Risk and Prognosis of Thyroid Cancer in Patients with Graves’ Disease: An Umbrella Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Umbrella Review Methods
2.2. Search Strategy and Selection Criteria
2.3. Eligibility Criteria
2.4. Data Extraction
2.5. Evaluation of the Strength of Evidence
2.6. Quality Assessment
2.7. Statistical Analysis
3. Results
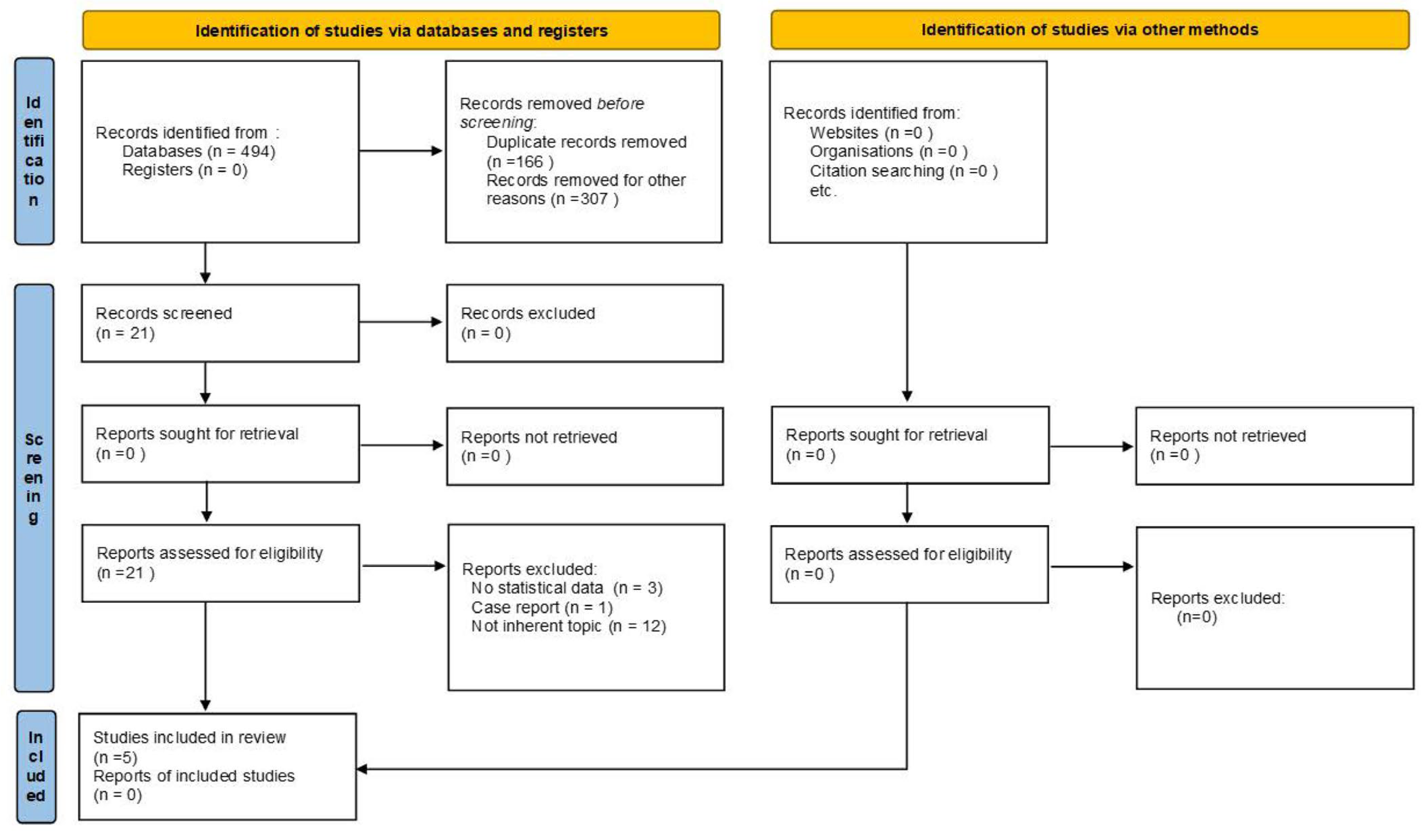
3.1. Search Strategy Outcome
3.2. Quality Assessment and Bias
3.3. Risk and Prognosis (Mortality and Recurrence/Persistence) of Thyroid Cancer in Patients with Graves’ Disease
3.3.1. Risk of Thyroid Cancer in Patients with Graves’ Disease by Comparison Groups
Risk of Thyroid Cancer in Patients with Graves’ Disease vs. Multinodular Toxic Goiter (MTG), Uninodular Toxic Goiter (UTG), or Unspecified Toxic Nodular Goiter (uTNG)
Risk of Thyroid Cancer in Patients with Graves’ Disease with Nodular vs. Those without Nodules
Number of Thyroid Nodules in Graves’ Disease Patients and Risk of Differentiated Thyroid Cancer
3.3.2. Prognosis (Mortality) of Thyroid Cancer in Patients with Graves’ Disease by Comparison Groups
Graves’ Disease vs. No Graves’ Disease Hyperthyroid Patients
Graves’ Disease Hyperthyroid Patients Compared with Euthyroid Subjects
Graves’ Disease Patients Compared with Those without Graves’ Disease (Including Both Euthyroid and Hyperthyroid Patients)
3.3.3. Prognosis (Recurrence/Persistence) of Thyroid Cancer in Patients with Graves’ Disease by Comparison Groups
Graves’ Disease Compared to Non-Graves’ Disease Hyperthyroid Patients
Patients with Graves’ Disease Compared with Euthyroid Patients
Patients with Graves’ Disease Compared to Non-Graves’ Disease Patients (Including Both Euthyroid and Hyperthyroid Patients)
4. Discussion
4.1. Main Findings and Interpretation Considering Evidence
- “Strong” evidence of thyroid cancer risk in patients with GD and thyroid nodules compared to patients with GD without nodules.
- “Modest” evidence of thyroid cancer risk in GD patients compared to MTG patients and in GD patients with solitary nodules compared with GD patients with multiple nodules.
- “Strong evidence of increased thyroid cancer risk mortality in GD patients compared with non-GD patients (including both euthyroid and hyperthyroid patients) that increased after excluding the low-rate incidental cancers and also after stratifying by continent (Europe higher than Asia) [41].
- “Moderate” evidence of higher risk of recurrence/persistence among patients with GD compared to those without GD (including both euthyroid and hyperthyroid patients) by continents (Europa and America vs. Asia).
4.2. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mclever, B.; Morris, J.C. The pathogenesis of Graves’ disease. Endocrinol. Metab. Clin. N. Am. 1998, 27, 73–89. [Google Scholar]
- Subekti, I.; Pramono, L.A. Current Diagnosis and Management of Graves’ Disease. Indones. J. Intern. Med. 2018, 50, 2. [Google Scholar]
- Davies, T.F.; Andersen, S.; Latif, R.; Nagayama, Y.; Barbesino, G.; Brito, M.; Eckstein, A.K.; Stagnaro-Green, A.; Hahaly, G.J. Graves’ disease. Mat. Rev. Dis. Prim. 2020, 6, 52. [Google Scholar] [CrossRef]
