Efficacy and Safety of High-Dose Chemotherapy with Treosulfan and Melphalan in Multiple Myeloma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Endpoints, and Patient Cohort
2.2. HDCT Treatment Schedule
2.3. Assessment of Treosulfan Pharmacokinetics
2.4. Response Assessment
2.5. Statistical Analysis
3. Results
3.1. Patient Baseline Characteristics
3.2. Induction and Maintenance Therapy
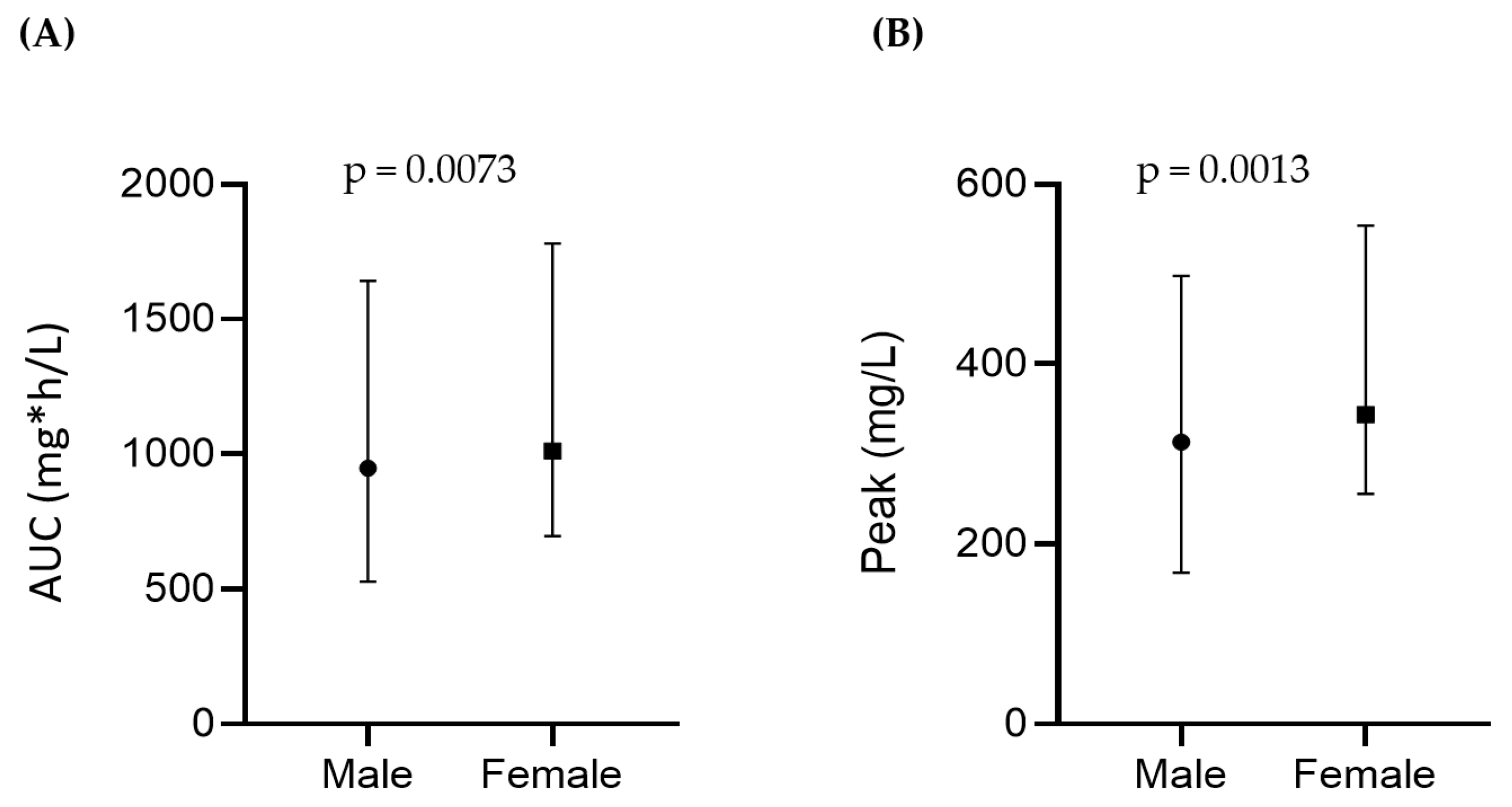
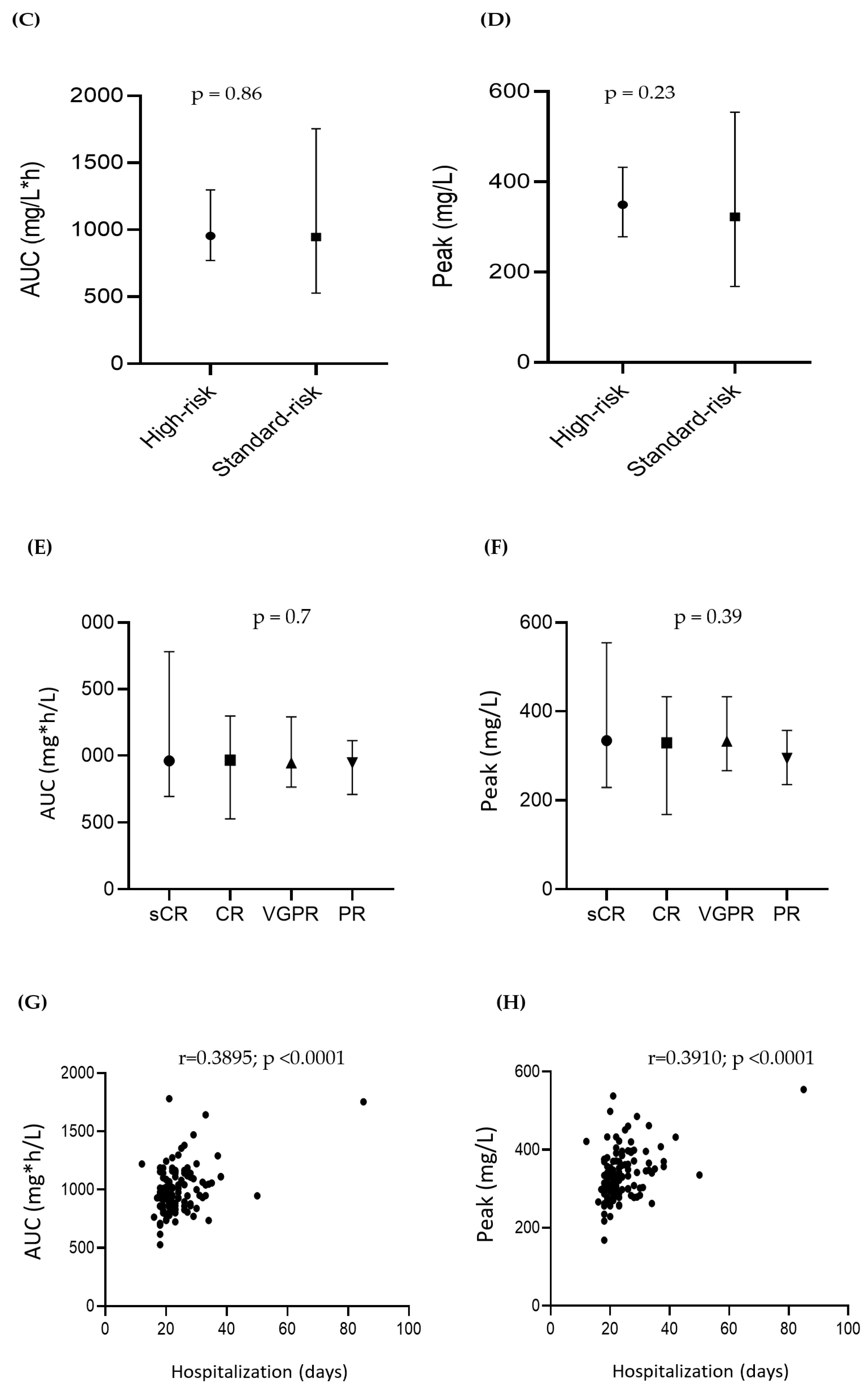
3.3. Treosulfan Pharmacokinetics
3.4. Adverse Events
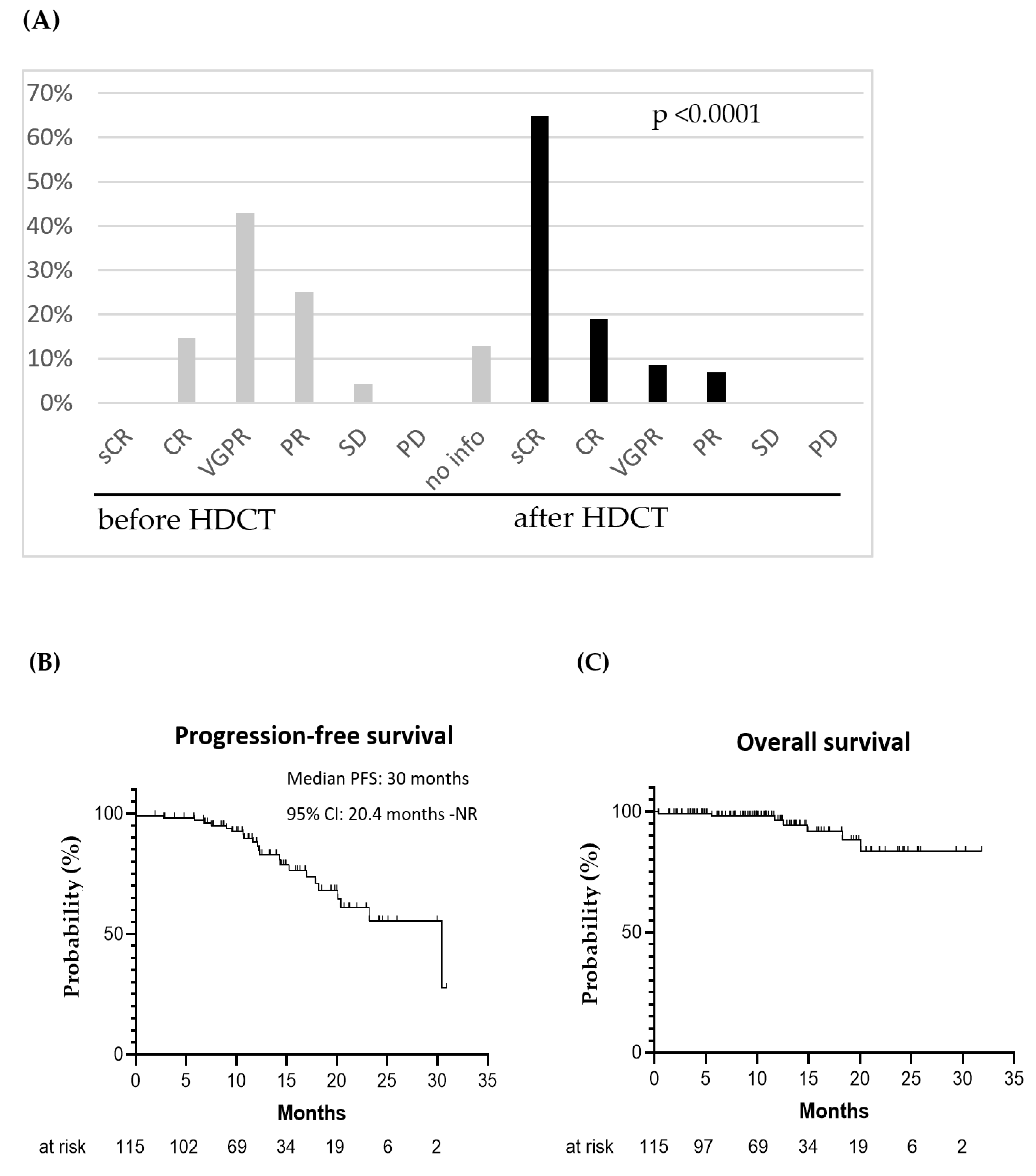
3.5. Outcome of HDCT and ASCT
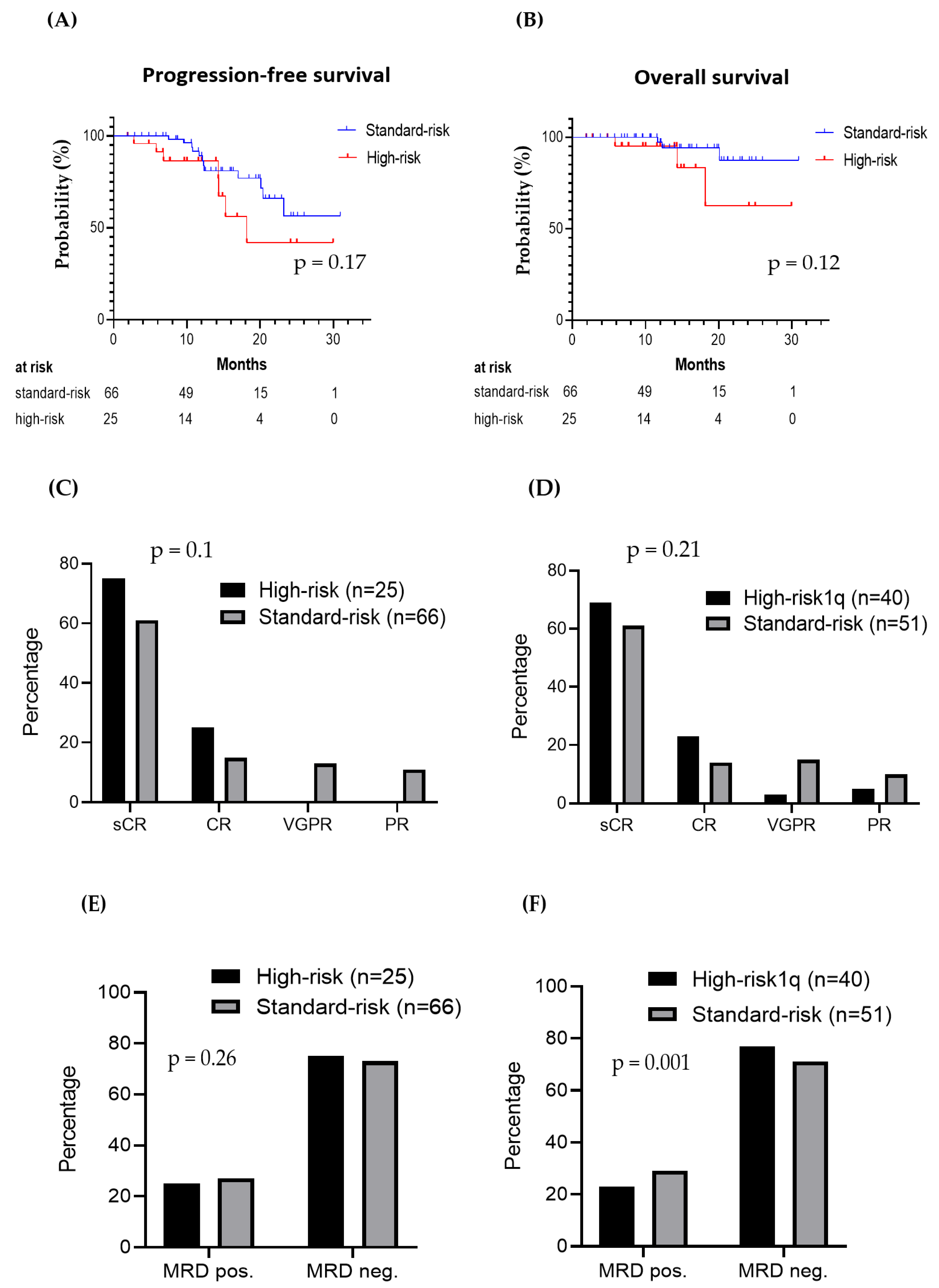
3.6. Clinical Outcomes in Patients with Standard vs. High-Risk Cytogenetics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mishima, Y.; Mishima, Y.; Shirouchi, Y.; Nishimura, N.; Yokoyama, M.; Okabe, T.; Inoue, N.; Uryu, H.; Fukuta, T.; Hatake, K.; et al. The clonal evolution during long-term clinical course of multiple myeloma. Int. J. Hematol. 2020, 113, 279–284. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute at the National Institutes of Health. Surveillance, Epidemiology, and End Results (SEER) Program. 2023. Available online: https://seer.cancer.gov/statfacts/html/mulmy.html (accessed on 14 March 2023).
- Cowan, A.J.; Green, D.J.; Kwok, M.; Lee, S.; Coffey, D.G.; Holmberg, L.A.; Tuazon, S.; Gopal, A.K.; Libby, E.N. Diagnosis and Management of Multiple Myeloma. JAMA 2022, 327, 464–477. [Google Scholar] [CrossRef] [PubMed]
- The International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: A report of the International Myeloma Working Group. Br. J. Haematol. 2003, 121, 749–757. [Google Scholar] [CrossRef]
- Palumbo, A.; Anderson, K. Multiple Myeloma. N. Engl. J. Med. 2011, 364, 1046–1060. [Google Scholar] [CrossRef] [PubMed]
- Alexanian, R. Treatment for multiple myeloma. Combination chemotherapy with different melphalan dose regimens. JAMA 1969, 208, 1680–1685. [Google Scholar] [CrossRef]
