Low CD8 T Cell Counts Predict Benefit from Hypoxia-Modifying Therapy in Muscle-Invasive Bladder Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. BCON Cohort
2.2. Multiplex Staining Protocol
2.3. Multispectral Scanning and Unmixing
2.4. Data Analysis
2.5. Statistical Methods
3. Results
3.1. Study Cohort
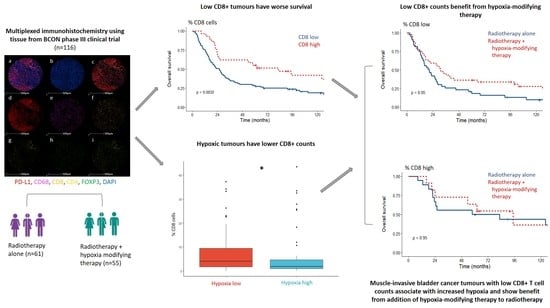
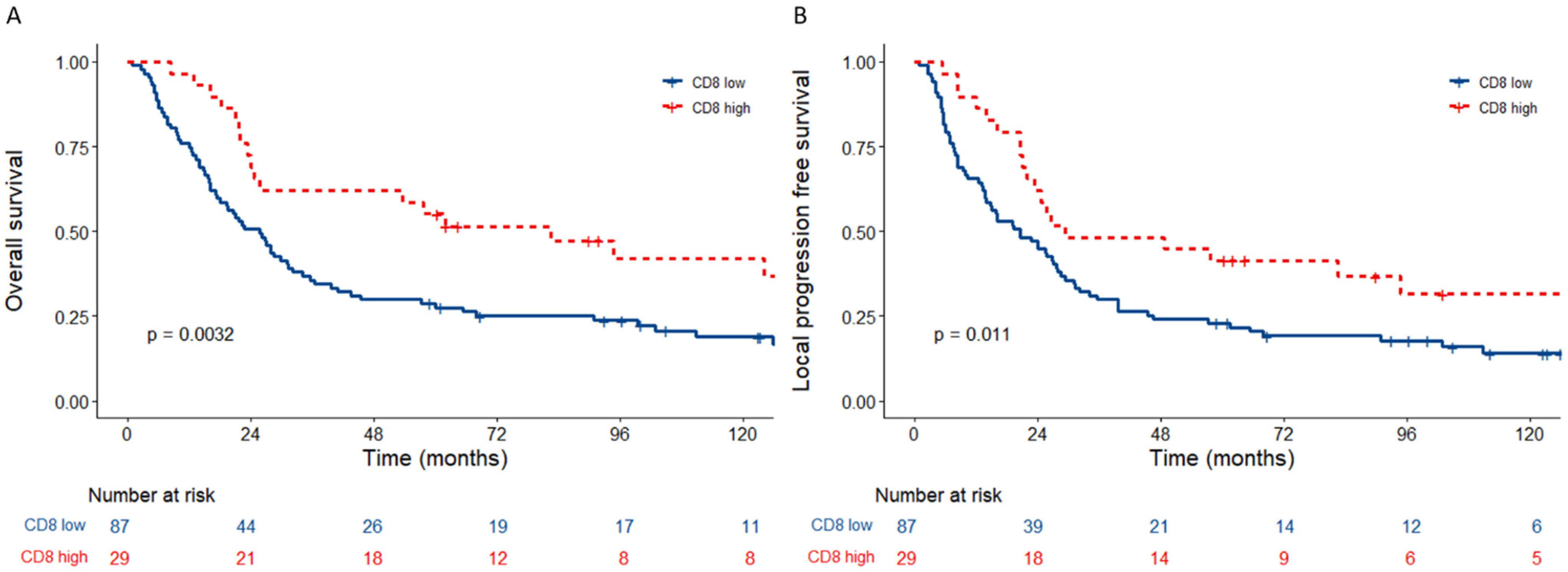
3.2. Tumours with Low Tumour CD8+ Cells Associate with a Poor Prognosis
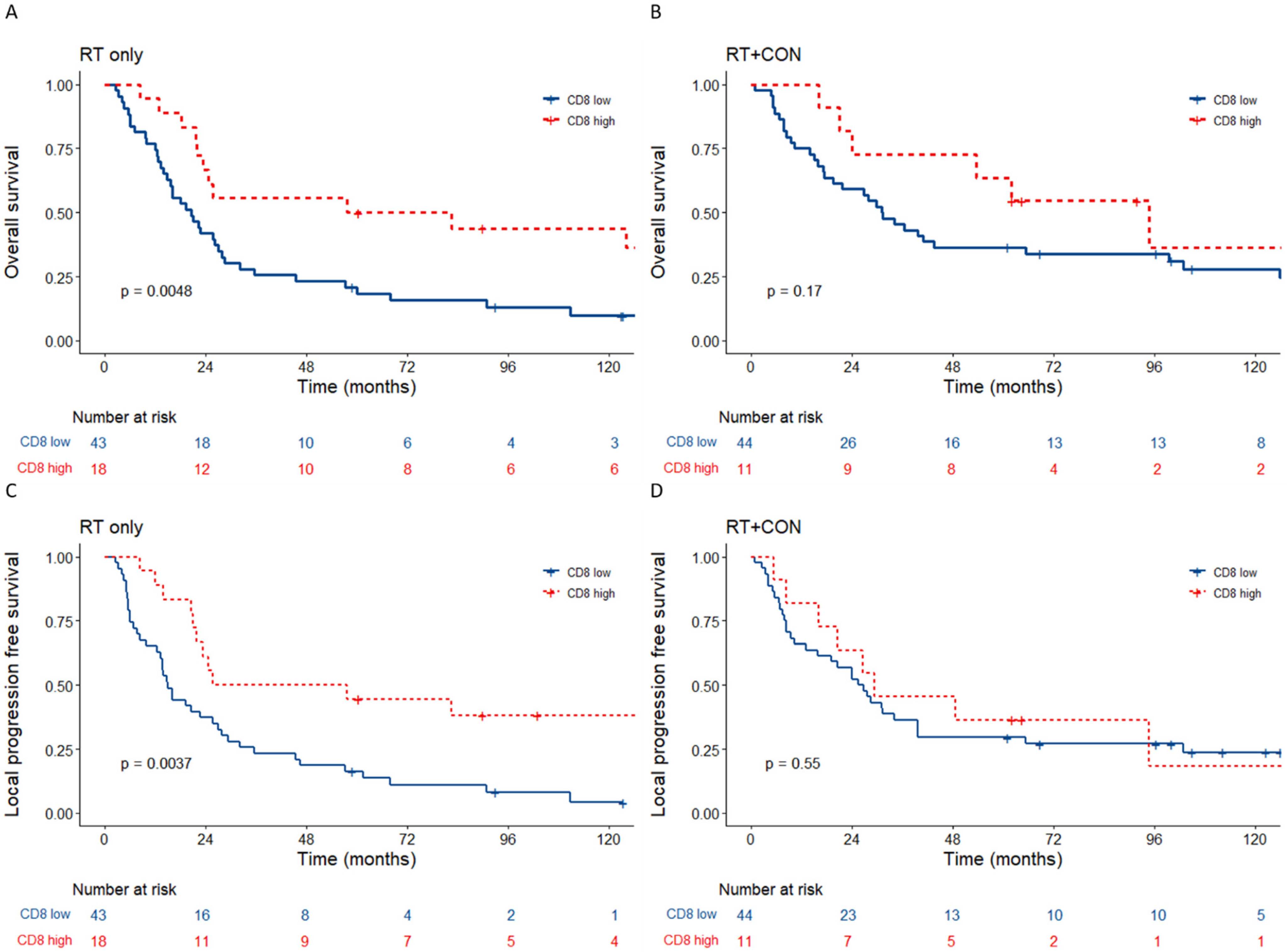
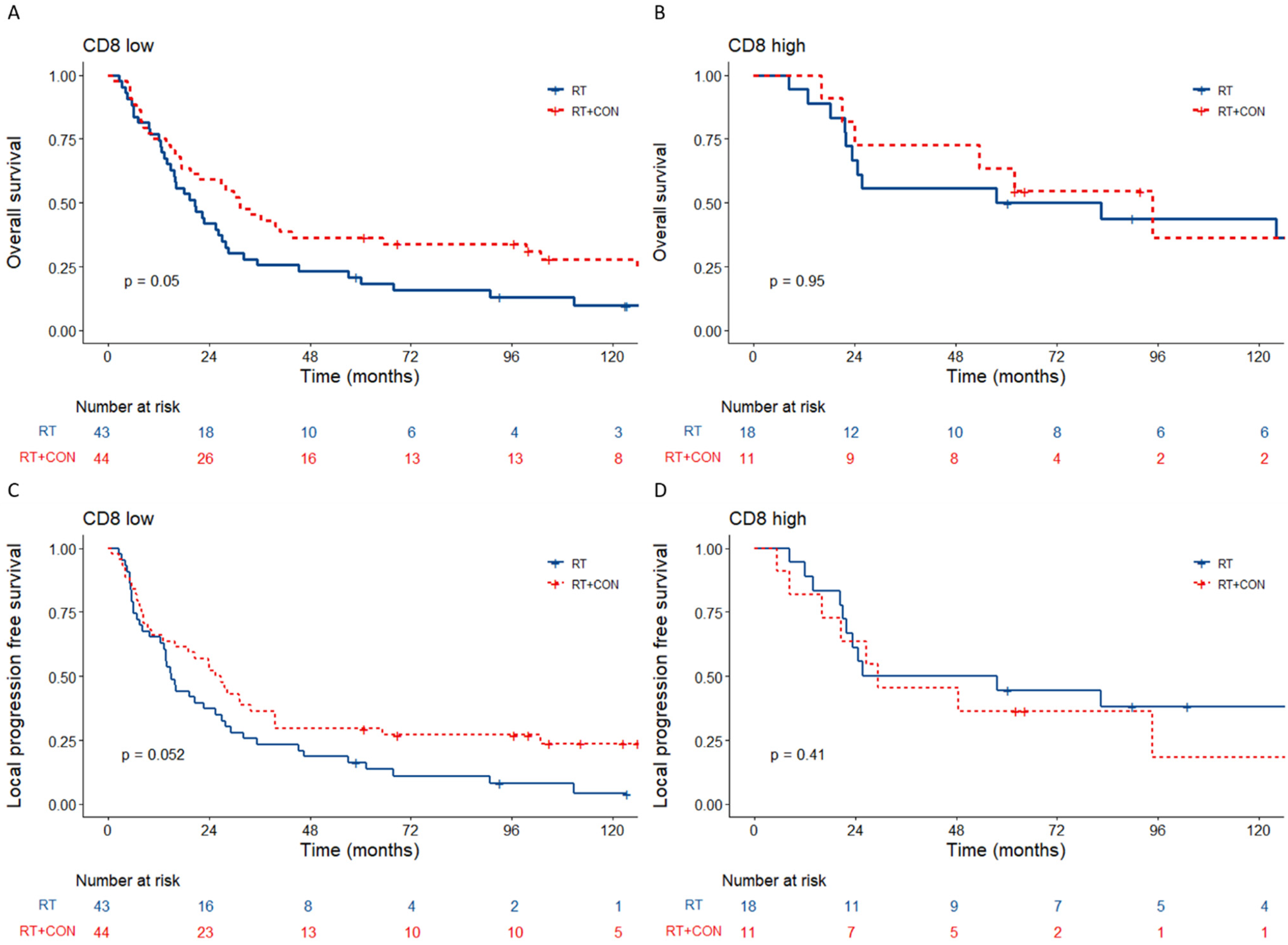
