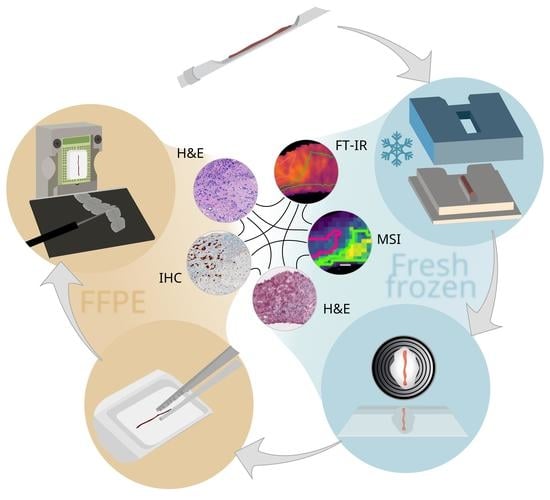
Spatial Omics Imaging of Fresh-Frozen Tissue and Routine FFPE Histopathology of a Single Cancer Needle Core Biopsy: A Freezing Device and Multimodal Workflow
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Specimens and Needle Biopsies
2.2. Biopsy Freezing Device, Preparation of Fresh-Frozen Samples
2.3. MALDI Mass Spectrometry Imaging (MSI) and FT-IR Imaging (IRI)
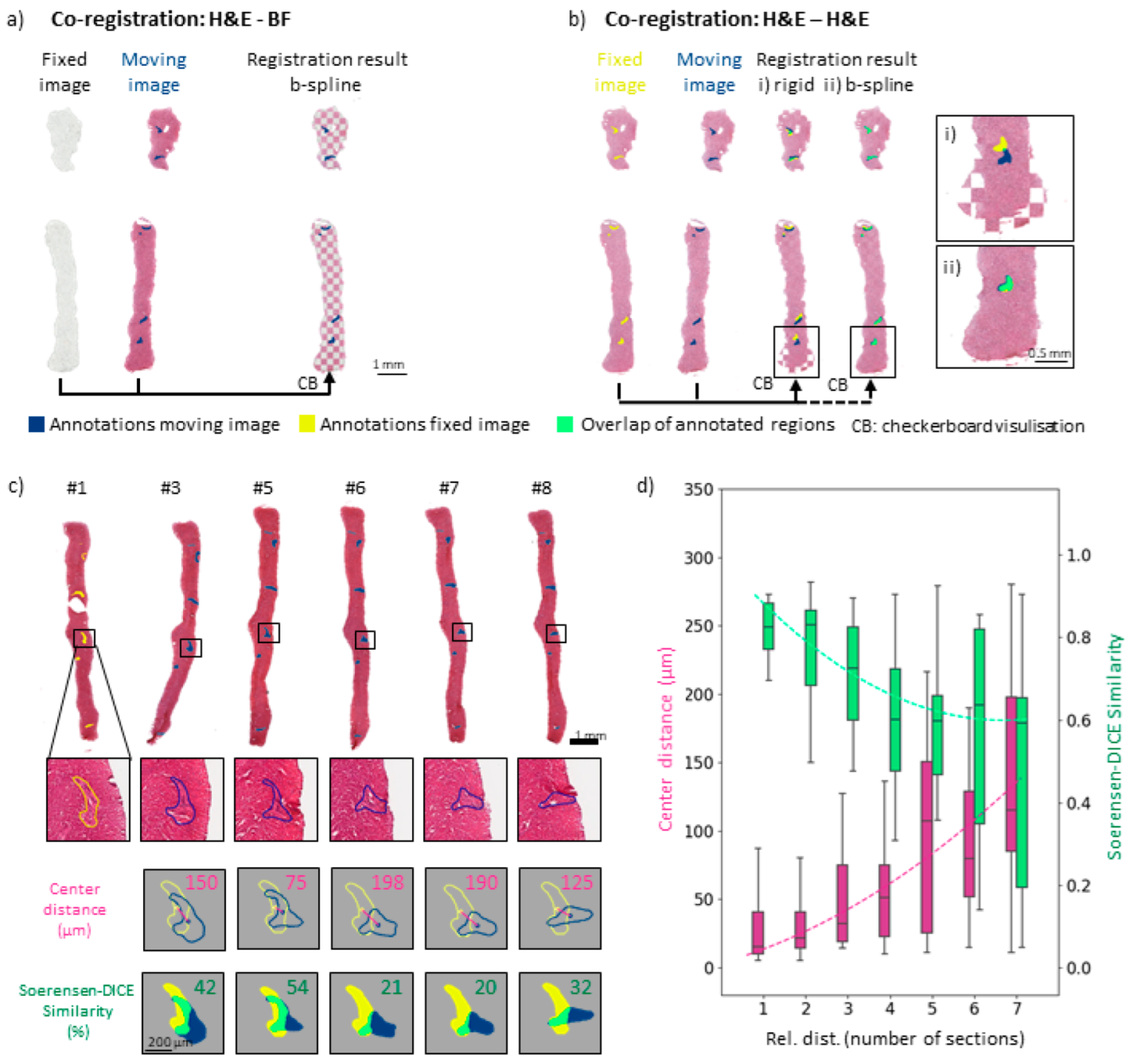
2.4. Precise Co-Registration
2.5. Conversion of the Remaining FF Samples to FFPE and Clinical Pathology Analysis
3. Results and Discussion
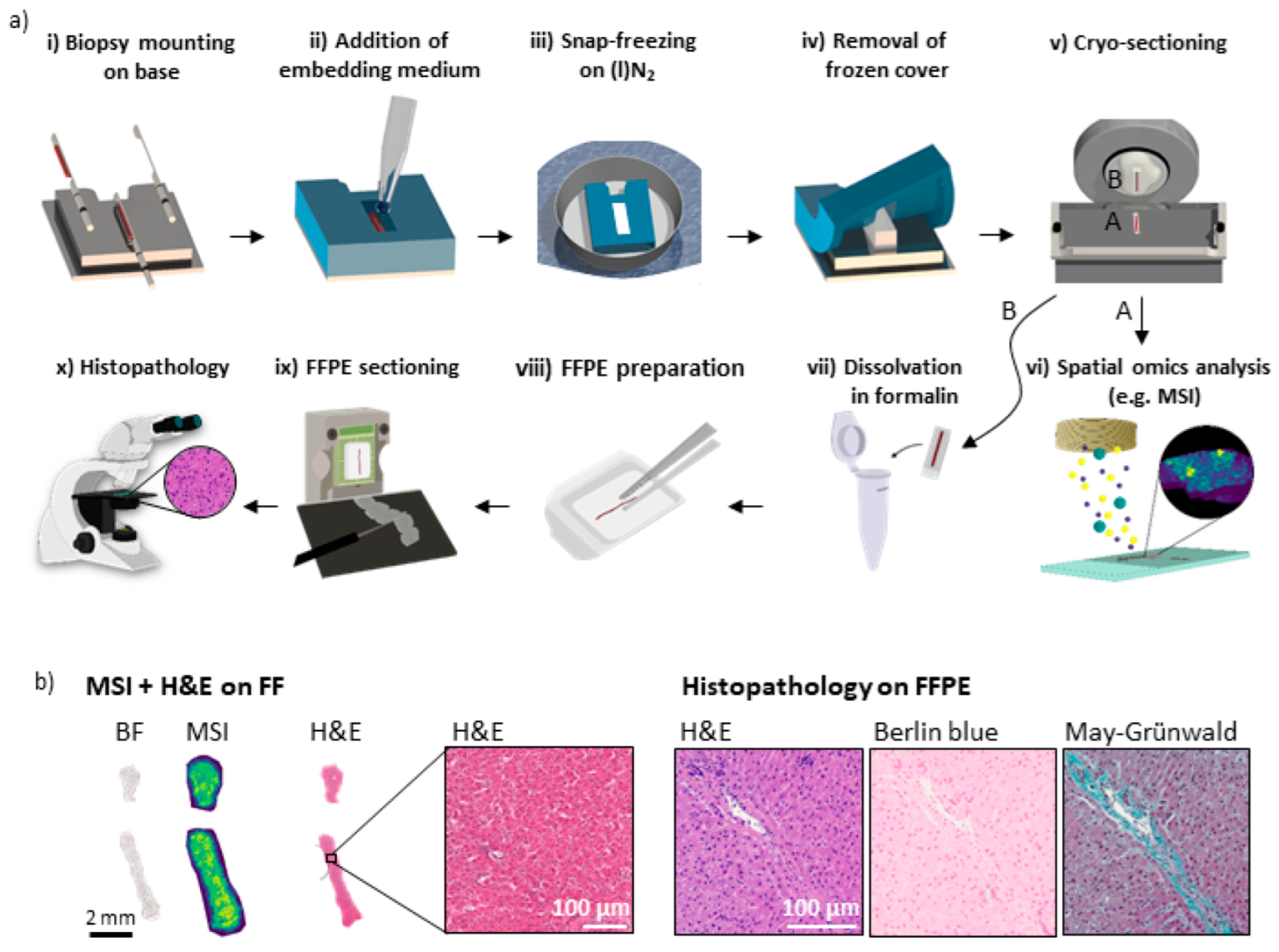
3.1. A 3D-Printed Flexible Embedding and Freezing Device for the Longitudinal Sectioning of Needle Biopsies
3.2. Sample Preparation Workflow for Multimodal Multi-Omics Analysis of Needle Biopsies
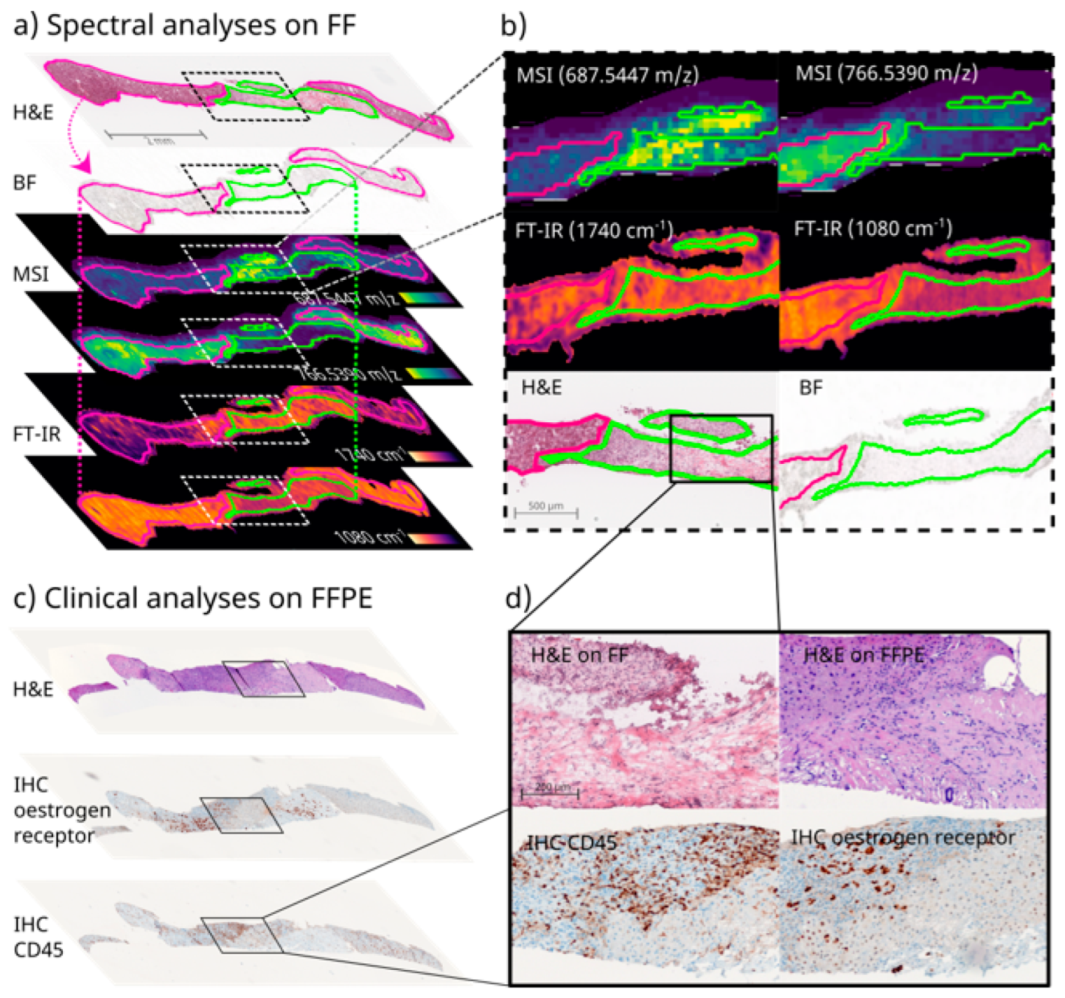
3.3. Image Co-Registration for Multimodal Spectral Analyses of Needle Biopsies
3.4. Clinical Proof of Concept of FF and FFPE Tissue Multimodal Spatial Omics Analysis of the Same Needle Biopsy Core
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Birgin, E.; Yang, C.; Hetjens, S.; Reissfelder, C.; Hohenberger, P.; Rahbari, N.N. Core needle biopsy versus incisional biopsy for differentiation of soft-tissue sarcomas: A systematic review and meta-analysis. Cancer 2020, 126, 1917–1928. [Google Scholar] [CrossRef] [PubMed]
- Meattini, I.; Bicchierai, G.; Saieva, C.; De Benedetto, D.; Desideri, I.; Becherini, C.; Abdulcadir, D.; Vanzi, E.; Boeri, C.; Gabbrielli, S.; et al. Impact of molecular subtypes classification concordance between preoperative core needle biopsy and surgical specimen on early breast cancer management: Single-institution experience and review of published literature. Eur. J. Surg. Oncol. 2017, 43, 642–648. [Google Scholar] [CrossRef] [PubMed]
- de Gonzalez, A.K.; Lagana, S.M. Update on Ancillary Testing in the Evaluation of High-Grade Liver Tumors. Surg. Pathol. Clin. 2018, 11, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Torres, R.; Celli, R.; Koelmel, J.; Charkoftaki, G.; Vasiliou, V. Evolution of the liver biopsy and its future. Transl. Gastroenterol. Hepatol. 2021, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Connolly, L.; Jamzad, A.; Kaufmann, M.; Farquharson, C.E.; Ren, K.; Rudan, J.F.; Fichtinger, G.; Mousavi, P. Combined Mass Spectrometry and Histopathology Imaging for Perioperative Tissue Assessment in Cancer Surgery. J. Imaging 2021, 7, 203. [Google Scholar] [CrossRef]