- Ferrari, S.M.; Fallahi, P.; Elia, G.; Ragusa, F.; Ruffilli, I.; Paparo, S.R.; Antonelli, A. Thyroid autoimmune disorders and cancer. Semin. Cancer Biol. 2020, 64, 135–146. [Google Scholar] [CrossRef]
- Dias Lopes, N.M.; Mendonca Lens, H.H.; Armani, A.; Marinello, P.C.; Cecchini, A.L. Thyroid cancer and thyroid autoimmune disease: A review of molecular aspects and clinical outcomes. Pathol. Res. Pract. 2020, 216, 153098. [Google Scholar] [CrossRef] [PubMed]
- Laha, D.; Nilubol, N.; Boufragech, M. New Therapies for Advanced Thyroid Cancer. Front. Endocrinol. 2020, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Chmielik, E.; Rusinek, D.; Oczko-Wojciechowska, M.; Jarzab, M.; Krajewska, J.; Czarniecka, A.; Jarzab, B. Heterogeneity of Thyroid Cancer. Pathobiology 2018, 85, 117–129. [Google Scholar] [CrossRef]
- Feldt-Rasmussen, U. Hashimoto’s thyroiditis as a risk factor for thyroid cancer. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 364–371. [Google Scholar] [CrossRef]
- Yoon, J.H.; Jin, M.; Kim, M.; Hong, A.R.; Kim, H.K.; Kim, B.H.; Kim, W.B.; Shong, Y.K.; Jeon, M.J.; Kang, H.C. Clinical Characteristics and Prognosis of Coexisting Thyroid Cancer in Patients with Graves’ Disease: A Retrospective Multicenter Study. Endocrinol. Metab. 2021, 36, 1268–1276. [Google Scholar] [CrossRef]
- Gabriele, R.; Letizia, C.; Borghese, M.; De Toma, G.; Celi, M.; Izzo, L.; Cavallaro, A. Thyroid cancer in patients with hyperthyroidism. Horm. Res. 2003, 60, 79–83. [Google Scholar] [CrossRef]
- Rieger, R.; Pimpl, W.; Money, S.; Rettenbacher, L.; Galvan, G. Hyperthyroidism and concurrent thyroid malignancies. Surgery 1989, 106, 6–10. [Google Scholar] [PubMed]
- Pazaitou-Panayiotou, K.; Michalakis, K.; Paschke, R. Thyroid cancer in patients with hyperthyroidism. Horm. Metab. Res. 2012, 44, 255–262. [Google Scholar] [CrossRef]
- Keskin, C.; Sahin, M.; Hasanov, R.; Aydogan, B.I.; Demir, O.; Emral, R.; Gullu, S.; Erdogan, M.F.; Gedik, V.; Uysal, A.R.; et al. Frequency of thyroid nodules and thyroid cancer in thyroidectomized patients with Graves’ disease. Arch. Med. Sci. 2019, 16, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Di Cristofano, A. The Year in Basic Thyroid Cancer Research. Thyroid 2022, 32, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Moscatello, C.; Di Marcantonio, M.C.; Savino, L.; D’Amico, E.; Spacco, G.; Simeone, P.; Lanuti, P.; Muraro, R.; Mincione, G.; Cotellese, R.; et al. Emerging Role of Oxidative Stress on EGFR and OGG1-BER Cross-Regulation: Implications in Thyroid Physiopathology. Cells 2022, 11, 822. [Google Scholar] [CrossRef]
- Ozaki, O.; Ito, K.; Kobayashi, K.; Toshima, K.; Iwasaki, H.; Yashiro, T. Thyroid carcinoma in Graves’ disease. World J. Surg. 1990, 14, 437–441. [Google Scholar] [CrossRef]
- Kasuga, Y.; Sugenoya, A.; Kobayashi, S.; Masuda, H.; Iida, F. The outcome of patients with thyroid carcinoma and Graves’ disease. Surg. Today 1993, 23, 9–12. [Google Scholar] [CrossRef]
- Hales, I.B.; McElduff, A.; Crummer, P.; Clifton-Bligh, P.; Delbridge, L.; Hoschl, R.; Poole, A.; Reeve, T.S.; Wilmshurst, E.; Wiseman, J. Does Graves’ disease or thyrotoxicosis affect the prognosis of thyroid cancer. J. Clin. Endocrinol. Metab. 1992, 75, 886–889. [Google Scholar]