3.3. Low Tumour CD8+ Cell Counts Predict Benefit from Hypoxia Modification
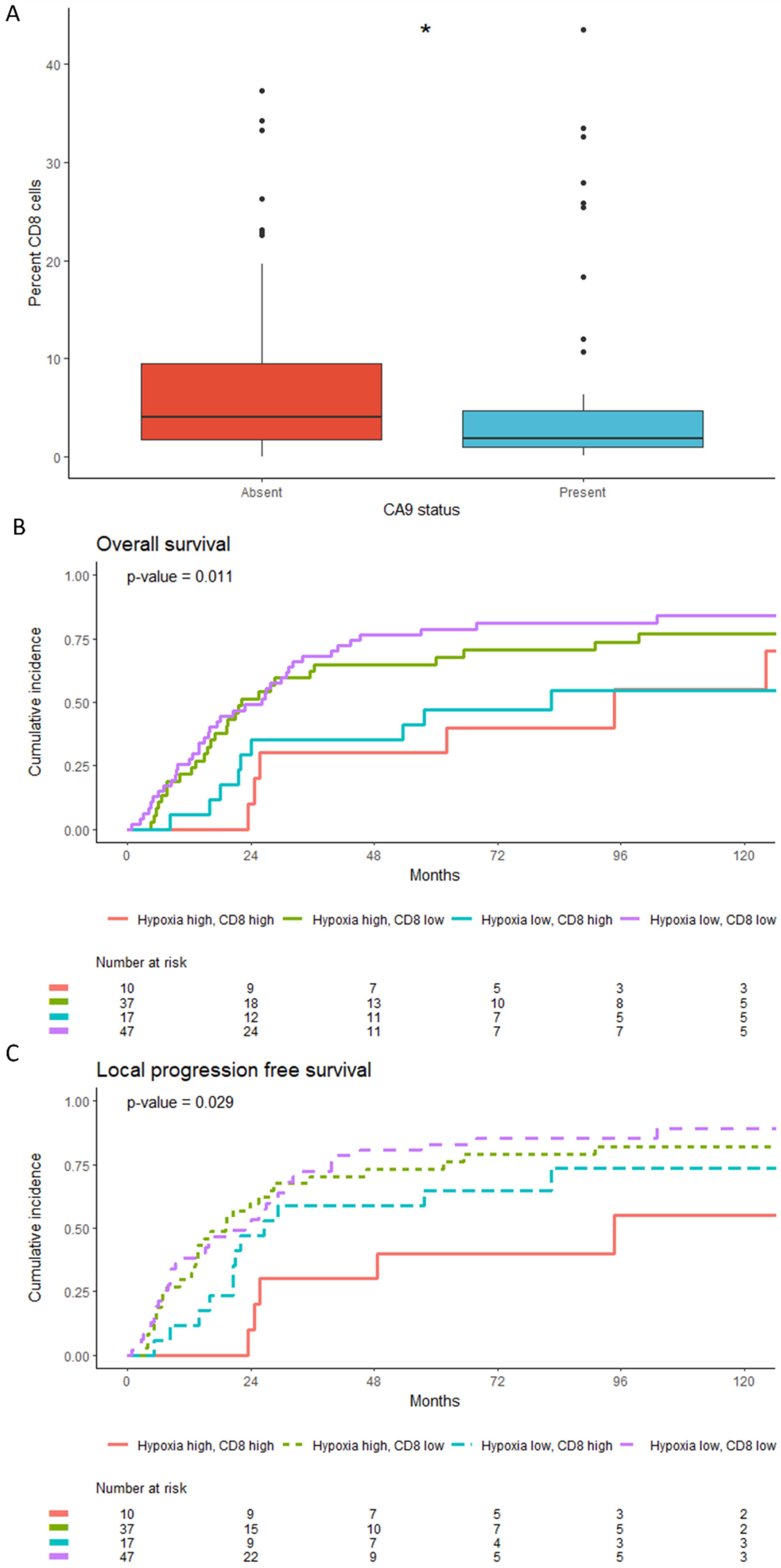
3.4. Low CD8+T Cell Counts Associate with CA9 Positivity but Retain Independent Prognostic Significance
3.5. Tumours with Low CD8+ Counts Are More Likely to Have a Luminal Molecular Subtype
3.6. Exploratory Analyses with PD-L1, Macrophages and Other T Cell Types
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.B.F. Global Cancer Observatory: Cancer Today. Available online: https://gco.iarc.fr/today (accessed on 27 May 2022).
