Introduction of a Polyethylene Glycol Linker Improves Uptake of 67Cu-NOTA-Conjugated Lactam-Cyclized Alpha-Melanocyte-Stimulating Hormone Peptide in Melanoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
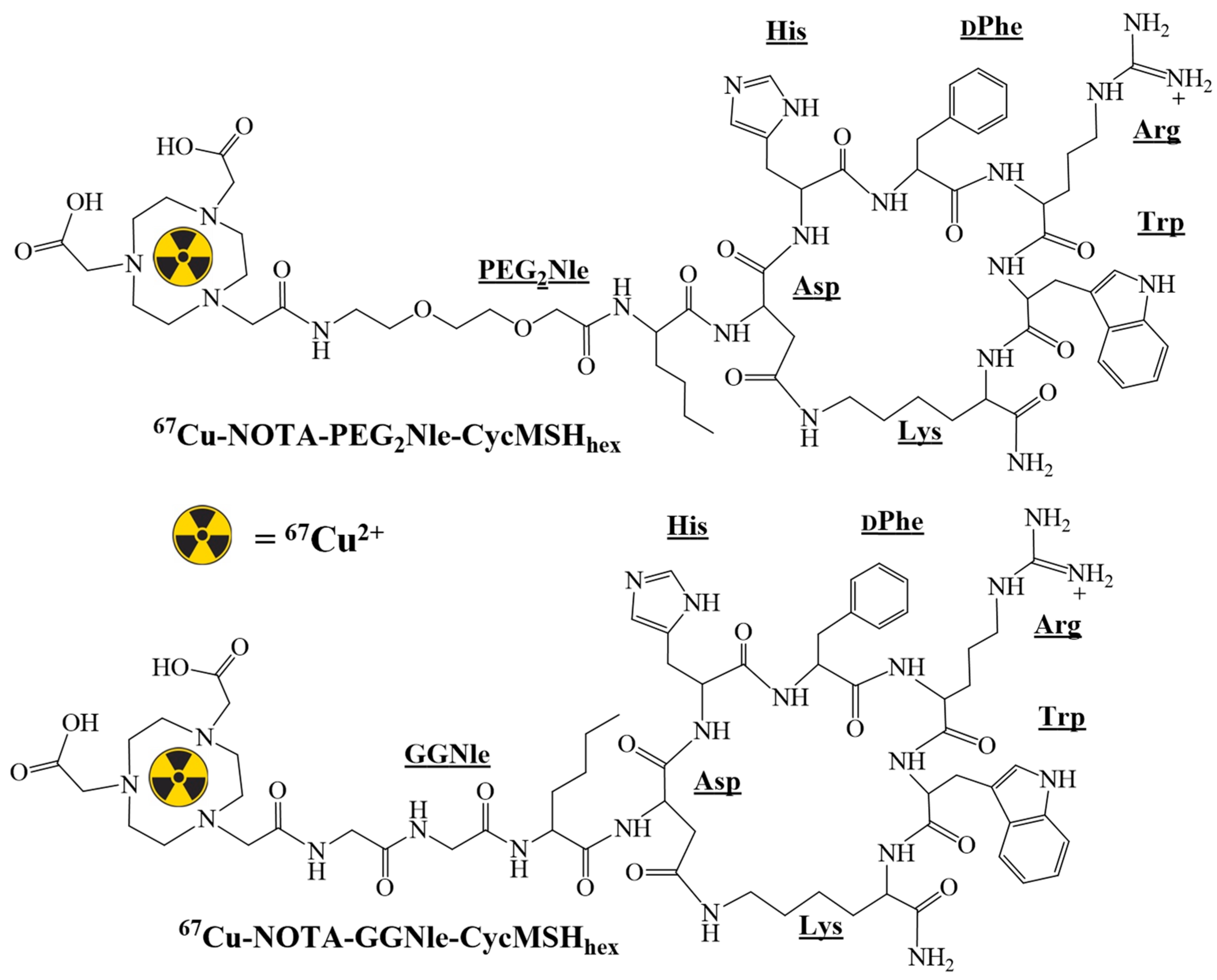
2.2. Peptide Synthesis and Radiolabeling
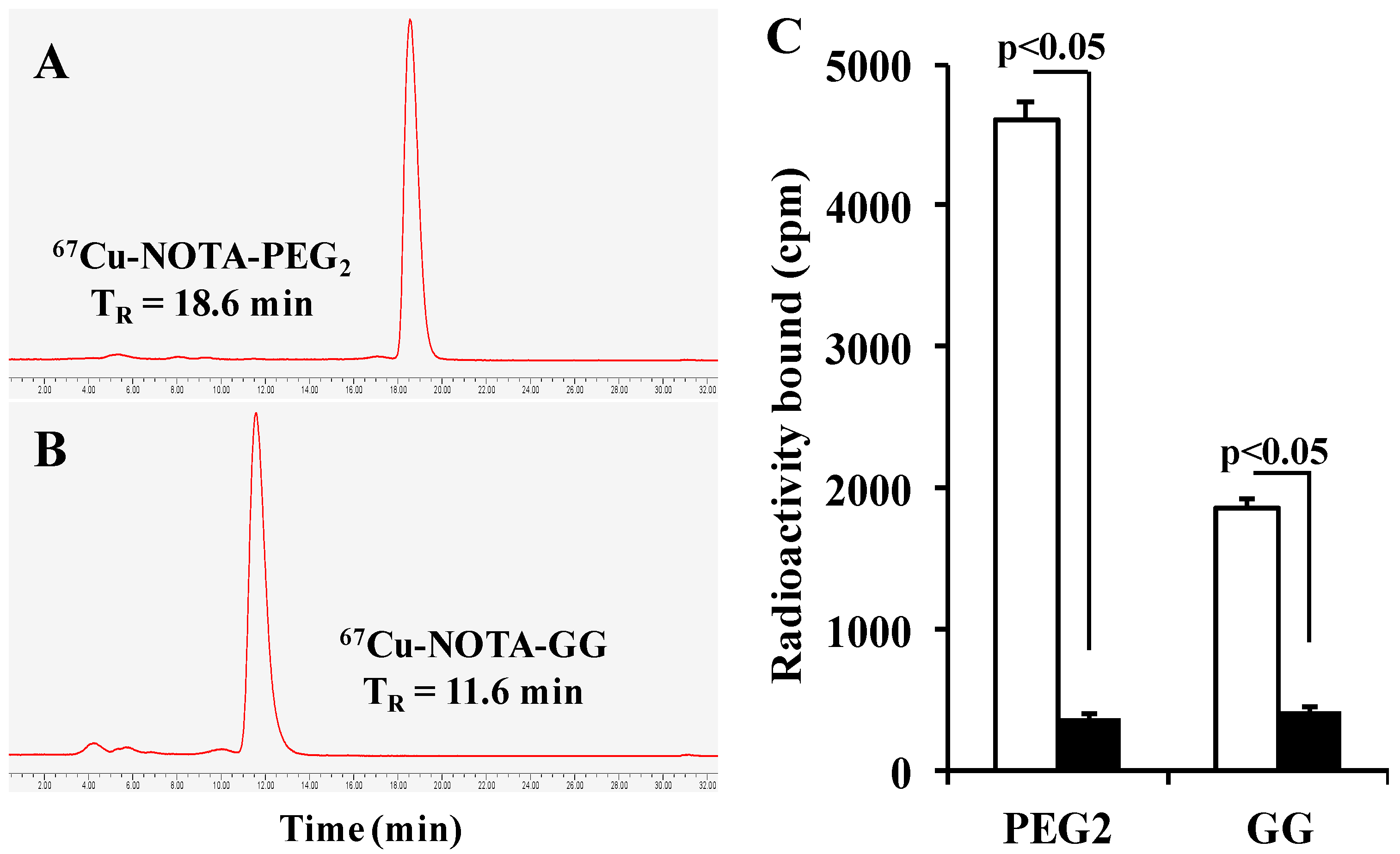
2.3. Specific Binding
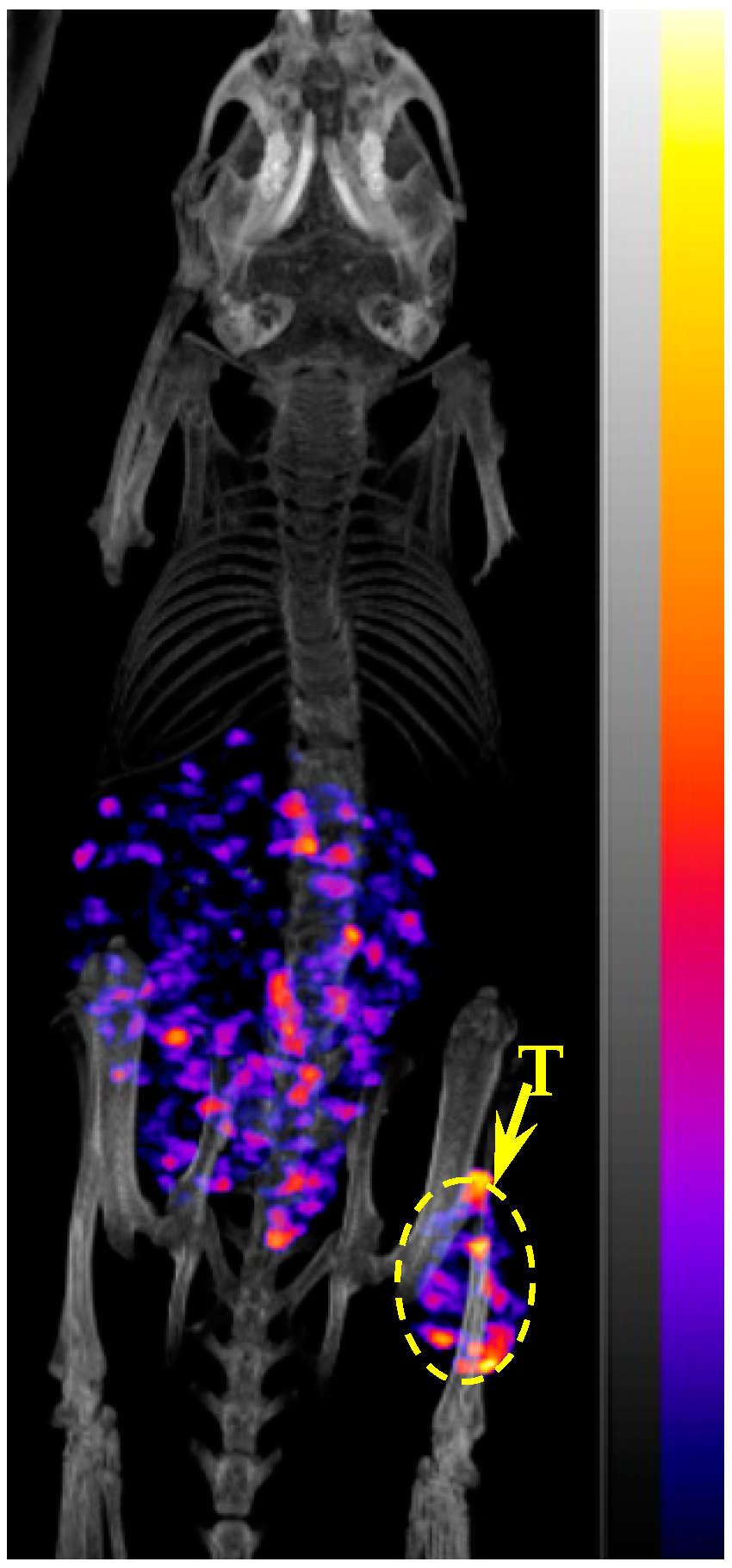
2.4. Biodistribution and Imaging Studies
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. BRIM-3 Study Group. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef] [PubMed]
- Sosman, J.A.; Kim, K.B.; Schuchter, L.; Gonzalez, R.; Pavlick, A.C.; Weber, J.S.; McArthur, G.A.; Hutson, T.E.; Moschos, S.J.; Flaherty, K.T.; et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N. Engl. J. Med. 2012, 366, 707–714. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.S.; O’day, S.; Urba, W.; Powderly, J.; Nichol, G.; Yellin, M.; Snively, J.; Hersh, E. Phase I/II study of ipilimumab for patients with metastatic melanoma. J. Clin. Oncol. 2008, 26, 5950–5956. [Google Scholar] [CrossRef]
- Topalian, S.L.; Sznol, M.; McDermott, D.F.; Kluger, H.M.; Carvajal, R.D.; Sharfman, W.H.; Brahmer, J.R.; Lawrence, D.P.; Atkins, M.B.; Powderly, J.D.; et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. 2014, 32, 1020–1030. [Google Scholar] [CrossRef]
- Weiss, S.A.; Wolchok, J.D.; Sznol, M. Immunotherapy of melanoma: Facts and hopes. Clin. Cancer Res. 2019, 25, 5191–5201. [Google Scholar] [CrossRef]