- Kikuchi, S.; Noguchi, S.; Yamashita, H.; Uchino, S.; Kawamoto, H. Prognosis of small thyroid cancer in patients with Graves’ disease. Br. J. Surg. 2006, 93, 434–439. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Radua, J. Ten simple rules for conducting umbrella reviews. Evid. Based Ment. Health 2018, 21, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, N160. [Google Scholar] [CrossRef] [PubMed]
- Bartalena, L. Graves’ Disease: Complications. [Updated 2018 Feb 20]. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Damaskos, C.; Garmpis, N.; Dimitroulis, D.; Kyriakos, G.; Diamantis, E. Is There a Correlation of TSI Levels and Incidental Papillary Thyroid Carcinoma in Graves Disease? A Review of the Latest Evidence. Acta Med. 2021, 64, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, Y. Thyroid Autoimmunity and Thyroid Cancer—The Pathogenic Connection: A 2018 Update. Horm. Metab. Res. 2018, 50, 922–931. [Google Scholar] [CrossRef]
- Al Eyadeh, A.A.; Al-Sarihin, K.; Etewi, S.; Al-Omari, A.; Al-Asa’d, R.A.; Haddad, F.H. Thyroid cancer post radioactive iodine treatment for hyperthyroidism—Case series and review of the literature. Endokrynol. Pol. 2017, 68, 561–566. [Google Scholar] [CrossRef]
- Bonnema, S.J.; Hegedüs, L. Radioiodine therapy in benign thyroid diseases: Effects, side effects, and factors affecting therapeutic outcome. Endocr. Rev. 2012, 33, 920–980. [Google Scholar] [CrossRef]
- Alhashemi, A.; Goldstein, D.P.; Sawka, A.M. A systematic review of primary active surveillance management of low-risk papillary carcinoma. Curr. Opin. Oncol. 2016, 28, 11–17. [Google Scholar] [CrossRef]
- Medas, F.; Erdas, E.; Canu, G.L.; Longheu, A.; Pisano, G.; Tuveri, M.; Calò, P.G. Does hyperthyroidism worsen prognosis of thyroid carcinoma? A retrospective analysis on 2820 consecutive thyroidectomies. J. Otolaryngol. Head Neck Surg. 2018, 47, 6. [Google Scholar] [CrossRef]
- Varadharajan, K.; Choudhury, N. A systematic review of the incidence of thyroid carcinoma in patients undergoing thyroidectomy for thyrotoxicosis. Clin. Otolaryngol. 2020, 45, 538–544. [Google Scholar] [CrossRef]
- Arosemena, M.A.; Cipriani, N.A.; Dumitrescu, A.M. Graves’ disease and papillary thyroid carcinoma: Case report and literature review of a single academic center. BMC Endocr. Disord. 2022, 22, 199. [Google Scholar] [CrossRef]
- Anastasilakis, A.D.; Ruggeri, R.M.; Polyzos, S.A.; Makras, P.; Molyva, D.; Campennì, A.; Gkiomisi, A.; Balaris, C.; Fotiadis, P.P.; Tuccari, G.; et al. Coexistence of Graves’ disease, papillary thyroid carcinoma and unilateral benign struma ovarii: Case report and review of the literature. Metabolism 2013, 62, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Pacini, F. Thyroid microcarcinoma. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Kyrgiou, M.; Kalliala, I.; Markozannes, G.; Gunter, M.J.; Paraskevaidis, E.; Gabra, H.; Martin-Hirsch, P.; Tsilidis, K.K. Adiposity and cancer at major anatomical sites: Umbrella review of the literature. BMJ 2017, 356, j477. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P.A. The Importance of Predefined Rules and Prespecified Statistical Analyses: Do Not Abandon Significance. JAMA 2019, 321, 2067–2068. [Google Scholar] [CrossRef]