- Neuberger, J.; Patel, J.; Caldwell, H.; Davies, S.; Hebditch, V.; Hollywood, C.; Hubscher, S.; Karkhanis, S.; Lester, W.; Roslund, N.; et al. Guidelines on the use of liver biopsy in clinical practice from the British Society of Gastroenterology, the Royal College of Radiologists and the Royal College of Pathology. Gut 2020, 69, 1382–1403. [Google Scholar] [CrossRef]
- Shmatko, A.; Laleh, N.G.; Gerstung, M.; Kather, J.N. Artificial intelligence in histopathology: Enhancing cancer research and clinical oncology. Nat. Cancer 2022, 3, 1026–1038. [Google Scholar] [CrossRef]
- Khoo, A.; Liu, L.Y.; Nyalwidhe, J.O.; Semmes, O.J.; Vesprini, D.; Downes, M.R.; Boutros, P.C.; Liu, S.K.; Kislinger, T. Proteomic discovery of non-invasive biomarkers of localized prostate cancer using mass spectrometry. Nat. Rev. Urol. 2021, 18, 707–724. [Google Scholar] [CrossRef]
- Satpathy, S.; Jaehnig, E.J.; Krug, K.; Kim, B.-J.; Saltzman, A.B.; Chan, D.W.; Holloway, K.R.; Anurag, M.; Huang, C.; Singh, P.; et al. Microscaled proteogenomic methods for precision oncology. Nat. Commun. 2020, 11, 532. [Google Scholar] [CrossRef]
- Mitsa, G.; Guo, Q.; Goncalves, C.; Preston, S.E.J.; Lacasse, V.; Aguilar-Mahecha, A.; Benlimame, N.; Basik, M.; Spatz, A.; Batist, G.; et al. A Non-Hazardous Deparaffinization Protocol Enables Quantitative Proteomics of Core Needle Biopsy-Sized Formalin-Fixed and Paraffin-Embedded (FFPE) Tissue Specimens. Int. J. Mol. Sci. 2022, 23, 4443. [Google Scholar] [CrossRef]
- Gonçalves, J.P.L.; Bollwein, C.; Schwamborn, K. Mass Spectrometry Imaging Spatial Tissue Analysis toward Personalized Medicine. Life 2022, 12, 1037. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Fernández, F.M. Advances in mass spectrometry imaging for spatial cancer metabolomics. Mass Spectrom. Rev. 2022, e21804. [Google Scholar] [CrossRef] [PubMed]
- Engel, K.M.; Prabutzki, P.; Leopold, J.; Nimptsch, A.; Lemmnitzer, K.; Vos, D.N.; Hopf, C.; Schiller, J. A new update of MALDI-TOF mass spectrometry in lipid research. Prog. Lipid Res. 2022, 86, 101145. [Google Scholar] [CrossRef] [PubMed]
