Direct-Acting Oral Anticoagulant Therapy in Cancer Patients—A Review
Abstract
Simple Summary
Abstract
1. Introduction
2. DOACs: Revolution in Anticoagulant Treatment
- (A)
- Rivaroxaban is a direct inhibitor of factor Xa, binding directly to the active site of this factor [25]. It also has the ability to inhibit the activity of prothrombinase [26]. Rivaroxaban is a drug that is quickly absorbed after oral administration, with peak concentrations appearing after 2–4 h [27]. Current recommendations to use rivaroxaban include: prevention of atherothrombotic events in adult patients after an acute coronary syndrome with elevated cardiac biomarkers; prevention of atherothrombotic events in adult patients with coronary artery disease or symptomatic peripheral artery disease at a high risk of ischaemic events; prevention of VTE in adult patients undergoing elective hip or knee replacement surgery; treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE); prevention of recurrent DVT and PE in adults; prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation (NVAF) with one or more risk factors; and treatment of VTE and prevention of VTE recurrence in children and adolescents under 18 years of age [28,29,30].
- (B)
- Apixaban is a drug approved by the US Food and Drug Administration (FDA) in 2012. It is a highly selective inhibitor of factor Xa. It does not affect platelet aggregation. Its total bioavailability stands at about 50%, with peak plasma concentrations after 3–4 h [31]. Therapeutic indications of apixaban include: prevention of VTE events in adult patients who have undergone elective hip or knee replacement surgery; prevention of stroke and systemic embolism in adult patients with NVAF who have one or more risk factors; treatment of DVT and PE; and prevention of recurrent DVT and PE in adults [32,33,34].
- (C)
- Edoxaban is a direct and specific inhibitor of factor Xa, with selectivity towards factor Xa nearly 10,000 times higher than thrombin [26]. Edoxaban was registered by the FDA in 2015. The highest concentration in plasma is noted after 1–2 h, with the half-life of the molecule being 10–12 h [35]. Currently, edoxaban is registered for the following therapeutic indications: prevention of stroke and systemic embolism in adult patients with NVAF with one or more risk factors; treatment of DVT and pulmonary embolism (PE); and prevention of recurrent DVT and PE in adults [36,37,38].
- (D)
- Dabigatran etexilate is a factor IIa inhibitor. It is produced in the form of a prodrug that must be transformed into its active form by microsomal carboxylesterases in the liver. Due to its poor availability (around 6%), there is a need for the administration of high dabigatran dosages [39]. The half-life of dabigatran particles is 12–17 h, with the highest serum concentration achievable between 1 and 2 h after admission. It is important to mention that dabigatran is mainly eliminated by the kidneys; therefore, it is contraindicated in patients with renal failure [40]. Therapeutic indications of dabigatran include primary prevention of VTE events in adult patients who have undergone elective total hip or total knee replacement surgery; prevention of stroke and systemic embolism in adult patients with NVAF who have one or more risk factors; treatment of DVT and PE; and prevention of recurrent DVT and PE in adults [41,42,43].
3. DOACs’ Potential to Replace Classical VKAs in the Therapy of Patients with Cancer
4. Could DOACs Be Used as a Substitute for Classic Anticoagulant LMWH Therapy?
5. Clinical Trials
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mulder, F.I.; Horvàth-Puhó, E.; van Es, N.; Van Laarhoven, H.W.M.; Pedersen, L.; Moik, F.; Ay, C.; Büller, H.R.; Sørensen, H.T. Venous thromboembolism in cancer patients: A population-based cohort study. Blood 2021, 137, 1959–1969. [Google Scholar] [CrossRef] [PubMed]
- Pastori, D.; Cormaci, V.M.; Marucci, S.; Franchino, G.; Del Sole, F.; Capozza, A.; Fallarino, A.; Corso, C.; Valeriani, E.; Menichelli, D.; et al. A Comprehensive Review of Risk Factors for Venous Thromboembolism: From Epidemiology to Pathophysiology. Int. J. Mol. Sci. 2023, 24, 3169. [Google Scholar] [CrossRef]
- Santoro, C.; Capone, V.; Canonico, M.E.; Gargiulo, G.; Esposito, R.; Sanna, G.D.; Parodi, G.; Esposito, G. Single, dual, and triple antithrombotic therapy in cancer patients with coronary artery disease: Searching for evidence and personalized approaches. In Seminars in Thrombosis and Hemostasis; Thieme Medical Publishers, Inc.: Stuttgart, Germany, 2021; Volume 47, pp. 950–961. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Gong, S.; Zhao, J.; Yao, C.; Zhu, H.; Xiao, R.; Qin, Y.; Li, R.; Sun, N.; et al. Platelets promote CRC by activating the C5a/C5aR1 axis via PSGL-1/JNK/STAT1 signaling in tumor-associated macrophages. Theranostics 2023, 13, 2040–2056. [Google Scholar] [CrossRef] [PubMed]
- Mezouar, S.; Frère, C.; Darbousset, R.; Mege, D.; Crescence, L.; Dignat-George, F.; Panicot-Dubois, L.; Dubois, C. Role of platelets in cancer and cancer-associated thrombosis: Experimental and clinical evidences. Thromb. Res. 2016, 139, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.G.; Sandim, V.; Almeida, V.H.; Rondon, A.M.; Succar, B.B.; Hottz, E.D.; Leal, A.C.; Verçoza, B.R.F.; Rodrigues, J.C.F.; Bozza, P.T.; et al. Breast-cancer extracellular vesicles induce platelet activation and aggregation by tissue factor-independent and -dependent mechanisms. Thromb. Res. 2017, 159, 24–32. [Google Scholar] [CrossRef]
- Stone, R.L.; Nick, A.M.; McNeish, I.A.; Balkwill, F.; Han, H.D.; Bottsford-Miller, J.; Rupaimoole, R.; Armaiz-Pena, G.N.; Pecot, C.V.; Coward, J.; et al. Paraneoplastic thrombocytosis in ovarian cancer. N. Engl. J. Med. 2012, 366, 610–618. [Google Scholar] [CrossRef]
- Suzuki, A.; Takahashi, T.; Nakamura, K.; Tsuyuoka, R.; Okuno, Y.; Enomoto, T.; Fukumoto, M.; Imura, H. Thrombocytosis in patients with tumors producing colony-stimulating factor. Blood 1992, 80, 2052–2059. [Google Scholar] [CrossRef]
- Mege, D.; Aubert, M.; Lacroix, R.; Dignat-George, F.; Panicot-Dubois, L.; Dubois, C. Involvement of Platelets in Cancers. In Seminars in Thrombosis and Hemostasis; Thieme Medical Publishers, Inc.: Stuttgart, Germany, 2019; Volume 45, pp. 569–575. [Google Scholar] [CrossRef]
- Laslo, C.L.; Bacalbasa, N.; Stanescu, A.M.A.; Carsote, M.; Bungau, S.; Rus, M.; Bratu, O.G.; Diaconu, C.C. New oral anticoagulants—Possible extension to other indications (Review). Exp. Ther. Med. 2020, 20, 2401–2405. [Google Scholar] [CrossRef]
