Simple Summary
We performed a meta-analysis of all clinical trials of first-line combination therapies for advanced biliary tract cancer and concluded that combining immunotherapy with chemotherapies could improve survival for patients with aBTCs by increasing the objective response rate.
Abstract
Background: Biliary tract cancer is one of the most aggressive and fatal tumours. Gemcitabine with cisplatin chemotherapy has long been the first-line treatment, but the prognosis is poor. In recent years, targeted treatment and immunotherapy have produced encouraging outcomes requiring a thorough review and meta-analysis. Method: For this systematic review and meta-analysis, we searched four databases, starting from the inception dates of databases to 11 January 2022. This study comprised randomised clinical trials and cohort studies that used immunotherapy or targeted treatment as the first line of treatment for patients with biliary tract cancer. Results: From the 888 studies extracted, 33 trials were examined and found to meet the criteria. These included 3087 patients, 16 single-arm trials, 13 RCTs, one nRCT, a prospective single-arm pilot study, and a clinical setting in the real world. From 2010 to 2020, 33 studies were conducted using targeted treatment or immunologic therapies as first-line treatments for BTC patients, and 18 of those studies had positive outcomes. Conclusion: This study demonstrates that immunotherapy combined with chemotherapy as first-line treatment can provide survival benefits by improving the objective response rate for patients with unresectable biliary tract cancer. The potential for combination therapy to become a new trend in clinical treatment is promising but needs further clinical evaluation.
1. Introduction
Biliary tract cancer (BTC), which includes intrahepatic cholangiocarcinoma (ICC) and extrahepatic cholangiocarcinoma (ECC), is one of the most malignant and lethal tumours (ECC). The epidemiological characteristics of ICC and ECC differ. ECC is the most common subtype of cholangiocarcinoma in general. However, ICC is more common in some East Asian countries, accounting for 15% to 20% of all liver cancers and typically invading the bile duct wall [1]. In Western countries, the incidence and mortality rate of ICC are increasing [2]. There are significant gender and ethnic differences in ICC incidence and mortality. Men are 1.5 times more likely than women to develop ICC. Asians have a 2.0 times higher incidence rate than whites and blacks. Southeast Asia and China have the highest prevalence of ICC worldwide. Indian Americans, Alaska Natives, and Asians have the highest ICC mortality rates, while Caucasians and blacks have the lowest [3].
Most BTC patients cannot be resected at the time of diagnosis, and the prognosis is poor, with a median survival of 3–6 months, compared to 10% and 0% 5-year survival rates for stage III and IV BTC, respectively [2].
Currently, surgery is the only curable treatment option for cholangiocarcinoma that has not spread beyond the primary site. The objective response rate (ORR) is 15–26%, with a median survival time of less than one year, and drug resistance is common [1]. Most patients with ICC are initially diagnosed with local invasion or distant metastasis and do not have the option of undergoing radical surgery. In recent years, the morbidity and incidence of intrahepatic BTC have increased, while extrahepatic BTC has decreased.
Cisplatin and gemcitabine are the standard first-line chemotherapy treatment for BTC [4]. Most BTC patients have no other treatment options after developing resistance to first-line chemotherapy, and their disease often worsens rapidly. The dismal median overall survival (median-OS) of 11–13 months under systemic palliative therapy with gemcitabine and cisplatin highlights the urgent need to expand the limited therapeutic measures available to date for patients with advanced BTC [5].
Patients have benefited from the emergence of targeted therapeutic agents in recent years. Mutations in FGFR, IDH, BRAF, and NTRK are all linked to the development of BTC. Fibroblast growth factor receptor (FGFR) and neurotrophic tyrosine receptor kinase (NTRK) have been linked to cholangiocarcinoma development and are expected to be important targeted therapies [6,7]. Recent sequencing results from multiple sources have shown that up to 11–45% of patients with ICC contain FGFR2 fusion mutations. The binding proteins of fusion mutations include ARID1A-, PBRM1-, and TP53-. In addition, 24 patients with FGFR2 fusion mutations were reported in the MSKCC 10,000 sequencing data, including 18 cases of cholangiocarcinoma (242 cases in total), accounting for 75% of all FGFR2 fusion mutations. It is evident that FGFR2 fusion mutations are relatively highly enriched in cholangiocarcinoma. To summarise previous reports, IDH mutations in cholangiocarcinoma have the following characteristics: (1) IDH1 mutations are more frequent than IDH2 mutations; the hotspot mutation of IDH1 is located at R132, while the hotspot mutation of IDH2 is located at R172; (2) the proportion of mutations is higher in ICC than in ECC; and (3) IDH1/2 mutations lose normal enzymatic activity and generate new activity, which can produce the oncogenic metabolite 2-hydroxyglutarate (2-HG). 2-HG can be detected in tumours or blood and can be used as one of the PD indicators in clinical trials [7]. Other targets, such as anti-angiogenesis, EGFR amp, WNT/a-catenin, Hedgehog, and HGF/c-MET, have been reported in cholangiocarcinoma, but most of these pathways can be found in most tumour types, and multiple previous clinical trials in cholangiocarcinoma have shown limited effectiveness.
