regeneration Template. Plast. Reconstr. Surg. 2019, 144, 966–970. [Google Scholar] [CrossRef]
- Steinbichler, T.B.; Golm, L.; Dejaco, D.; Riedl, D.; Kofler, B.; Url, C.; Wolfram, D.; Riechelmann, H. Surgical rescue for persistent head and neck cancer after first-line treatment. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 1437–1448. [Google Scholar] [CrossRef] [PubMed]
- Steinbichler, T.B.; Lichtenecker, M.; Anegg, M.; Dejaco, D.; Kofler, B.; Schartinger, V.H.; Kasseroler, M.T.; Forthuber, B.; Posch, A.; Riechelmann, H. Persistent Head and Neck Cancer Following First-Line Treatment. Cancers 2018, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Riechelmann, H.; Dejaco, D.; Steinbichler, T.B.; Lettenbichler-Haug, A.; Anegg, M.; Ganswindt, U.; Gamerith, G.; Riedl, D. Functional Outcomes in Head and Neck Cancer Patients. Cancers 2022, 14, 2135. [Google Scholar] [CrossRef] [PubMed]
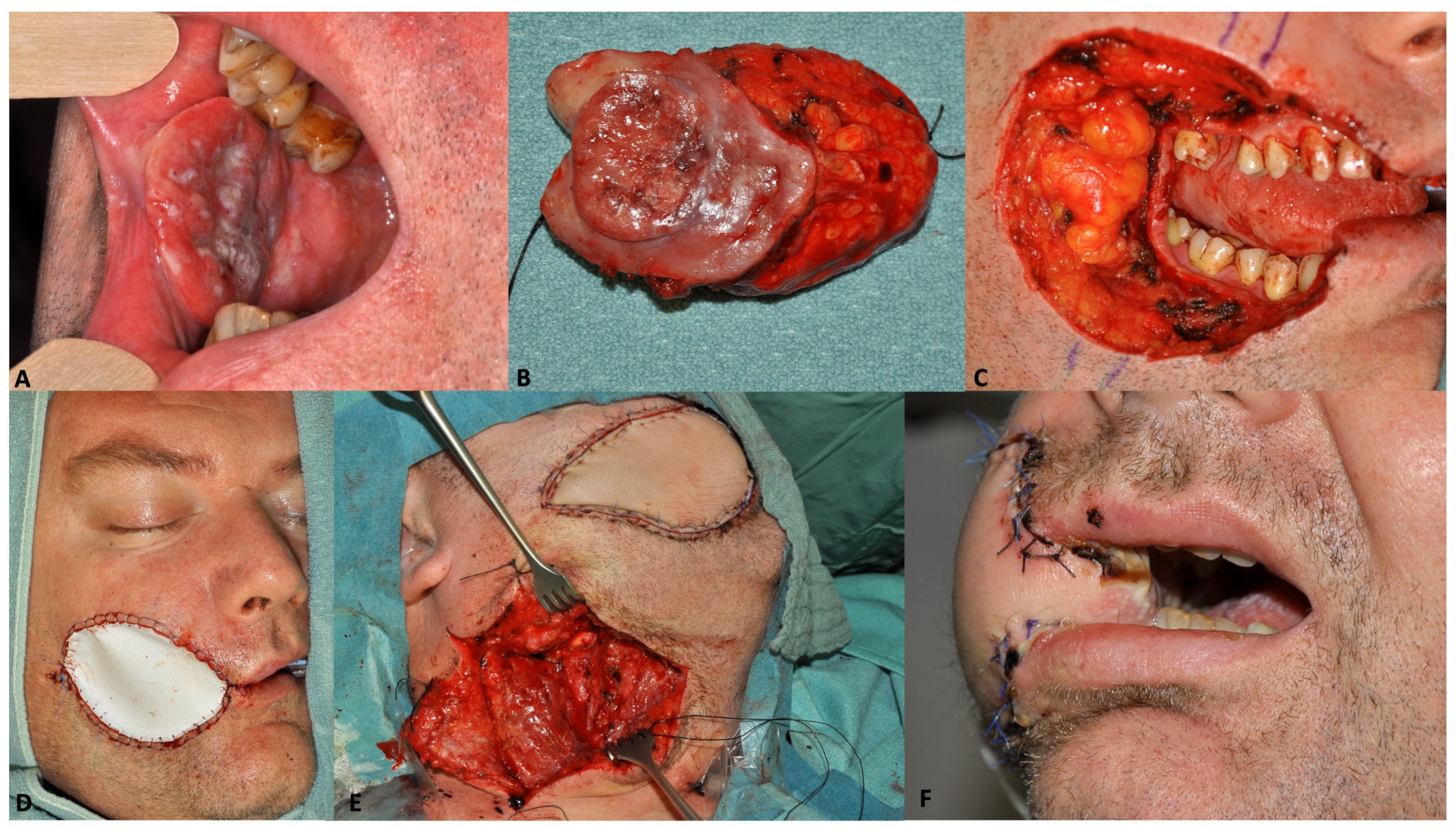
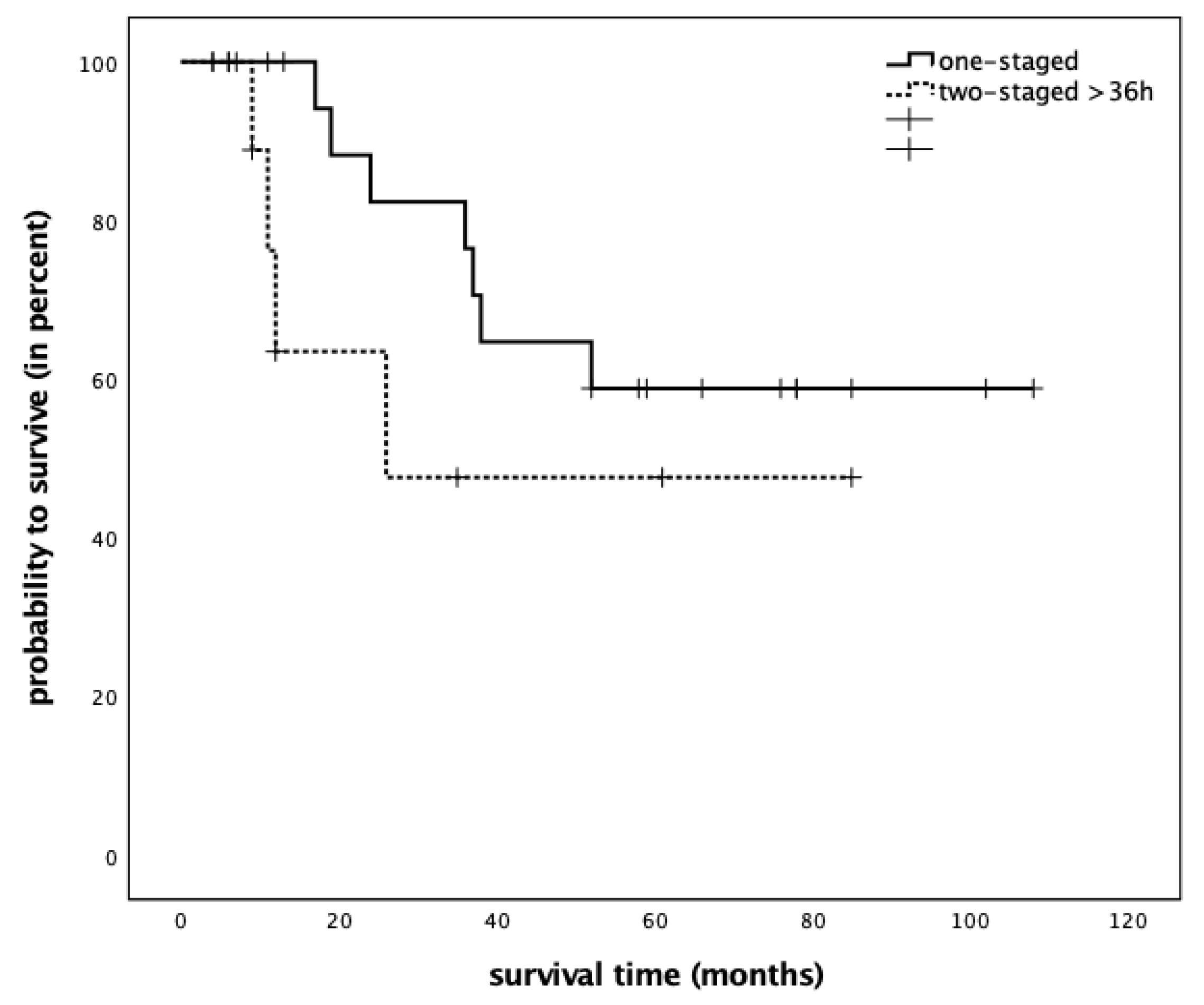
| Variable | Value | Immediate Reconstruction | Delayed Reconstruction | ||
|---|---|---|---|---|---|
| n = 20 | Percent | n = 13 | Percent | ||
| Sex | Male | 15 | 75.0% | 10 | 76.9% |
| Female | 5 | 25.0% | 3 | 23.1% | |
| Age at diagnosis | ≤50 | 4 | 20.0% | 3 | 23.1% |
| 51–60 | 6 | 30.0% | 4 | 30.8% | |
| 61–70 | 5 | 25.0% | 3 | 23.1% | |
| 71–80 | 5 | 25.0% | 2 | 15.4% | |
| ASA score | ASA I/II | 10 | 50.0% | 7 | 53.8% |
| ASA III/IV | 4 | 20.0% | 1 | 7.7% | |
| Missing | 6 | 30.0% | 5 | 38.5% | |