- Wasserstein, R.L.; Schirm, A.L.; Lazar, N.A. Moving to a World Beyond “p < 0.05”. Am. Stat. 2020, 73, 1–19. [Google Scholar]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Mekraksakit, P.; Rattanawong, P.; Karnchanasorn, R.; Kanitsoraphan, C.; Leelaviwat, N.; Poonsombudlert, K.; Kewcharoen, J.; Dejhansathit, S.; Samoa, R. Prognosis of differentiated thyroid carcinoma in patients with graves disease: A systematic review and meta-analysis. Endocr. Pract. 2019, 25, 1323–1337. [Google Scholar] [CrossRef]
- Papanastasiou, A.; Sapalidis, K.; Goulis, D.G.; Michalopoulos, N.; Mareti, E.; Mantalovas, S.; Kesisoglou, I. Thyroid nodules as a risk factor for thyroid cancer in patients with Graves’ disease: A systematic review and meta-analysis of observational studies in surgically treated patients. Clin. Endocrinol. 2019, 91, 571–577. [Google Scholar] [CrossRef]
- Staniforth, J.U.L.; Erdirimanne, S.; Eslick, G.D. Thyroid carcinoma in Graves’ disease: A meta-analysis. Int. J. Surg. 2016, 27, 118–125. [Google Scholar] [CrossRef]
- Song, Y.; Fu, L.; Wang, P.; Ning, S.; Qiu, X.; Li, J.; Zheng, S.; Ren, S.; Ding, X.; Li, L.; et al. Effect of Graves’ disease on the prognosis of differentiated thyroid carcinoma: A meta-analysis. Endocrine 2020, 67, 516–525. [Google Scholar] [CrossRef]
- Jia, Q.; Li, X.; Liu, Y.; Li, L.; Kwong, J.S.W.; Ren, K.; Jiang, Y.; Sun, X.; Tian, H.; Li, S. Incidental thyroid carcinoma in surgery-treated hyperthyroid patients with Graves’ disease: A systematic review and meta-analysis of cohort studies. Cancer Manag. Res. 2018, 10, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Peacock, J.L.; Peacock, P.J. Oxford Handbook of Medical Statistics; Oxford University: Oxford, UK, 2020. [Google Scholar]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Belfiore, A.; Russo, D.; Vigneri, R.; Filetti, S. Graves’ disease, thyroid nodules and thyroid cancer. Clin. Endocrinol. 2001, 55, 711–718. [Google Scholar] [CrossRef]
- Pohl, M.; Grabellus, F.; Worm, K.; Arnold, G.; Walz, M.; Schmid, K.W.; Sheu-Grabellus, S.Y. Intermediate microRNA expression profile in Graves’ disease falls between that of normal thyroid tissue and papillary thyroid carcinoma. J. Clin. Pathol. 2017, 70, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Brandt, F.; Thvilum, M.; Almind, D.; Christensen, K.; Green, A.; Hegedus, L.; Heiberg Brix, T. Morbidity before and after the diagnosis of hyperthyroidism: A nationwide register-based study. PLoS ONE 2013, 8, e66711. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Nam, K.H.; Chung, W.Y.; Soh, E.Y.; Park, C.S. Clinicopathologic features and treatment outcomes in differentiated thyroid carcinoma patients with concurrent Graves’ disease. J. Korean Med. Sci. 2008, 23, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Marazuela, M.; García-López, M.A.; Figueroa-Vega, N.; de la Fuente, H.; Alvarado-Sánchez, B.; Monsiváis-Urenda, A.; Sánchez-Madrid, F.; González-Amaro, R. Regulatory T cells in human autoimmune thyroid disease. J. Clin. Endocrinol. Metab. 2006, 91, 3639–3646. [Google Scholar] [CrossRef]