- He, M.J.; Pu, W.; Wang, X.; Zhang, W.; Tang, D.; Dai, Y. Comparing DESI-MSI and MALDI-MSI Mediated Spatial Metabolomics and Their Applications in Cancer Studies. Front. Oncol. 2022, 12, 891018. [Google Scholar] [CrossRef]
- Abu Sammour, D.; Cairns, J.L.; Boskamp, T.; Marsching, C.; Kessler, T.; Guevara, C.R.; Panitz, V.; Sadik, A.; Cordes, J.; Schmidt, S.; et al. Spatial probabilistic mapping of metabolite ensembles in mass spectrometry imaging. Nat. Commun. 2023, 14, 1823. [Google Scholar] [CrossRef]
- Dewez, F.; Oejten, J.; Henkel, C.; Hebeler, R.; Neuweger, H.; De Pauw, E.; Heeren, R.M.A.; Balluff, B. MS Imaging-Guided Microproteomics for Spatial Omics on a Single Instrument. Proteomics 2020, 20, e1900369. [Google Scholar] [CrossRef]
- Mund, A.; Coscia, F.; Kriston, A.; Hollandi, R.; Kovács, F.; Brunner, A.-D.; Migh, E.; Schweizer, L.; Santos, A.; Bzorek, M.; et al. Deep Visual Proteomics defines single-cell identity and heterogeneity. Nat. Biotechnol. 2022, 40, 1231–1240. [Google Scholar] [CrossRef]
- Palla, G.; Spitzer, H.; Klein, M.; Fischer, D.; Schaar, A.C.; Kuemmerle, L.B.; Rybakov, S.; Ibarra, I.L.; Holmberg, O.; Virshup, I.; et al. Squidpy: A scalable framework for spatial omics analysis. Nat. Methods 2022, 19, 171–178. [Google Scholar] [CrossRef]
- Kuppe, C.; Flores, R.O.R.; Li, Z.; Hayat, S.; Levinson, R.T.; Liao, X.; Hannani, M.T.; Tanevski, J.; Wünnemann, F.; Nagai, J.S.; et al. Spatial multi-omic map of human myocardial infarction. Nature 2022, 608, 766–777. [Google Scholar] [CrossRef]
- Thomas, A.; Patterson, N.H.; Marcinkiewicz, M.M.; Lazaris, A.; Metrakos, P.; Chaurand, P. Histology-Driven Data Mining of Lipid Signatures from Multiple Imaging Mass Spectrometry Analyses: Application to Human Colorectal Cancer Liver Metastasis Biopsies. Anal. Chem. 2013, 85, 2860–2866. [Google Scholar] [CrossRef]
- Abu Sammour, D.; Marsching, C.; Geisel, A.; Erich, K.; Schulz, S.; Guevara, C.R.; Rabe, J.-H.; Marx, A.; Findeisen, P.; Hohenberger, P.; et al. Quantitative Mass Spectrometry Imaging Reveals Mutation Status-independent Lack of Imatinib in Liver Metastases of Gastrointestinal Stromal Tumors. Sci. Rep. 2019, 9, 10698. [Google Scholar] [CrossRef] [PubMed]
