Recent Trends and Advancements in the Diagnosis and Management of Gastric Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Diagnosis
2.1. Biomarkers
2.1.1. Proteins
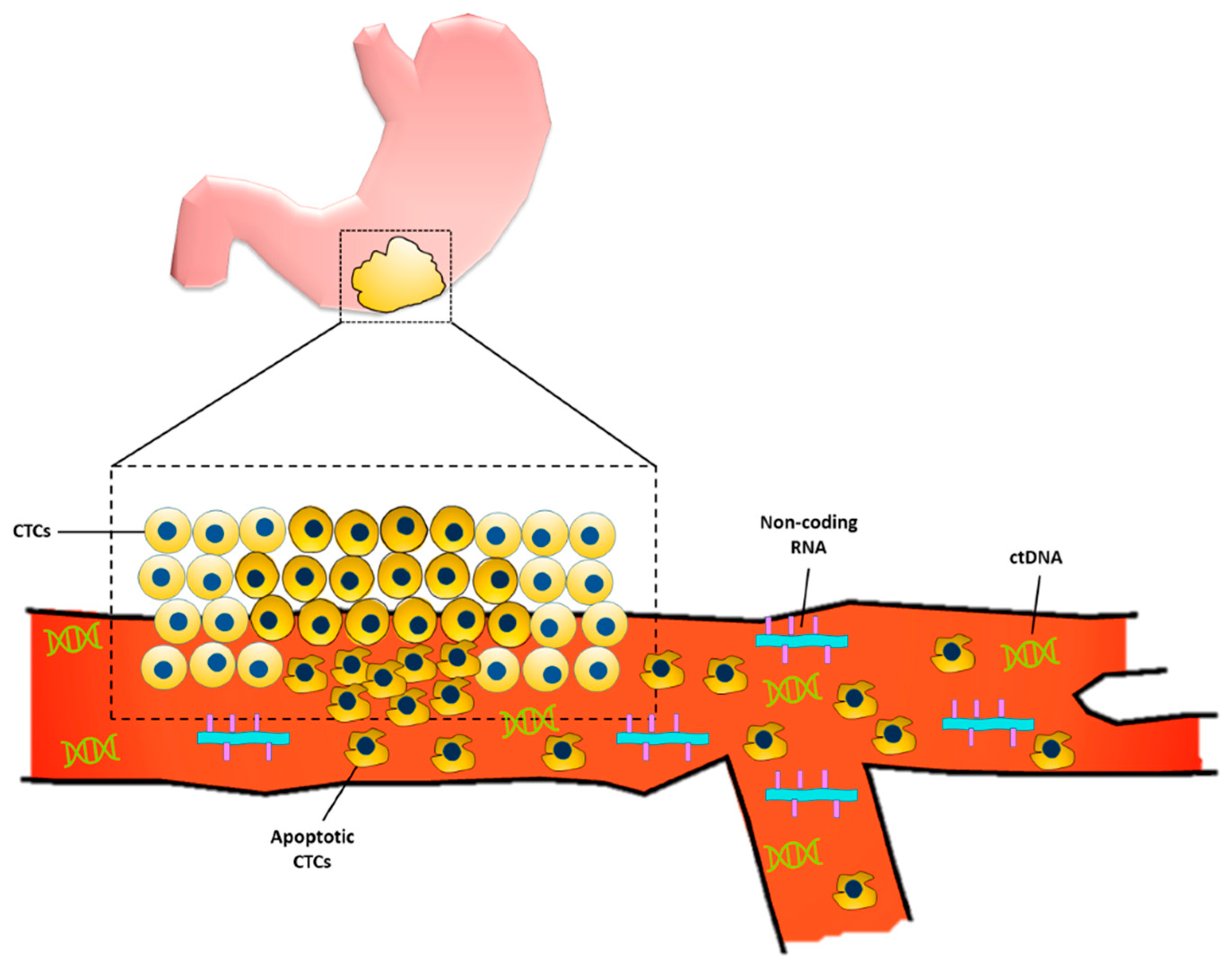
2.1.2. Circulating Tumor Cells
2.1.3. Circulating Tumor DNA
2.1.4. Non-Coding RNA
2.1.5. Circulating Extracellular Vesicles
3. Treatment
3.1. Epidermal Growth Factor Receptors
3.1.1. HER-1
3.1.2. HER-2
3.2. Angiogenesis
3.3. Immune Checkpoint Inhibitors
3.4. Anti-DNA Synthesis
3.5. Anti-Hepatocyte Growth Factor Receptor (Anti-HGFR)
3.6. Anti-FGFR
3.7. PARP Inhibitors
3.8. Anti-MMP-9
3.9. mTOR Inhibitors
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.; Arnold, M.; Camargo, M.C.; Gini, A.; Kunzmann, A.T.; Matsuda, T.; Meheus, F.; Verhoeven, R.H.A.; Vignat, J.; Laversanne, M.; et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020-40: A population-based modelling study. EClinicalMedicine 2022, 47, 101404. [Google Scholar] [CrossRef] [PubMed]
- Tsugane, S.; Sasazuki, S. Diet and the risk of gastric cancer: Review of epidemiological evidence. Gastric Cancer 2007, 10, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.T.; Zeng, L.; Yang, J.; Zeng, C.; Chen, Y. Analysis of the Incidence and Survival of Gastric Cancer Based on the Lauren Classification: A Large Population-Based Study Using SEER. Front. Oncol. 2020, 10, 1212. [Google Scholar] [CrossRef] [PubMed]
- Noh, C.-K.; Lee, E.; Lee, G.H.; Kang, J.K.; Lim, S.G.; Park, B.; Park, J.B.; Shin, S.J.; Cheong, J.Y.; Kim, J.H.; et al. Association of Intensive Endoscopic Screening Burden With Gastric Cancer Detection. JAMA Netw. Open 2021, 4, e2032542. [Google Scholar] [CrossRef]
- Ebigbo, A.; Messmann, H.; Römmele, C. Endoscopic Upper GI Screening. Visc. Med. 2019, 35, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, C.G.; Hussain, S.; Trapani, D.; El Bairi, K.; Altuna, S.C.; Seeber, A.; Odhiambo, A.; Habeeb, B.S.; Seid, F. The Emerging Role of Liquid Biopsy in Gastric Cancer. J. Clin. Med. 2021, 10, 2108. [Google Scholar] [CrossRef]
- Virgilio, E.; Montali, F.; Annicchiarico, A.; Salvemini, C.; Baldinu, M.; Giarnieri, E.; Montagnini, M.; Villani, S.; Proietti, A.; D’Urso, R.; et al. Exosomal Functional Cargoes from Liquid Biopsy of Gastric Cancer: A Systematic Review of Studies With Potential Clinical Relevance. Anticancer Res. 2022, 42, 2249–2259. [Google Scholar] [CrossRef]
- Feng, F.; Tian, Y.; Xu, G.; Liu, Z.; Liu, S.; Zheng, G.; Guo, M.; Lian, X.; Fan, D.; Zhang, H. Diagnostic and prognostic value of CEA, CA19–9, AFP and CA125 for early gastric cancer. BMC Cancer 2017, 17, 737. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Zhang, Z.; Huang, D. Association of multiple tumor markers with newly diagnosed gastric cancer patients: A retrospective study. PeerJ 2022, 10, e13488. [Google Scholar] [CrossRef]
- Namikawa, T.; Kawanishi, Y.; Fujisawa, K.; Munekage, E.; Iwabu, J.; Munekage, M.; Maeda, H.; Kitagawa, H.; Kobayashi, M.; Hanazaki, K. Serum carbohydrate antigen 125 is a significant prognostic marker in patients with unresectable advanced or recurrent gastric cancer. Surg. Today 2018, 48, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, D.; Pinto, E.; De Stefano, A.; Farnetani, M.; Garosi, L.; Roviello, F. Clinical utility of CEA, CA 19-9, and CA 72-4 in the follow-up of patients with resectable gastric cancer. Am. J. Surg. 2001, 181, 16–19. [Google Scholar] [CrossRef]
- Kim, N.; Jung, H.C. The Role of Serum Pepsinogen in the Detection of Gastric Cancer. Gut Liver 2010, 4, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Wu, Y.; Song, Z.; Yu, Y.; Yu, X. The potential value of serum pepsinogen for the diagnosis of atrophic gastritis among the health check-up populations in China: A diagnostic clinical research. BMC Gastroenterol. 2017, 17, 88. [Google Scholar] [CrossRef]
- Lee, S.-Y. Endoscopic gastritis, serum pepsinogen assay, and Helicobacter pylori infection. Korean J. Intern. Med. 2016, 31, 835–844. [Google Scholar] [CrossRef]
- Cha, J.H.; Jang, J.S. Clinical correlation between serum pepsinogen level and gastric atrophy in gastric neoplasm. Korean J. Intern. Med. 2020, 35, 550–558. [Google Scholar] [CrossRef]
- Miki, K. Gastric cancer screening using the serum pepsinogen test method. Gastric Cancer 2006, 9, 245–253. [Google Scholar] [CrossRef]
- Miftahussurur, M.; Waskito, L.A.; Aftab, H.; Vilaichone, R.-K.; Subsomwong, P.; Nusi, I.A.; Syam, A.F.; Ratanachu-Ek, T.; Doohan, D.; Siregar, G.; et al. Serum pepsinogens as a gastric cancer and gastritis biomarker in South and Southeast Asian populations. PLoS ONE 2020, 15, e0230064. [Google Scholar] [CrossRef]
- Aikou, S.; Ohmoto, Y.; Gunji, T.; Matsuhashi, N.; Ohtsu, H.; Miura, H.; Kubota, K.; Yamagata, Y.; Seto, Y.; Nakajima, A.; et al. Tests for Serum Levels of Trefoil Factor Family Proteins Can Improve Gastric Cancer Screening. Gastroenterology 2011, 141, 837–845. [Google Scholar] [CrossRef]
- Zhang, C.-X.; Wu, C.-T.; Xiao, L.; Tang, S.-H. The diagnostic and clinicopathological value of trefoil factor 3 in patients with gastric cancer: A systematic review and meta-analysis. Biomarkers 2021, 26, 95–102. [Google Scholar] [CrossRef]
- Kaise, M.; Miwa, J.; Tashiro, J.; Ohmoto, Y.; Morimoto, S.; Kato, M.; Urashima, M.; Ikegami, M.; Tajiri, H. The combination of serum trefoil factor 3 and pepsinogen testing is a valid non-endoscopic biomarker for predicting the presence of gastric cancer: A new marker for gastric cancer risk. J. Gastroenterol. 2011, 46, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, H.; Tageldin, O.; Hasak, S.; Lee, H. AFP-producing gastric carcinoma. Hum. Pathol. Rep. 2022, 28, 300640. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Q.; Cao, H.; Gao, Z.; Qian, G.; Lu, Q.; Wu, Y. Prognostic value of serum alpha-fetoprotein levels in patients with gastric cancer: A meta-analysis. J. Int. Med. Res. 2020, 48, 030006051989978. [Google Scholar] [CrossRef]
- Zhan, Z. Elevated serum alpha-fetoprotein is a significant prognostic factor for gastric cancer patients: Results based on a large-scale retrospective study. J. Clin. Oncol. 2022, 40 (Suppl. 16), e16059. [Google Scholar] [CrossRef]
- Gong, W.; Su, Y.; Liu, A.; Liu, J.; Sun, D.; Jiang, T.; Xiang, J.; Chi, C.; Sun, P. Clinical characteristics and treatments of patients with alpha-fetoprotein producing gastric carcinoma. Neoplasma 2018, 65, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Neumann, M.H.D.; Bender, S.; Krahn, T.; Schlange, T. ctDNA and CTCs in Liquid Biopsy–Current Status and Where We Need to Progress. Comput. Struct. Biotechnol. J. 2018, 16, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Leja, M.; Linē, A. Early detection of gastric cancer beyond endoscopy—new methods. Best Pract. Res. Clin. Gastroenterol. 2021, 50–51, 101731. [Google Scholar] [CrossRef]
- Necula, L.; Matei, L.; Dragu, D.; Neagu, A.I.; Mambet, C.; Nedeianu, S.; Bleotu, C.; Diaconu, C.C.; Chivu-Economescu, M. Recent advances in gastric cancer early diagnosis. World J. Gastroenterol. 2019, 25, 2029–2044. [Google Scholar] [CrossRef]
- Nakamura, K.; Iwatsuki, M.; Kurashige, J.; Ishimoto, T.; Baba, Y.; Miyamoto, Y.; Yoshida, N.; Watanabe, M.; Baba, H. Circulating tumor cells in gastric cancer. J. Cancer Metastasis Treat. 2018, 4, 32. [Google Scholar] [CrossRef]
- Lee, M.W.; Kim, G.H.; Jeon, H.K.; Park, S.J. Clinical Application of Circulating Tumor Cells in Gastric Cancer. Gut Liver 2019, 13, 394–401. [Google Scholar] [CrossRef]
- Sudhakar, P.; Sanapala, P.; Naidu, B.P. Overview of Early Detection of Gastrointestinal Cancer. In Recent Advancements in Biomarkers and Early Detection of Gastrointestinal Cancers; Springer: Singapore, 2020; pp. 117–129. [Google Scholar]
- Uchôa Guimarães, C.T.; Ferreira Martins, N.N.; Cristina Da Silva Oliveira, K.; Almeida, C.M.; Pinheiro, T.M.; Gigek, C.O.; Roberto De Araújo Cavallero, S.; Assumpção, P.P.; Cardoso Smith, M.A.; Burbano, R.R.; et al. Liquid biopsy provides new insights into gastric cancer. Oncotarget 2018, 9, 15144–15156. [Google Scholar] [CrossRef] [PubMed]
- Vasseur, A.; Kiavue, N.; Bidard, F.C.; Pierga, J.Y.; Cabel, L. Clinical utility of circulating tumor cells: An update. Mol. Oncol. 2021, 15, 1647–1666. [Google Scholar] [CrossRef] [PubMed]
- Pernot, S.; Badoual, C.; Terme, M.; Castan, F.; Cazes, A.; Bouche, O.; Bennouna, J.; Francois, E.; Ghiringhelli, F.; De La Fouchardiere, C.; et al. Dynamic evaluation of circulating tumour cells in patients with advanced gastric and oesogastric junction adenocarcinoma: Prognostic value and early assessment of therapeutic effects. Eur. J. Cancer 2017, 79, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.A. Parallel progression of primary tumours and metastases. Nat. Rev. Cancer 2009, 9, 302–312. [Google Scholar] [CrossRef]
- Fang, W.-L.; Lan, Y.-T.; Huang, K.-H.; Liu, C.-A.; Hung, Y.-P.; Lin, C.-H.; Jhang, F.-Y.; Chang, S.-C.; Chen, M.-H.; Chao, Y.; et al. Clinical significance of circulating plasma DNA in gastric cancer. Int. J. Cancer 2016, 138, 2974–2983. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, K.; Xi, H.; Cai, A.; Wu, X.; Cui, J.; Li, J.; Qiao, Z.; Wei, B.; Chen, L. Diagnostic and prognostic value of circulating tumor DNA in gastric cancer: A meta-analysis. Oncotarget 2017, 8, 6330–6340. [Google Scholar] [CrossRef]
- Wu, J.; Li, G.; Wang, Z.; Yao, Y.; Chen, R.; Pu, X.; Wang, J. Circulating MicroRNA-21 Is a Potential Diagnostic Biomarker in Gastric Cancer. Dis. Mrk. 2015, 2015, 435656. [Google Scholar] [CrossRef]
- Hung, P.-S.; Chen, C.-Y.; Chen, W.-T.; Kuo, C.-Y.; Fang, W.-L.; Huang, K.-H.; Chiu, P.-C.; Lo, S.-S. miR-376c promotes carcinogenesis and serves as a plasma marker for gastric carcinoma. PLoS ONE 2017, 12, e0177346. [Google Scholar] [CrossRef]
- Tsai, M.M.; Wang, C.S.; Tsai, C.Y.; Huang, C.G.; Lee, K.F.; Huang, H.W.; Lin, Y.H.; Chi, H.C.; Kuo, L.M.; Lu, P.H.; et al. Circulating microRNA-196a/b are novel biomarkers associated with metastatic gastric cancer. Eur. J. Cancer 2016, 64, 137–148. [Google Scholar] [CrossRef]