- Attal, M.; Harousseau, J.-L.; Stoppa, A.-M.; Sotto, J.-J.; Fuzibet, J.-G.; Rossi, J.-F.; Casassus, P.; Maisonneuve, H.; Facon, T.; Ifrah, N.; et al. A Prospective, Randomized Trial of Autologous Bone Marrow Transplantation and Chemotherapy in Multiple Myeloma. N. Engl. J. Med. 1996, 335, 91–97. [Google Scholar] [CrossRef]
- Child, J.A.; Morgan, G.J.; Davies, F.E.; Owen, R.G.; Bell, S.E.; Hawkins, K.; Brown, J.; Drayson, M.T.; Selby, P.J.; Medical Research Council Adult Leukaemia Working Party. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N. Engl. J. Med. 2003, 348, 1875–1883. [Google Scholar] [CrossRef]
- Bird, S.; Cairns, D.; Menzies, T.; Boyd, K.; Davies, F.; Cook, G.; Drayson, M.; Gregory, W.; Jenner, M.; Jones, J.; et al. Sex Differences in Multiple Myeloma Biology but not Clinical Outcomes: Results from 3894 Patients in the Myeloma XI Trial. Clin. Lymphoma Myeloma Leuk. 2021, 21, 667–675. [Google Scholar] [CrossRef]
- Stettler, J.; Novak, U.; Baerlocher, G.M.; Seipel, K.; Taleghani, B.M.; Pabst, T. Autologous stem cell transplantation in elderly patients with multiple myeloma: Evaluation of its safety and efficacy. Leuk. Lymphoma 2016, 58, 1076–1083. [Google Scholar] [CrossRef]
- Attal, M.; Lauwers-Cances, V.; Hulin, C.; Leleu, X.; Caillot, D.; Escoffre, M.; Arnulf, B.; Macro, M.; Belhadj, K.; Garderet, L.; et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. N. Engl. J. Med. 2017, 376, 1311–1320. [Google Scholar] [CrossRef]
- Richardson, P.G.; Jacobus, S.J.; Weller, E.A.; Hassoun, H.; Lonial, S.; Raje, N.S.; Medvedova, E.; McCarthy, P.L.; Libby, E.N.; Voorhees, P.M.; et al. Triplet Therapy, Transplantation, and Maintenance until Progression in Myeloma. N. Engl. J. Med. 2022, 387, 132–147. [Google Scholar] [CrossRef] [PubMed]
- Roussel, M.; Lauwers-Cances, V.; Macro, M.; Leleu, X.; Royer, B.; Hulin, C.; Karlin, L.; Perrot, A.; Touzeau, C.; Chrétien, M.-L.; et al. Bortezomib and high-dose melphalan conditioning regimen in frontline multiple myeloma: An IFM randomized phase 3 study. Blood 2022, 139, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