- DelaCourt, A.; Black, A.; Angel, P.; Drake, R.; Hoshida, Y.; Singal, A.; Lewin, D.; Taouli, B.; Lewis, S.; Schwarz, M.; et al. N-Glycosylation Patterns Correlate with Hepatocellular Carcinoma Genetic Subtypes. Mol. Cancer Res. 2021, 19, 1868–1877. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Huang, P.; Chen, C.; Lee, C.; Hsu, C. Next-generation pathology practices with mass spectrometry imaging. Mass Spectrom. Rev. 2022, e21795. [Google Scholar] [CrossRef] [PubMed]
- Iakab, S.A.; Ràfols, P.; Correig-Blanchar, X.; García-Altares, M. Perspective on Multimodal Imaging Techniques Coupling Mass Spectrometry and Vibrational Spectroscopy: Picturing the Best of Both Worlds. Anal. Chem. 2021, 93, 6301–6310. [Google Scholar] [CrossRef] [PubMed]
- Rabe, J.-H.; Sammour, D.A.; Schulz, S.; Munteanu, B.; Ott, M.; Ochs, K.; Hohenberger, P.; Marx, A.; Platten, M.; Opitz, C.A.; et al. Fourier Transform Infrared Microscopy Enables Guidance of Automated Mass Spectrometry Imaging to Predefined Tissue Morphologies. Sci. Rep. 2018, 8, 313. [Google Scholar] [CrossRef] [PubMed]
- Neumann, E.K.; Djambazova, K.V.; Caprioli, R.M.; Spraggins, J.M. Multimodal Imaging Mass Spectrometry: Next Generation Molecular Mapping in Biology and Medicine. J. Am. Soc. Mass Spectrom. 2020, 31, 2401–2415. [Google Scholar] [CrossRef]
- Tuck, M.; Blanc, L.; Touti, R.; Patterson, N.H.; Van Nuffel, S.; Villette, S.; Taveau, J.-C.; Römpp, A.; Brunelle, A.; Lecomte, S.; et al. Multimodal Imaging Based on Vibrational Spectroscopies and Mass Spectrometry Imaging Applied to Biological Tissue: A Multiscale and Multiomics Review. Anal. Chem. 2020, 93, 445–477. [Google Scholar] [CrossRef]
- Mittal, S.; Yeh, K.; Leslie, L.S.; Kenkel, S.; Kajdacsy-Balla, A.; Bhargava, R. Simultaneous cancer and tumor microenvironment subtyping using confocal infrared microscopy for all-digital molecular histopathology. Proc. Natl. Acad. Sci. USA 2018, 115, E5651–E5660. [Google Scholar] [CrossRef]
- Kümmel, T.; van Marwick, B.; Rittel, M.; Guevara, C.R.; Wühler, F.; Teumer, T.; Wängler, B.; Hopf, C.; Rädle, M. Rapid brain structure and tumour margin detection on whole frozen tissue sections by fast multiphotometric mid-infrared scanning. Sci. Rep. 2021, 11, 11307. [Google Scholar] [CrossRef]