| Tumor site | Oral cavity | 8 | 40.0% | 12 | 92.3% |
| Pharynx | 10 | 50.0% | 1 | 7.7% | |
| Esophagus | 2 | 10.0% | 0 | 0.0% | |
| UICC stage | Stage I | 1 | 5.0% | 0 | 0.0% |
| Stage II | 4 | 20.0% | 0 | 0.0% | |
| Stage III | 2 | 10.0% | 4 | 30.8% | |
| Stage IV | 11 | 55.0% | 7 | 53.8% | |
| Missing | 2 | 10.0% | 2 | 15.4% | |
| p16 IHC | Negative | 13 | 65.0% | 7 | 53.8% |
| Positive | 1 | 5.0% | 1 | 7.7% | |
| Missing | 6 | 30.0% | 5 | 38.5% | |
| Pack years | <10 pack years | 9 | 45.0% | 2 | 15.4% |
| >10 pack years | 6 | 30.0% | 6 | 46.2% | |
| Missing | 5 | 25.0% | 5 | 38.5% | |
| Prior irradiation | No | 13 | 65.0% | 10 | 76.9% |
| Yes | 7 | 35.0% | 3 | 23.1% | |
| Phase of disease | First diagnosis | 9 | 45.0% | 8 | 61.5% |
| Persistent disease | 1 | 5.0% | 2 | 15.4% | |
| Recurrent disease | 8 | 40.0% | 3 | 23.1% | |
| Second tumor | 2 | 10.0% | 0 | 0.0% | |
| Immediate Reconstruction | Delayed Reconstruction | ||||
|---|---|---|---|---|---|
| Surgical Specification | n = 20 | Percent | n = 13 | Percent | |
| Type of free flap | Radial forearm flap | 12 | 60.0% | 9 | 69.2% |
| Anterior lateral thigh flap | 4 | 20.0% | 3 | 23.1% | |
| Latissimus dorsi flap | 0 | 0.0% | 1 | 7.7% | |
| Jejunal flap | 4 | 20.0% | 0 | 0.0% | |
| Neck dissection | No | 4 | 20.0% | 0 | 0.0% |
| Unilateral | 8 | 40.0% | 6 | 46.2% | |
| Bilateral | 8 | 40.0% | 7 | 53.8% | |
| Tracheotomy | Yes | 16 | 80.0% | 11 | 84.6% |
| No | 0 | 0.0% | 2 | 15.4% | |
| Prior tracheostomy | 4 | 20.0% | 0 | 0.0% | |