- Voorhees, P.M.; Kaufman, J.L.; Laubach, J.P.; Sborov, D.W.; Reeves, B.; Rodriguez, C.; Chari, A.; Silbermann, R.; Costa, L.J.; Anderson, L.D., Jr.; et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: The GRIFFIN trial. Blood 2020, 136, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Prediletto, I.; Farag, S.A.; Bacher, U.; Jeker, B.; Taleghani, B.M.; Brégy, R.; Zander, T.; Betticher, D.; Egger, T.; Novak, U.; et al. High incidence of reversible renal toxicity of dose-intensified bendamustine-based high-dose chemotherapy in lymphoma and myeloma patients. Bone Marrow Transplant. 2019, 54, 1923–1925. [Google Scholar] [CrossRef]
- Ludwig, H.; Durie, B.G.M.; Bolejack, V.; Turesson, I.; Kyle, R.A.; Blade, J.; Fonseca, R.; Dimopoulos, M.; Shimizu, K.; Miguel, J.S.; et al. Myeloma in patients younger than age 50 years presents with more favorable features and shows better survival: An analysis of 10 549 patients from the International Myeloma Working Group. Blood 2008, 111, 4039–4047. [Google Scholar] [CrossRef]
- Poczta, A.; Rogalska, A.; Marczak, A. Treatment of Multiple Myeloma and the Role of Melphalan in the Era of Modern Therapies—Current Research and Clinical Approaches. J. Clin. Med. 2021, 10, 1841. [Google Scholar] [CrossRef]
- Farag, S.; Bacher, U.; Jeker, B.; Legros, M.; Rhyner, G.; Lüthi, J.-M.; Schardt, J.; Zander, T.; Daskalakis, M.; Mansouri, B.; et al. Adding bendamustine to melphalan before ASCT improves CR rate in myeloma vs. melphalan alone: A randomized phase-2 trial. Bone Marrow Transplant. 2022, 57, 990–997. [Google Scholar] [CrossRef]
- Gran, C.; Wang, J.; Nahi, H.; Koster, L.; Gahrton, G.; Einsele, H.; Niittyvoupio, R.; Edinger, M.; Beelen, D.; Ciceri, F.; et al. Treosulfan conditioning for allogeneic transplantation in multiple myeloma—Improved overall survival in first line haematopoietic stem cell transplantation—A large retrospective study by the Chronic Malignancies Working Party of the EBMT. Br. J. Haematol. 2020, 189, e213–e217. [Google Scholar] [CrossRef]
- Gurevich, E.; Hayoz, M.; Aebi, Y.; Largiadèr, C.R.; Taleghani, B.M.; Bacher, U.; Pabst, T. Comparison of Melphalan Combined with Treosulfan or Busulfan as High-Dose Chemotherapy before Autologous Stem Cell Transplantation in AML. Cancers 2022, 14, 1024. [Google Scholar] [CrossRef]