- Hong, K.S. Non-Vitamin K Antagonist Oral Anticoagulants in Medical Conditions at High Risk of Thromboembolism beyond Atrial Fibrillation. J. Stroke 2019, 21, 259–275. [Google Scholar] [CrossRef]
- Weitz, J.I.; Harenberg, J. New developments in anticoagulants: Past, present and future. Thromb. Haemost. 2017, 117, 1283–1288. [Google Scholar] [CrossRef]
- Lee, L.H. DOACs—Advances and limitations in real world. Thromb. J. 2016, 14 (Suppl. 1), 17. [Google Scholar] [CrossRef] [PubMed]
- Weronska, A.; Papuga-Szela, E.; Broniatowska, E.; Undas, A. Nonvitamin K Antagonist Oral Anticoagulant in Patients With Venous Thromboembolism and Polycythemia Vera or Essential Thrombocythemia: A Cohort Study. J. Cardiovasc. Pharmacol. 2021, 78, e743–e748. [Google Scholar] [CrossRef] [PubMed]
- Etxeandia-Ikobaltzeta, I.; Zhang, Y.; Brundisini, F.; Florez, I.D.; Wiercioch, W.; Nieuwlaat, R.; Begum, H.; Cuello, C.A.; Roldan, Y.; Chen, R.; et al. Patient values and preferences regarding VTE disease: A systematic review to inform American Society of Hematology guidelines. Blood Adv. 2020, 4, 953–968, Erratum in Blood Adv. 2020, 4, 1219. [Google Scholar] [CrossRef] [PubMed]
- Noble, S.; Matzdorff, A.; Maraveyas, A.; Holm, M.V.; Pisa, G. Assessing patients’ anticoagulation preferences for the treatment of cancer-associated thrombosis using conjoint methodology. Haematologica 2015, 100, 1486–1492. [Google Scholar] [CrossRef] [PubMed]
- Overvad, T.F.; Larsen, T.B.; Søgaard, M.; Albertsen, I.E.; Ording, A.G.; Noble, S.; Højen, A.A.; Nielsen, P.B. Cancer-associated venous thromboembolism and the non-vitamin K antagonist oral anticoagulants: A review of clinical outcomes and patient perspectives. Expert Rev. Cardiovasc. Ther. 2020, 18, 791–800. [Google Scholar] [CrossRef]
- Hutchinson, A.; Rees, S.; Young, A.; Maraveyas, A.; Date, K.; Johnson, M.J. Oral anticoagulation is preferable to injected, but only if it is safe and effective: An interview study of patient and carer experience of oral and injected anticoagulant therapy for cancer-associated thrombosis in the select-d trial. Palliat. Med. 2019, 33, 510–517. [Google Scholar] [CrossRef]
- Picker, N.; Lee, A.Y.; Cohen, A.T.; Maraveyas, A.; Beyer-Westendorf, J.; Mantovani, L.G.; Abdelgawwad, K.; Fatoba, S.; Thate-Waschke, I.M.; Bach, M.; et al. Anticoagulation Treatment in Cancer-Associated Venous Thromboembolism: Assessment of Patient Preferences Using a Discrete Choice Experiment (COSIMO Study). Thromb. Haemost. 2021, 121, 206–215. [Google Scholar] [CrossRef]
- Lanéelle, D.; Le Brun, C.; Mauger, C.; Guillaumat, J.; Le Pabic, E.; Omarjee, L.; Mahé, G.; SFMV VTE Study Group. Patient Characteristics and Preferences Regarding Anticoagulant Treatment in Venous Thromboembolic Disease. Front. Cardiovasc. Med. 2021, 8, 675969. [Google Scholar] [CrossRef]
- Moyer, G.C.; Bannow, B.S.; Thornburg, C.; Rosovsky, R.; Wang, T.F.; Woller, S.; Thornhill, D.; Kreuziger, L.B. Venous Thromboembolism: A Survey of Oral Anticoagulant Preferences in the Treatment of Challenging Patient Populations. Clin. Appl. Thromb. Hemost. 2018, 24 (Suppl. 9), 209S–216S. [Google Scholar] [CrossRef]
- Zhu, J.; Alexander, G.C.; Nazarian, S.; Segal, J.B.; Wu, A.W. Trends and Variation in Oral Anticoagulant Choice in Patients with Atrial Fibrillation, 2010–2017. Pharmacotherapy 2018, 38, 907–920. [Google Scholar] [CrossRef]
- Shah, S.B.; Pahade, A.; Chawla, R. Novel reversal agents and laboratory evaluation for direct-acting oral anticoagulants (DOAC): An update. Indian J. Anaesth. 2019, 63, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare, M.; Jarvis, J.D.; Scarlatescu, O.; Leng, X.; Zaidel, E.J.; Burrone, E.; Eiselé, J.L.; Prabhakaran, D.; Silwa, K. NOACs Added to WHO’s Essential Medicines List: Recommendations for Future Policy Actions. Global Heart 2020, 15, 67. [Google Scholar] [CrossRef] [PubMed]
- Roehrig, S.; Straub, A.; Pohlmann, J.; Lampe, T.; Pernerstorfer, J.; Schlemmer, K.H.; Reinemer, P.; Perzborn, E. Discovery of the novel antithrombotic agent 5-chloro-N-([(5S)-2-oxo-3-[4-(3-oxomorpholin-4-yl)phenyl]-1, 3-oxazolidin-5-yl]methyl)thiophene-2-carboxamide (BAY 59–7939): An oral, direct Factor Xa inhibitor. J. Med. Chem. 2005, 48, 5900–5908. [Google Scholar] [CrossRef] [PubMed]
- Samama, M.M. The mechanism of action of rivaroxaban-an oral, direct Factor Xa inhibitor-Compared with other anticoagulants. Thromb. Res. 2011, 127, 497–504. [Google Scholar] [CrossRef]
- Kubitza, D.; Becka, M.; Voith, B.; Zuehlsdorf, M.; Wensing, G. Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59-7939, an oral, direct factor Xa inhibitor. Clin. Pharmacol. Ther. 2005, 78, 412–421. [Google Scholar] [CrossRef]
- European Medicines Agency: Xarelto. European Medicines Agency. 2023. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/xarelto#authorisation-details-section (accessed on 27 February 2023).
- Janssen Pharmaceuticals, Inc. Xarelto (Rivaroxaban) Prescribing Information. Revised 02/2023. Available online: https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/XARELTO-pi.pdf (accessed on 17 February 2023).
- Xarelto: EPAR-Product Information. Last Updated 23/02/2023. Available online: https://www.ema.europa.eu/en/documents/overview/xarelto-epar-medicine-overview_en.pdf (accessed on 17 April 2023).
- Agrawal, A.; Kerndt, C.C.; Manna, B. Apixaban; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- European Medicines Agency: Eliquis. European Medicines Agency. 2023. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/eliquis (accessed on 27 February 2023).
- Eliquis Prescribing Information. Revised 04/2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/202155s032lbl.pdf (accessed on 17 April 2023).
- Eliquis: EPAR-Product Information. Last Updated 09/09/2022. Available online: https://www.ema.europa.eu/en/documents/product-information/eliquis-epar-product-information_en.pdf (accessed on 17 April 2023).
- Padda, I.S.; Chowdhury, Y.S. Chowdhury. Edoxaban; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- European Medicines Agency: Lixiana. European Medicines Agency. 2023. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/lixiana (accessed on 27 February 2023).
- Savaysa Prescribing Information. Revised 01/2015. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206316lbl.pdf (accessed on 17 April 2023).
- Lixiana: EPAR-Product Information. Last Updated 23/04/2021. Available online: https://www.ema.europa.eu/en/documents/product-information/lixiana-epar-product-information_en.pdf (accessed on 17 April 2023).