- Valladares-Ayerbes, M.; Reboredo, M.; Medina-Villaamil, V.; Iglesias-Díaz, P.; Lorenzo-Patiño, M.J.; Haz, M.; Santamarina, I.; Blanco, M.; Fernández-Tajes, J.; Quindós, M.; et al. Circulating miR-200c as a diagnostic and prognostic biomarker for gastric cancer. J. Transl. Med. 2012, 10, 186. [Google Scholar] [CrossRef]
- Ranjbar, R.; Hesari, A.; Ghasemi, F.; Sahebkar, A. Expression of microRNAs and IRAK1 pathway genes are altered in gastric cancer patients with Helicobacter pylori infection. J. Cell Biochem. 2018, 119, 7570–7576. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Kwong, A.; Sihoe, A.; Chu, K.M. Plasma miR-940 may serve as a novel biomarker for gastric cancer. Tumour Biol. 2016, 37, 3589–3597. [Google Scholar] [CrossRef] [PubMed]
- Qu, A.; Wang, W.; Yang, Y.; Zhang, X.; Dong, Y.; Zheng, G.; Wu, Q.; Zou, M.; Du, L.; Wang, Y.; et al. A serum piRNA signature as promising non-invasive diagnostic and prognostic biomarkers for colorectal cancer. Cancer Manag. Res. 2019, 11, 3703–3720. [Google Scholar] [CrossRef]
- Cui, L.; Lou, Y.; Zhang, X.; Zhou, H.; Deng, H.; Song, H.; Yu, X.; Xiao, B.; Wang, W.; Guo, J. Detection of circulating tumor cells in peripheral blood from patients with gastric cancer using piRNAs as markers. Clin. Biochem. 2011, 44, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, I.; Pérez-Moreno, P.; Letelier, P.; Brebi, P.; Roa, J.C. The Emerging Role of PIWI-Interacting RNAs (piRNAs) in Gastrointestinal Cancers: An Updated Perspective. Cancers 2021, 14, 202. [Google Scholar] [CrossRef]
- Jin, C.; Shi, W.; Wang, F.; Shen, X.; Qi, J.; Cong, H.; Yuan, J.; Shi, L.; Zhu, B.; Luo, X.; et al. Long non-coding RNA HULC as a novel serum biomarker for diagnosis and prognosis prediction of gastric cancer. Oncotarget 2016, 7, 51763–51772. [Google Scholar] [CrossRef]
- Arita, T.; Ichikawa, D.; Konishi, H.; Komatsu, S.; Shiozaki, A.; Shoda, K.; Kawaguchi, T.; Hirajima, S.; Nagata, H.; Kubota, T.; et al. Circulating long non-coding RNAs in plasma of patients with gastric cancer. Anticancer Res. 2013, 33, 3185–3193. [Google Scholar]
- Kalfon, T.; Loewenstein, S.; Gerstenhaber, F.; Leibou, S.; Geller, H.; Sher, O.; Nizri, E.; Lahat, G. Gastric Cancer-Derived Extracellular Vesicles (EVs) Promote Angiogenesis via Angiopoietin-2. Cancers 2022, 14, 2953. [Google Scholar] [CrossRef]
- Nederveen, J.P.; Warnier, G.; Di Carlo, A.; Nilsson, M.I.; Tarnopolsky, M.A. Extracellular Vesicles and Exosomes: Insights From Exercise Science. Front. Physiol. 2020, 11, 604274. [Google Scholar] [CrossRef]
- Fu, M.; Gu, J.; Jiang, P.; Qian, H.; Xu, W.; Zhang, X. Exosomes in gastric cancer: Roles, mechanisms, and applications. Mol. Cancer 2019, 18, 41. [Google Scholar] [CrossRef]
- Im, K.; Baek, J.; Kwon, W.S.; Rha, S.Y.; Hwang, K.W.; Kim, U.; Min, H. The Comparison of Exosome and Exosomal Cytokines between Young and Old Individuals with or without Gastric Cancer. Int. J. Gerontol. 2018, 12, 233–238. [Google Scholar] [CrossRef]
- Wang, X.; Huang, J.; Chen, W.; Li, G.; Li, Z.; Lei, J. The updated role of exosomal proteins in the diagnosis, prognosis, and treatment of cancer. Exp. Mol. Med. 2022, 54, 1390–1400. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Ren, W.; Zhang, D. Research progress on exosomal proteins as diagnostic markers of gastric cancer (review article). Clin. Exp. Med. 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Zhong, H.; Yang, Y.; Ma, S.; Xiu, F.; Cai, Z.; Zhao, H.; Du, L. Induction of a tumour-specific CTL response by exosomes isolated from heat-treated malignant ascites of gastric cancer patients. Int. J. Hyperth. 2011, 27, 604–611. [Google Scholar] [CrossRef]
- Yamamoto, H.; Watanabe, Y.; Oikawa, R.; Morita, R.; Yoshida, Y.; Maehata, T.; Yasuda, H.; Itoh, F. BARHL2 Methylation Using Gastric Wash DNA or Gastric Juice Exosomal DNA is a Useful Marker For Early Detection of Gastric Cancer in an H. pylori-Independent Manner. Clin. Transl. Gastroenterol. 2016, 7, e184. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H. Detection of DNA methylation of gastric juice-derived exosomes in gastric cancer. Integr. Mol. Med. 2014, 1, 17–21. [Google Scholar] [CrossRef]
- Ren, J.; Zhou, Q.; Li, H.; Li, J.; Pang, L.; Su, L.; Gu, Q.; Zhu, Z.; Liu, B. Characterization of exosomal RNAs derived from human gastric cancer cells by deep sequencing. Tumor Biol. 2017, 39, 101042831769501. [Google Scholar] [CrossRef]
- Wang, N.; Wang, L.; Yang, Y.; Gong, L.; Xiao, B.; Liu, X. A serum exosomal microRNA panel as a potential biomarker test for gastric cancer. Biochem. Biophys. Res. Commun. 2017, 493, 1322–1328. [Google Scholar] [CrossRef]
- Huang, Z.; Zhu, D.; Wu, L.; He, M.; Zhou, X.; Zhang, L.; Zhang, H.; Wang, W.; Zhu, J.; Cheng, W.; et al. Six Serum-Based miRNAs as Potential Diagnostic Biomarkers for Gastric Cancer. Cancer Epidemiol. Biomark. Prev. 2017, 26, 188–196. [Google Scholar] [CrossRef]
- Tokuhisa, M.; Ichikawa, Y.; Kosaka, N.; Ochiya, T.; Yashiro, M.; Hirakawa, K.; Kosaka, T.; Makino, H.; Akiyama, H.; Kunisaki, C.; et al. Exosomal miRNAs from Peritoneum Lavage Fluid as Potential Prognostic Biomarkers of Peritoneal Metastasis in Gastric Cancer. PLoS ONE 2015, 10, e0130472. [Google Scholar] [CrossRef]
- Chen, K.B.; Chen, J.; Jin, X.L.; Huang, Y.; Su, Q.M.; Chen, L. Exosome-mediated peritoneal dissemination in gastric cancer and its clinical applications (Review). Biomed. Rep. 2018, 8, 503–509. [Google Scholar] [CrossRef]
- Arienti, C.; Pignatta, S.; Tesei, A. Epidermal Growth Factor Receptor Family and its Role in Gastric Cancer. Front. Oncol. 2019, 9, 1308. [Google Scholar] [CrossRef] [PubMed]
- Dulak, A.M.; Schumacher, S.E.; Van Lieshout, J.; Imamura, Y.; Fox, C.; Shim, B.; Ramos, A.H.; Saksena, G.; Baca, S.C.; Baselga, J. Gastrointestinal adenocarcinomas of the esophagus, stomach, and colon exhibit distinct patterns of genome instability and oncogenesis. Cancer Res. 2012, 72, 4383–4393. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.A.; Lee, H.S.; Lee, H.E.; Jeon, Y.K.; Yang, H.K.; Kim, W.H. EGFR in gastric carcinomas: Prognostic significance of protein overexpression and high gene copy number. Histopathology 2008, 52, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Boku, N. HER2-positive gastric cancer. Gastric Cancer 2014, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Starling, N.; Cunningham, D.; Sumpter, K.; Gilligan, D.; Ruhstaller, T.; Valladares-Ayerbes, M.; Wilke, H.; Archer, C.; Kurek, R.; et al. Matuzumab plus epirubicin, cisplatin and capecitabine (ECX) compared with epirubicin, cisplatin and capecitabine alone as first-line treatment in patients with advanced oesophago-gastric cancer: A randomised, multicentre open-label phase II study. Ann. Oncol. 2010, 21, 2213–2219. [Google Scholar] [CrossRef] [PubMed]
- Richards, D.; Kocs, D.M.; Spira, A.I.; David McCollum, A.; Diab, S.; Hecker, L.I.; Cohn, A.; Zhan, F.; Asmar, L. Results of docetaxel plus oxaliplatin (DOCOX) ± cetuximab in patients with metastatic gastric and/or gastroesophageal junction adenocarcinoma: Results of a randomised Phase 2 study. Eur. J. Cancer 2013, 49, 2823–2831. [Google Scholar] [CrossRef]
- Tebbutt, N.C.; Price, T.J.; Ferraro, D.A.; Wong, N.; Veillard, A.-S.; Hall, M.; Sjoquist, K.M.; Pavlakis, N.; Strickland, A.; Varma, S.C.; et al. Panitumumab added to docetaxel, cisplatin and fluoropyrimidine in oesophagogastric cancer: ATTAX3 phase II trial. Br. J. Cancer 2016, 114, 505–509. [Google Scholar] [CrossRef]
- Kentepozidis, N. Panitumumab in combination with modified docetaxel/cisplatin/5-fluorouracil as first-line treatment in gastric and gastroesophageal junction adenocarcinomas: A multicenter phase II study by the Hellenic Oncology Research Group. Ann. Gastroenterol. 2018, 31, 698–704. [Google Scholar] [CrossRef]
- Malka, D.; François, E.; Penault-Llorca, F.; Castan, F.; Bouché, O.; Bennouna, J.; Ghiringhelli, F.; de la Fouchardière, C.; Borg, C.; Samalin, E.; et al. FOLFOX alone or combined with rilotumumab or panitumumab as first-line treatment for patients with advanced gastroesophageal adenocarcinoma (PRODIGE 17-ACCORD 20-MEGA): A randomised, open-label, three-arm phase II trial. Eur. J. Cancer 2019, 115, 97–106. [Google Scholar] [CrossRef]
- Du, F.; Zheng, Z.; Shi, S.; Jiang, Z.; Qu, T.; Yuan, X.; Sun, Y.; Song, Y.; Yang, L.; Zhao, J.; et al. S-1 and Cisplatin With or Without Nimotuzumab for Patients With Untreated Unresectable or Metastatic Gastric Cancer: A Randomized, Open-Label Phase 2 Trial. Medicine 2015, 94, e958. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Lin, L.-S.; Dicarlo, B.; Dao, K.M.; Patel, R.; Park, D.J.; Wang, H.-J.; Elashoff, R.; Ryba, N.; Hecht, J.R. Phase II trial of modified FOLFOX6 and erlotinib in patients with metastatic or advanced adenocarcinoma of the oesophagus and gastro-oesophageal junction. Br. J. Cancer 2011, 105, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Waddell, T.; Chau, I.; Cunningham, D.; Gonzalez, D.; Okines, A.F.; Okines, C.; Wotherspoon, A.; Saffery, C.; Middleton, G.; Wadsley, J.; et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): A randomised, open-label phase 3 trial. Lancet Oncol. 2013, 14, 481–489. [Google Scholar] [CrossRef]
- Lordick, F.; Kang, Y.-K.; Chung, H.-C.; Salman, P.; Oh, S.C.; Bodoky, G.; Kurteva, G.; Volovat, C.; Moiseyenko, V.M.; Gorbunova, V.; et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): A randomised, open-label phase 3 trial. Lancet Oncol. 2013, 14, 490–499. [Google Scholar] [CrossRef]
- Makiyama, A.; Sagara, K.; Kawada, J.; Kashiwada, T.; Hosokawa, A.; Horie, Y.; Satake, H.; Yamamoto, Y.; Tanioka, H.; Shinozaki, K. A randomized phase II study of weekly paclitaxel±trastuzumab in patients with HER2-positive advanced gastric or gastro-esophageal junction cancer refractory to trastuzumab combined with fluoropyrimidine and platinum: WJOG7112G (T-ACT). Am. Soc. Clin. Oncol. 2018, 36, 4011. [Google Scholar] [CrossRef]