- Cancer Research UK Cancer Survival Statistics. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bladder-cancer#heading-Zero (accessed on 3 May 2022).
- National Institute for Health and Care Excellence Bladder Cancer: Diagnosis and Management. Available online: https://www.nice.org.uk/guidance/ng2/chapter/1-Recommendations#treating-muscle-invasive-bladder-cancer-2 (accessed on 8 March 2022).
- Vashistha, V.; Wang, H.; Mazzone, A.; Liss, M.A.; Svatek, R.S.; Schleicher, M.; Kaushik, D. Radical Cystectomy Compared to Combined Modality Treatment for Muscle-Invasive Bladder Cancer: A Systematic Review and Meta-Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 1002–1020. [Google Scholar] [CrossRef]
- Flaig, T.W. NCCN Guidelines Updates: Management of Muscle-Invasive Bladder Cancer. J. Natl. Compr. Canc. Netw. 2019, 17, 591–593. [Google Scholar]
- Theodoropoulos, V.E.; Lazaris, A.C.; Sofras, F.; Gerzelis, I.; Tsoukala, V.; Ghikonti, I.; Manikas, K.; Kastriotis, I. Hypoxia-Inducible Factor 1α Expression Correlates with Angiogenesis and Unfavorable Prognosis in Bladder Cancer. Eur. Urol. 2004, 46, 200–208. [Google Scholar] [CrossRef]
- Walshaw, R.C.; Honeychurch, J.; Illidge, T.M.; Choudhury, A. The Anti-PD-1 Era—An Opportunity to Enhance Radiotherapy for Patients with Bladder Cancer. Nat. Rev. Urol. 2018, 15, 251–259. [Google Scholar] [CrossRef]
- Song, Y.P.; Mistry, H.; Irlam, J.; Valentine, H.; Yang, L.; Lane, B.; West, C.; Choudhury, A.; Hoskin, P.J. Long-Term Outcomes of Radical Radiation Therapy with Hypoxia Modification with Biomarker Discovery for Stratification: 10-Year Update of the BCON (Bladder Carbogen Nicotinamide) Phase 3 Randomized Trial (ISRCTN45938399). Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 1407–1415. [Google Scholar] [CrossRef]
- Hoskin, P.J.; Rojas, A.M.; Bentzen, S.M.; Saunders, M.I. Radiotherapy with Concurrent Carbogen and Nicotinamide in Bladder Carcinoma. J. Clin. Oncol. 2010, 28, 4912–4918. [Google Scholar] [CrossRef]
- Yang, L.; Taylor, J.; Eustace, A.; Irlam, J.J.; Denley, H.; Hoskin, P.J.; Alsner, J.; Buffa, F.M.; Harris, A.L.; Choudhury, A.; et al. A Gene Signature for Selecting Benefit from Hypoxia Modification of Radiotherapy for High-Risk Bladder Cancer Patients. Clin. Cancer Res. 2017, 23, 4761–4768. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, Q.; Zhang, Y.; Liu, Z.; Zheng, Z.; Liu, S.; Meng, L.; Xin, Y.; Jiang, X. Targeting Hypoxia in the Tumor Microenvironment: A Potential Strategy to Improve Cancer Immunotherapy. J. Exp. Clin. Cancer Res. 2021, 40, 1–16. [Google Scholar] [CrossRef]
- Hatfield, S.M.; Kjaergaard, J.; Lukashev, D.; Belikoff, B.; Schreiber, T.H.; Sethumadhavan, S.; Abbott, R.; Philbrook, P.; Thayer, M.; Shujia, D.; et al. Systemic Oxygenation Weakens the Hypoxia and Hypoxia Inducible Factor 1α-Dependent and Extracellular Adenosine-Mediated Tumor Protection. J. Mol. Med. 2014, 92, 1283–1292. [Google Scholar] [CrossRef]