- Schwarb, H.; Tsakiris, D.A. New Direct Oral Anticoagulants (DOAC) and Their Use Today. Dent. J. 2016, 4, 5. [Google Scholar] [CrossRef]
- Nagarakanti, R.; Ellis, C.R. Dabigatran in clinical practice. Clin. Ther. 2012, 34, 2051–2060. [Google Scholar] [CrossRef]
- European Medicines Agency: Pradaxa. European Medicines Agency. 2023. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/pradaxa (accessed on 28 February 2023).
- Pradaxa Prescribing Information. Revised 06/2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214358s000lbl.pdf (accessed on 17 April 2023).
- Pradaxa: EPAR-Product Information. Last Updated 25/07/2022. Available online: https://www.ema.europa.eu/en/documents/product-information/pradaxa-epar-product-information_en.pdf (accessed on 17 April 2023).
- Baglin, T. Chapter 29—Drugs and haemostasis. In Clinical Pharmacology, 11th ed.; Bennett, P.N., Brown, M.J., Sharma, P., Eds.; Churchill Livingstone: London, UK, 2012; pp. 482–495. ISBN 9780702040849. [Google Scholar] [CrossRef]
- Van den Heuvel, J.M.; Hövels, A.M.; Büller, H.R.; Mantel-Teeuwisse, A.K.; De Boer, A.; Maitland-Van Der Zee, A.H. NOACs replace VKA as preferred oral anticoagulant among new patients: A drug utilization study in 560 pharmacies in The Netherlands. Thromb. J. 2018, 16, 7. [Google Scholar] [CrossRef]
- Lee, S.I.; Sayers, M.; Lip, G.Y.H.; Lane, D.A. Use of non-vitamin K antagonist oral anticoagulants in atrial fibrillation patients: Insights from a specialist atrial fibrillation clinic. Int. J. Clin. 2015, 69, 1341–1348. [Google Scholar] [CrossRef]
- Mocek, A.; Weber, V.; Schmölders, J.; Witt, H.; Gothe, H. Preferences for and use of oral anticoagulants for stroke prevention in atrial fibrillation under real-world conditions in Germany: A survey among physicians. Prev. Med. Rep. 2022, 28, 101861. [Google Scholar] [CrossRef]
- Ravikumar, R.; Lim, C.S.; Davies, A.H. The Role of New Oral Anticoagulants (NOACs) in Cancer Patients. Adv. Exp. Med. Biol. 2017, 906, 137–148. [Google Scholar] [CrossRef]
- Vedovati, M.C.; Germini, F.; Agnelli, G.; Becattini, C. Direct oral anticoagulants in patients with VTE and cancer: A systematic review and meta-analysis. Chest 2015, 147, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.B.; Nielsen, P.B.; Skjøth, F.; Rasmussen, L.H.; Lip, G.Y. Non-vitamin K antagonist oral anticoagulants and the treatment of venous thromboembolism in cancer patients: A semi systematic review and meta-analysis of safety and efficacy outcomes. PLoS ONE 2014, 9, e114445. [Google Scholar] [CrossRef] [PubMed]
- van der Hulle, T.; den Exter, P.L.; Kooiman, J.; van der Hoeven, J.J.; Huisman, M.V.; Klok, F.A. Meta-analysis of the efficacy and safety of new oral anticoagulants in patients with cancer-associated acute venous thromboembolism. J. Thromb. Haemost. 2014, 12, 1116–1120. [Google Scholar] [CrossRef]
- Ebner, M.; Lankeit, M. Antithrombotische Therapie bei Lungenembolie [Antithrombotic Treatment of Pulmonary Embolism]. Dtsch. Med. Wochenschr. 2020, 145, 970–977. (In German) [Google Scholar] [CrossRef]
- Yan, Y.D.; Zhang, C.; Shen, L.; Su, Y.J.; Liu, X.Y.; Wang, L.W.; Gu, Z.C. Net Clinical Benefit of Non-vitamin K Antagonist Oral Anticoagulants for Venous Thromboembolism Prophylaxis in Patients with Cancer: A Systematic Review and Trade-Off Analysis from 9 Randomized Controlled Trials. Front Pharmacol. 2018, 9, 575. [Google Scholar] [CrossRef]
- Malavasi, V.L.; Vitolo, M.; Proietti, M.; Diemberger, I.; Fauchier, L.; Marin, F.; Nabauer, M.; Potpara, T.S.; Dan, G.A.; Kalarus, Z.; et al. Impact of malignancy on outcomes in European patients with atrial fibrillation: A report from the ESC-EHRA EURObservational research programme in atrial fibrillation general long-term registry. Eur. J. Clin. Investig. 2022, 52, e13773. [Google Scholar] [CrossRef] [PubMed]
- Wumaier, K.; Li, W.; Cui, J. New Oral Anticoagulants Open New Horizons for Cancer Patients with Venous Thromboembolism. Drug Des. Devel. Ther. 2022, 16, 2497–2507. [Google Scholar] [CrossRef]
- Mohamed, M.F.H.; ElShafei, M.N.; Ahmed, M.B.; Abdalla, L.O.; Ahmed, I.; Elzouki, A.N.; Danjuma, M.I. The Net Clinical Benefit of Rivaroxaban Compared to Low-Molecular-Weight Heparin in the Treatment of Cancer-Associated Thrombosis: Systematic Review and Meta-Analysis. Clin. Appl. Thromb. Hemost. 2021, 27, 1076029620940046. [Google Scholar] [CrossRef]
- Prandoni, P.; Lensing, A.W.; Piccioli, A.; Bernardi, E.; Simioni, P.; Girolami, B.; Marchiori, A.; Sabbion, P.; Prins, M.H.; Noventa, F.; et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002, 100, 3484–3488. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, J.; Zhang, W.; Jiang, M.; Liu, J.; Xu, L.; Liu, G.; Zhao, Z. Quality Appraisal of Guidelines on Cancer-Associated Thrombosis Using AGREE II Instrument and Analysis of Current Status of New Oral Anticoagulants. Clin. Appl. Thromb. Hemost. 2019, 25, 1076029619846562. [Google Scholar] [CrossRef]
- Mekaj, Y.H.; Mekaj, A.Y.; Duci, S.B.; Miftari, E.I. New oral anticoagulants: Their advantages and disadvantages compared with vitamin K antagonists in the prevention and treatment of patients with thromboembolic events. Ther. Clin. Risk Manag. 2015, 11, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, Y.S.; Kim, T.H.; Cha, M.J.; Lee, J.M.; Park, J.; Park, J.K.; Kang, K.W.; Shim, J.; Uhm, J.S.; et al. A prospective survey of the persistence of warfarin or NOAC in nonvalvular atrial fibrillation: A comparison study of Drugs for symptom control and complication prevention of Atrial Fibrillation (CODE-AF). Korean J. Intern. Med. 2020, 35, 99–108. [Google Scholar] [CrossRef]