- Chua, C.; Tan, I.B.; Yamada, Y.; Rha, S.Y.; Yong, W.P.; Ong, W.S.; Tham, C.K.; Ng, M.; Tai, D.W.M.; Iwasa, S.; et al. Phase II study of trastuzumab in combination with S-1 and cisplatin in the first-line treatment of human epidermal growth factor receptor HER2-positive advanced gastric cancer. Cancer Chemother. Pharmacol. 2015, 76, 397–408. [Google Scholar] [CrossRef]
- Ryu, M.H.; Yoo, C.; Kim, J.G.; Ryoo, B.Y.; Park, Y.S.; Park, S.R.; Han, H.S.; Chung, I.J.; Song, E.K.; Lee, K.H.; et al. Multicenter phase II study of trastuzumab in combination with capecitabine and oxaliplatin for advanced gastric cancer. Eur. J. Cancer 2015, 51, 482–488. [Google Scholar] [CrossRef]
- Wang, F.; Liu, T.S.; Yuan, X.L.; Luo, H.Y.; Gu, K.S.; Yuan, Y.; Deng, Y.H.; Xu, J.M.; Bai, Y.X.; Wang, Y.; et al. Trastuzumab plus docetaxel and capecitabine as a first-line treatment for HER2-positive advanced gastric or gastroesophageal junction cancer: A phase II, multicenter, open-label, single-arm study. Am. J. Cancer Res. 2020, 10, 3037–3046. [Google Scholar]
- Takahari, D.; Chin, K.; Ishizuka, N.; Takashima, A.; Minashi, K.; Kadowaki, S.; Nishina, T.; Nakajima, T.E.; Amagai, K.; Machida, N.; et al. Multicenter phase II study of trastuzumab with S-1 plus oxaliplatin for chemotherapy-naïve, HER2-positive advanced gastric cancer. Gastric Cancer 2019, 22, 1238–1246. [Google Scholar] [CrossRef]
- Catenacci, D.V.T.; Kang, Y.K.; Park, H.; Uronis, H.E.; Lee, K.W.; Ng, M.C.H.; Enzinger, P.C.; Park, S.H.; Gold, P.J.; Lacy, J.; et al. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22-05): A single-arm, phase 1b-2 trial. Lancet Oncol. 2020, 21, 1066–1076. [Google Scholar] [CrossRef]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Shah, M.A.; Xu, R.-H.; Bang, Y.-J.; Hoff, P.M.; Liu, T.; Herráez-Baranda, L.A.; Xia, F.; Garg, A.; Shing, M.; Tabernero, J. HELOISE: Phase IIIb Randomized Multicenter Study Comparing Standard-of-Care and Higher-Dose Trastuzumab Regimens Combined With Chemotherapy as First-Line Therapy in Patients With Human Epidermal Growth Factor Receptor 2–Positive Metastatic Gastric or Gast. J. Clin. Oncol. 2017, 35, 2558–2567. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Hoff, P.M.; Shen, L.; Ohtsu, A.; Shah, M.A.; Cheng, K.; Song, C.; Wu, H.; Eng-Wong, J.; Kim, K.; et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): Final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018, 19, 1372–1384. [Google Scholar] [CrossRef]
- Shitara, K.; Bang, Y.-J.; Iwasa, S.; Sugimoto, N.; Ryu, M.-H.; Sakai, D.; Chung, H.-C.; Kawakami, H.; Yabusaki, H.; Lee, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N. Engl. J. Med. 2020, 382, 2419–2430. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Liu, T.; Wei, J.; Wang, A.; He, Y.; Yang, L.; Zhang, X.; Fan, N.; Luo, S.; Li, Z.; et al. Efficacy and safety of a novel anti-HER2 therapeutic antibody RC48 in patients with HER2-overexpressing, locally advanced or metastatic gastric or gastroesophageal junction cancer: A single-arm phase II study. Cancer Commun. 2021, 41, 1173–1182. [Google Scholar] [CrossRef]
- Thuss-Patience, P.C.; Shah, M.A.; Ohtsu, A.; Van Cutsem, E.; Ajani, J.A.; Castro, H.; Mansoor, W.; Chung, H.C.; Bodoky, G.; Shitara, K.; et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): An international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 2017, 18, 640–653. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Lee, K.-W.; Cho, J.Y.; Kang, W.K.; Im, S.-A.; Kim, J.W.; Bang, Y.-J. Phase II trial of dacomitinib in patients with HER2-positive gastric cancer. Gastric Cancer 2016, 19, 1095–1103. [Google Scholar] [CrossRef]
- Iqbal, S.; Goldman, B.; Fenoglio-Preiser, C.M.; Lenz, H.J.; Zhang, W.; Danenberg, K.D.; Shibata, S.I.; Blanke, C.D. Southwest Oncology Group study S0413: A phase II trial of lapatinib (GW572016) as first-line therapy in patients with advanced or metastatic gastric cancer. Ann. Oncol. 2011, 22, 2610–2615. [Google Scholar] [CrossRef]
- Hecht, J.R.; Bang, Y.-J.; Qin, S.K.; Chung, H.C.; Xu, J.M.; Park, J.O.; Jeziorski, K.; Shparyk, Y.; Hoff, P.M.; Sobrero, A.; et al. Lapatinib in Combination With Capecitabine Plus Oxaliplatin in Human Epidermal Growth Factor Receptor 2–Positive Advanced or Metastatic Gastric, Esophageal, or Gastroesophageal Adenocarcinoma: TRIO-013/LOGiC—A Randomized Phase III Trial. J. Clin. Oncol. 2016, 34, 443–451. [Google Scholar] [CrossRef]
- Satoh, T.; Xu, R.-H.; Chung, H.C.; Sun, G.-P.; Doi, T.; Xu, J.-M.; Tsuji, A.; Omuro, Y.; Li, J.; Wang, J.-W.; et al. Lapatinib Plus Paclitaxel Versus Paclitaxel Alone in the Second-Line Treatment ofHER2-Amplified Advanced Gastric Cancer in Asian Populations: TyTAN—A Randomized, Phase III Study. J. Clin. Oncol. 2014, 32, 2039–2049. [Google Scholar] [CrossRef]
- Strickler, J.H. EGFR Amplification as a Target in Gastroesophageal Adenocarcinoma: Do Anti-EGFR Therapies Deserve a Second Chance? Cancer Discov. 2018, 8, 679–681. [Google Scholar] [CrossRef]
- Kandel, C.; Leclair, F.; Bou-Hanna, C.; Laboisse, C.L.; Mosnier, J.F. Association of HER1 amplification with poor prognosis in well differentiated gastric carcinomas. J. Clin. Pathol. 2014, 67, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yang, J.M.; Hu, T.T.; Xu, T.J.; Yan, G.; Hu, S.L.; Wei, W.; Xu, W.P. Prognostic role of human epidermal growth factor receptor in gastric cancer: A systematic review and meta-analysis. Arch. Med. Res. 2013, 44, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Aydin, K.; Okutur, S.K.; Bozkurt, M.; Turkmen, I.; Namal, E.; Pilanci, K.; Ozturk, A.; Akcali, Z.; Dogusoy, G.; Demir, O.G. Effect of epidermal growth factor receptor status on the outcomes of patients with metastatic gastric cancer: A pilot study. Oncol. Lett. 2014, 7, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Maron, S.B.; Alpert, L.; Kwak, H.A.; Lomnicki, S.; Chase, L.; Xu, D.; O’Day, E.; Nagy, R.J.; Lanman, R.B.; Cecchi, F.; et al. Targeted Therapies for Targeted Populations: Anti-EGFR Treatment for EGFR-Amplified Gastroesophageal Adenocarcinoma. Cancer Discov. 2018, 8, 696–713. [Google Scholar] [CrossRef]
- Seshacharyulu, P.; Ponnusamy, M.P.; Haridas, D.; Jain, M.; Ganti, A.K.; Batra, S.K. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin.Ther. Targets 2012, 16, 15–31. [Google Scholar] [CrossRef]
- Apicella, M.; Corso, S.; Giordano, S. Targeted therapies for gastric cancer: Failures and hopes from clinical trials. Oncotarget 2017, 8, 57654–57669. [Google Scholar] [CrossRef]
- Smyth, E.C.; Vlachogiannis, G.; Hedayat, S.; Harbery, A.; Hulkki-Wilson, S.; Salati, M.; Kouvelakis, K.; Fernandez-Mateos, J.; Cresswell, G.D.; Fontana, E.; et al. EGFR amplification and outcome in a randomised phase III trial of chemotherapy alone or chemotherapy plus panitumumab for advanced gastro-oesophageal cancers. Gut 2021, 70, 1632–1641. [Google Scholar] [CrossRef]
- Kahraman, S.; Yalcin, S. Recent Advances in Systemic Treatments for HER-2 Positive Advanced Gastric Cancer. OncoTargets Ther. 2021, 14, 4149–4162. [Google Scholar] [CrossRef]
- Park, D.I.; Yun, J.W.; Park, J.H.; Oh, S.J.; Kim, H.J.; Cho, Y.K.; Sohn, C.I.; Jeon, W.K.; Kim, B.I.; Yoo, C.H.; et al. HER-2/neu Amplification Is an Independent Prognostic Factor in Gastric Cancer. Dig. Dis. Sci. 2006, 51, 1371–1379. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Procter, M.; De Azambuja, E.; Zardavas, D.; Benyunes, M.; Viale, G.; Suter, T.; Arahmani, A.; Rouchet, N.; Clark, E.; et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N. Engl. J. Med. 2017, 377, 122–131. [Google Scholar] [CrossRef]
- Kotani, D.; Shitara, K. Trastuzumab deruxtecan for the treatment of patients with HER2-positive gastric cancer. Ther. Adv. Med. Oncol. 2021, 13, 175883592098651. [Google Scholar] [CrossRef] [PubMed]
- Voigtlaender, M.; Schneider-Merck, T.; Trepel, M. Lapatinib. In Small Molecules in Oncology; Springer International Publishing: Cham, Switherlands, 2018; pp. 19–44. [Google Scholar]
- Jung, Y.D.; Mansfield, P.F.; Akagi, M.; Takeda, A.; Liu, W.; Bucana, C.D.; Hicklin, D.J.; Ellis, L.M. Effects of combination anti-vascular endothelial growth factor receptor and anti-epidermal growth factor receptor therapies on the growth of gastric cancer in a nude mouse model. Eur. J. Cancer 2002, 38, 1133–1140. [Google Scholar] [CrossRef]
- Lieto, E.; Ferraraccio, F.; Orditura, M.; Castellano, P.; Mura, A.L.; Pinto, M.; Zamboli, A.; De Vita, F.; Galizia, G. Expression of Vascular Endothelial Growth Factor (VEGF) and Epidermal Growth Factor Receptor (EGFR) is an Independent Prognostic Indicator of Worse Outcome in Gastric Cancer Patients. Ann. Surg. Oncol. 2008, 15, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Jhawer, M.; Ilson, D.H.; Lefkowitz, R.A.; Robinson, E.; Capanu, M.; Kelsen, D.P. Phase II Study of Modified Docetaxel, Cisplatin, and Fluorouracil With Bevacizumab in Patients With Metastatic Gastroesophageal Adenocarcinoma. J. Clin. Oncol. 2011, 29, 868–874. [Google Scholar] [CrossRef]
- Enzinger, P.C.; Ryan, D.P.; Regan, E.M.; Lehman, N.; Abrams, T.A.; Hezel, A.F.; Fidias, P.; Sequist, L.V.; Blaszkowsky, L.S.; Fuchs, C.S. Phase II trial of docetaxel, cisplatin, irinotecan, and bevacizumab in metastatic esophagogastric cancer. J. Clin. Oncol. 2008, 26 (Suppl. 15), 4552. [Google Scholar] [CrossRef]
- El-Rayes, B.F.; Zalupski, M.; Bekai-Saab, T.; Heilbrun, L.K.; Hammad, N.; Patel, B.; Urba, S.; Shields, A.F.; Vaishampayan, U.; Dawson, S.; et al. A phase II study of bevacizumab, oxaliplatin, and docetaxel in locally advanced and metastatic gastric and gastroesophageal junction cancers. Ann. Oncol. 2010, 21, 1999–2004. [Google Scholar] [CrossRef]
- Shah, M.A.; Ramanathan, R.K.; Ilson, D.H.; Levnor, A.; D’Adamo, D.; O’Reilly, E.; Tse, A.; Trocola, R.; Schwartz, L.; Capanu, M.; et al. Multicenter Phase II Study of Irinotecan, Cisplatin, and Bevacizumab in Patients With Metastatic Gastric or Gastroesophageal Junction Adenocarcinoma. J. Clin. Oncol. 2006, 24, 5201–5206. [Google Scholar] [CrossRef]
- Uronis, H.E.; Bendell, J.C.; Altomare, I.; Blobe, G.C.; Hsu, S.D.; Morse, M.A.; Pang, H.; Zafar, S.Y.; Conkling, P.; Favaro, J.; et al. A Phase II Study of Capecitabine, Oxaliplatin, and Bevacizumab in the Treatment of Metastatic Esophagogastric Adenocarcinomas. Oncologist 2013, 18, 271–272. [Google Scholar] [CrossRef][Green Version]