ICIs monotherapy has achieved some efficacy in patients with cholangiocarcinoma. On this basis, several clinical trials are underway investigating the combination of ICIs or ICIs with other types of immunotherapies. CTLA-4 regulates early immune responses, and PD-L1 mainly regulates immune responses in advanced peripheral tissues. Based on this regulatory mechanism, the combination of immunosuppressive agents of PD-1, PD-L1, and CTLA-4 can be made to achieve antitumour therapy through complementary mechanisms with synergistic effects. It has been shown that the combination of CTLA-4 and PD-1 inhibitors is more effective than single therapy, probably because the synergistic effect leads to an increase in the number of tumour-infiltrating lymphocytes, a decrease in regulatory T cells, and an overall improvement in the inhibition of tumour growth, with an overall efficiency of 10.8% (7/65, all in partial remission), a disease control rate of 32.2%, and an overall survival time for cholangiocarcinoma was 10.1 months [8].
Furthermore, while there is no evidence of adjuvant therapy, many studies on treating BTC with immune checkpoint inhibitors have been conducted (ICI). For the first-line treatment of advanced BTC, a combination of ICI and chemotherapy or targeted therapies are still being studied in clinical trials [9].
However, there is no comprehensive systematic review or meta-analysis of the efficacy of updated BTC treatment. Only EGFR inhibitors were studied in Alexandro Rizzo’s study [10]. Although data on treatment, including systemic chemotherapy and radiation therapy, were analysed in Michael N’s study, the data were only available up to 2013 and did not focus on targeted therapies [11]. As a result, there is a need for more recent and comprehensive meta-analysis in this field.
Through systematic review and meta-analysis, this study aimed to summarise the evidence from randomised controlled trials (RCTs) and cohort studies comparing the efficacy and safety of immunotherapy and targeted therapy for BTCs. The findings of this study are expected to provide evidence for managing patients with BTCs.
2. Methods
2.1. Study Design
This systematic review and meta-analysis were conducted and reported under the Preferred Reporting Items for System Review and Meta-Analysis Scenarios (PRISMA) [12]. It was registered with PROSPERO (CRD42022336576).
Extensive searches of databases for clinical trials related to BTC were conducted. The inclusion/exclusion criteria for this study were developed in accordance with the PICOS Principles [13].
2.2. Inclusion Criteria for Study Selection
2.2.1. Types of Studies
We focused on RCT and cohort studies. Case-control studies, letters, reviews, case reports, and articles that do not provide raw data were not included. When data came from different phases of the same experiment, only studies with the most complete and up-to-date data were retained. No restrictions were placed on the language of the article. We also searched CNKI but did not find any more additional Chinese studies.
2.2.2. Population
This study targeted populations who needed to meet all the following criteria:
(1) Unresectable gallbladder cancer and cholangiocarcinoma diagnosed by histopathology or cytology.
(2) The patient has not received systemic treatment for unresectable biliary cancer.
(3) The patient has at least one measurable lesion.
2.2.3. Interventions
Targeted and immunologic agents used alone or in combination with chemotherapeutic agents were included in this study. The drug targets include MEK1/2, EGFR, VEGF, mTOR, PD-1/PD-L1, MET, and CTLA-4 (as shown in Table 1).

Table 1.
Types of interventions.
2.2.4. Outcome Measures
Primary Outcomes
The primary outcomes were the objective response rate (ORR), disease control rate (DCR), overall survival (OS), and progression-free survival (PFS).
Overall Survival (OS) is the time between randomisation and the onset of death by any cause. Objective Response Rate (ORR) is the proportion of patients whose tumour volume decreases to a predetermined value. ORR equals the ratio of complete response (CR) to partial response (PR), or ORR = CR + PR. ORR excludes both stable disease (SD) and the effect of the disease’s natural progression. Smaller sample sizes and shorter follow-up periods are required. Partial response (PR) is defined as a volume reduction of at least 30 percent in all tumours that can be measured. The Disease Control Rate (DCR) is the proportion of patients whose cancer diminishes or stabilises over time. DCR equals the sum of the rates of complete remission, partial remission, and stable disease. PFS is the time between randomisation and the onset of objective tumour progression or death from any cause, which is a surrogate endpoint for OS.
Secondary Outcomes
Secondary outcomes were treatment-related adverse events (TRAE).
2.3. Data Sources and Search Strategy
Two investigators searched PubMed, Web of Science, Embase, Cochrane Library, and clinicaltrials.gov, starting from the inception dates of databases to 11 September 2022. The terms “biliary tract cancer”, “cholangiocarcinoma”, gene mutation type such as “EGFR”, and drug names such as “pembrolizumab” were used as keywords to search titles or abstracts (See Appendix A for a detailed search strategy).