- Pilli, T.; Toti, P.; Occhini, R.; Castagna, M.G.; Cantara, S.; Caselli, M.; Cardinale, S.; Barbagli, L.; Pacini, F. Chronic lymphocytic thyroiditis (CLT) has a positive prognostic value in papillary thyroid cancer (PTC) patients: The potential key role of Foxp3+ T lymphocytes. J. Endocrinol. Investig. 2018, 41, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Vasileiadis, I.; Boutzios, G.; Charitoudis, G.; Koukoulioti, E.; Karatzas, T. Thyroglobulin antibodies could be a potential predictive marker for papillary thyroid carcinoma. Ann. Surg. Oncol. 2014, 8, 2725–2732. [Google Scholar] [CrossRef] [PubMed]
- French, J.D.; Weber, Z.J.; Fretwell, D.L.; Said, S.; Klopper, J.P.; Haugen, B.R. Tumor-associated lymphocytes and increased FoxP3+ regulatory T cell frequency correlate with more aggressive papillary thyroid cancer. J. Clin. Endocrinol. Metab. 2010, 95, 2325–2333. [Google Scholar] [CrossRef]
- Lubin, D.; Baraban, E.; Lisby, A.; Jalali-Farahani, S.; Zhang, P.; Livolsi, V. Papillary Thyroid Carcinoma Emerging from Hashimoto Thyroiditis Demonstrates Increased PD-L1 Expression, Which Persists with Metastasis. Endocr. Pathol. 2018, 29, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.X.; Denhardt, D.T. Osteopontin: Role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008, 19, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Joshi, B.H.; Leland, P.; Lababidi, S.; Varrichio, F.; Puri, R.K. Interleukin-4 receptor alpha overexpression in human bladder cancer correlates with the pathological grade and stage of the disease. Cancer Med. 2014, 3, 1615–1628. [Google Scholar] [CrossRef]
- Venmar, K.T.; Carter, K.J.; Hwang, D.G.; Dozier, E.A.; Fingleton, B. IL4 receptor ILR4α regulates metastatic colonization by mammary tumors through multiple signaling pathways. Cancer Res. 2014, 15, 4329–4340. [Google Scholar] [CrossRef]
- Pellegriti, G.; Mannarino, C.; Russo, M.; Terranova, R.; Marturano, I.; Vigneri, R.; Belfiore, A. Increased mortality in patients with differentiated thyroid cancer associated with Graves’ disease. J. Clin. Endocrinol. Metab. 2013, 98, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Sapalidis, K.; Papanastasiou, A.; Michalopoulos, N.; Mantalovas, S.; Giannakidis, D.; Koimtzis, G.D.; Florou, M.; Poulios, C.; Mantha, N.; Kesisoglou, I.I. A Rare Coexistence of Medullary Thyroid Cancer with Graves Disease: A Case Report and Systematic Review of the Literature. Am. J. Case Rep. 2019, 20, 1398–1401. [Google Scholar] [CrossRef]
| Evidence | Criteria Used |
|---|---|
| Strong | OR * > 2; p ** < 10−6; >1000 cases; p < 0.05 of largest study in meta-analysis; I2 *** < 50%; no small study effect; prediction interval excludes null value **; no excess significance bias |
| Moderate | OR > 1.5; p * < 10−6; >1000 cases; p < 0.05 of largest study in meta-analysis |
| Modest | OR >1.2; p * < 10−3 |
| Weak | OR > 1; p < 0.05 |
| Mekraksakit et al., 2019 [38] | Papanastasiou et al., 2019 [39] | Staniforth et al., 2015 [40] | Song et al., 2019 [41] | Jia et al., 2018 [42] | |
|---|---|---|---|---|---|
| Yes | Yes | Yes | Yes | Yes |
| Partial Yes | Partial Yes | Partial yes | Partial Yes | Partial Yes |
| Yes | No | Yes | Yes | Yes |