- Kuepper, C.; Kallenbach-Thieltges, A.; Juette, H.; Tannapfel, A.; Großerueschkamp, F.; Gerwert, K. Quantum Cascade Laser-Based Infrared Microscopy for Label-Free and Automated Cancer Classification in Tissue Sections. Sci. Rep. 2018, 8, 7717. [Google Scholar] [CrossRef]
- Tiwari, S.; Falahkheirkhah, K.; Cheng, G.; Bhargava, R. Colon Cancer Grading Using Infrared Spectroscopic Imaging-Based Deep Learning. Appl. Spectrosc. 2022, 76, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.P.L.; Bollwein, C.; Schlitter, A.M.; Martin, B.; Märkl, B.; Utpatel, K.; Weichert, W.; Schwamborn, K. The Impact of Histological Annotations for Accurate Tissue Classification Using Mass Spectrometry Imaging. Metabolites 2021, 11, 752. [Google Scholar] [CrossRef] [PubMed]
- Pietrowska, M.; Gawin, M.; Polańska, J.; Widłak, P. Tissue fixed with formalin and processed without paraffin embedding is suitable for imaging of both peptides and lipids by MALDI-IMS. Proteomics 2016, 16, 1670–1677. [Google Scholar] [CrossRef] [PubMed]
- Buck, A.; Ly, A.; Balluff, B.; Sun, N.; Gorzolka, K.; Feuchtinger, A.; Janssen, K.-P.; Kuppen, P.J.; van de Velde, C.J.; Weirich, G.; et al. High-resolution MALDI-FT-ICR MS imaging for the analysis of metabolites from formalin-fixed, paraffin-embedded clinical tissue samples. J. Pathol. 2015, 237, 123–132. [Google Scholar] [CrossRef]
- Casadonte, R.; Longuespée, R.; Kriegsmann, J. MALDI IMS and Cancer Tissue Microarrays. Adv. Cancer Res. 2017, 134, 173–200. [Google Scholar] [CrossRef]
- Cazares, L.H.; Troyer, D.; Mendrinos, S.; Lance, R.A.; Nyalwidhe, J.O.; Beydoun, H.A.; Clements, M.A.; Drake, R.R.; Semmes, O.J. Imaging Mass Spectrometry of a Specific Fragment of Mitogen-Activated Protein Kinase/Extracellular Signal-Regulated Kinase Kinase Kinase 2 Discriminates Cancer from Uninvolved Prostate Tissue. Clin. Cancer Res. 2009, 15, 5541–5551. [Google Scholar] [CrossRef]
- Shiraishi, T.; Inui, S.; Inoue, Y.; Saito, Y.; Taga, H.; Kaneko, M.; Tsuji, K.; Ueda, S.; Ueda, T.; Matsugasumi, T.; et al. Usefulness of a novel device to divide core needle biopsy specimens in a spatially matched fashion. Sci. Rep. 2020, 10, 17098. [Google Scholar] [CrossRef]
- Dannhorn, A.; Kazanc, E.; Ling, S.; Nikula, C.; Karali, E.; Serra, M.P.; Vorng, J.-L.; Inglese, P.; Maglennon, G.; Hamm, G.R.; et al. Universal Sample Preparation Unlocking Multimodal Molecular Tissue Imaging. Anal. Chem. 2020, 92, 11080–11088. [Google Scholar] [CrossRef]