- Siegrist, W.; Solca, F.; Stutz, S.; Giuffrè, L.; Carrel, S.; Girard, J.; Eberle, A.N. Characterization of receptors for alpha-melanocyte-stimulating hormone on human melanoma cells. Cancer Res. 1989, 49, 6352–6358. [Google Scholar]
- Tatro, J.B.; Wen, Z.; Entwistle, M.L.; Atkins, M.B.; Smith, T.J.; Reichlin, S.; Murphy, J.R. Interaction on an α-melanocyte stimulating hormone-diptheria toxin fusion protein with melanotropin receptors in human metastases. Cancer Res. 1992, 52, 2545–2548. [Google Scholar]
- Yang, J.; Xu, J.; Gonzalez, R.; Lindner, T.; Kratochwil, C.; Miao, Y. 68Ga-DOTA-GGNle-CycMSHhex targets the melanocortin-1 receptor for melamoma imaging. Sci. Transl. Med. 2018, 10, eaau4445. [Google Scholar] [CrossRef]
- Guo, H.; Yang, J.; Gallazzi, F.; Miao, Y. Reduction of the ring size of radiolabeled lactam bridge-cyclized alpha-MSH peptide resulting in enhanced melanoma uptake. J. Nucl. Med. 2010, 51, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Yang, J.; Gallazzi, F.; Miao, Y. Effects of the amino acid linkers on melanoma-targeting and pharmacokinetic properties of Indium-111-labeled lactam bridge-cyclized α-MSH peptides. J. Nucl. Med. 2011, 52, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Gallazzi, F.; Miao, Y. Ga-67-labeled lactam bridge-cyclized alpha-MSH peptides with enhanced melanoma uptake and reduced renal uptake. Bioconjug. Chem. 2012, 23, 1341–1348. [Google Scholar] [CrossRef]
- Guo, H.; Miao, Y. Cu-64-labeled lactam bridge-cyclized alpha-MSH peptides for PET imaging of melanoma. Mol. Pharm. 2012, 9, 2322–2330. [Google Scholar] [CrossRef]