| Partial Yes | Partial Yes | Partial Yes | Partial Yes | Partial Yes |
| Yes | Yes | No | Yes | Yes |
| Yes | Yes | No | Yes | Yes |
| No | No | No | No | No |
| Yes | Partial Yes | No | Yes | Partial Yes |
| Yes | Yes | Yes | Partial Yes | Partial Yes |
| Yes | Yes | Yes | Yes | Yes |
| Yes | Yes | Yes | Yes | Yes |
| Yes | Yes | Yes | Yes | Yes |
| Yes | Yes | Yes | Yes | Yes |
| Yes | Yes | Yes | Yes | No |
| Yes | Yes | Yes | No | Yes |
| Yes | Yes | Yes | Yes | Yes |
| Total of yes | 13/16 (75.0%) | 11/16 (68.7%) | 10/16 (75.0%) | 11/16 (68.7%) | 10/16 (62.5%) |
| Rating overall confidence | Moderate | Moderate | Low | Low | Moderate |
| Author, Year | N. and Study Design in Meta-Analyses | Graves’ Patients (GD) (Number of Thyroid Cancer Cases) | Sex by Comparison Groups | Comparison Groups (N) | Age (Years) | Type of Thyroid Cancer | Country | Period of Primary Studies’ Publication | Exposure Time Period/ Follow-Up | Effect Size by Comparing Group (Thyroid Cancer Risk in GD Patients, Mortality, Recurrence/Persistence) | Heterogenicity (p-Value) * | Publication Bias |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mekraksakit et al., 2019 [38] | 15 retrospective cohorts 9 case-control 1 prospective cohort | GD: 2892(2892) | F: 1320 M: 1572 | -DTC patients with non-Graves’ hyperthyroidism -DTC patients with euthyroidism -Non-specified DTC patients -DTC with TNMG -DTC with TA | from 5 to 81 | 2662 PTC, 213 FTC, 16 mixed PTC and FTC 1 CCC | 1 Germany, 4 USA, 7 Italy, 2 Taiwan, 2 Turkey, 1 Oman, 1 Greece, 1 U.K., 1 Spain, 1 Australia, 2 Japan, 1 India | from 1988 to 2018 | from 1 to 30 years | GD vs. no GD hyperthyroidism | Yes | |
| OR for mortality = 0.79 (95% CI 0.17–3.67) | 0.0% (0.50) | |||||||||||
| OR for recurrence/persistence 2.66 (95% CI 0.94–7.54) | 1.8% (0.41) | |||||||||||
| GD vs. euthyroid | ||||||||||||
| OR for mortality = 2.69 (95%CI 0.70–10.40) | 22.9% (0.27) | |||||||||||
| OR for recurrence/persistence = 1.39 (95% CI 0.52–3.76) | 64.2% (0.04) | |||||||||||
| GD vs. Non-specified DTC OR for recurrence/persistence = 0.91 (95% CI 0.18–4.58) | 73.3% (0.01) | |||||||||||
| Papanastasiou et al., 2019 [39] | 7 retrospectivecohorts | GD:2582(297) | F: 1368 M: 517 (this number is partly due to the lack of data in some of the included studies) | -GD patients without thyroid nodules -GD patients without thyroid nodules (without malignant or suspicious cytology) -GD patients with multiple nodules | from 27 to 58 | 297 DTC | 1 France, 4 Turkey, 1 USA, 1 China | from 1988 to 2018 | Notapplicable | GD with thyroid nodules vs. GD without thyroid nodules OR incidence for thyroid cancer risk = 5.30 (95% CI 2.43–11.59) | 83%(0.00) | Not evaluated because of the insufficient number of included studies |
| GD with thyroid nodules vs. GD without malignant or suspicious cytology OR incidence for thyroid cancer risk = 4.02 (95% CI 1.24–12.99) | 89% (0.00) | |||||||||||
| GD with thyroid nodules vs. number of nodules OR incidence for thyroid cancer risk = 1.39 (95% CI 0.85–2.29) | 0% (0.76) | |||||||||||