- Iorga, R.A.; Bratu, O.G.; Marcu, R.D.; Constantin, T.; Mischianu, D.L.D.; Socea, B.; Gaman, M.A.; Diaconu, C.C. Venous thromboembolism in cancer patients: Still looking for answers. Exp. Ther. Med. 2019, 18, 5026–5032. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Zhang, Y.; Li, Y.; Li, Y.; Miao, Y.; Zhao, R.; Zhai, S. Direct Oral Anticoagulant for the Treatment of VTE in Cancer Patients: A Systematic Review and Meta-analysis. Ann. Pharmacother. 2021, 55, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Raskob, G.E.; van Es, N.; Segers, A.; Angchaisuksiri, P.; Oh, D.; Boda, Z.; Lyons, R.M.; Meijer, K.; Gudz, I.; Weitz, J.I.; et al. Edoxaban for venous thromboembolism in patients with cancer: Results from a non-inferiority subgroup analysis of the Hokusai-VTE randomised, double-blind, double-dummy trial. Lancet Haematol. 2016, 3, e379–e387. [Google Scholar] [CrossRef] [PubMed]
- Karakatsanis, S.J.; Roumpi, A.; Syrigos, K.N. The use of novel oral anticoagulants in cancer patients with venous thromboembolism. Semin. Oncol. 2016, 43, 655–665. [Google Scholar] [CrossRef]
- Wang, C.X.; Wu, D.; Yang, P.P.; Wu, Q.H. Efficacy and safety of non-vitamin K antagonist versus vitamin K antagonist oral anticoagulants in the prevention and treatment of thrombotic disease in active cancer patients: A systematic review and meta-analysis of randomized controlled trials. Zhonghua Xin Xue Guan Bing Za Zhi 2020, 48, 689–696. (In Chinese) [Google Scholar] [CrossRef]
- Verso, M.; Agnelli, G.; Prandoni, P. Pros and cons of new oral anticoagulants in the treatment of venous thromboembolism in patients with cancer. Intern. Emerg. Med. 2015, 10, 651–656. [Google Scholar] [CrossRef]
- Haas, S.; Farjat, A.E.; Pieper, K.; Ageno, W.; Angchaisuksiri, P.; Bounameaux, H.; Goldhaber, S.Z.; Goto, S.; Mantovani, L.; Prandoni, P.; et al. On-treatment Comparative Effectiveness of Vitamin K Antagonists and Direct Oral Anticoagulants in GARFIELD-VTE, and Focus on Cancer and Renal Disease. TH Open 2022, 6, e354–e364. [Google Scholar] [CrossRef]
- Barbarawi, M.; Barbarawi, O.; Corcoran, J.; Obeidat, K.; Al-Abdouh, A.; Mhanna, M.; Al Kasasbeh, M.; Pickett, C.C. Efficacy and Safety of the Non-Vitamin K Antagonist Oral Anticoagulant Among Patients with Nonvalvular Atrial Fibrillation and Cancer: A Systematic Review and Network Meta-analysis. Curr. Probl. Cardiol. 2022, 47, 101346. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.H.; Chao, T.F.; Lee, H.F.; Chen, S.W.; Li, P.R.; Liu, J.R.; Wu, L.S.; Chang, S.H.; Yeh, Y.H.; Kuo, C.T.; et al. Clinical Outcomes in Atrial Fibrillation Patients with a History of Cancer Treated with Non-Vitamin K Antagonist Oral Anticoagulants: A Nationwide Cohort Study. Stroke 2021, 52, 3132–3141. [Google Scholar] [CrossRef] [PubMed]
- Clemens, A.; Strack, A.; Noack, H.; Konstantinides, S.; Brueckmann, M.; Lip, G.Y. Anticoagulant-related gastrointestinal bleeding--could this facilitate early detection of benign or malignant gastrointestinal lesions? Ann. Med. 2014, 46, 672–678. [Google Scholar] [CrossRef]
- Papanastasiou, A.; Morsi-Yeroyannis, A.; Karagiannidis, E.; Kartas, A.; Doundoulakis, I.; Karvounis, H.; Giannakoulas, G. Association of anticoagulant-related bleeding events with cancer detection in atrial fibrillation: A systematic review and meta-analysis. Hell. J. Cardiol. 2021, 62, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.Y.; Chan, Y.H.; Chiang, C.E.; Lin, Y.J.; Chang, S.L.; Lo, L.W.; Hu, Y.F.; Tuan, T.C.; Liao, J.N.; Chung, F.P.; et al. Risks and outcomes of gastrointestinal malignancies in anticoagulated atrial fibrillation patients experiencing gastrointestinal bleeding: A nationwide cohort study. Heart Rhythm. 2020, 17, 1745–1751. [Google Scholar] [CrossRef]
- Antunes, L.F. New Oral Anticoagulants (NOACs) are The Gold Standard Invenous Thromboembolism. Rev. Port. Cir. Cardiotorac. Vasc. 2020, 27, 33–37. [Google Scholar]
- Choi, Y.J.; Choi, Y.W.; Chae, J.W.; Yun, H.Y.; Shin, S. Clinical Benefits of Oral Anticoagulant Use in Cancer Patients at Increased Risk for Venous Thromboembolism per Khorana Index. Risk Manag. Healthc. Policy 2021, 14, 1855–1867. [Google Scholar] [CrossRef]
- Chen, Y.; Mao, M.; Chang, J.; Yan, J.; Yang, T.; Liu, Y.; Luo, M.; Hu, Y.; Yang, Q.; Zhou, L.; et al. Safety and efficacy of new oral anticoagulants compared to those of warfarin in AF patients with cancer: A meta-analysis of randomized clinical trials and observational studies. Eur. J. Clin. Pharmacol. 2021, 77, 849–857. [Google Scholar] [CrossRef]
- Wu, V.C.; Wang, C.L.; Huang, Y.T.; Lan, W.C.; Wu, M.; Kuo, C.F.; Chen, S.W.; Chu, P.H.; Wen, M.S.; Kuo, C.C.; et al. Novel Oral Anticoagulant versus Warfarin in Cancer Patients with Atrial Fibrillation: An 8-Year Population-Based Cohort Study. J. Cancer 2020, 11, 92–99. [Google Scholar] [CrossRef]
- Kim, K.; Lee, Y.J.; Kim, T.H.; Uhm, J.S.; Pak, H.N.; Lee, M.H.; Joung, B. Effect of Non-vitamin K Antagonist Oral Anticoagulants in Atrial Fibrillation Patients with Newly Diagnosed Cancer. Korean Circ. J. 2018, 48, 406–417. [Google Scholar] [CrossRef]
- Atterman, A.; Asplund, K.; Friberg, L.; Engdahl, J. Use of oral anticoagulants after ischemic stroke in patients with atrial fibrillation and cancer. J. Intern. Med. 2020, 288, 457–468. [Google Scholar] [CrossRef]
- Solari, F.; Varacallo, M. Low Molecular Weight Heparin (LMWH); StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Mulloy, B.; Hogwood, J.; Gray, E.; Lever, R.; Page, C.P. Pharmacology of Heparin and Related Drugs. Pharmacol. Rev. 2016, 68, 76–141. [Google Scholar] [CrossRef]
- Howard, L.S. Non-vitamin K antagonist oral anticoagulants for pulmonary embolism: Who, where and for how long? Expert Rev. Respir. Med. 2018, 12, 387–402. [Google Scholar] [CrossRef]
- Short, N.J.; Connors, J.M. New oral anticoagulants and the cancer patient. Oncologist 2014, 19, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Enea, I.; Roncon, L.; Gulizia, M.M.; Azzarito, M.; Becattini, C.; Bongarzoni, A.; Casazza, F.; Cuccia, C.; D’Agostino, C.; Rugolotto, M.; et al. ANMCO Position Paper: The use of non-vitamin K dependent new oral anticoagulant(s) in pulmonary embolism therapy and prevention. Eur. Heart J. Suppl. 2017, 19 (Suppl. D), D293–D308. [Google Scholar] [CrossRef] [PubMed]
- Al-Samkari, H.; Connors, J.M. The Role of Direct Oral Anticoagulants in Treatment of Cancer-Associated Thrombosis. Cancers 2018, 10, 271. [Google Scholar] [CrossRef]