- Kunz, P.L.; Nandoskar, P.; Koontz, M.Z.; Ji, H.; Ford, J.M.; Balise, R.R.; Kamaya, A.; Rubin, D.; Fisher, G.A. A phase II study of capecitabine, carboplatin, and bevacizumab for metastatic or unresectable gastroesophageal junction and gastric adenocarcinoma. J. Clin. Oncol. 2014, 32 (Suppl. 3), 115. [Google Scholar] [CrossRef]
- Brenner, B.; Sarfaty, M.; Purim, O.; Kundel, Y.; Amit, L.; Abramovich, A.; Sadeh Gonik, U.; Idelevich, E.; Gordon, N.; Medalia, G.; et al. A Phase Ib/II Study Evaluating the Combination of Weekly Docetaxel and Cisplatin Together with Capecitabine and Bevacizumab in Patients with Advanced Esophago-Gastric Cancer. PLoS ONE 2016, 11, e0157548. [Google Scholar] [CrossRef]
- Meulendijks, D.; Beerepoot, L.V.; Boot, H.; de Groot, J.W.; Los, M.; Boers, J.E.; Vanhoutvin, S.A.; Polee, M.B.; Beeker, A.; Portielje, J.E.; et al. Trastuzumab and bevacizumab combined with docetaxel, oxaliplatin and capecitabine as first-line treatment of advanced HER2-positive gastric cancer: A multicenter phase II study. Investig. New Drugs 2016, 34, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Meulendijks, D.; De Groot, J.W.B.; Los, M.; Boers, J.E.; Beerepoot, L.V.; Polee, M.B.; Beeker, A.; Portielje, J.E.A.; Goey, S.H.; De Jong, R.S.; et al. Bevacizumab combined with docetaxel, oxaliplatin, and capecitabine, followed by maintenance with capecitabine and bevacizumab, as first-line treatment of patients with advanced HER2-negative gastric cancer: A multicenter phase 2 study. Cancer 2016, 122, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Wöll, E.; Thaler, J.; Keil, F.; Gruenberger, B.; Hejna, M.; Eisterer, W.; Fridrik, M.A.; Ulmer, H.; Trommet, V.; Huemer, F.; et al. Oxaliplatin/Irinotecan/Bevacizumab Followed by Docetaxel/Bevacizumab in Inoperable Locally Advanced or Metastatic Gastric Cancer Patients—AGMT_GASTRIC-3. Anticancer Res. 2017, 37, 5553–5558. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kortmansky, J.S.; Saif, M.; Fischbach, N.A.; Ravage-Mass, L.; Elligers, K.; Hahn, C.; Cohenuram, M.K.; Lacy, J. Phase II study of mFOLFOX6 with bevacizumab (Bev) in metastatic gastric and esophageal (GE) adenocarcinoma. J. Clin. Oncol. 2010, 28 (Suppl. 15), TPS203. [Google Scholar] [CrossRef]
- Yoon, H.H.; Bendell, J.C.; Braiteh, F.S.; Firdaus, I.; Philip, P.A.; Cohn, A.L.; Lewis, N.; Anderson, D.M.; Arrowsmith, E.; Schwartz, J.D.; et al. Ramucirumab combined with FOLFOX as front-line therapy for advanced esophageal, gastroesophageal junction, or gastric adenocarcinoma: A randomized, double-blind, multicenter Phase II trial. Ann. Oncol. 2016, 27, 2196–2203. [Google Scholar] [CrossRef]
- Muro, K.; Yoshikawa, T.; Shitara, K.; Oh, D.-Y.; Kang, Y.-K.; Chung, H.C.; Kudo, T.; Chin, K.; Kadowaki, S.; Hamamoto, Y.; et al. Randomized, double-blind, phase 2 study of S-1 plus oxaliplatin (SOX) with or without ramucirumab (RAM) as first-line therapy followed by paclitaxel plus RAM as second-line therapy in patients with advanced gastric or gastroesophageal junction adenocarcinoma (AGC). J. Clin. Oncol. 2018, 36 (Suppl. 15), 4036. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Fujitani, K.; Nagashima, F.; Omuro, Y.; Machida, N.; Nishina, T.; Koue, T.; Tsujimoto, M.; Maeda, K.; Satoh, T. Ramucirumab for the treatment of metastatic gastric or gastroesophageal junction adenocarcinoma following disease progression on first-line platinum- or fluoropyrimidine-containing combination therapy in Japanese patients: A phase 2, open-label study. Gastric Cancer 2018, 21, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Ohtsu, A.; Shah, M.A.; Van Cutsem, E.; Rha, S.Y.; Sawaki, A.; Park, S.R.; Lim, H.Y.; Yamada, Y.; Wu, J.; Langer, B.; et al. Bevacizumab in Combination With Chemotherapy As First-Line Therapy in Advanced Gastric Cancer: A Randomized, Double-Blind, Placebo-Controlled Phase III Study. J. Clin. Oncol. 2011, 29, 3968–3976. [Google Scholar] [CrossRef]
- Shen, L.; Li, J.; Xu, J.; Pan, H.; Dai, G.; Qin, S.; Wang, L.; Wang, J.; Yang, Z.; Shu, Y.; et al. Bevacizumab plus capecitabine and cisplatin in Chinese patients with inoperable locally advanced or metastatic gastric or gastroesophageal junction cancer: Randomized, double-blind, phase III study (AVATAR study). Gastric Cancer 2015, 18, 168–176. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Shitara, K.; Di Bartolomeo, M.; Lonardi, S.; Al-Batran, S.E.; Van Cutsem, E.; Ilson, D.H.; Alsina, M.; Chau, I.; Lacy, J.; et al. Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 420–435. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Tomasek, J.; Yong, C.J.; Dumitru, F.; Passalacqua, R.; Goswami, C.; Safran, H.; Dos Santos, L.V.; Aprile, G.; Ferry, D.R.; et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014, 383, 31–39. [Google Scholar] [CrossRef]
- Wilke, H.; Muro, K.; Van Cutsem, E.; Oh, S.C.; Bodoky, G.; Shimada, Y.; Hironaka, S.; Sugimoto, N.; Lipatov, O.; Kim, T.Y.; et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol. 2014, 15, 1224–1235. [Google Scholar] [CrossRef]
- Sun, W.; Powell, M.; O’Dwyer, P.J.; Catalano, P.; Ansari, R.H.; Benson, A.B. Phase II Study of Sorafenib in Combination With Docetaxel and Cisplatin in the Treatment of Metastatic or Advanced Gastric and Gastroesophageal Junction Adenocarcinoma: ECOG 5203. J. Clin. Oncol. 2010, 28, 2947–2951. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Lee, K.H.; Shen, L.; Yeh, K.; Hong, Y.S.; Park, Y.I.; Yang, S.H.; Shin, D.B.; Zang, D.Y.; Kang, W.K.; et al. 615O–Randomized Phase Ii Study of Capecitabine and Cisplatin with or Without Sorafenib in Patients with Metastatic Gastric Cancer: Stargate Study. Ann. Oncol. 2014, 25, iv210. [Google Scholar] [CrossRef]
- Martin-Richard, M.; Gallego, R.; Pericay, C.; Foncillas, J.G.; Queralt, B.; Casado, E.; Barriuso, J.; Iranzo, V.; Juez, I.; Visa, L.; et al. Multicenter phase II study of oxaliplatin and sorafenib in advanced gastric adenocarcinoma after failure of cisplatin and fluoropyrimidine treatment. A gemcad study. Investig. New Drugs 2013, 31, 1573–1579. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Vakiani, E.; Ku, G.Y.; Herrera, J.M.; Tang, L.H.; Bouvier, N.; Viale, A.; Socci, N.D.; Capanu, M.; Berger, M.; et al. Phase II Trial of Sorafenib in Patients with Chemotherapy Refractory Metastatic Esophageal and Gastroesophageal (GE) Junction Cancer. PLoS ONE 2015, 10, e0134731. [Google Scholar] [CrossRef]
- Bang, Y.-J.; Kang, Y.-K.; Kang, W.K.; Boku, N.; Chung, H.C.; Chen, J.-S.; Doi, T.; Sun, Y.; Shen, L.; Qin, S.; et al. Phase II study of sunitinib as second-line treatment for advanced gastric cancer. Investig. New Drugs 2011, 29, 1449–1458. [Google Scholar] [CrossRef]
- Yi, J.H.; Lee, J.; Lee, J.; Park, S.H.; Park, J.O.; Yim, D.-S.; Park, Y.S.; Lim, H.Y.; Kang, W.K. Randomised phase II trial of docetaxel and sunitinib in patients with metastatic gastric cancer who were previously treated with fluoropyrimidine and platinum. Br. J. Cancer 2012, 106, 1469–1474. [Google Scholar] [CrossRef]
- Moehler, M.; Gepfner-Tuma, I.; Maderer, A.; Thuss-Patience, P.C.; Ruessel, J.; Hegewisch-Becker, S.; Wilke, H.; Al-Batran, S.-E.; Rafiyan, M.-R.; Weißinger, F.; et al. Sunitinib added to FOLFIRI versus FOLFIRI in patients with chemorefractory advanced adenocarcinoma of the stomach or lower esophagus: A randomized, placebo-controlled phase II AIO trial with serum biomarker program. BMC Cancer 2016, 16, 699. [Google Scholar] [CrossRef]
- Moehler, M.; Mueller, A.; Hartmann, J.T.; Ebert, M.P.; Al-Batran, S.E.; Reimer, P.; Weihrauch, M.; Lordick, F.; Trarbach, T.; Biesterfeld, S.; et al. An open-label, multicentre biomarker-oriented AIO phase II trial of sunitinib for patients with chemo-refractory advanced gastric cancer. Eur. J. Cancer 2011, 47, 1511–1520. [Google Scholar] [CrossRef]
- Alsina, M.; Ko, A.H.; Garcia De Paredes, M.; Rivera, F.; Schwartzberg, L.S.; Fattaey, A.; Kunkel, L.A.; Tabernero, J.; Ajani, J.A. Clinical and pharmacodynamic (PD) results of TEL0805 trial: A phase II study of telatinib (TEL) in combination with capecitabine (X) and cisplatin (P) as first-line treatment in patients (pts) with advanced gastric or gastroesophageal junction (GEJ) cancer. J. Clin. Oncol. 2011, 29 (Suppl. 15), 4122. [Google Scholar] [CrossRef]
- Koizumi, W.; Yamaguchi, K.; Hosaka, H.; Takinishi, Y.; Nakayama, N.; Hara, T.; Muro, K.; Baba, H.; Sasaki, Y.; Nishina, T.; et al. Randomised phase II study of S-1/cisplatin plus TSU-68 vs S-1/cisplatin in patients with advanced gastric cancer. Br. J. Cancer 2013, 109, 2079–2086. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qin, S.; Xu, J.; Guo, W.; Xiong, J.; Bai, Y.; Sun, G.; Yang, Y.; Wang, L.; Xu, N.; et al. Apatinib for Chemotherapy-Refractory Advanced Metastatic Gastric Cancer: Results From a Randomized, Placebo-Controlled, Parallel-Arm, Phase II Trial. J. Clin. Oncol. 2013, 31, 3219–3225. [Google Scholar] [CrossRef]
- Kim, S.T.; Lee, J.; Lee, S.J.; Park, S.H.; Jung, S.-H.; Park, Y.S.; Lim, H.Y.; Kang, W.K.; Park, J.O. Prospective phase II trial of pazopanib plus CapeOX (capecitabine and oxaliplatin) in previously untreated patients with advanced gastric cancer. Oncotarget 2016, 7, 24088–24096. [Google Scholar] [CrossRef] [PubMed]
- Thuss-Patience, P.C.; Al-Batran, S.-E.; Siveke, J.T.; Homann, N.; Malfertheiner, P.; Glaeser, D.; Stein, A.; Tamm, I.; Daum, S.; Potenberg, J.; et al. Pazopanib and 5-FU/oxaliplatin as first-line treatment in advanced gastric cancer: PaFLO, a randomized phase II study from the AIO (Arbeitsgemeinschaft Internistische Onkologie). J. Clin. Oncol. 2015, 33 (Suppl. 15), 4033. [Google Scholar] [CrossRef]
- Moy, R.H.; Dos Santos Fernandes, G.; Jonsson, P.; Chou, J.F.; Basunia, A.; Ku, G.Y.; Chalasani, S.B.; Boyar, M.S.; Goldberg, Z.; Desai, A.M.; et al. Regorafenib in Combination with First-Line Chemotherapy for Metastatic Esophagogastric Cancer. Oncologist 2020, 25, e68–e74. [Google Scholar] [CrossRef]