2.4. Literature Selection
Endnote 20 software was used to import all the search results. First, duplicates were removed. Second, two researchers independently screened titles and abstracts to determine inclusion eligibility. Third, full manuscripts of potentially eligible trials were read to determine which studies should be included. During the literature selection process, disagreements were resolved through discussion between the two researchers with the assistance of a third researcher as needed [14].
2.5. Data Extraction
Two researchers extracted and compiled data. The first author, study method, publication time, journal of publication, follow-up time, number of patients, baseline level of patients, observed indicators, and interventions were all extracted from the study data. If there were any disagreements, the researchers discussed them. If two researchers cannot reach an agreement, a third researcher makes the final decision. We recorded the data in Microsoft Excel.
2.6. Quality Assessment
The PRISMA guidelines were followed when conducting the systematic review. The quality of the included literature was assessed using Version 2 of the Cochrane risk-of-bias tool for randomised trials and plotted in the quality evaluation table [15]. Non-randomised intervention studies were evaluated using ROBINS-I (Risk Of Bias In Non-randomised Studies of Interventions) [16].
2.7. Statistical Analysis
We used the “meta” package in R environment to perform the meta-analysis using frequentist approach [17]. Heterogeneity was assessed with I2. A fixed-effects model was used for small heterogeneity (I2 < 25%), and a random-effects model was used for a large one (I2 > 25%) [18]. Bias is tested using funnel plots if the heterogeneity between studies in the meta was too large (I2 > 75%) [19]. We determined the rank of all interventions by using the ‘netrank’ function in the ‘netmeta’ package in R to obtain P-scores. The P-score ranged from 0 to 1, showing a progressive rise in the efficacy of the included medications based on the estimation result and confidence interval of the effect value [18].
3. Results
3.1. Study Inclusion
We initially identified a total of 888 articles from four databases. After excluding 179 duplicates, 709 articles remained. Then, we screened titles and abstracts to exclude the types of articles that did not meet the criteria, leaving 128 articles. Full-text screening was performed to exclude 73 articles with immunotherapies or targeted therapies as second-line therapy, Seven articles with a mix of first-line and second-line therapy, and eight articles whose studies included other diseases were excluded, leaving 33 included. After searching clinicaltrial.gov and checking the reference of the included literature, 17 additional studies were identified, resulting in a final 33 studies that met all the inclusion criteria (Figure 1). The total number of patients included in the trials is 3087.
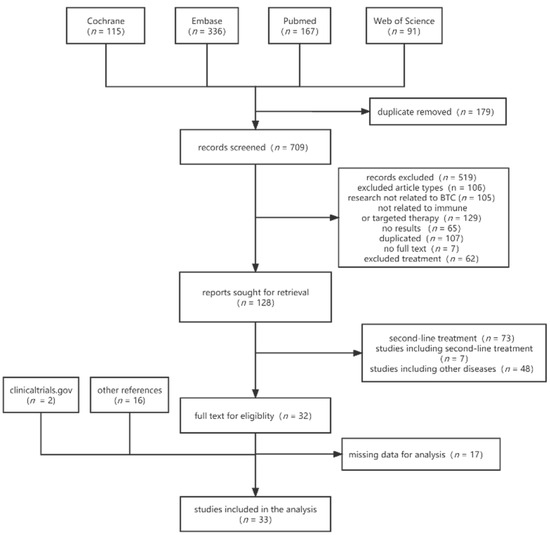
Figure 1.
Flowchart of study selection.
3.2. Characteristics of the Included Studies
3.2.1. Targeted Therapies
This study included 22 trials focusing on targeted therapies, with a total of 1658 patients. The basic characteristics of patients are listed in Table 2.
Among the 22 studies evaluating targeted therapy, 11 studies were single-arm phase II trials; 10 studies were randomised parallel phase II trials, and one study was a phase III trial. The publication years ranged from 2010 to 2021, with the earliest trial starting in 2006. There were 11 single-arm trials, 11 controlled trials, one with three groups (Valle et al., 2020 [20]), and three trials were blinded (Santoro et al., 2015 [21]; Valle et al., 2015 [22]; Moehler et al., 2014 [23]). One nRCT (Factorial assignment) and 10 RCTs were included. Seven trials were completed in the United States; five were multicenter trials, and the others were distributed in Italy, Australia, Austria, France, Korea, Taiwan, Denmark, and Germany.

Table 2.
Basic characteristics of targeted therapies.
Table 2.