- Hatfield, S.M.; Kjaergaard, J.; Lukashev, D.; Schreiber, T.H.; Belikoff, B.; Abbott, R.; Sethumadhavan, S.; Philbrook, P.; Ko, K.; Cannici, R.; et al. Immunological Mechanisms of the Antitumor Effects of Supplemental Oxygenation. Sci. Transl. Med. 2015, 7, 277ra30. [Google Scholar] [CrossRef]
- Colombani, T.; Eggermont, L.J.; Hatfield, S.M.; Rogers, Z.J.; Rezaeeyazdi, M.; Memic, A.; Sitkovsky, M.V.; Bencherif, S.A. Oxygen-Generating Cryogels Restore T Cell Mediated Cytotoxicity in Hypoxic Tumors. Adv. Funct. Mater. 2021, 31, 2102234. [Google Scholar] [CrossRef]
- Liu, X.; Ye, N.; Liu, S.; Guan, J.; Deng, Q.; Zhang, Z.; Xiao, C.; Ding, Z.Y.; Zhang, B.X.; Chen, X.P.; et al. Hyperbaric Oxygen Boosts PD-1 Antibody Delivery and T Cell Infiltration for Augmented Immune Responses Against Solid Tumors. Adv. Sci. 2021, 8, 2100233. [Google Scholar] [CrossRef]
- Cheng, W.; Fu, D.; Xu, F.; Zhang, Z. Unwrapping the Genomic Characteristics of Urothelial Bladder Cancer and Successes with Immune Checkpoint Blockade Therapy. Oncogenesis 2018, 7, 2. [Google Scholar] [CrossRef]
- Wołącewicz, M.; Hrynkiewicz, R.; Grywalska, E.; Suchojad, T.; Leksowski, T.; Roliński, J.; Niedźwiedzka-Rystwej, P. Immunotherapy in Bladder Cancer: Current Methods and Future Perspectives. Cancers 2020, 12, 1181. [Google Scholar] [CrossRef]
- Li, F.; Li, C.; Cai, X.; Xie, Z.; Zhou, L.; Cheng, B.; Zhong, R.; Xiong, S.; Li, J.; Chen, Z.; et al. The Association between CD8+ Tumor-Infiltrating Lymphocytes and the Clinical Outcome of Cancer Immunotherapy: A Systematic Review and Meta-Analysis. eClinicalMedicine 2021, 41, 101134. [Google Scholar] [CrossRef]
- Aouali, N.; Bosseler, M.; Sauvage, D.; Van Moer, K.; Berchem, G.; Janji, B. The Critical Role of Hypoxia in Tumor-Mediated Immunosuppression. In Hypoxia and Human Diseases; InTech: London, UK, 2017. [Google Scholar]
- Smith, V.; Mukherjee, D.; Lunj, S.; Choudhury, A.; Hoskin, P.; West, C.; Illidge, T. The Effect of Hypoxia on PD-L1 Expression in Bladder Cancer. BMC Cancer 2021, 21, 1–11. [Google Scholar] [CrossRef]
- van Wilpe, S.; Gerretsen, E.C.F.; van der Heijden, A.G.; de Vries, I.J.M.; Gerritsen, W.R.; Mehra, N. Prognostic and Predictive Value of Tumor-Infiltrating Immune Cells in Urothelial Cancer of the Bladder. Cancers 2020, 12, 2692. [Google Scholar] [CrossRef]
- Eustace, A.; Irlam, J.J.; Taylor, J.; Denley, H.; Agrawal, S.; Choudhury, A.; Ryder, D.; Ord, J.J.; Harris, A.L.; Rojas, A.M.; et al. Necrosis Predicts Benefit from Hypoxia-Modifying Therapy in Patients with High Risk Bladder Cancer Enrolled in a Phase III Randomised Trial. Radiother. Oncol. 2013, 108, 40. [Google Scholar] [CrossRef]