| Surgical approach | External approach | 7 | 35.0% | 8 | 61.5% |
| Transoral | 7 | 35.0% | 4 | 30.8% | |
| Total laryngectomy | 6 | 30.0% | 1 * | 7.7% | |
| Immediate Reconstruction | Delayed Reconstruction | |||
|---|---|---|---|---|
| Type of Complication | n = 20 * | Percent | n = 13 * | Percent |
| Bleeding | 7 | 35.0% | 5 | 38.5% |
| Wound infection | 4 | 20.0% | 3 | 23.1% |
| Fistula | 1 | 5.0% | 1 | 7.7% |
| Pneumonia | 2 | 10.0% | 1 | 7.7% |
| Thrombosis | 1 | 5.0% | 0 | 0.0% |
| Other | 2 | 10.0% | 1 | 7.7% |
| Immediate Reconstruction | Delayed Reconstruction | |||
|---|---|---|---|---|
| Clavien–Dindo Score | n = 20 | Percent | n = 13 | Percent |
| 0 | 5 | 25.0% | 4 | 30.8% |
| 1 | 4 | 20.0% | 1 | 7.7% |
| 2 | 4 | 20.0% | 1 | 7.7% |
| 3 | 7 | 35.0% | 6 | 46.2% |
| 4 | 0 | 0.0% | 1 | 7.7% |
| Immediate Reconstruction | Delayed Reconstruction | |||
|---|---|---|---|---|
| Functional Domain | Functional Integrity | n = 13 | n = 8 | p-Values |
| Food intake | Impaired | 10 (76.9%) | 4 (50.0%) | p = 0.35 |
| Normal/near normal | 3 (23.1%) | 4 (50.0%) | ||
| Breathing | Impaired | 4 (30.8%) | 0 (0.0%) | p = 0.13 |
| Normal/near normal | 9 (69.2%) | 8 (100%) | ||
| Speech | Impaired | 8 (61.5%) | 3 (37.5%) | p = 0.39 |
| Normal/near normal | 5 (38.5%) | 5 (62.5%) | ||
| Pain | Impaired | 1 (7.7%) | 1 (12.5%) | p = 0.72 |
| Normal/near normal | 12 (92.3%) | 7 (87.5%) | ||
| Mood | Impaired | 0 (0.0%) | 1 (12.5%) | p = 0.38 |
| Normal/near normal | 13 (100%) | 7 (87.5%) | ||