- Guo, H.; Gallazzi, F.; Miao, Y. Design and evaluation of new Tc-99m-labeled lactam bridge-cyclized alpha-MSH peptides for melanoma imaging. Mol. Pharm. 2013, 10, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Miao, Y. Introduction of an aminooctanoic acid linker enhances uptake of Tc-99m-labeled lactam bridge-cyclized alpha-MSH peptide in melanoma. J. Nucl. Med. 2014, 55, 2057–2063. [Google Scholar] [CrossRef]
- Guo, H.; Miao, Y. Melanoma targeting property of a Lu-177-labeled lactam bridge-cyclized alpha-MSH peptide. Bioorg. Med. Chem. Lett. 2013, 23, 2319–2323. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xu, J.; Cheuy, L.; Gonzalez, R.; Fisher, D.R.; Miao, Y. Evaluation of a novel Pb-203-labeled lactam-cyclized alpha-melanocyte-stimulating hormone peptide for melanoma targeting. Mol. Pharm. 2019, 16, 1694–1702. [Google Scholar] [CrossRef]
- Xu, J.; Yang, J.; Gonzalez, R.; Fisher, D.R.; Miao, Y. Melanoma-targeting property of Y-90-labeled lactam-cyclized alpha-melanocyte-stimulating hormone peptide. Cancer Biother. Radiopharm. 2019, 34, 597–603. [Google Scholar]
- Qiao, Z.; Xu, J.; Gonzalez, R.; Miao, Y. Novel [99mTc]-tricarbonyl-NOTA-conjugated lactam-cyclized alpha-MSH peptide with enhanced melanoma uptake and reduced renal uptake. Mol. Pharm. 2020, 17, 3581–3588. [Google Scholar] [CrossRef]