- Kamoun, A.; de Reyniès, A.; Allory, Y.; Sjödahl, G.; Robertson, A.G.; Seiler, R.; Hoadley, K.A.; Groeneveld, C.S.; Al-Ahmadie, H.; Choi, W.; et al. A Consensus Molecular Classification of Muscle-Invasive Bladder Cancer. Eur. Urol. 2020, 77, 420–433. [Google Scholar] [CrossRef]
- Sharma, P.; Shen, Y.; Wen, S.; Yamada, S.; Jungbluth, A.A.; Gnjatic, S.; Bajorin, D.F.; Reuter, V.E.; Herr, H.; Old, L.J.; et al. CD8 Tumor-Infiltrating Lymphocytes Are Predictive of Survival in Muscle-Invasive Urothelial Carcinoma. Proc. Natl. Acad. Sci. USA 2007, 104, 3967–3972. [Google Scholar] [CrossRef]
- Deng, B.; Park, J.; Ren, L.; Yew, P.Y.; Kiyotani, K.; Antic, T.; O’Connor, K.; O’Donnell, P.H.; Nakamura, Y. CD8 Lymphocytes in Tumors and Nonsynonymous Mutational Load Correlate with Prognosis of Bladder Cancer Patients Treated with Immune Checkpoint Inhibitors. Cancer Rep. 2018, 1, e1002. [Google Scholar] [CrossRef]
- Hunter, B.A.; Eustace, A.; Irlam, J.J.; Valentine, H.R.; Denley, H.; Oguejiofor, K.K.; Swindell, R.; Hoskin, P.J.; Choudhury, A.; West, C.M. Expression of Hypoxia-Inducible Factor-1α Predicts Benefit from Hypoxia Modification in Invasive Bladder Cancer. Br. J. Cancer 2014, 111, 437. [Google Scholar] [CrossRef]
- Wang, L.; Saci, A.; Szabo, P.M.; Chasalow, S.D.; Castillo-Martin, M.; Domingo-Domenech, J.; Siefker-Radtke, A.; Sharma, P.; Sfakianos, J.P.; Gong, Y.; et al. EMT- A Nd Stroma-Related Gene Expression and Resistance to Pd-1 Blockade in Urothelial Cancer. J. Urol. 2019, 202, 458. [Google Scholar]
- Sikic, D.; Weyerer, V.; Geppert, C.I.; Bertz, S.; Lange, F.; Taubert, H.; Wach, S.; Schmitz-Draeger, B.J.; Wullich, B.; Hartmann, A.; et al. Utility of Stromal Tumor Infiltrating Lymphocyte Scoring (STILs) for Risk Stratification of Patients with Muscle-Invasive Urothelial Bladder Cancer after Radical Cystectomy. Urol. Oncol. Semin. Orig. Investig. 2022, 40, 63.e19–63.e26. [Google Scholar] [CrossRef]
- Van Limbergen, E.J.; De Ruysscher, D.K.; Olivo Pimentel, V.; Marcus, D.; Berbee, M.; Hoeben, A.; Rekers, N.; Theys, J.; Yaromina, A.; Dubois, L.J.; et al. Combining Radiotherapy with Immunotherapy: The Past, the Present and the Future. Br. J. Radiol. 2017, 90, 20170157. [Google Scholar] [CrossRef]
- Barker, H.E.; Paget, J.T.E.; Khan, A.A.; Harrington, K.J. The Tumour Microenvironment after Radiotherapy: Mechanisms of Resistance and Recurrence. Nat. Rev. Cancer 2015, 15, 409–425. [Google Scholar] [CrossRef]
- Efstathiou, J.A.; Mouw, K.W.; Gibb, E.A.; Liu, Y.; Wu, C.L.; Drumm, M.R.; da Costa, J.B.; du Plessis, M.; Wang, N.Q.; Davicioni, E.; et al. Impact of Immune and Stromal Infiltration on Outcomes Following Bladder-Sparing Trimodality Therapy for Muscle-Invasive Bladder Cancer(Figure Presented.). Eur. Urol. 2019, 76, 59–68. [Google Scholar] [CrossRef]