- Baker, M.J.; Trevisan, J.; Bassan, P.; Bhargava, R.; Butler, H.J.; Dorling, K.M.; Fielden, P.R.; Fogarty, S.W.; Fullwood, N.J.; Heys, K.A.; et al. Using Fourier transform IR spectroscopy to analyze biological materials. Nat. Protoc. 2014, 9, 1771–1791. [Google Scholar] [CrossRef]
- Friedrich, M.; Sankowski, R.; Bunse, L.; Kilian, M.; Green, E.; Guevara, C.R.; Pusch, S.; Poschet, G.; Sanghvi, K.; Hahn, M.; et al. Tryptophan metabolism drives dynamic immunosuppressive myeloid states in IDH-mutant gliomas. Nat. Cancer 2021, 2, 723–740. [Google Scholar] [CrossRef]
- Lowekamp, B.C.; Chen, D.T.; Eibanez, L.; Eblezek, D. The Design of SimpleITK. Front. Neuroinformatics 2013, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Marstal, K.; Berendsen, F.; Staring, M.; Klein, S. SimpleElastix: A User-Friendly, Multi-lingual Library for Medical Image Registration. In Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition Workshops (CVPRW), Las Vegas, NV, USA, 27–30 June 2016. [Google Scholar]
- Klein, S.; Staring, M.; Murphy, K.; Viergever, M.A.; Pluim, J.P.W. elastix: A Toolbox for Intensity-Based Medical Image Registration. IEEE Trans. Med. Imaging 2010, 29, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Rohlfing, T. Image Similarity and Tissue Overlaps as Surrogates for Image Registration Accuracy: Widely Used but Unreliable. IEEE Trans. Med. Imaging 2012, 31, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Ucal, Y.; Tokat, F.; Duren, M.; Ince, U.; Ozpinar, A. Peptide Profile Differences of Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features, Encapsulated Follicular Variant, and Classical Papillary Thyroid Carcinoma: An Application of Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry Imaging. Thyroid 2019, 29, 1125–1137. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Chen, F.; Macosko, E.Z. The expanding vistas of spatial transcriptomics. Nat. Biotechnol. 2022. [Google Scholar] [CrossRef]
- Balluff, B.; Heeren, R.M.; Race, A.M. An overview of image registration for aligning mass spectrometry imaging with clinically relevant imaging modalities. J. Mass Spectrom. Adv. Clin. Lab 2022, 23, 26–38. [Google Scholar] [CrossRef]