| Neck and shoulder mobility | Impaired | 5 (38.5%) | 3 (37.5%) | p = 0.96 |
| Normal/near normal | 8 (61.5%) | 5 (62.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steinbichler, T.B.; Rauchenwald, T.; Rajsic, S.; Fischer, H.T.; Wolfram, D.; Runge, A.; Dejaco, D.; Prossliner, H.; Pierer, G.; Riechelmann, H. Delayed Reconstruction after Major Head and Neck Cancer Resection: An Interdisciplinary Feasibility Study. Cancers 2023, 15, 2777. https://doi.org/10.3390/cancers15102777
Steinbichler TB, Rauchenwald T, Rajsic S, Fischer HT, Wolfram D, Runge A, Dejaco D, Prossliner H, Pierer G, Riechelmann H. Delayed Reconstruction after Major Head and Neck Cancer Resection: An Interdisciplinary Feasibility Study. Cancers. 2023; 15(10):2777. https://doi.org/10.3390/cancers15102777
Chicago/Turabian StyleSteinbichler, Teresa B., Tina Rauchenwald, Sasa Rajsic, Hannes T. Fischer, Dolores Wolfram, Annette Runge, Daniel Dejaco, Harald Prossliner, Gerhard Pierer, and Herbert Riechelmann. 2023. "Delayed Reconstruction after Major Head and Neck Cancer Resection: An Interdisciplinary Feasibility Study" Cancers 15, no. 10: 2777. https://doi.org/10.3390/cancers15102777
APA StyleSteinbichler, T. B., Rauchenwald, T., Rajsic, S., Fischer, H. T., Wolfram, D., Runge, A., Dejaco, D., Prossliner, H., Pierer, G., & Riechelmann, H. (2023). Delayed Reconstruction after Major Head and Neck Cancer Resection: An Interdisciplinary Feasibility Study. Cancers, 15(10), 2777. https://doi.org/10.3390/cancers15102777