Basic characteristics of targeted therapies.
| No. | Study | Phase | pts | Location | Intervention | Dose | Chemotherapy | Mean Age | Gender | Race |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Khoueiry (2012) [24] | II | 31 | US | Sorafenib | 400 mg po twice daily continuously. | NA | 57.8 (33–81) | male: 15 (48%) |
|
| 2 | Khoueiry (2014) [25] | II | 34 | US | Sorafenib | 400 mg BID and 100 mg daily | NA | 63 | male: 13 (38%) |
|
| 3 | Hezel (2014) [26] | II | 31 | US | Panitumumab | 6 mg/kg | GEMOX | NA | NA | NA |
| 4 | Santoro (2015) [21] | II | 173 | Italy | Vandetanib | Vandetanib (300 mg or 100 mg) or placebo was given in single oral daily doses. | Gemcitabine | 63.6 (sd: 9.5) | male: 81 (46.8) |
|
| 5 | Zhu (2010) [25] | II | 35 | US | Bevacizumab | 10 m g/kg | GEMOX | NA | NA | NA |
| 6 | Gruenberger (2010) [27] | II | 30 | Austria | Cetuximab | 500 mg/m2 | GEMOX | median age: 68 years (IQR 62–73) | NA | NA |
| 7 | Lau (2018) [28] | II | 27 | Australia | Everolimus | 10 mg/d | NA | NA | NA | NA |
| 8 | Malka (2014) [29] | II | 150 | France | Cetuximab | GEMOX | NA | NA | NA | |
| 9 | Sohal (2013) [30] | II | 35 | US | Panitumumab | 9 mg/kg | Gemcitabine Irinotecan | NA | NA | NA |
| 10 | Borbath (2013) [31] | II | 44 | Multi- center | Cetuximab | 400 mg/m2 at week 1, then 250 mg/m2/week | GEM | median age: 61.5 | NA | NA |
| 11 | Lee (2013) [32] | II | 39 | US | Sorafenib | 400 mg twice daily | GEMCIS | NA | NA | NA |
| 12 | Lee (2012) [33] | III | −135 −133 | Korea | Erlotinib | GEMOX | chemotherapy alone: 61 (55–68) C + T: 59 (54–66) | male: A: 79 (59%) B: 91 (67%) | NA | |
| 13 | Chen (2015) [34] | II | −62 −60 | Taiwan | Cetuximab | C-GEMOX (500 mg/m2 cetuximab plus GEMOX) every 2 weeks | GEMOX | C-GEMOX: 61 (32–78) GEMOX: 59 (32–80) | male: C-GEMOX: 28 (45%) GEMOX: 30 (50%) | NA |
| 14 | Valle (2015) [22] | II | 62 | Multi -center | Cediranib | GEMCIS | NA | NA | NA | |
| 15 | Valle (2020) [20] | II | 106 102 101 | Multi -center | Ramucirumab | GEMCIS | NA | NA | NA | |
| 16 | Jensen (2012) [35] | II | 46 | Denmark | Panitumumab | GEMOX | NA | NA | NA | |
| 17 | Leone (2016) [36] | II | 45 44 | Italy | Panitumumab | GEMOX | NA | NA | NA | |
| 18 | Lowery (2019) [37] | II | 41 | US | Binimetinib | 45 mg orally twice daily | GEMCIS | 66 (45–83) | male: 21 (51.2%) | NA |
| 19 | Moehler (2014) [23] | II | 102 | Germany | Sorafenib | 400 mg bid orally continuously | GEM | Sorafenib: 64.0 placebo: 64.5 | sorafenib: male: 20 Gemcitabine: male: 23 |
|
| 20 | Iyer (2018) [38] | II | 50 | Multi -center | Bevacizumab | Gemcitabine Capecitabine | NA | NA | NA | |
| 21 | Lubner (2010) [39] | II | 53 | Multi -center | Bevacizumab Erlotinib | NA | 63 (31–87) | male: 23 (43%) | NA | |
| 22 | Vogel (2018) [40] | II | −62 −28 | Germany | Panitumumab | 9 mg/kg BW at day 1 | GEMCIS | NA | NA |
|
The primary tumour sites are shown in Table 3 below. 694 patients with IHC, 384 patients with gallbladder cancer, 235 patients with EHC, 44 patients with hilar cholangiocarcinoma, and 17 patients with Vater ampulla carcinoma were included. Three studies (consisting of 219 patients) did not specify the disease type in patients with BTC, and 48 patients were classified as “other disease types” in the original literature, which included patients with liver metastases.

Table 3.
Primary target sites of targeted-therapy studies.
3.2.2. Immunotherapies
As shown in Table 4, the included immunotherapy-related studies were published between 2018 and 2021. The trial of Oh et al., 2020 [41] of Bintrafusp alfa is a phase 2/3 trial. Oh et al., 2022 [42] is a phase 3 trial, demonstrating the effectiveness of Durvalumab in combination with chemotherapy therapies. No immunotherapy was used alone in any of the study’s regimens, which all combined both immunotherapy and chemotherapy as the first-line treatment. A total of 1286 patients were included.

Table 4.