- Pavlakis, N.; Sjoquist, K.M.; Martin, A.J.; Tsobanis, E.; Yip, S.; Kang, Y.-K.; Bang, Y.-J.; Alcindor, T.; O’Callaghan, C.J.; Burnell, M.J.; et al. Regorafenib for the Treatment of Advanced Gastric Cancer (INTEGRATE): A Multinational Placebo-Controlled Phase II Trial. J. Clin. Oncol. 2016, 34, 2728–2735. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.X.; Shen, L.; Li, J.; Huang, J.; Su, W.G.; Zhang, D.S.; Xu, R.H. A phase Ib/II study of fruquintinib in combination with paclitaxel as the second-line therapy for advanced gastric cancer. Cancer Commun. 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Kawazoe, A.; Fukuoka, S.; Nakamura, Y.; Kuboki, Y.; Mikamoto, Y.; Shima, H.; Fujishiro, N.; Higuchi, T.; Wakabayashi, M.; Nomura, S.; et al. An open-label phase II study of lenvatinib plus pembrolizumab in patients with advanced gastric cancer (EPOC1706). J. Clin. Oncol. 2020, 38 (Suppl. 4), 374. [Google Scholar] [CrossRef]
- Chung, H.C.; Lwin, Z.; Gomez-Roca, C.; Longo, F.; Yanez, E.; Castanon Alvarez, E.; Graham, D.; Doherty, M.; Cassier, P.; Lopez, J.S.; et al. LEAP-005: A phase II multicohort study of lenvatinib plus pembrolizumab in patients with previously treated selected solid tumors—Results from the gastric cancer cohort. J. Clin. Oncol. 2021, 39 (Suppl. 3), 230. [Google Scholar] [CrossRef]
- Li, J.; Qin, S.; Xu, J.; Xiong, J.; Wu, C.; Bai, Y.; Liu, W.; Tong, J.; Liu, Y.; Xu, R.; et al. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J. Clin. Oncol. 2016, 34 (Suppl. 3), 1448–1454. [Google Scholar] [CrossRef]
- Cleary, J.M.; Horick, N.K.; Mccleary, N.J.; Abrams, T.A.; Yurgelun, M.B.; Azzoli, C.G.; Rubinson, D.A.; Brooks, G.A.; Chan, J.A.; Blaszkowsky, L.S.; et al. FOLFOX plus ziv-aflibercept or placebo in first-line metastatic esophagogastric adenocarcinoma: A double-blind, randomized, multicenter phase 2 trial. Cancer 2019, 125, 2213–2221. [Google Scholar] [CrossRef] [PubMed]
- Presta, L.G.; Chen, H.; O’Connor, S.J.; Chisholm, V.; Meng, Y.G.; Krummen, L.; Winkler, M.; Ferrara, N. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997, 57, 4593–4599. [Google Scholar] [PubMed]
- Shah, M.A.; Van Cutsem, E.; Kang, Y.-K.; Dakhil, S.R.; Satoh, T.; Chin, K.; Bang, Y.-J.; Bu, L.; Bilic, G.; Ohtsu, A. Survival analysis according to disease subtype in AVAGAST: First-line capecitabine and cisplatin plus bevacizumab (bev) or placebo in patients (pts) with advanced gastric cancer. J. Clin. Oncol. 2012, 30 (Suppl. 4), 5. [Google Scholar] [CrossRef]
- Tada, Y.; Togashi, Y.; Kotani, D.; Kuwata, T.; Sato, E.; Kawazoe, A.; Doi, T.; Wada, H.; Nishikawa, H.; Shitara, K. Targeting VEGFR2 with Ramucirumab strongly impacts effector/activated regulatory T cells and CD8(+) T cells in the tumor microenvironment. J. Immunother. Cancer 2018, 6, 106. [Google Scholar] [CrossRef]
- Selim, J.H.; Shaheen, S.; Sheu, W.-C.; Hsueh, C.-T. Targeted and novel therapy in advanced gastric cancer. Exp. Hematol. Oncol. 2019, 8, 25. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Bass, A.J.; Thorsson, V.; Shmulevich, I.; Reynolds, S.M.; Miller, M.; Bernard, B.; Hinoue, T.; Laird, P.W.; Curtis, C.; Shen, H. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202. [Google Scholar]
- Magalhães, H.; Fontes-Sousa, M.; Machado, M. Immunotherapy in Advanced Gastric Cancer: An Overview of the Emerging Strategies. Can. J. Gastroenterol. Hepatol. 2018, 2018, 2732408. [Google Scholar] [CrossRef]
- Garattini, S.K.; Basile, D.; Cattaneo, M.; Fanotto, V.; Ongaro, E.; Bonotto, M.; Negri, F.V.; Berenato, R.; Ermacora, P.; Cardellino, G.G. Molecular classifications of gastric cancers: Novel insights and possible future applications. World J. Gastrointest. Oncol. 2017, 9, 194. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Doi, T.; Jang, R.W.; Muro, K.; Satoh, T.; Machado, M.; Sun, W.; Jalal, S.I.; Shah, M.A.; Metges, J.-P.; et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer. JAMA Oncol. 2018, 4, e180013. [Google Scholar] [CrossRef]
- Rha, S.Y.; Lee, C.-k.; Kim, H.S.; Kang, B.; Jung, M.; Kwon, W.S.; Bae, W.K.; Koo, D.-H.; Shin, S.-J.; Jeung, H.-C.; et al. A multi-institutional phase Ib/II trial of first-line triplet regimen (Pembrolizumab, Trastuzumab, Chemotherapy) for HER2-positive advanced gastric and gastroesophageal junction cancer (PANTHERA Trial): Molecular profiling and clinical update. J. Clin. Oncol. 2021, 39 (Suppl. 3), 218. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Maron, S.B.; Chatila, W.K.; Millang, B.; Chavan, S.S.; Alterman, C.; Chou, J.F.; Segal, M.F.; Simmons, M.Z.; Momtaz, P.; et al. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: An open-label, single-arm, phase 2 trial. Lancet Oncol. 2020, 21, 821–831. [Google Scholar] [CrossRef]
- Klempner, S.J.; Bendell, J.C.; Villaflor, V.M.; Tenner, L.L.; Stein, S.; Naik, G.S.; Sirard, C.A.; Kagey, M.; Chaney, M.F.; Strickler, J.H. DKN-01 in combination with pembrolizumab in patients with advanced gastroesophageal adenocarcinoma (GEA): Tumoral DKK1 expression as a predictor of response and survival. J. Clin. Oncol. 2020, 38 (Suppl. 4), 357. [Google Scholar] [CrossRef]
- Nakajima, T.E.; Kadowaki, S.; Minashi, K.; Nishina, T.; Yamanaka, T.; Hayashi, Y.; Izawa, N.; Muro, K.; Hironaka, S.; Kajiwara, T.; et al. Multicenter Phase I/II Study of Nivolumab Combined with Paclitaxel Plus Ramucirumab as Second-line Treatment in Patients with Advanced Gastric Cancer. Clin. Cancer Res. 2021, 27, 1029–1036. [Google Scholar] [CrossRef]
- Hara, H.; Shoji, H.; Takahari, D.; Esaki, T.; Machida, N.; Nagashima, K.; Aoki, K.; Honda, K.; Miyamoto, T.; Boku, N.; et al. Phase I/II study of ramucirumab plus nivolumab in patients in second-line treatment for advanced gastric adenocarcinoma (NivoRam study). J. Clin. Oncol. 2019, 37 (Suppl. 4), 129. [Google Scholar] [CrossRef]
- Shen, L.; Peng, Z.; Zhang, Y.-Q.; Wei, J.; Wang, F.; Ying, J.; Deng, Y.; Gu, K.; Cheng, Y.; Yuan, X.; et al. Camrelizumab combined with capecitabine and oxaliplatin followed by camrelizumab and apatinib as first-line therapy for advanced or metastatic gastric or gastroesophageal junction cancer: Updated results from a multicenter, open label phase II trial. J. Clin. Oncol. 2019, 37 (Suppl. 15), 4031. [Google Scholar] [CrossRef]
- Jiang, H.; Yu, X.; Kong, M.; Ma, Z.; Zhou, D.; Wang, W.; Wang, H.; Li, N.; Wang, H.; He, K.; et al. Sintilimab plus oxaliplatin/capecitabine (CapeOx) as neoadjuvant therapy in patients with locally advanced, resectable gastric (G)/esophagogastric junction (GEJ) adenocarcinoma. J. Clin. Oncol. 2021, 39 (Suppl. 3), 211. [Google Scholar] [CrossRef]
- Wang, F.; Wei, X.L.; Wang, F.H.; Xu, N.; Shen, L.; Dai, G.H.; Yuan, X.L.; Chen, Y.; Yang, S.J.; Shi, J.H.; et al. Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Ann. Oncol. 2019, 30, 1479–1486. [Google Scholar] [CrossRef]
- Xu, J.; Bai, Y.; Xu, N.; Li, E.; Wang, B.; Wang, J.; Li, X.; Wang, X.; Yuan, X. Tislelizumab Plus Chemotherapy as First-line Treatment for Advanced Esophageal Squamous Cell Carcinoma and Gastric/Gastroesophageal Junction Adenocarcinoma. Clin. Cancer Res. 2020, 26, 4542–4550. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Özgüroğlu, M.; Bang, Y.-J.; Di Bartolomeo, M.; Mandalà, M.; Ryu, M.-h.; Fornaro, L.; Olesinski, T.; Caglevic, C.; Chung, H.C.; et al. Pembrolizumab versus paclitaxel for previously treated patients with PD-L1–positive advanced gastric or gastroesophageal junction cancer (GC): Update from the phase III KEYNOTE-061 trial. J. Clin. Oncol. 2020, 38 (Suppl. 15), 4503. [Google Scholar] [CrossRef]
- Shitara, K.; Van Cutsem, E.; Bang, Y.-J.; Fuchs, C.; Wyrwicz, L.; Lee, K.-W.; Kudaba, I.; Garrido, M.; Chung, H.C.; Lee, J.; et al. Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients With First-line, Advanced Gastric Cancer. JAMA Oncol. 2020, 6, 1571. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.C.; Bang, Y.-J.; S Fuchs, C.; Qin, S.-K.; Satoh, T.; Shitara, K.; Tabernero, J.; Van Cutsem, E.; Alsina, M.; Cao, Z.A.; et al. First-line pembrolizumab/placebo plus trastuzumab and chemotherapy in HER2-positive advanced gastric cancer: KEYNOTE-811. Future Oncol. 2021, 17, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Boku, N.; Satoh, T.; Ryu, M.-H.; Chao, Y.; Kato, K.; Chung, H.C.; Chen, J.-S.; Muro, K.; Kang, W.K.; Yeh, K.-H.; et al. Nivolumab in previously treated advanced gastric cancer (ATTRACTION-2): 3-year update and outcome of treatment beyond progression with nivolumab. Gastric Cancer 2021, 24, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Campos Bragagnoli, A.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef]
- Kelly, R.J.; Ajani, J.A.; Kuzdzal, J.; Zander, T.; Van Cutsem, E.; Piessen, G.; Mendez, G.; Feliciano, J.; Motoyama, S.; Lièvre, A.; et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N. Engl. J. Med. 2021, 384, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Moehler, M.; Dvorkin, M.; Boku, N.; Özgüroğlu, M.; Ryu, M.-H.; Muntean, A.S.; Lonardi, S.; Nechaeva, M.; Bragagnoli, A.C.; Coşkun, H.S.; et al. Phase III Trial of Avelumab Maintenance After First-Line Induction Chemotherapy Versus Continuation of Chemotherapy in Patients With Gastric Cancers: Results From JAVELIN Gastric 100. J. Clin. Oncol. 2021, 39, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Moehler, M.H.; Cho, J.Y.; Kim, Y.H.; Kim, J.W.; Di Bartolomeo, M.; Ajani, J.A.; Yamaguchi, K.; Balogh, A.; Kong-Sanchez, M.T.; Bang, Y.-J. A randomized, open-label, two-arm phase II trial comparing the efficacy of sequential ipilimumab (ipi) versus best supportive care (BSC) following first-line (1L) chemotherapy in patients with unresectable, locally advanced/metastatic (A/M) gastric or gastro-esophageal junction (G/GEJ) cancer. J. Clin. Oncol. 2016, 34 (Suppl. 15), 4011. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Bendell, J.; Calvo, E.; Kim, J.W.; Ascierto, P.A.; Sharma, P.; Ott, P.A.; Peltola, K.; Jaeger, D.; Evans, J.; et al. CheckMate-032 Study: Efficacy and Safety of Nivolumab and Nivolumab Plus Ipilimumab in Patients With Metastatic Esophagogastric Cancer. J. Clin. Oncol. 2018, 36 (Suppl. 15), 2836–2844. [Google Scholar] [CrossRef]