- Qiao, Z.; Xu, J.; Gonzalez, R.; Miao, Y. Novel 64Cu-labeled NOTA-conjugated lactam-cyclized alpha-melanocyte-stimulating hormone peptides with enhanced tumor to kidney uptake ratios. Mol. Pharm. 2022, 19, 2535–2541. [Google Scholar] [CrossRef] [PubMed]
- Cullinane, C.; Jeffery, C.M.; Roselt, P.D.; van Dam, E.M.; Jackson, S.; Kuan, K.; Jackson, P.; Binns, D.; van Zuylekom, J.; Harris, M.J.; et al. Peptide receptor radionuclide therapy with 67Cu-CuSarTATE is highly efficacious against a somatostatin positive neuroendocrine tumor model. J. Nucl. Med. 2020, 61, 1800–1805. [Google Scholar] [CrossRef] [PubMed]
- Keinänen, O.; Fung, K.; Brennan, J.M.; Zia, N.; Harris, M.; van Dam, E.; Biggin, C.; Hedt, A.; Stoner, J.; Donnelly, P.S.; et al. Harnessing 64Cu/67Cu for a theranostic approach to pretargeted radioimmunotherapy. Proc. Natl. Acad. Sci. USA 2020, 117, 28316–28327. [Google Scholar] [CrossRef]
- Kelly, J.M.; Ponnala, S.; Amor-Coarasa, A.; Zia, N.A.; Nikolopoulou, A.; Williams, C., Jr.; Schlyer, D.J.; DiMagno, S.G.; Donnelly, P.S.; Babich, J.W. Preclinical evaluation of a high-affinity sarcophagine-containing PSMA ligand for 64Cu/67Cu-based theranostics in prostate cancer. Mol. Pharm. 2020, 17, 1954–1962. [Google Scholar] [CrossRef]
- Hao, G.; Mastren, T.; Silvers, W.; Hassan, G.; Öz, O.K.; Sun, X. Copper-67 radioimmunotheranostics for simultaneous immunotherapy and immuno-SPECT. Sci. Rep. 2021, 11, 3622. [Google Scholar] [CrossRef]
- Huynh, T.T.; van Dam, E.M.; Sreekumar, S.; Mpoy, C.; Blyth, B.J.; Muntz, F.; Harris, M.J.; Rogers, B.E. Copper-67-Labeled Bombesin Peptide for Targeted Radionuclide Therapy of Prostate Cancer. Pharmaceuticals 2022, 15, 728. [Google Scholar] [CrossRef] [PubMed]