Basic characteristics of immunotherapies.
The primary tumour sites of immunotherapy in the studies are listed in Table 5, among which most were ICC, 460 cases, accounting for 50.9% of the total.

Table 5.
Primary target sites of immunotherapy studies.
3.3. Quality Assessment
Figure 2 and Figure 3 depict the quality of the included RCTs. Overall, the included randomised controlled trials were of high quality. El-Khoueiry (2012) [24] was terminated after the first phase of accrual because the primary objectives were not met. Iyer (2018) [38] is a meeting abstract lacking detailed data.
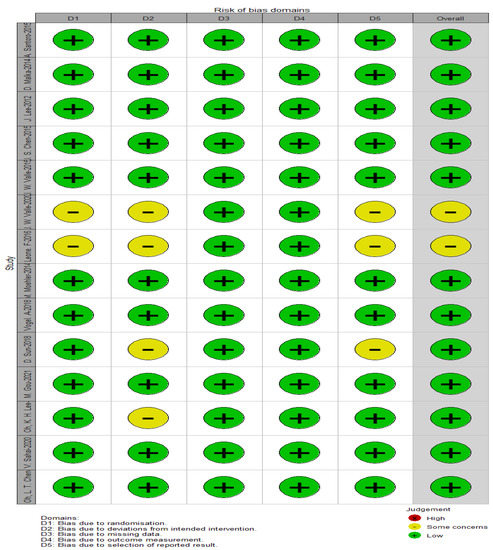
Figure 2.
Assessment of the risk of bias using ROB-2.
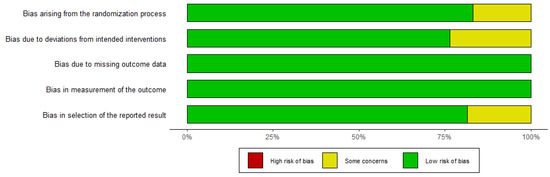
Figure 3.
Assessment of the risk of bias using ROB-2 (traffic light figure).
As shown in Table 6, ROBINS-1 assessed the quality of 16 single-arm studies and observational studies. All studies documented the definition of controls and the comparability of cases and controls, except for one study that did not report case definitions (Khoueiry, 2012 [24]).

Table 6.
ROBINS-1 of non-RCT (risk of bias).
3.4. Systematic Review
3.4.1. Targeted Therapies
Zhu et al., 2010 [52] showed that patients with BTC treated with bevacizumab + GEMOX obtained a median PFS of 7.0 months (95% CI 5.3–10.3) and a 6-month PFS of 63% (47–79), below the set target rate of 70%, with objective responses recorded in 19 patients and an overall disease control rate of 80%. The trial by Gruenberger et al., 2010 [27] had an overall disease control rate of 80%, progression-free survival of 8.8 months (95% CI 5.1–12.5), and median overall survival of 15.2 months (9.9–20.5) for all treated patients. The trial by Lau et al., 2018 [28], using Everolimus 10 mg/d alone, had a median PFS of 5.5 months (95% confidence interval (CI: 2.1–10.0 months) and a median OS of 9.5 months (95% CI: 5.5–16.6 months). Notably, gallbladder cancer had a significantly worse DCR at 12 weeks than other anatomic sites and a trend toward worse PFS and OS, but the treatment was well tolerated.
Sohal et al., 2013 [30] added Irinotecan and Panitumumab to Gemcitabine, which had a median PFS of 9.7 months and a median OS of 12.9 months, showing encouraging efficacy and good tolerability of this regimen. The trial by Borbath et al., 2013 [31] met the primary endpoint with a median PFS time of 5.8 months (95% CI 3.6–8.5 months), median OS time of 13.5 months (95% CI 9.8–31.8 months), and 53.7% of patients remained alive at 1 year, suggesting that Gemcitabine-Cetuximab has activity in BTC and that KRAS status is not associated with PFS and, unlike cutaneous toxic effects, may serve as a surrogate marker of efficacy. Lubner et al., 2010 [39] showed that 87% of patients showed disease progression, with a median time to disease progression of 4.4 months and a median OS of 9.9 months. This study concluded that the combination of bevacizumab and erlotinib demonstrated significant activity in treating advanced BTC, with few adverse events of grades 3 or 4. Bevacizumab and Erlotinib demonstrated significant activity in advanced BTC with few Grade 3 or 4 adverse events. The trial by Leone et al., 2016 [36] added Panitumumab to GEMOX, and the results confirmed a marginal effect of anti-EGFR therapy in WT-KRAS-selected BTC.