- Ji, J.; Shen, L.; Li, Z.; Xu, N.; Liu, T.; Chen, Y.; Li, C.; Gao, X.; Ji, K.; Mao, C.; et al. AK104 (PD-1/CTLA-4 bispecific) combined with chemotherapy as first-line therapy for advanced gastric (G) or gastroesophageal junction (GEJ) cancer: Updated results from a phase Ib study. J. Clin. Oncol. 2021, 39, 232. [Google Scholar] [CrossRef]
- Kelly, R.J.; Lee, J.; Bang, Y.-J.; Almhanna, K.; Blum Murphy, M.A.; Catenacci, D.V.T.; Chung, H.C.; Wainberg, Z.A.; Gibson, M.; Lee, K.W.; et al. Safety and efficacy of durvalumab in combination with tremelimumab, durvalumab monotherapy, and tremelimumab monotherapy in patients with advanced gastric cancer. J. Clin. Oncol. 2018, 36, 4031. [Google Scholar] [CrossRef]
- Takei, S.; Kawazoe, A.; Shitara, K. The New Era of Immunotherapy in Gastric Cancer. Cancers 2022, 14, 1054. [Google Scholar] [CrossRef] [PubMed]
- Markham, A. Margetuximab: First Approval. Drugs 2021, 81, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Catenacci, D.V.; Rosales, M.; Chung, H.C.; Yoon, H.H.; Shen, L.; Moehler, M.; Kang, Y.K. MAHOGANY: Margetuximab combination in HER2+ unresectable/metastatic gastric/gastroesophageal junction adenocarcinoma. Future Oncol. 2021, 17, 1155–1164. [Google Scholar] [CrossRef]
- Kawazoe, A.; Shitara, K.; Boku, N.; Yoshikawa, T.; Terashima, M. Current status of immunotherapy for advanced gastric cancer. Jpn. J. Clin. Oncol. 2021, 51, 20–27. [Google Scholar] [CrossRef]
- Mehta, R.; Kim, R.D.; Shah, N.; Carballido, E.M.; Kim, Y.; Imanirad, I.; Kim, D.W. A phase II study of TAS-102 in combination with ramucirumab in advanced, refractory gastric or gastroesophageal junction (GEJ) adenocarcinoma. J. Clin. Oncol. 2019, 37, TPS4149. [Google Scholar] [CrossRef]
- Shitara, K.; Doi, T.; Dvorkin, M.; Mansoor, W.; Arkenau, H.T.; Prokharau, A.; Alsina, M.; Ghidini, M.; Faustino, C.; Gorbunova, V.; et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018, 19 (Suppl. 15), 1437–1448. [Google Scholar] [CrossRef]
- Tabernero, J.; Shitara, K.; Zaanan, A.; Doi, T.; Lorenzen, S.; Van Cutsem, E.; Fornaro, L.; Catenacci, D.V.T.; Fougeray, R.; Moreno, S.R.; et al. Trifluridine/tipiracil versus placebo for third or later lines of treatment in metastatic gastric cancer: An exploratory subgroup analysis from the TAGS study. ESMO Open 2021, 6, 100200. [Google Scholar] [CrossRef]
- Fostea, R.M.; Arkenau, H.-T. Trifluridine/tipiracil in the treatment of gastric cancer. Future Oncol. 2022, 18, 1511–1517. [Google Scholar] [CrossRef]
- Bando, H.; Doi, T.; Muro, K.; Yasui, H.; Nishina, T.; Yamaguchi, K.; Takahashi, S.; Nomura, S.; Kuno, H.; Shitara, K.; et al. A multicenter phase II study of TAS-102 monotherapy in patients with pre-treated advanced gastric cancer (EPOC1201). Eur. J. Cancer 2016, 62, 46–53. [Google Scholar] [CrossRef][Green Version]
- Thiel, A.; Ristimäki, A. Targeted therapy in gastric cancer. Apmis 2015, 123, 365–372. [Google Scholar] [CrossRef]
- Shao, Z.; Pan, H.; Tu, S.; Zhang, J.; Yan, S.; Shao, A. HGF/c-Met Axis: The Advanced Development in Digestive System Cancer. Front. Cell Dev. Biol. 2020, 8, 801. [Google Scholar] [CrossRef] [PubMed]
- Iveson, T.; Donehower, R.C.; Davidenko, I.; Tjulandin, S.; Deptala, A.; Harrison, M.; Nirni, S.; Lakshmaiah, K.; Thomas, A.; Jiang, Y.; et al. Rilotumumab in combination with epirubicin, cisplatin, and capecitabine as first-line treatment for gastric or oesophagogastric junction adenocarcinoma: An open-label, dose de-escalation phase 1b study and a double-blind, randomised phase 2 study. Lancet Oncol. 2014, 15, 1007–1018. [Google Scholar] [CrossRef]
- Shah, M.A.; Cho, J.-Y.; Tan, I.B.; Tebbutt, N.C.; Yen, C.-J.; Kang, A.; Shames, D.S.; Bu, L.; Kang, Y.-K. A Randomized Phase II Study of FOLFOX With or Without the MET Inhibitor Onartuzumab in Advanced Adenocarcinoma of the Stomach and Gastroesophageal Junction. Oncologist 2016, 21, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Sakai, D.; Chung, H.C.; Oh, D.-Y.; Park, S.H.; Kadowaki, S.; Kim, Y.H.; Tsuji, A.; Komatsu, Y.; Kang, Y.-K.; Uenaka, K.; et al. A non-randomized, open-label, single-arm, Phase 2 study of emibetuzumab in Asian patients with MET diagnostic positive, advanced gastric cancer. Cancer Chemother. Pharmacol. 2017, 80, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.J.; Zhu, A.X.; Bauer, T.M.; Choueiri, T.K.; Drilon, A.; Voss, M.H.; Fuchs, C.S.; Abou-Alfa, G.K.; Wijayawardana, S.R.; Wang, X.A.; et al. A Phase Ib/II Study of Ramucirumab in Combination with Emibetuzumab in Patients with Advanced Cancer. Clin. Cancer Res. 2019, 25, 5202–5211. [Google Scholar] [CrossRef] [PubMed]
- Catenacci, D.V.T.; Tebbutt, N.C.; Davidenko, I.; Murad, A.M.; Al-Batran, S.-E.; Ilson, D.H.; Tjulandin, S.; Gotovkin, E.; Karaszewska, B.; Bondarenko, I.; et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1467–1482. [Google Scholar] [CrossRef]
- Shah, M.A.; Bang, Y.-J.; Lordick, F.; Alsina, M.; Chen, M.; Hack, S.P.; Bruey, J.M.; Smith, D.; Mccaffery, I.; Shames, D.S.; et al. Effect of Fluorouracil, Leucovorin, and Oxaliplatin With or Without Onartuzumab in HER2-Negative, MET-Positive Gastroesophageal Adenocarcinoma. JAMA Oncol. 2017, 3, 620. [Google Scholar] [CrossRef]
- Shah, M.A.; Wainberg, Z.A.; Catenacci, D.V.T.; Hochster, H.S.; Ford, J.; Kunz, P.; Lee, F.-C.; Kallender, H.; Cecchi, F.; Rabe, D.C.; et al. Phase II Study Evaluating 2 Dosing Schedules of Oral Foretinib (GSK1363089), cMET/VEGFR2 Inhibitor, in Patients with Metastatic Gastric Cancer. PLoS ONE 2013, 8, e54014. [Google Scholar] [CrossRef]
- Pant, S.; Patel, M.; Kurkjian, C.; Hemphill, B.; Flores, M.; Thompson, D.; Bendell, J. A Phase II Study of the c-Met Inhibitor Tivantinib in Combination with FOLFOX for the Treatment of Patients with Previously Untreated Metastatic Adenocarcinoma of the Distal Esophagus, Gastroesophageal Junction, or Stomach. Cancer Investig. 2017, 35, 463–472. [Google Scholar] [CrossRef]
- Lengyel, C.G.; Hussain, S.; Seeber, A.; Jamil Nidhamalddin, S.; Trapani, D.; Habeeb, B.S.; Elfaham, E.; Mazher, S.A.; Seid, F.; Khan, S.Z.; et al. FGFR Pathway Inhibition in Gastric Cancer: The Golden Era of an Old Target? Life 2022, 12, 81. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Enzinger, P.C.; Kang, Y.-K.; Yamaguchi, K.; Qin, S.; Lee, K.-W.; Oh, S.C.; Li, J.; Turk, H.M.; Teixeira, A.C.; et al. Randomized double-blind placebo-controlled phase 2 study of bemarituzumab combined with modified FOLFOX6 (mFOLFOX6) in first-line (1L) treatment of advanced gastric/gastroesophageal junction adenocarcinoma (FIGHT). J. Clin. Oncol. 2021, 39 (Suppl. 3), 160. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Bang, Y.J.; Mansoor, W.; Petty, R.D.; Chao, Y.; Cunningham, D.; Ferry, D.R.; Smith, N.R.; Frewer, P.; Ratnayake, J.; et al. A randomized, open-label study of the efficacy and safety of AZD4547 monotherapy versus paclitaxel for the treatment of advanced gastric adenocarcinoma with FGFR2 polysomy or gene amplification. Ann. Oncol. 2017, 28, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, K.; Huang, Y.; Xiong, H.; Su, J.; Chen, R.; Zou, Y. PARP inhibitors in gastric cancer: Beacon of hope. J. Exp. Clin. Cancer Res. 2021, 40, 211. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.-J.; Im, S.-A.; Lee, K.-W.; Cho, J.Y.; Song, E.-K.; Lee, K.H.; Kim, Y.H.; Park, J.O.; Chun, H.G.; Zang, D.Y.; et al. Randomized, Double-Blind Phase II Trial With Prospective Classification by ATM Protein Level to Evaluate the Efficacy and Tolerability of Olaparib Plus Paclitaxel in Patients With Recurrent or Metastatic Gastric Cancer. J. Clin. Oncol. 2015, 33, 3858–3865. [Google Scholar] [CrossRef]
- Bang, Y.J.; Xu, R.H.; Chin, K.; Lee, K.W.; Park, S.H.; Rha, S.Y.; Shen, L.; Qin, S.; Xu, N.; Im, S.A.; et al. Olaparib in combination with paclitaxel in patients with advanced gastric cancer who have progressed following first-line therapy (GOLD): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1637–1651. [Google Scholar] [CrossRef]
- Ciardiello, F.; Bang, Y.-J.; Bendell, J.C.; Cervantes, A.; Dvorkin, M.; Lopez, C.D.; Metges, J.-P.; Sanchez, A.; Calvo, M.; Strickland, A.; et al. PARALLEL 303: Phase 2 randomized study of pamiparib vs placebo as maintenance therapy in patients (pts) with inoperable locally advanced or metastatic gastric cancer that responded to platinum-based first-line (1L) chemotherapy. J. Clin. Oncol. 2021, 39 (Suppl. 15), 3109. [Google Scholar] [CrossRef]
- Shah, M.A.; Cunningham, D.; Metges, J.-P.; Van Cutsem, E.; Wainberg, Z.; Elboudwarej, E.; Lin, K.-W.; Turner, S.; Zavodovskaya, M.; Inzunza, D.; et al. Randomized, open-label, phase 2 study of andecaliximab plus nivolumab versus nivolumab alone in advanced gastric cancer identifies biomarkers associated with survival. J. ImmunoTherapy Cancer 2021, 9, e003580. [Google Scholar] [CrossRef]
- Shah, M.A.; Bodoky, G.; Starodub, A.; Cunningham, D.; Yip, D.; Wainberg, Z.A.; Bendell, J.; Thai, D.; He, J.; Bhargava, P.; et al. Phase III Study to Evaluate Efficacy and Safety of Andecaliximab With mFOLFOX6 as First-Line Treatment in Patients With Advanced Gastric or GEJ Adenocarcinoma (GAMMA-1). J. Clin. Oncol. 2021, 39, 990–1000. [Google Scholar] [CrossRef]
- Ohtsu, A.; Ajani, J.A.; Bai, Y.-X.; Bang, Y.-J.; Chung, H.-C.; Pan, H.-M.; Sahmoud, T.; Shen, L.; Yeh, K.-H.; Chin, K.; et al. Everolimus for Previously Treated Advanced Gastric Cancer: Results of the Randomized, Double-Blind, Phase III GRANITE-1 Study. J. Clin. Oncol. 2013, 31, 3935–3943. [Google Scholar] [CrossRef]
- Doi, T.; Muro, K.; Boku, N.; Yamada, Y.; Nishina, T.; Takiuchi, H.; Komatsu, Y.; Hamamoto, Y.; Ohno, N.; Fujita, Y.; et al. Multicenter Phase II Study of Everolimus in Patients With Previously Treated Metastatic Gastric Cancer. J. Clin. Oncol. 2010, 28, 1904–1910. [Google Scholar] [CrossRef]
- Hudler, P.; Komel, R. Clinical Implications of Molecular Heterogeneity of Gastric Cancer. In Gastric Cancer; InTech: London, UK, 2017. [Google Scholar] [CrossRef][Green Version]