- Dearling, J.L.J.; van Dam, E.M.; Harris, M.J.; Packard, A.B. Detection and therapy of neuroblastoma minimal residual disease using [64/67Cu] Cu-SARTATE in a preclinical model of hepatic metastases. EJNMMI Res. 2021, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.L.; Willowson, K.P.; Harris, M.; Biggin, C.; Aslani, A.; Lengkeek, N.A.; Stoner, J.; Eslick, M.E.; Marquis, H.; Parker, M.; et al. 64Cu treatment planning and 67Cu therapy with radiolabelled SARTATE ([64Cu/67Cu]MeCOSAR-Octreotate) in subjects with unresectable multifocal meningioma–initial results for human imaging, safety, biodistribution and radiation dosimetry. J. Nucl. Med. 2023, 64, 704–710. [Google Scholar] [CrossRef]
- Dash, A.; Pillai, M.R.A.; Knapp, F.F. Production of 177Lu for targeted radionuclide therapy: Available options. Nucl. Med. Mol. Imaging 2015, 49, 85–107. [Google Scholar] [CrossRef]
- Ehst, D.A.; Smith, N.A.; Bowers, D.L.; Makarashvili, V. Copper-67 production on electron linacs—Photonuclear technology development. AIP Conf. Proc. 2012, 1509, 157–161. [Google Scholar]
- Stoner, J.; Gardner, T.; Gardner, T. A comparison of DOTA and DiamSar chelates of high specific activity eLINAC produced 67Cu. J. Nucl. Med. 2016, 57, 1107. [Google Scholar]
| Tissues | 0.5 h | 2 h | 4 h | 24 h | 2 h NDP Blockade |
|---|---|---|---|---|---|
| Percent injected dose/gram (%ID/g) | |||||
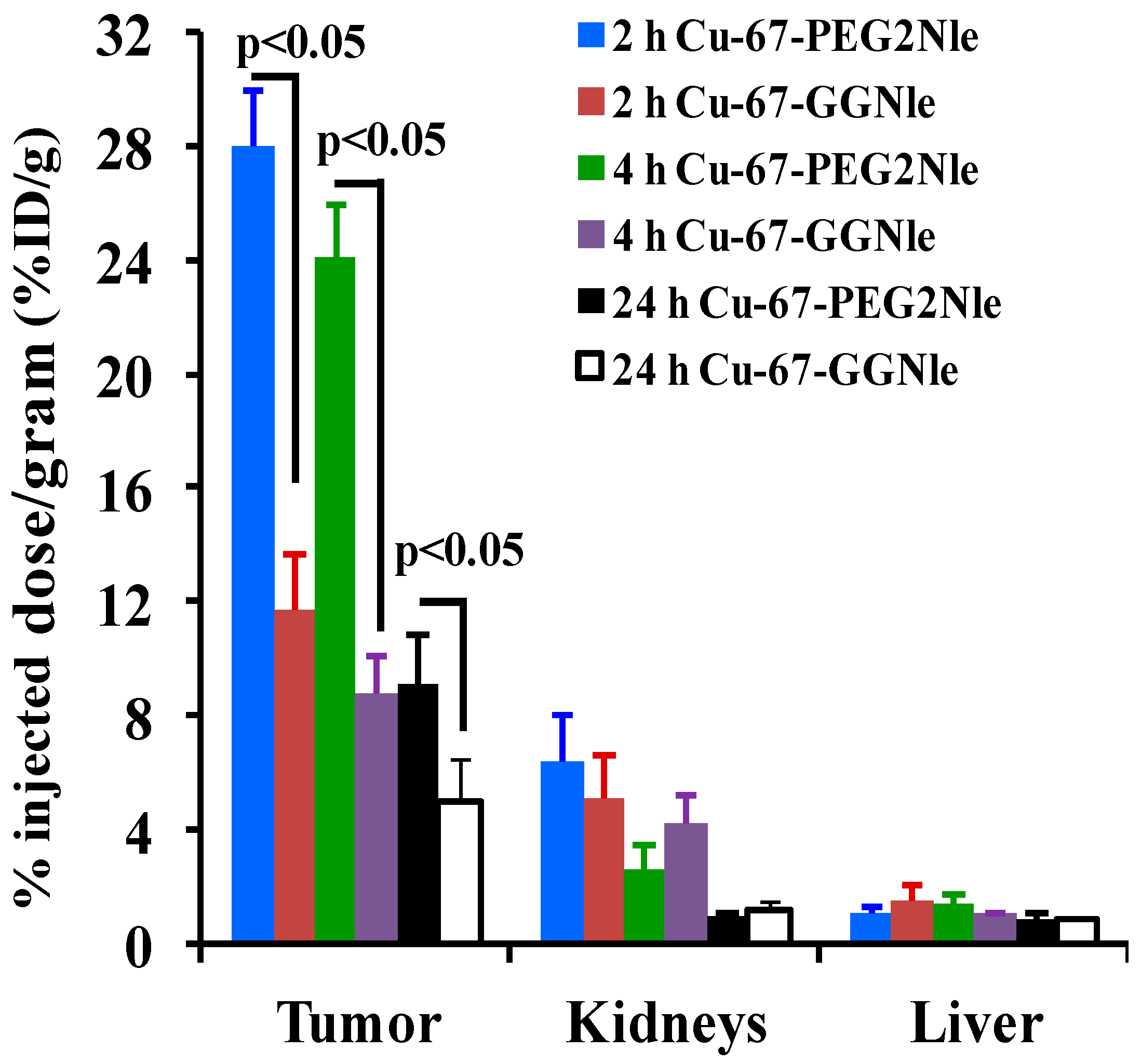