Lee et al., 2012 [33] combined erlotinib with GEMOX and showed no significant difference in progression-free survival between the groups. Still, adding erlotinib to gemcitabine and oxaliplatin showed antitumour activity: significantly more patients had objective responses in the chemotherapy plus erlotinib group than in the chemotherapy alone group (40 patients versus 21 patients; p = 0.005), but median overall survival was the same in both groups. The trial by Chen et al., 2015 [34] showed a trend toward improved PFS was observed, but the addition of cetuximab did not significantly improve the ORR of GEMOX chemotherapy in advanced BTC, and KRAS mutations did not affect the trend in ORR and PFS differences between C-GEMOX and GEMOX. In the study by Jensen et al., 2012 [35], the addition of Panitumumab to chemotherapy resulted in a 6-month progression-free survival (PFS) rate of 31/42 [74%; 95% confidence interval (CI) 58% to 84%], a disease control rate of 86%, a median PFS of 8.3 months (95% CI 6.7–8.7 months), and a median overall survival of 10.0 months (95% CI. 7.4–12.7 months). Hezel et al., 2014 [26] used a combination of gemcitabine, oxaliplatin, and panitumumab for KRAS wild-type metastatic BTC and achieved a remission rate of 45% and a disease control rate of 90%. Its median PFS was 10.6 months (95% CI 5–24 months), and median overall survival was 20.3 months (95% CI 9–25 months).
Other trials did not meet the expected endpoints but were still informative. Khoueiry et al., 2012 [24], as a phase II study of sorafenib in patients with advanced BTC based on the role of the RAS-RAF-MEK-ERK pathway and the VEGF axis in BTC, was terminated after phase I due to failure to meet the primary objective. The trial by Khoueiry et al., 2014 [25] to study sorafenib and erlotinib was also terminated after Phase I enrollment. Lee (2012) [33] added sorafenib to gemcitabine and cisplatin for biliary tract adenocarcinoma, which did not improve efficacy compared with historical data and had increased toxicity. In the trial of Santoro et al., 2015 [21], patients were randomised in a 1:1:1. The results showed no statistical difference between secondary endpoints except for ORR, and the V/G combination was slightly outperformed by the other treatments. Patients in the three groups reported similar rates of adverse effects. Malka et al., 2014 [29] concluded that adding cetuximab to gemcitabine and oxaliplatin did not appear to enhance chemotherapeutic activity in patients with advanced BTC, although it was well tolerated.
The trial by Valle et al., 2015 [22] showed that Cediranib did not improve progression-free survival in patients with advanced BTC in combination with cisplatin and gemcitabine. Valle et al., 2020 [20] added Ramucirumab or Merestinib to GEM + CIS standard chemotherapy and showed no improvement in PFS, OS, or ORR. Lowery et al., 2019 [37] demonstrated that Binimetinib, combined with Gemcitabine and Cisplatin, had no effect on PFS-6-month or RR. Moehler et al., 2014 [23] similarly demonstrated that adding Sorafenib to Gemcitabine did not improve outcomes in patients with advanced BTC, but biomarker subgroup analysis suggested that some patients may benefit from the combination. Iyer et al., 2018 [38] demonstrated that adding Bevacizumab to Gemcitabine/Capecitabine did not improve prognosis in unselected patients with advanced BTC compared to historical controls. Vogel et al., 2018 [40] concluded that combining Panitumumab with chemotherapy did not improve ORR, PFS, or OS in patients with KRAS wild-type advanced BTC.
3.4.2. Immunotherapies
The trial of Yu et al., 2021 [43] resulted in an ORR of 14.3% (95% CI: 1.8 to 42.8), a DCR of 64.3% (95% CI: 41.7 to 86.9), a median PFS of 6.5 months (95% CI: 3.8 to 9.2), PFS rates of 61.6% and 12.3% at 6 and 12 months, respectively, and a median OS of 9.9 months (95% CI: 7.6 to 12.2), concluding that Camrelizumab in combination with chemotherapy as first-line treatment for metastatic BTC demonstrated acceptable safety and efficacy. Chen et al., 2015 [34] also concluded that Camrelizumab plus GEMOX as first-line treatment for patients with advanced BTC looked promising, with a median PFS that was 6.1 months and median OS that was 11.8 months. Sun et al., 2018 [44] concluded that the combination of PD-1 antagonist plus chemotherapy or targeted therapy was effective and tolerable as first-line treatment for advanced BTC. OS was significantly longer in the group treated with the combination drug than in the chemotherapy group (median, 8.2 vs. 3.6 months, HR 0.47 [0.20–1.10], p = 0.011), as was PFS (median, 3.9 vs. 2.0 months, HR 0.58 [0.28–1.19], p = 0.034), p = 0.034), and no significant ORR difference was observed.