- Miao, Z.-F.; Chen, H.; Wang, Z.-N.; Ji, J.-F.; Liang, H.; Xu, H.-M.; Wang, J. Progress and remaining challenges in comprehensive gastric cancer treatment. Holist. Integr. Oncol. 2022, 1, 4. [Google Scholar] [CrossRef]
| Drug | Trial | Phase | Method | mOS (p-Value) | mPFS (p-Value) | ORR (p-Value) |
|---|---|---|---|---|---|---|
| Matuzumab | MATRIX EG (first-line) [67] | II | Chemotherapy with or without matazumub | 9.4 M vs. 12.2 M (p = 0.945) | 4.8 M vs. 7.1 M (p = 0.678) | 31.0% vs. 58.0% (p = 0.994) |
| Cetuximab | DOCOX + C/second-line [68] | II | Chemotherapy (docetaxel + oxaliplatin) with or without cetuximab | 9.4 M vs. 8.5 M (p > 0.050) | 5.1 M vs. 4.7 M (p > 0.050) | 38.0% vs. 26.0% (p > 0.050) |
| Panitumumab | ATTAX3/second-line [69] | II | Chemotherapy with or without panitumumab | 10.0 M vs. 11.7 M (p > 0.050) | 6.0 M vs. 6.9 M (p > 0.050) | 57.9% vs. 48.7% (p > 0.050) |
| Panitumumab | first-line [70] | II | Single-arm: Chemotherapy with panitumumab | 11.3 M | 6.9 M | 35.0% |
| Panitumumab | MEGA/first-line [71] | II | Chemotherapy with or without either panitumumab or rilotumumab | 8.3 M (P) vs. 11.5 M (R) vs. 13.1 M (C) | 4-month PFS rate:57% (P) vs. 61% (R) vs. 71% (C) | 43% (P) vs. 49% (R) vs. 52% (C) |
| Nimotuzumab | NCS/first-line [72] | II | Chemotherapy with or without nimotuzumab | 10.2 M vs. 14.3 M (p = 0.062) | 4.8 M vs. 7.2 M (p = 0.011) | 54.8% vs. 58.1% (p = 0.798) |
| Erlotinib | first-line [73] | II | Single arm: Chemotherapy with erlotinib | 11.0 M | 5.5 M | 51.5% |
| Panitumumab | REAL3/first-line [74] | III | Chemotherapy with or without Panitumumab | 8.8 M vs. 11.3 M (p = 0.013) | 6.0 M vs. 7.4 M (p = 0.068) | 46% vs. 42% (p = 0.42) |
| Cetuximab | EXPAND/first-line [75] | III | Chemotherapy with or without cetuximab | 9.4 M vs. 10.7 M (p = 0.95) | 4.4 M vs. 5.6 M (p = 0.032) | 30% vs. 29% (p = 0.77) |
| Drug Class | Drug | Trial | Phase | Method | mOS (p-Value) | mPFS (p-Value) | ORR (p-Value) |
|---|---|---|---|---|---|---|---|
| Monoclonal antibody | Trastuzumab | WJOG7112G (T-ACT)/second-line [76] | II | Chemotherapy (paclitaxel) with or without trastuzumab | 10.2 M vs. 9.95 M (p = 0.20) | 3.68 M vs. 3.19 M (p = 0.33) | 33.3% vs. 31.6% (p = 1.00) |
| Trastuzumab | first-line [77] | II | Single arm: Chemotherapy (S-1 + cisplatin) with trastuzumab | 14.6 M | 7.4 M | 53.3% | |
| Trastuzumab | first-line [78] | II | Single arm: chemotherapy (CapeOx) with trastuzumab | 21.0 M | 9.8 M | 67% | |
| Trastuzumab | first-line [79] | II | Single arm: chemotherapy (docetaxel + capecitiabine) | 20.9 M | 8.1 M | 67.8% | |
| Trastuzumab | first-line [80] | II | Single arm: chemotherapy (S-1 + oxaliplatin) with trastuzumab | 18.1 M | 8.8 M | 70.7% | |
| Margetuximab | second-line [81] | Ib/II | Margetuximab + pembrolizumab | 12.5 M | 2.73 M | 18.48% | |
| Trastuzumab | ToGA/first-line [82] | III | Chemotherapy (cisplatin + 5-FU) with or without trastuzumab | 13.8 M vs. 11.1 M (p = 0.0046) | 6.7 M vs. 5.5 M (p = 0.0002) | 47% vs. 35% (p = 0.0017) | |
| Trastuzumab | HELOISE/first-line [83] | IIIb | Chemotherapy (capecitabine + cisplatin) with standard-dose vs. high-dose trastuzumab | 12.5 M vs. 10.6 M (p = 0.2401) | 5.7 M vs. 5.6 M (p = 0.8222) | 58.9% vs. 56.9% (p = 0.76) | |
| Trastuzumab/Pertuzumab | JACOB/first-line [84] | III | Trastuzumab + pertuzumab + chemotherapy (capecitabine, cisplatin, or 5-FU) vs. trastuzumab + placebo + chemotherapy | 17.5 M vs. 14.2 M (p = 0.057) | 8.5 M vs. 7.0 M (p = 0.0001) | 56.7% vs. 48.3% (p = 0.026) | |
| ADCs | Trastuzumab deruxtexan | DESTINY-Gastric01/second-line [85] | II | Chemotherapy or trastuzumab deruxtecan | 12.5 M vs. 8.4 M (p = 0.01) | 5.6 M vs. 3.5 M (p = N/A) | 51% vs. 14% (p < 0.001) |
| RC48-ADC | second-line [86] | II | Single arm: RC48 | 7.9 M | 4.1 M | 18.1% | |
| Trastuzumab emtansine (T-DM1) | GATSBY/second-line [87] | III | T-DM1 vs. taxane | 7.9 M vs. 8.6 M (p = 0.86) | 2.7 M vs. 2.9 M (p = 0.31) | 20.6% vs. 19.6% (p = 0.846) | |
| TKIs | Dacomitinib | second-line [88] | II | Single arm: Dacomitinib | 7.1 M | 2.1 M | 7.4% |
| Lapatinib | first-line [89] | II | Single arm: lapatinib | 4.8 M | 1.9 M | 11% | |
| Lapatinib | LoGIC/first-line [90] | III | Chemotherapy with or without lapatinib | 12.2 M vs. 10.5 M (p = 0.91) | 6.0 M vs. 5.4 M (p = 0.0381) | 53% vs. 39% (p = 0.0031) | |
| Lapatinib | TyTAN/second-line [91] | III | Paclitaxel with or without lapatinib | 11.0 M vs. 8.9 M (p = 0.1044) | 5.4 M vs. 4.4 M (p = 0.2441) | 27% vs. 9% (p < 0.001) |
| Drug Class | Drug | Trial | Phase | Method | mOS (p-Value) | mPFS (p-Value) | ORR (p-Value) |
|---|---|---|---|---|---|---|---|
| Monoclonal antibody | Bevacizumab | first-line [107] | II | Single arm: Bevacizumab + docetaxel + 5-FU | 16.8 M | 6-month PFS rate: 79% | 67% |
| Bevacizumab | first-line [108] | II | Single arm: Bevacizumab + docetaxel + cisplatin + irinotecan | N/A | N/A | 69% | |
| Bevacizumab | first-line [109] | II | Single arm: Bevacizumab + docetaxel + oxaliplatin | 11.1 M | 6.6 M | 42% | |
| Bevacizumab | first-line [110] | II | Single arm: Bevacizumab + irinotecan + cisplatin | 12.3 M | 8.3 M | 65% | |
| Bevacizumab | first-line [111] | II | Single arm: Bevacizumab + capecitabine + oxaliplatin | 10.8 M | 7.2 M | 51.4% | |
| Bevacizumab | first-line [112] | II | Single arm: Bevacizumab + carboplatin + capecitabine | 14.3 M | 8.5 M | 51% | |
| Bevacizumab | first-line [113] | Ib/II | Single arm: bevacizumab + docetaxel + capecitabine + cisplatin | 13.9 M | 7.6 M | 54% | |
| Bevacizumab | first-line [114] | II | Single arm: Bevacizumab + docetaxel + oxaliplatin + capecitabine + trastuzumab | 17.9 M | 10.8 M | 74% | |
| Bevacizumab | first-line [115] | II | Single arm: Bevacizumab + docetaxel + oxaliplatin + capecitabine | 12.0 M | 8.3 M | 70% | |
| Bevacizumab | GASTRIC-3/first-line [116] | II | Single arm: oxaliplatin + irinotecan → bevacizumab + docetaxel | 11.0 M | 7.0 M | 51.5% | |
| Bevacizumab | first-line [117] | II | Single arm: mFOLFOX5 + Bevacizumab | 14.7 M | 7.8 M | 56.4% | |
| Ramucirumab | first-line [118] | II | Chemotherapy (mFOLFOX6) with or without ramucirumab | 11.7 M vs. 11.5 M (p = 0.712) | 6.4 M vs. 6.7 M (HR = 0.98, p = 0.886) | 45.2% vs. 46.4% (p = 0.830) | |
| Ramucirumab | RAINSTORM/first-line [119] | II | Chemotherapy (S-1 + oxaliplatin) with or without ramucirumab | N/A | 6.34 M vs. 6.74 M (p = 0.698) | 58% vs. 50% (p = 0.402) | |
| Ramucirumab | REGARD/second-line [120] | II | Single arm: Ramucirumab | 8.6 M | 6.6 weeks | 0% | |
| Bevacizumab | AVAGAST/first-line [121] | III | Chemotherapy (5-FU + cisplatin + capecitabine) with or without bevacizumab | 12.1 M vs. 10.1 M (p = 0.1002) | 6.7 M vs. 5.3 M (p = 0.0037) | 46.0% vs. 37.4% (p = 0.0315) | |
| Bevacizumab | AVATAR/first-line [122] | III | Chemotherapy (capecitabine + cisplatin) with or without bevacizumab | 10.5 M vs. 11.4 M (p = 0.5567) | 6.3 M vs. 6.0 M (p = 0.4709) | 40.7% vs. 33.7% (p > 0.05) | |
| Ramucirumab | RAINFALL/first-line [123] | III | Chemotherapy (cisplatin + 5-FU/capecitabine) with or without ramucirumab | 11.2 M vs. 10.7 M (p = 0.6757) | 5.7 M vs. 5.4 M (p = 0.0106) | 41% vs. 36% (p = 0.17) | |
| Ramucirumab | REGARD/second-line [124] | III | Ramucirumab vs. placebo | 5.2 M vs. 3.8 M (p = 0.047) | 2.1 M vs. 1.3 M (p < 0.0001) | 3% vs. 3% (p > 0.05) | |
| Ramucirumab | RAINBOW/second-line [125] | III | Chemotherapy (paclitaxel) with or without ramucirumab | 9.6 M vs. 7.4 M (p = 0.017) | 4.4 M vs. 2.9 M (p < 0.0001) | 28% vs. 16% (p = 0.0001) | |
| TKI | Sorafenib | ECOG5203/first-line [126] | II | Chemotherapy (docetaxel + cisplatin) with or without sorafenib | 13.6 M | 5.8 M | 41% |
| Sorafenib | first-line [127] | II | Chemotherapy (capecitabine + cisplatin) with or without sorafenib | 11.7 M vs. 10.8 M (p = 0.661) | 5.6 M vs. 5.3 M (p = 0.609) | 54% vs. 52% (p = 0.826) | |
| Sorafenib | GEMCAD/second-line [128] | II | Single arm: Chemotherapy (oxaliplatin) with sorafenib | 6.5 M | 3 M | N/A | |
| Sorafenib | ≥second-line [129] | II | Single arm: sorafenib | 9.7 M | 3.6 M | 3% | |
| Sunitinib | second-line [130] | II | Single arm: sunitinib | 6.8 M | 2.3 M | 2.6% | |
| Sunitinib | second-line [131] | II | Chemotherapy (docetaxel) with or without sunitinib | 8.0 M vs. 6.6 M (p = 0.802) | 3.9 M vs. 2.6 M (p = 0.206) | 41.1% vs. 14.3% (p = 0.002) | |
| Sunitinib | ≥second-line [132] | II | Chemotherapy (Na-FOLFIRI) with or without sunitinib | 10.4 M vs. 8.9 M (p = 0.21) | 3.5 M vs. 3.3 M (p = 0.66) | 20% vs. 29% (p = N/A) | |
| Sunitinib | ≥second-line [133] | II | Single arm: sunitinib | 5.81 M | 1.28 M | 3.9% | |
| Telatinib | TEL0805/first-line [134] | II | Single arm: chemotherapy (capecitabine + cisplatin) with telatinib | N/A | 4.7 M | 67% | |
| Orantinib | first-line [135] | II | Chemotherapy (S-1 + cisplatin) with or without oratinib | 16.6 M vs. 15.5 M (p = 0.213) | 6.9 M vs. 7.1 M (p = 0.424) | 62.2% vs. 56.5% (p = 0.671) | |
| Apatinib | ≥third-line [136] | II | Placebo vs. apatinib (850 mg) vs. apatinib (425 mg bid) | 2.50 M vs. 4.83 M vs. 4.27 M (p < 0.05) | 1.40 M vs. 3.67 M vs. 3.20 M (p < 0.001) | 0% vs. 6.38% vs. 13.04% (p = N/A) | |
| Pazopanib | first-line [137] | II | Single arm: chemotherapy (CapeOx) with pazopanib | 10.5 M | 6.5 M | 62.4% | |
| Pazopanib | first-line [138] | II | Chemotherapy (5-FU + oxaliplatin) with or without pazopanib | 10.1 M vs. 7.0 M (p = N/A) | 5.1 M vs. 3.9 M (p = N/A) | N/A | |
| Regorafenib | first-line [139] | II | Single arm: chemotherapy (mFOLFOX6) with regorafenib | 14.2 M | 7.1 M | 54% | |
| Regorafenib | ≥second-line [140] | II | Regorafenib vs. placebo | 5.8 M vs. 4.5 M (p = 0.147) | 2.6 M vs. 0.9 M (p < 0.001) | N/A | |
| Fruquintinib | second-line [141] | I/II | Single arm: fruquintinib with paclitaxel | 8.5 M | 4.0 M | 25.9% | |
| Lenvatinib | ≥first-line [142] | II | Single arm: Lenvatinib + pembrolizumab | N/A | 6.9 M | 69% | |
| Lenvatinib | ≥third-line [143] | II | Single arm: Lenvatinib + pembrolizumab | 5.9 M | 2.5 M | 10% | |