- Stepien, K.; Nowak, K.; Zalewski, J.; Pac, A.; Undas, A. Extended treatment with non-vitamin K antagonist oral anticoagulants versus low-molecular-weight heparins in cancer patients following venous thromboembolism. A pilot study. Vascul. Pharmacal. 2019, 120, 106567. [Google Scholar] [CrossRef]
- Tirandi, A.; Preda, A.; Carbone, F.; Montecucco, F.; Liberale, L. Pulmonary embolism in patients with cancer: An updated and operative guide for diagnosis and management. Int. J. Cardiol. 2022, 358, 95–102. [Google Scholar] [CrossRef]
- Asnani, A.; Manning, A.; Mansour, M.; Ruskin, J.; Hochberg, E.P.; Ptaszek, L.M. Management of atrial fibrillation in patients taking targeted cancer therapies. Cardiooncology 2017, 3, 2. [Google Scholar] [CrossRef]
- Jin, C.; Cui, C.; Seplowe, M.; Lee, K.I.; Vegunta, R.; Li, B.; Frishman, W.H.; Iwai, S. Anticoagulation for Atrial Fibrillation: A Review of Current Literature and Views. Cardiol. Rev. 2022. [Google Scholar] [CrossRef] [PubMed]
- Stevens, S.M.; Woller, S.C.; Kreuziger, L.B.; Bounameaux, H.; Doerschug, K.; Geersing, G.J.; Huisman, M.V.; Kearon, C.; King, C.S.; Knighton, A.J.; et al. Antithrombotic therapy for VTE disease: Second update of the CHEST guideline and expert panel report. Chest 2021, 160, e545–e608. [Google Scholar] [CrossRef] [PubMed]
- Gressel, G.M.; Marcus, J.Z.; Mullen, M.M.; Sinno, A.K. Direct oral anticoagulant use in gynecologic oncology: A Society of Gynecologic Oncology Clinical Practice Statement. Gynecol. Oncol. 2021, 160, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.W.; Zheng, X.; Han, X.H. Generic Methods for Simultaneous Analysis of Four Direct Oral Anticoagulants in Human Plasma and Urine by Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry. Molecules 2023, 28, 2254. [Google Scholar] [CrossRef]
- Lenihan, D.J.; Fradley, M.G.; Dent, S.; Brezden-Masley, C.; Carver, J.; Filho, R.K.; Neilan, T.G.; Blaes, A.; Melloni, C.; Herrmann, J.; et al. Proceedings from the Global Cardio-Oncology Summit: The Top 10 Priorities to Actualize for CardioOncology. JACC CardioOncol. 2019, 1, 256–272. [Google Scholar] [CrossRef]
- Khorana, A.A.; Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008, 111, 4902–4907. [Google Scholar] [CrossRef] [PubMed]
- Key, N.S.; Khorana, A.A.; Kuderer, N.M.; Bohlke, K.; Lee, A.Y.Y.; Arcelus, J.I.; Wong, S.L.; Balaban, E.P.; Flowers, C.R.; Francis, C.W.; et al. Venous Thromboembolism Prophylaxis and Treatment in Patients with Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2020, 38, 496–520. [Google Scholar] [CrossRef]
- Falanga, A.; Ay, C.; Di Nisio, M.; Gerotziafas, G.; Langer, F.; Lecumberri, R.; Mandala, M.; Maraveyas, A.; Pabinger, I.; Jara-Palomares, L.; et al. Venous thromboembolism in cancer patients: ESMO Clinical Practice Guideline. Ann. Oncol. 2023, 34, 452–467. [Google Scholar] [CrossRef]
- Streiff, M.B.; Holmstrom, B.; Angelini, D.; Ashrani, A.; Elshoury, A.; Fanikos, J.; Fertrin, K.Y.; Fogerty, A.E.; Gao, S.; Goldhaber, S.Z.; et al. Cancer-Associated Venous Thromboembolic Disease, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 1181–1201. [Google Scholar] [CrossRef]
- Lyman, G.H.; Carrier, M.; Ay, C.; Di Nisio, M.; Hicks, L.K.; Khorana, A.A.; Leavitt, A.D.; Lee, A.Y.Y.; Macbeth, F.; Morgan, R.L.; et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: Prevention and treatment in patients with cancer. Blood Adv. 2021, 5, 927–974. [Google Scholar] [CrossRef]
- Li, M.; Li, J.; Wang, X.; Hui, X.; Wang, Q.; Xie, S.; Yan, P.; Tian, J.; Li, J.; Xie, P.; et al. Oral direct thrombin inhibitors or oral factor Xa inhibitors versus conventional anticoagulants for the treatment of pulmonary embolism. Cochrane Database Syst. Rev. 2023, 4, CD010957. [Google Scholar] [CrossRef]
- Barca-Hernando, M.; Lopez-Ruz, S.; Marin-Romero, S.; Garcia-Garcia, V.; Elias-Hernandez, T.; Otero-Candelera, R.; Carrier, M.; Jara-Palomares, L. Risk of recurrent cancer-associated thrombosis after discontinuation of anticoagulant therapy. Res. Pract. Thromb. Haemost. 2023, 7, 100115. [Google Scholar] [CrossRef] [PubMed]
- Debourdeau, P.; Bertoletti, L.; Font, C.; López-Núñez, J.J.; Gómez-Cuervo, C.; Mahe, I.; Otero-Candelera, R.; Adarraga, M.D.; López-Miguel, P.; Monreal, M.; et al. Three-Month Outcomes in Cancer Patients with Superficial or Deep Vein Thrombosis in the Lower Limbs: Results from the RIETE Registry. Cancers 2023, 15, 2034. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.; Spectre, G.; Lishner, M.; Sharabi, O.; Robinson, E.; Hamburger Avnery, O.; Gafter-Gvili, A.; Raanani, P.; Leader, A. Direct oral anticoagulants in patients with venous thromboembolism and hematological malignancies. J. Thromb. Thrombolysis 2023, 55, 729–736. [Google Scholar] [CrossRef]
- Wiklund, P.; Medson, K.; Elf, J. Unreported incidental pulmonary embolism in patients with cancer: Radiologic natural history and risk of recurrent venous thromboembolism and death. Thromb. Res. 2023, 224, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Vedovati, M.C.; Giustozzi, M.; Munoz, A.; Bertoletti, L.; Cohen, A.T.; Klok, F.A.; Connors, J.M.; Bauersachs, R.; Brenner, B.; Campanini, M.; et al. Risk factors for recurrence and major bleeding in patients with cancer-associated venous thromboembolism. Eur. J. Intern. Med. 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Garcia, D.A.; Lyman, G.H.; Carrier, M. Direct oral anticoagulant (DOAC) versus low-molecular-weight heparin (LMWH) for treatment of cancer associated thrombosis (CAT): A systematic review and meta-analysis. Thromb. Res. 2019, 173, 158–163. [Google Scholar] [CrossRef]
- Lv, S.; Liu, Y.; Wei, G.; Shi, X.; Chen, S.; Zhang, X. The anticoagulants rivaroxaban and low molecular weight heparin prevent PICC-related upper extremity venous thrombosis in cancer patients. Medicine 2019, 98, e17894. [Google Scholar] [CrossRef]