Gou et al., 2021 [45] yielded results that in advanced BTC, anti-PD-1 therapy plus chemotherapy prolonged PFS compared to chemotherapy alone, and AE was tolerable. Oh et al., 2020 [46] showed that adding D + T immunotherapy to chemotherapy was tolerable and showed promising efficacy. Chiang et al., 2021 [48] concluded that Nivolumab in combination with a modified GS (gemcitabine and S-1) is a promising regimen with a good safety profile. Oh et al., 2020 [41] showed that Bintrafusp alfa was clinically active in Asian patients with BTC and had a durable response. Oh et al., 2022 [42] concluded that Durvalumab in combination with chemotherapy for BTC significantly improved OS compared to chemotherapy alone (D: 12.8 (11.1–14.0) vs. placebo: 11.5 (10.1–12.5)). The combination also greatly improved progression-free survival compared with chemotherapy alone. Median progression-free survival with durvalumab combined with gemcitabine and cisplatin was 7.2 months compared with 5.7 months with chemotherapy alone (HR = 0.75; p = 0.001). The proportion of progression-free patients was 34.8% and 24.6% at 9 months and 16.0% and 6.6% at 12 months, respectively. The ORR also improved, with an overall efficacy rate of 26.7% with durvalumab/chemotherapy compared with 18.7% with chemotherapy alone, with a superiority ratio of 1.60 for efficacy (p = 0.011). The trial by Sahai et al., 2020 [49] concluded that the combination of nivolumab with chemotherapy drugs failed to improve efficacy.
3.4.3. Combined Therapies
In the study by J. Zhou et al. [53], 30 patients with advanced ICC were included with an ORR of 80% (24/30; 95% CI: 61.4–92.3%) and a DCR of 93.3% (28/30; 95% CI: 77.4–99.2%). A complete response (CR) was scored 1. The median duration of follow-up was 8.4 months. Twelve patients experienced disease progression, and four patients died. Median PFS and OS had not been reached. The median duration of response has not been determined, and the 6-month OS rate was 90%. A quantity of 43% (13/30) of patients experienced grade 3 or higher adverse events (AEs). This study showed that ORR was significantly associated with PD-L1 expression and mutations associated with DNA damage repair (DDR) in tumour samples. In patients with advanced ICC, the combination of toripalimab, lenvatinib, and GEMOX chemotherapy was well tolerated and showed an encouraging ORR.
In the prospective phase II trial by Q. Zhang et al. [54], the efficacy and safety of first-line lenvatinib plus PD-1 inhibitor was similarly evaluated in patients with initially unresectable BTC, and the feasibility of translational surgery after this treatment was explored. The study included 38 patients, with a mean age of 62.5 years and 14 men, receiving PD-1 inhibitors, including Pembrolizumab (7.9%), Toripalimab 12 (31.6%), Tislelizumab 11 (28.9%), Sintilimab 11 (28.9%), and Camrelizumab 1 (2.6%), after a median follow-up of 13.7 (95% CI: 9.7 to 17.8) months, the 1-year OS rate was 47.4% (18/38), and 65.8% of patients were still alive. Median EFS was 8.0 months (95% CI: 4.6 to 11.4), and median OS was 17.7 months (95% CI: not estimable). Among the 13 patients who underwent conversion surgery, the median EFS was 13.5 months (95% CI: 13.0 to 14.0). Among patients who received only systemic therapy, the median EFS was 4.6 months (95% CI: 0.8 to 8.4). and the median OS was 12.4 months (95% CI: 8.5 to 16.3).
3.5. Meta-Analysis for OS
3.5.1. Meta-Analysis for OS of Targeted Therapy
As the heterogeneity was considerably high (I2 = 63%), a random effects model was used to obtain a pooled OS of 10.65 months for the targeted drug treatment group (Figure 4).
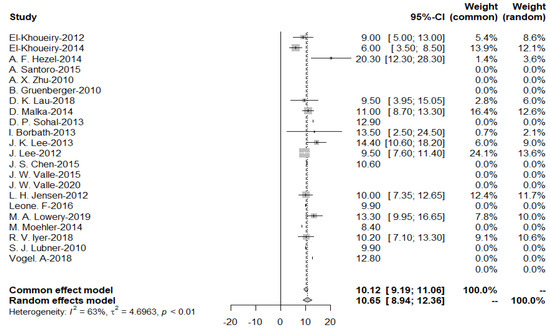
Figure 4.
Pooled overall survival with targeted therapy.
As evidenced by the funnel plot with a more symmetrical distribution, these studies have less publication bias. Egger’s test for a regression intercept gave a p-value of 0.1377 > 0.05, indicating no evidence of publication bias (Figure 5).
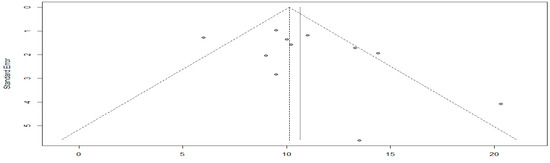
Figure 5.
Funnel plot of overall survival with targeted therapy.
A subgroup meta-analysis of the chemotherapy and combined therapy with targeted therapy, yielded I2 = 65%, p-value = 0.21 (Figure 6). So, a difference between the two groups could not be demonstrated.