| Apatinib | ≥third-line [144] | III | Apatinib vs. placebo | 6.5 M vs. 4.7 M (p = 0.0149) | 2.6 M vs. 1.8 M (p < 0.001) | 2.84% vs. 0.00% (p = 0.1695) | |
| Recombinant fusion protein | Ziv-aflibercept | first-line [145] | II | Chemotherapy (mFOLFOX6) with or without ziv-aflibercept | 14.5 M vs. 18.8 M (p = 0.45) | 9.7 M vs. 7.4 M (p = 0.72) | 61.1% vs. 75.0% (p = 0.53) |
| Drug Class | Drug | Trial | Phase | Method | mOS (p-Value) | mPFS (p-Value) | ORR (p-Value) |
|---|---|---|---|---|---|---|---|
| Anti-PD-1 | Pembrolizumab | KEYNOTE-059/≥second-line [154] | II | Single arm: pembrolizumab | 2.0 M | 5.6 M | PD-L1-positive tumor: 15.5% PD-L1-negative tumor: 6.4% |
| Pembrolizumab | PANTHERA/first-line [155] | Ib/II | Single arm: Chemotherapy (capecitabine + cisplatin) + pembrolizumab + trastuzumab | 19.3 M | 8.6 M | 76.7% | |
| Pembrolizumab | first-line [156] | II | Single arm: pembrolizumab + trastuzumab | 27.2 M | 13.0 M | 91% | |
| Pembrolizumab | EPOC1706/≥first-line [142] | II | Single arm:lenvatinib + pembrolizumab | NR | 6.9 M | 69% | |
| Pembrolizumab | ≥ third-line [143] | II | Single arm: Lenvatinib + pembrolizumab | 5.9 M | 2.5 M | 10% | |
| Pembrolizumab | second-line [81] | Ib/II | Margetuximab + pembrolizumab | 12.5 M | 2.73 M | 18.48% | |
| Pembrolizumab | ≥second-line [157] | Ib/II | Single arm: DKN-01 + pembrolizumab | DKK1 high: 7.3 M DKK1 low: 4.0 M | DKK1 high: 5.1 M DKK1 low: 1.4 M | DKK1 high: 50% DKK1 low: 0% | |
| Nivolumab | second-line [158] | Ib/II | Single arm: paclitaxel + nivolumab + ramucirumab | 13.1 M | 5.1 M | 37.2% | |
| Nivolumab | NivoRAM/second-line [159] | I/II | Single arm: nivolumab + ramucirumab | 9.0 M | 2.9 M | 26.7% | |
| Camrelizumab | first-line [160] | II | Single arm: CAPOX + camrelizumab → camrelizumab + apatinib | 14.9 M | 6.8 M | 58.3% | |
| Sintilimab | first-line [161] | II | Single arm: Chemotherapy (CAPOX) with sintilimab | N/A | N/A | 85.0% | |
| Toripalimab | first-line [162] | Ib/II | Toripalimab alone vs. chemotherapy (CAPOX) with toripalimab | 4.8 M vs. NR | 1.9 M vs. 5.8 M | 12.1% vs. 66.7% | |
| Tislelizumab | first-line [163] | II | Single arm: chemotherapy (CAPOX) + tislelizumab | NR | 6.1 M | 46.7% | |
| Pembrolizumab | KEYNOTE-061/second-line [164] | III | Pembrolizumab vs. paclitaxel | 9.1 M vs. 8.3 M (p = 0.0421) | 1.5 M vs. 4.1 M (p = N/A) | N/A | |
| Pembrolizumab | KEYNOTE-062/first-line [165] | IIIs | Pembrolizumab vs. chemotherapy (cisplatin + 5-FU/capecitabine) + pembrolizumab vs. chemotherapy with placebo | CPS ≥ 1: 10.6 M vs. 12.5 M vs. 11.1 M CPS ≥ 10: 17.4 M vs. 12.3 M vs. 10.8 M | CPS ≥ 1: 2.0 M vs. 6.9 M vs. 6.4 M CPS ≥ 10: 2.9 M vs. N/A vs. 6.1 M | CPS ≥ 1: 15% vs. 49% vs. 37% CPS ≥ 10: 25% vs. 53% vs. 38% | |
| Pembrolizumab | KEYNOTE-811/first-line [166] | III | Trastuzumab + chemotherapy (CAPOX/5-FU + cisplatin) with or without pembrolizumab | N/A | N/A | 74.4% vs. 51.9% (p = 0. 00006) | |
| Pembrolizumab | LEAP-005/≥third-line [143] | II | Single arm: Lenvatinib + pembrolizumab | 5.9 M | 2.5 M | 10% | |
| Nivolumab | ATTRACTION-2/≥second-line [167] | III | Nivolumab vs. placebo | 5.3 M vs. 4.1 M (p < 0.0001) | 1.61 M vs. 1.45 M (p < 0.0001) | 11.2% vs. 0% | |
| Nivolumab | CheckMate-649/first-line [168] | III | Nivolumab + chemotherapy (CAPOX or FOLFOX) vs. chemotherapy alone | 13.8 M vs. 11.6 M (p < 0.0002) | 7.7 M vs. 6.9 M (p = N/A) | 60% vs. 45% | |
| Nivolumab | CheckMate-577/adjuvant [169] | III | Nivolumab vs. placebo | DFS: 22.4 M vs. 11.0 M (p < 0.001) | N/A | N/A | |
| Anti-PD-L1 | Avelumab | JAVELIN Gastric 100/first-line [170] | III | Chemotherapy (5-FU + oxaliplatin) with or without avelumab | 10.4 M vs. 10.9 M (p = 0.1779) | 3.2 M vs. 4.4 M (p = N/A) | 13.3% vs. 14.4% (p = N/A) |
| Anti-CTLA-4 | Ipilimumab | first-line [171] | II | Chemotherapy (5-FU + platinum) with or without ipilimumab | 12.7 vs. 12.1 (p = N/A) | 2.7 M vs. 4.9 M (p = 0.034) | 1.8% vs. 7.0% (p = N/A) |
| Anti-PD-1/CTLA-4 | Nivolumab, ipilimumab | CheckMate-032/≥second-line [172] | I/II | Nivolumab (3 mg/kg) vs nivolumab (1 mg/kg) with ipilimumab (3 mg/kg) vs nivolumab (3 mg/kg) with ipilimumab (1 mg/kg) | 6.2 M vs. 6.9 M vs. 4.8 M | 1.4 M vs. 1.4 M vs. 1.6 M | 12% vs. 24% vs. 8% |
| Cadonilimab | AK104/first-line [173] | Ib/II | Single arm: Chemotherapy (CAPOX) + cadonilimab | 17.41 M | 7.10 M | 65.9% | |
| Anti-PD-L1/CTLA-4 | Durvalumab, tremelimumab | ≥second-line [174] | Ib/II | second-line durvalumab with tremelimumab vs. third-line durvalumab with tremelimumab vs. second-line durvalumab alone | 9.2 M vs. 10.6 M vs. 3.2 M | 1.8 M vs. 1.8 M vs. 1.6 M | 11.1% vs. 12.0% vs. 8.3% |
| Drug | Trial | Phase | Method | mOS (p-Value) | mPFS (p-Value) | ORR (p-Value) |
|---|---|---|---|---|---|---|
| TAS-102 (Trifluridine/tipiracil) | EPOC1201/≥ second-line [183] | II | Single arm: TAS-102 (trifluridine/tipiracil) | 8.7 M | 2.9 M | 3.4% |
| ≥second-line [179] | II | Single arm: TAS-102 + ramucirumab | 6.2 M | 4.9 M | N/A | |
| TAGS/≥ second-line [180] | III | Trifluridine/tipiracil vs. placebo | 5.7 M vs. 3.6 M (p = 0.00058) | 2.0 M vs. 1.8 M (p < 0.0001) | 4% vs. 2% (p = 0.28) | |
| TAGS/third-line [181] | III | Trifluridine/tipiracil vs. placebo | 6.8 M vs. 3.2 M (p = 0.0318) | 3.1 M vs. 1.9 M (p = 0.0004) | N/A | |
| TAGS/≥ fourth-line [181] | III | Trifluridine/tipiracil vs. placebo | 5.2 M vs. 3.7 M (p = 0.0192) | 1.9 M vs. 1.8 M (p < 0.0001) | N/A |
| Drug Class | Drug | Trial | Phase | Method | mOS (p-Value) | mPFS (p-Value) | ORR (p-Value) |
|---|---|---|---|---|---|---|---|
| Monoclonal antibody | Rilotumumab | first-line [186] | II | Chemotherapy (epirubicin + cisplatin + capecitabine) with either rilotumumab 15 mg/kg, rilotumumab 7.5 mg/kg, or placebo | N/A | 5.1 M vs. 6.8 M 4.2 M | N/A |
| Rilotumumab | MEGA/first-line [71] | II | Chemotherapy with or without either panitumumab or rilotumumab | 8.3 M (P) vs. 11.5 M (R) vs. 13.1 M (C) | 4-month PFS rate: 57% (P) vs. 61% (R) vs. 71% (C) | 43% (P) vs. 49% (R) vs. 52% (C) | |
| Onartuzumab | YO28252/first-line [187] | II | Chemotherapy (mFOLFOX6) with or without onartuzumab | 10.6 M vs. 11.3 M (p = 0.83) | 5.95 M vs. 6.80 M (p = 0.45) | 60.5% vs. 57.1% | |
| Emibetuzumab | ≥third-line [188] | II | Single arm: emibetuzumab | 3.9 M | 1.9 M | N/A | |
| Emibetuzumab | ≥first-line [189] | Ib/II | Single arm: ramuricumab + emibetuzumab | N/A | 1.6 M | 6% | |
| Rilotumumab | RILOMET-1/first-line [190] | III | Chemotherapy (epirubicin + cisplatin + capecitabine) with or without rilotumumab | 8.8 M vs. 10.7 M (p = 0.003) | 5.6 M vs. 6.0 M (p = 0.016) | 29.8% vs. 44.6% (p = 0.0005) | |
| Onartuzumab | METGastric/first-line [191] | III | Chemotherapy (mFOLFOX6) with or without onartuzumab | 11.0 M vs. 11.3 M (p = 0.24) | 6.7 M vs. 6.8 M (p = 0.43) | 46.1% vs. 40.6% (p = 0.25) | |
| TKIs | Foretinib | ≥first-line [192] | II | Single arm: foretinib intermittent dosing vs. daily dosing | Intermittent: 7.4 M Daily: 4.3 M | Intermittent: 1.6 M Daily: 1.8 M | 0% |
| Tivantinib | first-line [193] | II | Single arm: chemotherapy (FOLFOX) with tinvantinib | 9.6 M | 6.1 M | 38% |
| Drug Class | Drug | Trial | Phase | Method | mOS (p-Value) | mPFS (p-Value) | ORR (p-Value) |
|---|---|---|---|---|---|---|---|
| Monoclonal antibody | Bemarituzumab | FIGHT/first-line [195] | II | Chemotherapy (mFOLFOX6) with or without bemarituzumab | NR vs. 12.9 M (p = 0.03) | 9.5 M vs. 7.4 M (p = 0.07) | 53% vs. 40% |
| TKIs | AZD4547 | SHINE/second-line [196] | II | AZD4547 vs. chemotherapy (paclitaxel) | 5.5 M vs. 6.6 M (p = 0.82) | 1.8 M vs. 3.5 M (p = 0.96) | 2.6% vs. 23.3% (p = 0.99) |
| Drug | Trial | Phase | Method | mOS (p-Value) | mPFS (p-Value) | ORR (p-Value) |
|---|---|---|---|---|---|---|
| Pamiparib | PARALLEL 303/>first-line [200] | II | Pamiparib vs. placebo | 10.2 M vs. 12.0 M (p = N/A) | 3.7 M vs. 2.1 M (p = 0.14) | 7.7% vs. 6.3% |
| Olaparib | >first-line [198] | II | Chemotherapy (paclitaxel) with or without olaparib | 13.1 M vs. 8.3 M (p = 0.005) | 3.91 M vs. 3.55 M (p = 0.131) | 26.4% vs. 19.1% (p = 0.162) |
| GOLD/>first-line [199] | III | Chemotherapy (paclitaxel) with or without olaparib | 8.8 M vs. 6.9 M (p = 0.026) | 3.7 M vs. 3.2 M (p = 0.064) | 24% vs. 28% (p = 0.055) |
| Drug Class | Drug | Trial | Phase | Method | mOS (p-Value) | mPFS (p-Value) | ORR (p-Value) |
|---|---|---|---|---|---|---|---|
| ICI + monoclonal antibody | Andecaliximab | ≥first-line [201] | II | Andecaliximab + nivolumumab vs. nivolumumab alone | 7.1 M vs. 5.9 M (p = 0.23) | N/A (p = 0.10) | 9.7% vs. 6.9% (p = 0.8) |
| Monoclonal antibody | Andecaliximab | GAMMA-1/first-line [202] | III | Chemotherapy (mFOLFOX6) with or without andecaliximab | 12.5 M vs. 11.8 M (p = 0.56) | 7.5 M vs. 7.1 M (p = 0.10) | 50.5% vs. 41.1% |
| Drug | Trial | Phase | Method | mOS (p-Value) | mPFS (p-Value) | ORR (p-Value) |
|---|---|---|---|---|---|---|
| Everolimus | >first-line [204] | II | Single arm: everolimus | 10.1 M | 2.7 M | N/A |
| Everolimus | GRANITE-1/>first-line [203] | III | Everolimus vs. placebo | 5.4 M vs. 4.3 M (p = 0.124) | 1.7 M vs. 1.4 M (p = N/A) | 4.5% vs. 2.1% (p = N/A) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haque, E.; Esmail, A.; Muhsen, I.; Salah, H.; Abdelrahim, M. Recent Trends and Advancements in the Diagnosis and Management of Gastric Cancer. Cancers 2022, 14, 5615. https://doi.org/10.3390/cancers14225615
Haque E, Esmail A, Muhsen I, Salah H, Abdelrahim M. Recent Trends and Advancements in the Diagnosis and Management of Gastric Cancer. Cancers. 2022; 14(22):5615. https://doi.org/10.3390/cancers14225615
Chicago/Turabian StyleHaque, Emaan, Abdullah Esmail, Ibrahim Muhsen, Haneen Salah, and Maen Abdelrahim. 2022. "Recent Trends and Advancements in the Diagnosis and Management of Gastric Cancer" Cancers 14, no. 22: 5615. https://doi.org/10.3390/cancers14225615
APA StyleHaque, E., Esmail, A., Muhsen, I., Salah, H., & Abdelrahim, M. (2022). Recent Trends and Advancements in the Diagnosis and Management of Gastric Cancer. Cancers, 14(22), 5615. https://doi.org/10.3390/cancers14225615