- Longo de Oliveira, A.L.M.; de Oliveira Pereira, R.F.; Agati, L.B.; Ribeiro, C.M.; Kawamura Suguiura, G.Y.; Cioni, C.H.; Bermudez, M.; Pirani, M.B.; Caffaro, R.A.; Castelli, V., Jr.; et al. Rivaroxaban Versus Enoxaparin for Thromboprophylaxis After major Gynecological Cancer Surgery: The VALERIA Trial: Venous thromboembolism prophylAxis after gynecoLogical pElvic cancer surgery with RIvaroxaban versus enoxAparin (VALERIA trial). Clin. Appl. Thromb. Hemost. 2022, 28, 10760296221132556. [Google Scholar] [CrossRef]
- Chen, D.Y.; Tseng, C.N.; Hsieh, M.J.; Lan, W.C.; Chuang, C.K.; Pang, S.T.; Chen, S.W.; Chen, T.H.; Chang, S.H.; Hsieh, I.C.; et al. Comparison between Non-vitamin K Antagonist Oral Anticoagulants and Low-Molecular-Weight Heparin in Asian Individuals With Cancer-Associated Venous Thromboembolism. JAMA Netw. Open 2021, 4, e2036304. [Google Scholar] [CrossRef]
- Song, A.B.; Rosovsky, R.P.; Connors, J.M.; Al-Samkari, H. Direct oral anticoagulants for treatment and prevention of venous thromboembolism in cancer patients. Vasc. Health Risk Manag. 2019, 15, 175–186. [Google Scholar] [CrossRef]
- Camilli, M.; Lombardi, M.; Vescovo, G.M.; Del Buono, M.G.; Galli, M.; Aspromonte, N.; Zoccai, G.B.; Niccoli, G.; Montone, R.A.; Crea, F.; et al. Efficacy and safety of novel oral anticoagulants versus low molecular weight heparin in cancer patients with venous thromboembolism: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2020, 154, 103074. [Google Scholar] [CrossRef]
- Raskob, G.E.; van Es, N.; Verhamme, P.; Carrier, M.; Di Nisio, M.; Garcia, D.; Grosso, M.A.; Kakkar, A.K.; Kovacs, M.J.; Mercuri, M.F.; et al. Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. N. Engl. J. Med. 2018, 378, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.L.; Kao, Y.T.; Chang, W.T.; Chang, H.Y.; Huang, W.C.; Hsu, P.C.; Hsu, C.H.; Huang, C.L.; Hsieh, L.C.; Wang, C.Y.; et al. Management of Venous Thromboembolisms: Part, I.I. The Consensus for Pulmonary Embolism and Updates. Acta Cardiol. Sin. 2020, 36, 562–582. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.R.; Ali, F.S.; Verghese, D.; Myint, P.T.; Ahmed, M.; Gong, Z.; Gerais, Y.; Siddiqui, M.; Lin, J.J.; Troy, K. Factor Xa inhibitors versus low molecular weight heparin for the treatment of cancer associated venous thromboembolism; A meta-analysis of randomized controlled trials and non-randomized studies. Crit. Rev. Oncol. Hematol. 2022, 169, 103526. [Google Scholar] [CrossRef]
- Frere, C.; Farge, D.; Schrag, D.; Prata, P.H.; Connors, J.M. Direct oral anticoagulant versus low molecular weight heparin for the treatment of cancer-associated venous thromboembolism: 2022 updated systematic review and meta-analysis of randomized controlled trials. J. Hematol. Oncol. 2022, 15, 69. [Google Scholar] [CrossRef]
- Nam, K.W.; Kim, C.K.; Kim, T.J.; An, S.J.; Oh, K.; Ko, S.B.; Yoon, B.W. Treatment of Cryptogenic Stroke with Active Cancer with a New Oral Anticoagulant. J. Stroke Cerebrovasc. Dis. 2017, 26, 2976–2980. [Google Scholar] [CrossRef]
- Jiménez-Fonseca, P.; Gallardo, E.; Arranz Arija, F.; Blanco, J.M.; Callejo, A.; Lavin, D.C.; Costa Rivas, M.; Mosquera, J.; Rodrigo, A.; Sánchez Morillas, R.; et al. Consensus on prevention and treatment of cancer-associated thrombosis (CAT) in controversial clinical situations with low levels of evidence. Eur. J. Intern. Med. 2022, 100, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Delluc, A.; Wang, T.F.; Yap, E.S.; Ay, C.; Schaefer, J.; Carrier, M.; Noble, S. Anticoagulation of cancer patients with non-valvular atrial fibrillation receiving chemotherapy: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2019, 17, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Undas, A.; Drabik, L. Non-vitamin K antagonist oral anticoagulants (NOACs) in cancer patients with atrial fibrillation. Anatol. J. Cardiol. 2020, 23, 10–18. [Google Scholar] [CrossRef]
- Ferri, N.; Colombo, E.; Tenconi, M.; Baldessin, L.; Corsini, A. Drug-Drug Interactions of Direct Oral Anticoagulants (DOACs): From Pharmacological to Clinical Practice. Pharmaceutics 2022, 14, 1120. [Google Scholar] [CrossRef]
- Otten, L.S.; Piet, B.; van den Heuvel, M.M.; Marzolini, C.; van Geel, R.M.J.M.; Gulikers, J.L.; Burger, D.M.; Leentjens, J.; Ter Heine, R. Practical recommendations to combine small-molecule inhibitors and direct oral anticoagulants in patients with nonsmall cell lung cancer. Eur. Respir. Rev. 2022, 31, 220004. [Google Scholar] [CrossRef] [PubMed]
- Hellfritzsch, M.; Henriksen, J.N.; Holt, M.I.; Grove, E.L. Drug-Drug Interactions in the Treatment of Cancer-Associated Venous Thromboembolism with Direct Oral Anticoagulants. In Seminars in Thrombosis and Hemostasis; Thieme Medical Publishers, Inc.: Leipzig, Germany, 2023. [Google Scholar] [CrossRef]
- Rousseau, A.; Van Dreden, P.; Mbemba, E.; Elalamy, I.; Larsen, A.; Gerotziafas, G.T. Cancer cells BXPC3 and MCF7 differentially reverse the inhibition of thrombin generation by apixaban, fondaparinux and enoxaparin. Thromb. Res. 2015, 136, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Muñoz Martín, A.J.; Gallardo Díaz, E.; García Escobar, I.; Macías Montero, R.; Martínez-Marín, V.; Pachón Olmos, V.; Pérez Segura, P.; Quintanar Verdúguez, T.; Salgado Fernández, M. SEOM clinical guideline of venous thromboembolism (VTE) and cancer (2019). Clin. Transl. Oncol. 2020, 22, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.A.; Gibbs, H.; Merriman, E.; Curnow, J.L.; Young, L.; Bennett, A.; Tan, C.W.; Chunilal, S.D.; Ward, C.M.; Baker, R.; et al. New guidelines from the Thrombosis and Haemostasis Society of Australia and New Zealand for the diagnosis and management of venous thromboembolism. Med. J. Aust. 2019, 210, 227–235, Erratum in Med. J. Aust. 2019, 211, 94; Erratum in Med. J. Aust. 2020, 212, 108. [Google Scholar] [CrossRef]
- Nayak, A.L.; Zahrai, A.; Mallick, R.; Wang, T.F.; Delluc, A.; Castellucci, L.A.; Carrier, M.; Wells, P.S. Efficacy of primary prevention of venous thromboembolism among subgroups of cancer patients undergoing chemotherapy: A post- hoc analysis of the AVERT trial. Thromb. Res. 2021, 208, 79–82. [Google Scholar] [CrossRef]