- Lazzari, L.; Ruggeri, A.; Stanghellini, M.T.L.; Mastaglio, S.; Messina, C.; Giglio, F.; Lorusso, A.; Perini, T.; Piemontese, S.; Marcatti, M.; et al. Treosulfan-Based Conditioning Regimen Prior to Allogeneic Stem Cell Transplantation: Long-Term Results from a Phase 2 Clinical Trial. Front. Oncol. 2021, 11, 731478. [Google Scholar] [CrossRef]
- Scheulen, M.E.; Hilger, R.A.; Oberhoff, C.; Casper, J.; Freund, M.; Josten, K.M.; Bornhäuser, M.; Ehninger, G.; Berdel, W.E.; Baumgart, J.; et al. Clinical phase I dose escalation and pharmacokinetic study of high-dose chemotherapy with treosulfan and autologous peripheral blood stem cell transplantation in patients with advanced malignancies. Clin. Cancer Res. 2000, 6, 4209–4216. [Google Scholar] [PubMed]
- Bashir, Q.; Thall, P.F.; Milton, D.R.; Fox, P.S.; Kawedia, J.D.; Kebriaei, P.; Shah, N.; Patel, K.; Andersson, B.S.; Nieto, Y.L.; et al. Conditioning with busulfan plus melphalan versus melphalan alone before autologous haemopoietic cell transplantation for multiple myeloma: An open-label, randomised, phase 3 trial. Lancet Haematol. 2019, 6, e266–e275. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.O.; Al Hadidi, S. High dose (conditioning) regimens used prior to autologous stem cell transplantation in multiple myeloma. Transplant. Cell. Ther. 2022, 28, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Jain, T.; Sonbol, M.B.; Firwana, B.; Kolla, K.R.; Almader-Douglas, D.; Palmer, J.; Fonseca, R. High-Dose Chemotherapy with Early Autologous Stem Cell Transplantation Compared to Standard Dose Chemotherapy or Delayed Transplantation in Patients with Newly Diagnosed Multiple Myeloma: A Systematic Review and Meta-Analysis. Biol. Blood Marrow Transplant. 2018, 25, 239–247. [Google Scholar] [CrossRef]
- Knop, S.; Bauer, K.; Hebart, H.; Wandt, H.; Trumper, L.; Liebisch, P.; Maschmeyer, G.; Peest, D.; Wolf, H.H.; Kroger, N.; et al. A Randomized Comparison of Total-Marrow Irradiation, Busulfan and Cyclophosphamide with Tandem High-Dose Melphalan in Patients with Multiple Myeloma. Blood 2007, 110, 728. [Google Scholar] [CrossRef]