| Tumor | 8.83 ± 2.19 | 27.97 ± 1.98 | 24.10 ± 1.83 | 9.13 ± 1.66 | 1.66 ± 0.28 * |
| Brain | 0.04 ± 0.02 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.04 ± 0.03 | 0.02 ± 0.01 |
| Blood | 0.63 ± 0.26 | 0.20 ± 0.11 | 0.05 ± 0.02 | 0.02 ± 0.01 | 0.01 ± 0.01 |
| Heart | 0.58 ± 0.18 | 0.79 ± 0.06 | 0.34 ± 0.08 | 0.05 ± 0.05 | 0.11 ± 0.09 |
| Lung | 0.76 ± 0.16 | 0.53 ± 0.19 | 0.62 ± 0.18 | 0.35 ± 0.13 | 0.68 ± 0.29 |
| Liver | 0.95 ± 0.19 | 1.09 ± 0.14 | 1.41 ± 0.26 | 0.84 ± 0.15 | 1.70 ± 0.39 |
| Spleen | 0.37 ± 0.10 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.02 |
| Stomach | 1.13 ± 0.36 | 1.40 ± 0.51 | 0.77 ± 0.09 | 0.21 ± 0.09 | 0.65 ± 0.07 |
| Kidneys | 6.43 ± 1.31 | 6.34 ± 1.63 | 2.60 ± 0.79 | 0.90 ± 0.18 | 4.83 ± 0.72 |
| Muscle | 0.48 ± 0.12 | 0.81 ± 0.12 | 0.03 ± 0.03 | 0.01 ± 0.01 | 0.01 ± 0.01 |
| Pancreas | 0.10 ± 0.05 | 0.47 ± 0.06 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.08 ± 0.06 |
| Bone | 0.60 ± 0.35 | 0.07 ± 0.04 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 |
| Skin | 1.77 ± 0.28 | 0.59 ± 0.11 | 0.18 ± 0.07 | 0.03 ± 0.02 | 0.16 ± 0.04 |
| Percent injected dose (%ID) | |||||
| Intestines | 0.83 ± 0.09 | 0.95 ± 0.16 | 1.40 ± 0.34 | 0.77 ± 0.12 | 2.09 ± 0.96 |
| Urine | 78.31 ± 3.79 | 89.08 ± 4.96 | 91.05 ± 1.20 | 95.61 ± 0.59 | 89.34 ± 2.19 |
| Uptake ratio of tumor/normal tissue | |||||
| Tumor/blood | 14.02 | 139.85 | 482.0 | 456.50 | 166.0 |
| Tumor/kidney | 1.37 | 4.41 | 9.27 | 10.14 | 0.34 |
| Tumor/lung | 11.62 | 52.77 | 38.87 | 26.09 | 2.44 |