| Staniforth et al., 2015 [40] | 28 retrospective 1 cohort 3 prospective 1 case-control | GD: 10,594 (498) | GD: 451 M, 2456 F UTG: 674 M, 791 F MTG: 276 M, 491 F Hyperthyroidism/Thyrotoxicosis: 199 M, 573 F Goiter: 1613 M, 12,887 F (this number is partly due to the lack of data in some of the included studies) | Patients with non-Graves’ hyperthyroidism: -Any type of toxic nodular goiter -Toxic multinodulargoiter-Toxic uninodulargoiter-Unspecified toxic nodular goiter | from 3 to 82 | 325 out of 498 cases had the histological diagnosis: Papillary: 286 (88%) Follicular: 34 (10%) Mixed papillary-follicular: 2 (0.6%) Medullary: 2 (0.6%) Anaplastic: 1 (0.3%) | 7 Asia, 18 Europe, 2 Pacific Area, 6 USA | 1977–2014 | from 2 to 25 years | GD vs. any type of toxic nodular goiter OR incidence = 0.89 (95% CI 0.63–1.26) | 28.57% (0.10) | No publication bias (p = 0.98) |
| GD vs. toxic multinodular goiter OR incidence= 1.24 (95% CI 0.81–1.90) | 0.0% (0.82) | |||||||||||
| GD vs. toxic unimodular goiter OR incidence = 0.96 (95% CI 0.58–1.57) | 5.13% (0.39) | |||||||||||
| GD vs. unspecified toxic nodular goiter OR incidence = 0.43 (95% CI 0.14–1.33) | 71.73% (0.01) | |||||||||||
| Song et al., 2019 [41] | 12 retrospective | GD: 882 (36) | GD: 189 M, 1345 F UTG: 23 M, 141 F MTG: 166 M, 929 F Hyperthyroidism: 15 M, 68 F DTC: 22 M, 117 FEuthyroidism: 28 M, 181 F Thyroidectomy not GD and PTC: 104 M, 405 F Thyroidectomy not GD and STC: 33 M, 476 F (this number is partly due to the lack of data in some of the included studies) | -Non-Graves’ DTC patients (N: 2201) -Non-Graves’ hyperthyroidism DTC patients (N: 118) -Euthyroidism DTC patient (N: 697) | from 15 to 51 (meanage) | 2708 PTC 31 FTC 159 DTC | 3 Italy, 2 Greece, 2 USA, 1 India, 2 Japan, 1 U.K., 1 China | from 1988 to 2018 | Notapplicable | GD patients vs. not GD patients OR(recurrence/disease progress/persistence) = 1.07 (0.51–2.22) | 65% (0.00) | Not reported |
| Moderate–high quality subgroup OR(recurrence/disease progress/persistence) = 1.50 (95% Cl 0.60–3.79) | 64% (0.00) | |||||||||||
| Weak quality subgroup OR (recurrence/disease progress/persistence) = 0.53 (0.20–1.43) | 51% (0.13) | |||||||||||
| By K-M curves OR(recurrence/disease progress/persistence) = 2.02 (95% Cl 1.04–3.90) | 0% (0.04) | |||||||||||
| Europe OR (recurrence/disease progress/persistence) = 1.77 (95% Cl 0.99–3.16) | 0% (0.47) | |||||||||||
| Europe and America OR (recurrence/disease progress/persistence) = 1.74 (95% Cl 1.02–2.98) | N.A. | |||||||||||
| Asia OR (recurrence/disease progress/persistence) = 0.43 (95% Cl 0.25–0.77) | 80% (0.00) | |||||||||||
| Retrospective not randomized studies with subgroup OR(recurrence/disease progress/persistence) = 0.50 (95% Cl 0.30–0.85) | 76% (0.00) | |||||||||||
| Retrospective randomized studies subgroup OR(recurrence/disease progress/persistence) = 1.79 (95% Cl 1.01–3.18) | 0% (0.47) | |||||||||||
| High incidental carcinoma rate studies OR(recurrence/disease progress/persistence) = 1.75 (95% Cl 1.04–2.95) | 0% (0.81) | |||||||||||
| GD vs. not GD hyperthyroidism OR(recurrence/disease progress/persistence) = 3.56 (95% Cl 1.18–10.75) | 5% (0.37) | |||||||||||
| GD vs. euthyroid OR(recurrence/disease progress/persistence) = 0.86 (95% Cl 0.42–1.77) | 93% (0.00) | |||||||||||