- Reichle, A.; Burgmayer, N.; Holler, E.; Berand, A.; Andreesen, R. Inclusion of High-Dose Treosulfan Followed by Autologous Transplantation in the Tandem Transplantation for Patients with Multiple Myeloma <60 Years. Blood 2007, 110, 5098. [Google Scholar] [CrossRef]
- Koch, R.; Gelderblom, H.; Haveman, L.; Brichard, B.; Jürgens, H.; Cyprova, S.; Berg, H.V.D.; Hassenpflug, W.; Raciborska, A.; Ek, T.; et al. High-Dose Treosulfan and Melphalan as Consolidation Therapy Versus Standard Therapy for High-Risk (Metastatic) Ewing Sarcoma. J. Clin. Oncol. 2022, 40, 2307–2320. [Google Scholar] [CrossRef]
- Schmidt, T.M.; Fonseca, R.; Usmani, S.Z. Chromosome 1q21 abnormalities in multiple myeloma. Blood Cancer J. 2021, 11, 83. [Google Scholar] [CrossRef]
- Romański, M.; Wachowiak, J.; Główka, F.K. Treosulfan Pharmacokinetics and its Variability in Pediatric and Adult Patients Undergoing Conditioning Prior to Hematopoietic Stem Cell Transplantation: Current State of the Art, In-Depth Analysis, and Perspectives. Clin. Pharmacokinet. 2018, 57, 1255–1265. [Google Scholar] [CrossRef]
- Van Der Stoep, M.Y.E.C.; Bertaina, A.; Brink, M.H.T.; Bredius, R.G.; Smiers, F.J.; Wanders, D.C.M.; Moes, D.J.A.; Locatelli, F.; Guchelaar, H.-J.; Zwaveling, J.; et al. High interpatient variability of treosulfan exposure is associated with early toxicity in paediatric HSCT: A prospective multicentre study. Br. J. Haematol. 2017, 179, 772–780. [Google Scholar] [CrossRef]
- Bartelink, I.H.; Lalmohamed, A.; van Reij, E.M.L.; Dvorak, C.C.; Savic, R.M.; Zwaveling, J.; Bredius, R.G.M.; Egberts, A.C.G.; Bierings, M.; Kletzel, M.; et al. Association of busulfan exposure with survival and toxicity after haemopoietic cell transplantation in children and young adults: A multicentre, retrospective cohort analysis. Lancet Haematol. 2016, 3, e526–e536. [Google Scholar] [CrossRef] [PubMed]