| Tumor/liver | 9.29 | 25.66 | 17.09 | 10.87 | 0.98 |
| Tumor/muscle | 18.39 | 34.53 | 803.33 | 913.0 | 166.0 |
| Tissues | 0.5 h | 2 h | 4 h | 24 h | 2 h NDP Blockade |
|---|---|---|---|---|---|
| Percent injected dose/gram (%ID/g) | |||||
| Tumor | 16.58 ± 1.40 | 11.66 ± 1.94 | 8.79 ± 1.31 | 4.92 ± 1.58 | 0.61 ± 0.15 * |
| Brain | 0.07 ± 0.04 | 0.03 ± 0.02 | 0.02 ± 0.03 | 0.02 ± 0.02 | 0.01 ± 0.01 |
| Blood | 1.22 ± 0.08 | 0.03 ± 0.05 | 0.06 ± 0.07 | 0.01 ± 0.01 | 0.01 ± 0.01 |
| Heart | 0.71 ± 0.31 | 0.04 ± 0.04 | 0.07 ± 0.06 | 0.03 ± 0.03 | 0.04 ± 0.04 |
| Lung | 0.54 ± 0.69 | 0.41 ± 0.20 | 0.37 ± 0.09 | 0.27 ± 0.12 | 0.25 ± 0.16 |
| Liver | 1.15 ± 0.07 | 1.49 ± 0.56 | 1.02 ± 0.07 | 0.82 ± 0.12 | 0.66 ± 0.23 |
| Spleen | 0.52 ± 0.32 | 0.05 ± 0.03 | 0.12 ± 0.08 | 0.03 ± 0.01 | 0.09 ± 0.08 |
| Stomach | 1.53 ± 0.53 | 0.81 ± 0.14 | 0.71 ± 0.22 | 0.30 ± 0.17 | 0.65 ± 0.22 |
| Kidneys | 7.18 ± 2.63 | 5.08 ± 1.52 | 4.21 ± 0.92 | 1.19 ± 0.28 | 3.19 ± 0.89 |
| Muscle | 0.51 ± 0.18 | 0.04 ± 0.04 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 |
| Pancreas | 0.10 ± 0.08 | 0.12 ± 0.08 | 0.04 ± 0.04 | 0.02 ± 0.01 | 0.02 ± 0.02 |
| Bone | 0.68 ± 0.10 | 0.21 ± 0.24 | 0.06 ± 0.06 | 0.13 ± 0.16 | 0.01 ± 0.01 |
| Skin | 2.05 ± 0.46 | 0.50 ± 0.24 | 0.27 ± 0.19 | 0.02 ± 0.01 | 0.09 ± 0.06 |
| Percent injected dose (%ID) | |||||
| Intestines | 1.46 ± 0.49 | 1.30 ± 0.27 | 2.70 ± 0.26 | 0.94 ± 0.32 | 1.48 ± 0.17 |
| Urine | 79.77 ± 2.25 | 88.97 ± 2.48 | 86.99 ± 1.22 | 96.23 ± 1.03 | 95.14 ± 1.28 |
| Uptake ratio of tumor/normal tissue | |||||