- Ladha, D.; Mallick, R.; Wang, T.F.; Caiano, L.; Wells, P.S.; Carrier, M. Efficacy and safety of apixaban for primary prevention in gastrointestinal cancers: A post-hoc analysis of the AVERT trial. Thromb. Res. 2021, 202, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Brandt, W.; Brown, C.; Wang, T.F.; Tagalakis, V.; Shivakumar, S.; Ciuffini, L.A.; Mallick, R.; Wells, P.S.; Carrier, M. Efficacy and safety of apixaban for primary prevention of thromboembolism in patients with cancer and a central venous catheter: A subgroup analysis of the AVERT Trial. Thromb. Res. 2022, 216, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.F.; Mallick, R.; Carrier, M.; Wells, P.S. Safety and efficacy of apixaban thromboprophylaxis in ambulatory cancer patients according to renal function: A subgroup analysis of the AVERT trial. Thromb. Res. 2022, 211, 85–87. [Google Scholar] [CrossRef]
- Knoll, W.; Mallick, R.; Wells, P.S.; Carrier, M. Safety and efficacy of apixaban thromboprophylaxis in cancer patients with metastatic disease: A post-hoc analysis of the AVERT trial. Thromb. Res. 2021, 197, 13–15. [Google Scholar] [CrossRef]
- Zhang, J.; Atalla, M.; Mallick, R.; Wells, P.S.; Carrier, M. Thromboprophylaxis for patients with newly diagnosed vs. recurrent cancers: A post-hoc analysis of the avert trial. J. Thromb. Thrombolysis 2021, 51, 720–724. [Google Scholar] [CrossRef]
- Mones, J.V.; Streiff, M.B.; Khorana, A.A.; Bendheim, G.A.; Damaraju, C.V.; Wildgoose, P.; Burton, P.; Riess, H.; Soff, G.A. Rivaroxaban thromboprophylaxis for gastric/gastroesophageal junction tumors versus other tumors: A post hoc analysis of the randomized CASSINI trial. Res. Pract. Thromb. Haemost. 2021, 5, e12549. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; McNamara, M.G.; Kakkar, A.K.; Streiff, M.B.; Riess, H.; Vijapurkar, U.; Kaul, S.; Wildgoose, P.; Soff, G.A.; CASSINI Investigators. Assessing Full Benefit of Rivaroxaban Prophylaxis in High-Risk Ambulatory Patients with Cancer: Thromboembolic Events in the Randomized CASSINI Trial. TH Open 2020, 4, e107–e112. [Google Scholar] [CrossRef] [PubMed]
- Vadhan-Raj, S.; McNamara, M.G.; Venerito, M.; Riess, H.; O’Reilly, E.M.; Overman, M.J.; Zhou, X.; Vijapurkar, U.; Kaul, S.; Wildgoose, P.; et al. Rivaroxaban thromboprophylaxis in ambulatory patients with pancreatic cancer: Results from a pre-specified subgroup analysis of the randomized CASSINI study. Cancer Med. 2020, 9, 6196–6204. [Google Scholar] [CrossRef]
- Khorana, A.A.; Soff, G.A.; Kakkar, A.K.; Vadhan-Raj, S.; Riess, H.; Wun, T.; Streiff, M.B.; Garcia, D.A.; Liebman, H.A.; Belani, C.P.; et al. Rivaroxaban for Thromboprophylaxis in High-Risk Ambulatory Patients with Cancer. N. Engl. J. Med. 2019, 380, 720–728. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine (U.S.). Interaction between Direct Oral Anticoagulants and Drug-Metabolizing Enzyme Inducers. Identifier: NCT05750680. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT05750680 (accessed on 15 March 2023).
- National Library of Medicine (U.S.). Clinical Application Model of Direct Oral Anticoagulants (MACACOD). Comprehensive Management of ACOD from a Specialized Center in Antithrombotic Therapy and Its Area of Influence Identifier: NCT04042155. 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT04042155 (accessed on 15 March 2023).
- National Library of Medicine (U.S.). Direct Oral Anticoagulants (DOACs) Versus LMWH +/- Warfarin for VTE in Cancer: A Randomized Effectiveness Trial (CANVAS Trial). Identifier: NCT02744092. 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02744092 (accessed on 15 March 2023).
- National Library of Medicine (U.S.). VICTORIE (VTE In Cancer—Treatment, Outcomes and Resource Use in Europe). Identifier: NCT04618913. Available online: https://clinicaltrials.gov/ct2/show/NCT04618913 (accessed on 15 March 2023).
- Castle, J.; Blower, E.; Bundred, N.J.; Harvey, J.R.; Thachil, J.; Marshall, A.; Cox, K.; Cicconi, S.; Holcombe, C.; Palmieri, C.; et al. Rivaroxaban compared to no treatment in ER-negative stage I-III early breast cancer patients (the TIP Trial): Study protocol for a phase II preoperative window-of-opportunity study design randomised controlled trial. Trials 2020, 21, 749. [Google Scholar] [CrossRef]
- National Library of Medicine (U.S.). Observational Studies in Cancer Associated Thrombosis for Rivaroxaban in Sweden (OSCAR-SE). Identifier: NCT05150938. 2022. Available online: https://clinicaltrials.gov/ct2/show/study/NCT05150938 (accessed on 15 March 2023).
- National Library of Medicine (U.S.). Primary Thromboprophylaxis in Patients with Malignancy and Central Venous Catheters: A Randomized Controlled Trial. Identifier: NCT05029063. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05029063 (accessed on 15 March 2023).
- National Library of Medicine (U.S.). Bleeding Risk Guided VTE Prophylaxis Strategy for Hospitalized Patients with Lung Cancer: Rationale and Design for a Multicenter, Adjudicator-Blinded, Parallel, Randomized Clinical Trial in China. Identifier: NCT04158973. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04158973 (accessed on 15 March 2023).
- National Library of Medicine (U.S.). Venous Thromboembolism Prevention in Outpatients with Glioma. Identifier: NCT05683808. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT05683808 (accessed on 15 March 2023).
- National Library of Medicine (U.S.). Cost Comparison between Apixaban and Low Molecular Weight Heparin (LMWH) among Venous Thromboembolism (VTE) Cancer Patients. Identifier: NCT05643885. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05643885 (accessed on 15 March 2023).
- National Library of Medicine (U.S.). A Multicenter, Randomized, Open-Label, Blinded Endpoint Evaluation, Phase 3 Study Comparing the Effect of Abelacimab Relative to Apixaban on Venous Thromboembolism (VTE) Recurrence and Bleeding in Patients with Cancer Associated VTE. Identifier: NCT05171049. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05171049 (accessed on 15 March 2023).