- Soldin, O.P.; Mattison, D. Sex Differences in Pharmacokinetics and Pharmacodynamics. Clin. Pharmacokinet. 2009, 48, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Parmar, S.R.; Bookout, R.; Shapiro, J.F.; Tombleson, R.; Perkins, J.; Kim, J.; Yue, B.; Tomblyn, M.; Alsina, M.; Nishihori, T. Comparison of 1-day vs. 2-day dosing of high-dose melphalan followed by autologous hematopoietic cell transplantation in patients with multiple myeloma. Bone Marrow Transplant. 2014, 49, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Lisenko, K.; Sauer, S.; Bruckner, T.; Egerer, G.; Goldschmidt, H.; Hillengass, J.; Schmier, J.W.; Shah, S.; Witzens-Harig, M.; Ho, A.D.; et al. High-dose chemotherapy and autologous stem cell transplantation of patients with multiple myeloma in an outpatient setting. BMC Cancer 2017, 17, 151. [Google Scholar] [CrossRef] [PubMed]
- Aydin, M.; Tang, M.W.; Wondergem, M.J.; de Leeuw, D.C.; Wegman, J.J.; Biemond, B.J.; van de Donk, N.W.C.J.; Zweegman, S.; Meijer, E.; Nur, E. High-dose melphalan in 1 day versus over 2 days followed by autologous stem cell transplantation as consolidation treatment in patients with multiple myeloma. Br. J. Haematol. 2022, 196, e67–e70. [Google Scholar] [CrossRef] [PubMed]
- Hanamura, I. Multiple myeloma with high-risk cytogenetics and its treatment approach. Int. J. Hematol. 2022, 115, 762–777. [Google Scholar] [CrossRef]
- Hagen, P.; Zhang, J.; Barton, K. High-risk disease in newly diagnosed multiple myeloma: Beyond the R-ISS and IMWG definitions. Blood Cancer J. 2022, 12, 83. [Google Scholar] [CrossRef]

| Characteristics | All Patients (n = 115) |
|---|---|
| Median age, years (range) | 61 (36–73) |
| Males/females, n (ratio) | 73/42 (1.74) |
| Paraprotein subtype, n (%) | |
| kappa light chain | 75 (65%) |
| lambda light chain | 40 (35%) |
| IgG | 79 (69%) |
| IgA | 16 (14%) |
| IgM | 1 (1%) |
| light chain only | 19 (16%) |
| R-ISS Stage * | |
| I | 27 (25%) |
| II | 51 (48%) |
| III | 29 (27%) |
| MM diagnostic criteria | |
| Anemia (<110 g/L), n (%) | 46 (40%) |
| Hypercalcemia (>2.6 mmol/L), n (%) | 21 (18%) |
| Creatinine > 104 µmol/L, n (%) | 23 (20%) |
| Osteolytic lesions, n (%) | 88 (77%) |
| BM infiltration, median (range) | 60% (5–100%) |
| Cytogenetics | |