| GD vs. not GD OR for mortality = 2.93 (95% Cl 1.17–7.37) | 33% (0.20) | |||||||||||
| High incidental carcinoma rate studies OR for mortality = 7.17 (95% Cl 2.14–24.02) | 0% (0.51) | |||||||||||
| Europe OR for mortality = 4.89 (95% Cl 1.52–15.75) | 38% (0.20) | |||||||||||
| Asia OR for mortality = 1.13 (95% Cl 0.21–6.13) | 0% (0.34) | |||||||||||
| Retrospective not randomized studies with subgroup OR for mortality = 3.75 (95% Cl 1.29–10.90) | 57% (0.10) | |||||||||||
| Retrospective randomized studies subgroup OR for mortality = 1.36 (95% Cl 0.19–9.82) | 0% (0.43) | |||||||||||
| GD vs. euthyroid OR for mortality = 3.99 (95% Cl 1.19–13.39) | 78% (0.03) | |||||||||||
| GD vs. not GD hyperthyroidism OR for mortality= 1.36 (95% Cl 0.19–9.82) | 0% (0.43) | |||||||||||
| Jia et al., 2018 [42] | 11 cohorts | 10743 (207) | GD patients with PTC GD: 1065 F, 9678 M (this number is partly due to the lack of data in some of the included studies) | TA patients with TC TNG patients with TC non-GD patients with PTC | from 17 to 76 | 207 DTC | 2 USA, 1 Oman, 1 Greece, 1 Turkey, 1 India, 3 Italy, 1 Germany, 1 France | from 1946 to 2013 | Not reported | Surgery-hyperthyroid incidental thyroid cancer patients with GD vs. not GD OR incidence: 1.0 (0.68–1.46 p = 0.98). | 12% (0.33) | No publication bias (p = 0.77) |
| GD vs. toxic adenoma patients OR incidence: 0.53 (0.21–1.36 p = 0.18) | 40% (0.17) | |||||||||||
| GD vs. TNG patients OR incidence: 1.01 (0.65–1.57 p = 0.95) | 5% (0.39) | |||||||||||
| GD patients and non-GD patients OR incidence: 0.79 (0.24–2.64 p = 0.70) | 0% (0.97) | |||||||||||
| GD vs. Toxic multinodular goiter OR incidence = 1.24 (95% CI 0.81–1.90) | 0.0% (0.82) | |||||||||||
| GD vs. Toxic unimodular goiter OR incidence = 0.96 (95% CI 0.58–1.57) | 5.13% (0.39) | |||||||||||
| GD vs. unspecified toxic nodular goiter OR incidence = 0.43 (95% CI 0.14–1.33) | 71.73% (0.01) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palella, M.; Giustolisi, F.M.; Modica Fiascaro, A.; Fichera, M.; Palmieri, A.; Cannarella, R.; Calogero, A.E.; Ferrante, M.; Fiore, M. Risk and Prognosis of Thyroid Cancer in Patients with Graves’ Disease: An Umbrella Review. Cancers 2023, 15, 2724. https://doi.org/10.3390/cancers15102724
Palella M, Giustolisi FM, Modica Fiascaro A, Fichera M, Palmieri A, Cannarella R, Calogero AE, Ferrante M, Fiore M. Risk and Prognosis of Thyroid Cancer in Patients with Graves’ Disease: An Umbrella Review. Cancers. 2023; 15(10):2724. https://doi.org/10.3390/cancers15102724
Chicago/Turabian StylePalella, Marco, Francesca Maria Giustolisi, Adriana Modica Fiascaro, Martina Fichera, Antonella Palmieri, Rossella Cannarella, Aldo E. Calogero, Margherita Ferrante, and Maria Fiore. 2023. "Risk and Prognosis of Thyroid Cancer in Patients with Graves’ Disease: An Umbrella Review" Cancers 15, no. 10: 2724. https://doi.org/10.3390/cancers15102724
APA StylePalella, M., Giustolisi, F. M., Modica Fiascaro, A., Fichera, M., Palmieri, A., Cannarella, R., Calogero, A. E., Ferrante, M., & Fiore, M. (2023). Risk and Prognosis of Thyroid Cancer in Patients with Graves’ Disease: An Umbrella Review. Cancers, 15(10), 2724. https://doi.org/10.3390/cancers15102724