- Planquette, B.; Bertoletti, L.; Charles-Nelson, A.; Laporte, S.; Grange, C.; Mahé, I.; Pernod, G.; Elias, A.; Couturaud, F.; Falvo, N.; et al. Rivaroxaban vs. Dalteparin in Cancer-Associated Thromboembolism: A Randomized Trial. Chest 2022, 161, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Agnelli, G.; Becattini, C.; Meyer, G.; Muñoz, A.; Huisman, M.V.; Connors, J.M.; Cohen, A.; Bauersachs, R.; Brenner, B.; Torbicki, A.; et al. Apixaban for the Treatment of Venous Thromboembolism Associated with Cancer. N. Engl. J. Med. 2020, 382, 1599–1607. [Google Scholar] [CrossRef]
- Canonico, M.E.; Santoro, C.; Avvedimento, M.; Giugliano, G.; Mandoli, G.E.; Prastaro, M.; Franzone, A.; Piccolo, R.; Ilardi, F.; Cameli, M.; et al. Venous Thromboembolism and Cancer: A Comprehensive Review from Pathophysiology to Novel Treatment. Biomolecules 2022, 12, 259. [Google Scholar] [CrossRef]
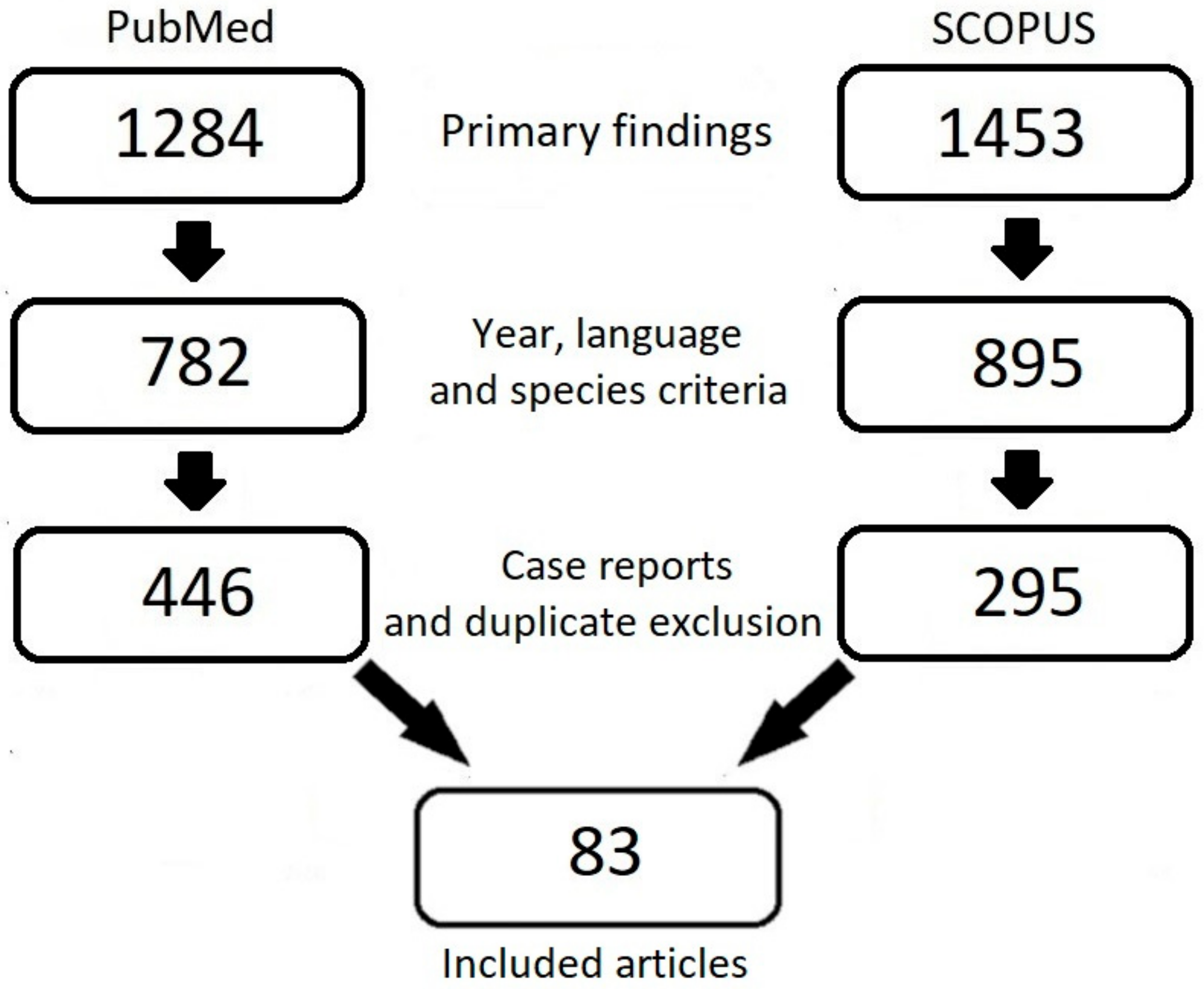

| Guidelines | SEOM 2019 [122] | THAZ 2019 [123] | ASCO 2020 [94] | ASH 2021 [97] | SGO 2021 [90] | NCCN 2021 [96] | ESMO 2023 [95] |
|---|---|---|---|---|---|---|---|
| Prophylaxis for VTE in hospitalized patients with cancer | - | - | Routine pharmacologic thromboprophylaxis may be offered | LMWHs | LMWHs | LMWHs, fondaparinux, UFHs | LMWHs, apixaban, rivaroxaban |
| Prophylaxis for VTE in ambulatory patients with cancer during systemic therapy | - | - | Routine pharmacologic thromboprophylaxis should not be offered. In high-risk patients, apixaban, rivaroxaban or LMWHs | LMWHs, fondaparinux | Rivaroxaban, apixaban, LMWHs | Apixaban, rivaroxaban, dalteparin and enoxaparin | - |
| Prophylaxis in patients with cancer undergoing surgery | - | - | Prophylaxis should be initiated preoperatively. LMWHs, UFHs | Prophylaxis should be initiated postoperatively. LMWHs | LMWHs, UFHs, apixaban | Apixaban, dalteparin and enoxaparin | LMWHs, UFHs |
| Prevention of rVTE | - | - | LMWHs, UFHs, fondaparinux or rivaroxaban. | - | - | - | - |
| Initial CAT treatment | LMWHs, rivaroxaban, UFHs, fondaparinux | - | - | LMWHs | - | - | LMWHs, UFHs, fondaparinux, apixaban, rivaroxaban |
| Short-term treatment for patients with active cancer | - | - | - | DOACs, LMWHs | - | - | - |
| Long-term treatment for patients with active cancer | LMWHs, DOACs | - | - | DOACs, LMWHs | LMWHs, apixaban, edoxaban or rivaroxaban | - | LMWHs, apixaban, edoxaban, rivaroxaban |
| CVCAT | LMWHs, DOACs | - | - | - | - | - | - |
| Incidental VTE | LMWHs, DOACs | Rivaroxaban, apixaban, dabigatran, warfarin, LMWHs | - | - | - | - | LMWHs, UFHs, fondaparinux |
| Recurrent VTE during anticoagulation therapy | LMWHs, DOACs | - | - | LMWHs | - | - | - |
| Central nervous system primary tumors and metastasis | LMWHs, DOACs | - | - | - | - | - | - |
| Anticoagulation in the absence of VTE to improve survival in cancer patients | Anticoagulant use in cancer patients should not be prescribed to improve survival | - | Anticoagulant use is not recommended to improve survival in patients with cancer without VTE | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górnicki, T.; Bułdyś, K.; Zielińska, D.; Chabowski, M. Direct-Acting Oral Anticoagulant Therapy in Cancer Patients—A Review. Cancers 2023, 15, 2697. https://doi.org/10.3390/cancers15102697
Górnicki T, Bułdyś K, Zielińska D, Chabowski M. Direct-Acting Oral Anticoagulant Therapy in Cancer Patients—A Review. Cancers. 2023; 15(10):2697. https://doi.org/10.3390/cancers15102697
Chicago/Turabian StyleGórnicki, Tomasz, Kacper Bułdyś, Dorota Zielińska, and Mariusz Chabowski. 2023. "Direct-Acting Oral Anticoagulant Therapy in Cancer Patients—A Review" Cancers 15, no. 10: 2697. https://doi.org/10.3390/cancers15102697
APA StyleGórnicki, T., Bułdyś, K., Zielińska, D., & Chabowski, M. (2023). Direct-Acting Oral Anticoagulant Therapy in Cancer Patients—A Review. Cancers, 15(10), 2697. https://doi.org/10.3390/cancers15102697