| Available, n (%) | 91 (79%) |
| High-risk (t(4;14), t(14;16), del(17p)) | 25 (28%) |
| Translocation (4;14) | 17 (19%) |
| Translocation (14;16) | 3 (3%) |
| Deletion (17p) | 4 (4%) |
| Standard-risk | 66 (72%) |
| 1q21+ | 22 (24%) |
| Parameter | Number (%) |
|---|---|
| Number of previous treatment lines | |
| 1 | 107 (93%) |
| 2 | 5 (4%) |
| 3 | 3 (3%) |
| Induction treatment regimens *a | |
| Bortezomib, lenalidomide, dexamethasone | 98 (85% *a) |
| Daratumomab, lenalidomide, bortezomib, dexamethasone | 11 (10% *a) |
| Bortezomib, cyclophosphamide, dexamethasone | 5 (4% *a) |
| Daratumomab, lenalidomide, dexamethasone | 3 (3% *a) |
| Daratumomab, carfilzomib, dexamethasone | 2 (2% *a) |
| Number of HDCT and ASCT | |
| 1 | 107 (93%) |
| 2 | 7 (6%) |
| 3 | 1 (1%) |
| Maintenance therapy after HDCT *b | |
| Lenalidomide | 97 (92%) |
| Daratumomab and lenalidomide | 2 (2%) |
| Rituximab and lenalidomide | 1 (1%) |
| None | 5 (5%) |
| Parameter | Number |
|---|---|
| Hospitalization days, median (range) | 22 (16–85) |
| Hematologic | |
| Duration of pancytopenia, median (range) | 8 (5–24) |
| Platelet transfusion required, n (%) | 109 (95%) |
| Platelet concentrates transfused, median (range) | 3 (1–30) |
| Erythrocyte transfusion required, n (%) | 58 (50%) |
| Erythrocyte concentrates transfused, median (range) | 1 (1–19) |
| Gastrointestinal | |
| Parenteral nutrition, n (%) | 101 (88%) |
| Days of TPN, median (range) | 9 (2–25) |
| Malnutrition (%) | 100 (87%) |
| Neutropenic entercolitis, n (%) | 72 (63%) |
| Refeeding syndrome, n (%) | 44 (38%) |
| Mucositis, n (%) | 4 (3%) |
| Renal | |
| Acute kidney injury | 8 (7%) |
| Stage * I, n (%) | 4 (3%) |
| Stage II, n (%) | 2 (2%) |
| Stage III, n (%) | 2 (2%) |
| Infections | |
| Febrile neutropenia, n (%) | 108 (94%) |
| Bacteremia, n (%) | 28 (24%) |
| Sepsis (SOFA ≥ 3) | 4 (3%) |
| Candidosis, n (%) | 14 (12%) |
| Deaths related to infectious complications | 2 (2%) |
| Neurological and mental disorders | |
| Polyneuropathy, n (%) | 22 (19%) |
| Delirium, n (%) | 5 (4%) |
| Metabolic disorders | |