| Tumor/blood | 13.59 | 388.67 | 146.50 | 492.0 | 61.0 |
| Tumor/kidney | 2.31 | 2.29 | 2.09 | 4.13 | 0.19 |
| Tumor/lung | 30.70 | 28.44 | 23.76 | 18.22 | 2.44 |
| Tumor/liver | 14.42 | 7.83 | 8.62 | 6.0 | 0.92 |
| Tumor/muscle | 32.51 | 291.50 | 879.0 | 492.0 | 61.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, Z.; Xu, J.; Fisher, D.R.; Gonzalez, R.; Miao, Y. Introduction of a Polyethylene Glycol Linker Improves Uptake of 67Cu-NOTA-Conjugated Lactam-Cyclized Alpha-Melanocyte-Stimulating Hormone Peptide in Melanoma. Cancers 2023, 15, 2755. https://doi.org/10.3390/cancers15102755
Qiao Z, Xu J, Fisher DR, Gonzalez R, Miao Y. Introduction of a Polyethylene Glycol Linker Improves Uptake of 67Cu-NOTA-Conjugated Lactam-Cyclized Alpha-Melanocyte-Stimulating Hormone Peptide in Melanoma. Cancers. 2023; 15(10):2755. https://doi.org/10.3390/cancers15102755
Chicago/Turabian StyleQiao, Zheng, Jingli Xu, Darrell R. Fisher, Rene Gonzalez, and Yubin Miao. 2023. "Introduction of a Polyethylene Glycol Linker Improves Uptake of 67Cu-NOTA-Conjugated Lactam-Cyclized Alpha-Melanocyte-Stimulating Hormone Peptide in Melanoma" Cancers 15, no. 10: 2755. https://doi.org/10.3390/cancers15102755
APA StyleQiao, Z., Xu, J., Fisher, D. R., Gonzalez, R., & Miao, Y. (2023). Introduction of a Polyethylene Glycol Linker Improves Uptake of 67Cu-NOTA-Conjugated Lactam-Cyclized Alpha-Melanocyte-Stimulating Hormone Peptide in Melanoma. Cancers, 15(10), 2755. https://doi.org/10.3390/cancers15102755