- Bowser, J.L.; Lee, J.W.; Yuan, X.; Eltzschig, H.K. The Hypoxia-Adenosine Link during Inflammation. J. Appl. Physiol. 2017, 123, 1303–1320. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Y.; Yang, M.; Zhang, Y.; Xie, Q.; Li, Z.; Dong, Z.; Yang, Y.; Deng, B.; Feng, A.; et al. Hypoxia Induces T-Cell Apoptosis by Inhibiting Chemokine C Receptor 7 Expression: The Role of Adenosine Receptor A2. Cell. Mol. Immunol. 2010, 7, 77–82. [Google Scholar] [CrossRef]
- Faraj, S.F.; Munari, E.; Guner, G.; Taube, J.; Anders, R.; Hicks, J.; Meeker, A.; Schoenberg, M.; Bivalacqua, T.; Drake, C.; et al. Assessment of Tumoral PD-L1 Expression and Intratumoral CD8+ T Cells in Urothelial Carcinoma. Urology 2015, 85, 703.e1–703.e6. [Google Scholar] [CrossRef]
- Hasmim, M.; Noman, M.Z.; Messai, Y.; Bordereaux, D.; Gros, G.; Baud, V.; Chouaib, S. Cutting Edge: Hypoxia-Induced Nanog Favors the Intratumoral Infiltration of Regulatory T Cells and Macrophages via Direct Regulation of TGF-Β1. J. Immunol. 2013, 191, 5802–5806. [Google Scholar] [CrossRef]

| Characteristic | HR 1 | 95% CI 1 | p-Value |
|---|---|---|---|
| Age | 1.05 | 1.02, 1.08 | <0.001 |
| Gender | |||
| Male | - | - | |
| Female | 1.24 | 0.65, 2.36 | 0.51 |
| Tumour stage | |||
| 2 | - | - | |
| 3, 4 | 1.03 | 0.61, 1.76 | 0.90 |
| Grade | |||
| 2 | - | - | |
| 3 | 0.79 | 0.41, 1.52 | 0.48 |
| Treatment | |||
| RT | - | - | |
| RT+CON | 0.60 | 0.38, 0.95 | 0.031 |
| Tumour de-bulking | |||
| Complete | - | - | |
| Partial | 0.92 | 0.54, 1.56 | 0.76 |
| Biopsy | 1.73 | 0.95, 3.16 | 0.075 |
| CA9 | |||
| Absent | - | - | |
| Present | 0.60 | 0.37, 0.99 | 0.045 |
| Necrosis | |||
| Absent | - | - | |
| Present | 2.12 | 1.27, 3.52 | 0.004 |
| Percent of CD8 cells | |||
| Low | - | - | |
| High | 0.33 | 0.19, 0.60 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, V.; Mukherjee, D.; Tsakiroglou, A.M.; Baker, A.; Mistry, H.; Choudhury, A.; Hoskin, P.; Illidge, T.; West, C.M.L. Low CD8 T Cell Counts Predict Benefit from Hypoxia-Modifying Therapy in Muscle-Invasive Bladder Cancer. Cancers 2023, 15, 41. https://doi.org/10.3390/cancers15010041
Smith V, Mukherjee D, Tsakiroglou AM, Baker A, Mistry H, Choudhury A, Hoskin P, Illidge T, West CML. Low CD8 T Cell Counts Predict Benefit from Hypoxia-Modifying Therapy in Muscle-Invasive Bladder Cancer. Cancers. 2023; 15(1):41. https://doi.org/10.3390/cancers15010041
Chicago/Turabian StyleSmith, Vicky, Debayan Mukherjee, Anna Maria Tsakiroglou, Alexander Baker, Hitesh Mistry, Ananya Choudhury, Peter Hoskin, Timothy Illidge, and Catharine M. L. West. 2023. "Low CD8 T Cell Counts Predict Benefit from Hypoxia-Modifying Therapy in Muscle-Invasive Bladder Cancer" Cancers 15, no. 1: 41. https://doi.org/10.3390/cancers15010041
APA StyleSmith, V., Mukherjee, D., Tsakiroglou, A. M., Baker, A., Mistry, H., Choudhury, A., Hoskin, P., Illidge, T., & West, C. M. L. (2023). Low CD8 T Cell Counts Predict Benefit from Hypoxia-Modifying Therapy in Muscle-Invasive Bladder Cancer. Cancers, 15(1), 41. https://doi.org/10.3390/cancers15010041