| Steroid induced diabetes, n (%) | 27 (23%) |
| Aggravated diabetes type II, n (%) | 8 (7%) |
| Hepatic disorders, n (%) | 7 (6%) |
| Cardiac disorders | |
| Atrial fibrillation, n (%) | 16 (14%) |
| Transfer to ICU, n (%) | 4 (3%) |
| Parameter | Number (%) |
|---|---|
| Best response after HDCT and ASCT *a | |
| sCR | 74 (65%) |
| CR | 22 (19%) |
| VGPR | 10 (9%) |
| PR | 8 (7%) |
| SD | 0 (0%) |
| PD | 0 (0%) |
| Remission status before HDCT and ASCT | |
| sCR | 0 (0%) *b |
| CR | 16 (14%) |
| VGPR | 50 (44%) |
| PR | 29 (25%) |
| SD | 5 (4%) |
| PD | 0 (0%) |
| not available | 15 (13%) |
| Relapse after HDCT and ASCT | 23 (20%) |
| Median PFS, months (range) | 30 (0.4–31) |
| OS, % | 83% |
| Deaths | 7 (6%) |
| due to disease progression | 4 (3%) |
| due to infectious complications | 3 (3%) |
| Median follow-up from start of TreoMel, months (range) | 12 (0.4–31) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gillich, C.; Akhoundova, D.; Hayoz, M.; Aebi, Y.; Largiadèr, C.R.; Seipel, K.; Daskalakis, M.; Bacher, U.; Pabst, T. Efficacy and Safety of High-Dose Chemotherapy with Treosulfan and Melphalan in Multiple Myeloma. Cancers 2023, 15, 2699. https://doi.org/10.3390/cancers15102699
Gillich C, Akhoundova D, Hayoz M, Aebi Y, Largiadèr CR, Seipel K, Daskalakis M, Bacher U, Pabst T. Efficacy and Safety of High-Dose Chemotherapy with Treosulfan and Melphalan in Multiple Myeloma. Cancers. 2023; 15(10):2699. https://doi.org/10.3390/cancers15102699
Chicago/Turabian StyleGillich, Cédric, Dilara Akhoundova, Michael Hayoz, Yolanda Aebi, Carlo R. Largiadèr, Katja Seipel, Michael Daskalakis, Ulrike Bacher, and Thomas Pabst. 2023. "Efficacy and Safety of High-Dose Chemotherapy with Treosulfan and Melphalan in Multiple Myeloma" Cancers 15, no. 10: 2699. https://doi.org/10.3390/cancers15102699
APA StyleGillich, C., Akhoundova, D., Hayoz, M., Aebi, Y., Largiadèr, C. R., Seipel, K., Daskalakis, M., Bacher, U., & Pabst, T. (2023). Efficacy and Safety of High-Dose Chemotherapy with Treosulfan and Melphalan in Multiple Myeloma. Cancers, 15(10), 2699. https://doi.org/10.3390/cancers15102699