- Neumann, E.K.; Comi, T.J.; Spegazzini, N.; Mitchell, J.W.; Rubakhin, S.S.; Gillette, M.U.; Bhargava, R.; Sweedler, J.V. Multimodal Chemical Analysis of the Brain by High Mass Resolution Mass Spectrometry and Infrared Spectroscopic Imaging. Anal. Chem. 2018, 90, 11572–11580. [Google Scholar] [CrossRef]
- Palmer, A.; Phapale, P.; Chernyavsky, I.; Lavigne, R.; Fay, D.; Tarasov, A.; Kovalev, V.; Fuchser, J.; Nikolenko, S.; Pineau, C.; et al. FDR-controlled metabolite annotation for high-resolution imaging mass spectrometry. Nat. Methods 2017, 14, 57–60. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis. Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Schulz, S.; Becker, M.; Groseclose, M.R.; Schadt, S.; Hopf, C. Advanced MALDI mass spectrometry imaging in pharmaceutical research and drug development. Curr. Opin. Biotechnol. 2019, 55, 51–59. [Google Scholar] [CrossRef]
- Finlayson, D.; Rinaldi, C.; Baker, M.J. Is Infrared Spectroscopy Ready for the Clinic? Anal. Chem. 2019, 91, 12117–12128. [Google Scholar] [CrossRef] [PubMed]
- Abdelmoula, W.M.; Lopez, B.G.-C.; Randall, E.C.; Kapur, T.; Sarkaria, J.N.; White, F.M.; Agar, J.N.; Wells, W.M.; Agar, N.Y.R. Peak learning of mass spectrometry imaging data using artificial neural networks. Nat. Commun. 2021, 12, 5544. [Google Scholar] [CrossRef] [PubMed]
- Tideman, L.E.; Migas, L.G.; Djambazova, K.V.; Patterson, N.H.; Caprioli, R.M.; Spraggins, J.M.; Van de Plas, R. Automated biomarker candidate discovery in imaging mass spectrometry data through spatially localized Shapley additive explanations. Anal. Chim. Acta 2021, 1177, 338522. [Google Scholar] [CrossRef]
- Seddiki, K.; Saudemont, P.; Precioso, F.; Ogrinc, N.; Wisztorski, M.; Salzet, M.; Fournier, I.; Droit, A. Cumulative learning enables convolutional neural network representations for small mass spectrometry data classification. Nat. Commun. 2020, 11, 5595. [Google Scholar] [CrossRef] [PubMed]
- St John, E.R.; Balog, J.; McKenzie, J.S.; Rossi, M.; Covington, A.; Muirhead, L.; Bodai, Z.; Rosini, F.; Speller, A.V.M.; Shousha, S.; et al. Rapid evaporative ionisation mass spectrometry of electrosurgical vapours for the identification of breast pathology: Towards an intelligent knife for breast cancer surgery. Breast Cancer Res. 2017, 19, 59. [Google Scholar] [CrossRef]
- Zhang, J.; Rector, J.; Lin, J.Q.; Young, J.H.; Sans, M.; Katta, N.; Giese, N.; Yu, W.; Nagi, C.; Suliburk, J.; et al. Nondestructive tissue analysis for ex vivo and in vivo cancer diagnosis using a handheld mass spectrometry system. Sci. Transl. Med. 2017, 9, eaan3968. [Google Scholar] [CrossRef]
- Matys, J.; Turska-Szewczuk, A.; Gieroba, B.; Kurzylewska, M.; Pękala-Safińska, A.; Sroka-Bartnicka, A. Evaluation of Proteomic and Lipidomic Changes in Aeromonas-Infected Trout Kidney Tissue with the Use of FT-IR Spectroscopy and MALDI Mass Spectrometry Imaging. Int. J. Mol. Sci. 2022, 23, 12551. [Google Scholar] [CrossRef]
- Moffitt, J.R.; Lundberg, E.; Heyn, H. The emerging landscape of spatial profiling technologies. Nat. Rev. Genet. 2022, 23, 741–759. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rittel, M.F.; Schmidt, S.; Weis, C.-A.; Birgin, E.; van Marwick, B.; Rädle, M.; Diehl, S.J.; Rahbari, N.N.; Marx, A.; Hopf, C. Spatial Omics Imaging of Fresh-Frozen Tissue and Routine FFPE Histopathology of a Single Cancer Needle Core Biopsy: A Freezing Device and Multimodal Workflow. Cancers 2023, 15, 2676. https://doi.org/10.3390/cancers15102676
Rittel MF, Schmidt S, Weis C-A, Birgin E, van Marwick B, Rädle M, Diehl SJ, Rahbari NN, Marx A, Hopf C. Spatial Omics Imaging of Fresh-Frozen Tissue and Routine FFPE Histopathology of a Single Cancer Needle Core Biopsy: A Freezing Device and Multimodal Workflow. Cancers. 2023; 15(10):2676. https://doi.org/10.3390/cancers15102676
Chicago/Turabian StyleRittel, Miriam F., Stefan Schmidt, Cleo-Aron Weis, Emrullah Birgin, Björn van Marwick, Matthias Rädle, Steffen J. Diehl, Nuh N. Rahbari, Alexander Marx, and Carsten Hopf. 2023. "Spatial Omics Imaging of Fresh-Frozen Tissue and Routine FFPE Histopathology of a Single Cancer Needle Core Biopsy: A Freezing Device and Multimodal Workflow" Cancers 15, no. 10: 2676. https://doi.org/10.3390/cancers15102676
APA StyleRittel, M. F., Schmidt, S., Weis, C.-A., Birgin, E., van Marwick, B., Rädle, M., Diehl, S. J., Rahbari, N. N., Marx, A., & Hopf, C. (2023). Spatial Omics Imaging of Fresh-Frozen Tissue and Routine FFPE Histopathology of a Single Cancer Needle Core Biopsy: A Freezing Device and Multimodal Workflow. Cancers, 15(10), 2676. https://doi.org/10.3390/cancers15102676