Figure 6.
Overall survival in the targeted chemotherapy combination group compared with the chemotherapy alone group.
The asymmetry of the funnel plot indicates a possible publication bias (Figure 7). The Egger’s test could not be applied because the sample size was less than 10.
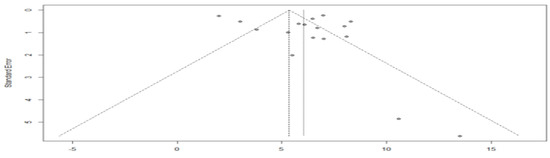
Figure 7.
Funnel plot of overall survival in the targeted chemotherapy combination group compared with the chemotherapy agent alone group.
3.5.2. Meta-Analysis for OS of Immunotherapy
The pooled overall survival is 15.62 months for the immunotherapy group, because the heterogeneity I2 = 84% > 50%, random effects model was applied (Figure 8).
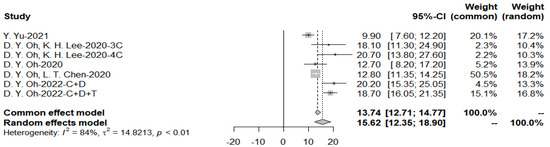
Figure 8.
Pooled overall survival with immunotherapy combined with chemotherapy.
3.6. Meta-Analysis for PFS
3.6.1. Meta-Analysis for PFS of Targeted Therapies
Data on PFS were available for six studies involving immunotherapy and 17 studies involving targeted treatment. Plots of each study’s individual PFS and its confidence intervals were made for the two groups (Figure 9). In the targeted therapy group, the median PFS was 6.02 months (95% CI: 5.01–7.03) (range: 2.0–13.5 months).

Figure 9.
Progression-free survival with targeted therapy.
As evidenced by the funnel plot with a more symmetrical distribution, these studies have less publication bias (Figure 10). In addition, Egger’s test for a regression intercept gave a p-value of 0.3583 > 0.05, indicating no evidence of publication bias.
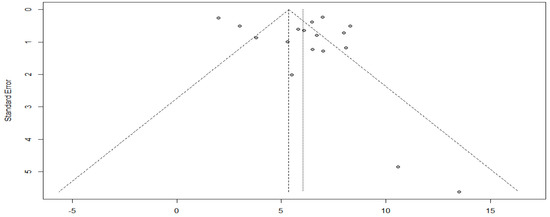
Figure 10.
Funnel plot of progression-free survival with targeted therapy.
Further subgroup analysis was performed to divide the studies of targeted therapies into two groups, EGFR and VEGF, and the pooled PFS was obtained from the forest plot (Figure 11 and Figure 12). After Egger’s test, the p-value of EGFR was 0.9437 > 0.05, and the p-value of VEGF was 0.3214 > 0.05, indicating no publication bias.
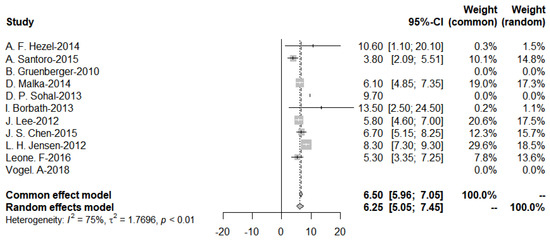
Figure 11.
Progression-Free-Survival of EGFR targeted therapies.
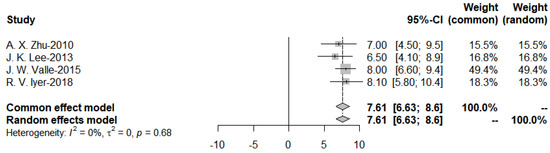
Figure 12.
Progression-Free-Survival of VEGF targeted therapies.
3.6.2. Meta-Analysis of PFS of Immunotherapies
Data on progression-free survival were available for seven studies in the immunotherapy group. Individual PFS and their confidence intervals were plotted for each study within the two groups (Figure 13). The median of PFS was 8.56 months (range: 2.5–12.3 months) (95% CI: 6.40–10.73) in the immunotherapy group.
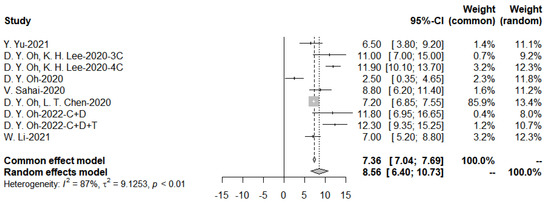
Figure 13.
Progression-free survival with immunotherapy and funnel plot.
The meta-analysis yielded a pooled PFS of 6.20 for the targeted drug treatment and 6.33 for the targeted combination chemotherapy group. Still, the p-value for the subgroup analysis was 0.60, which was not statistically different, with a considerable heterogeneity of I2 = 68% (Figure 14).

Figure 14.
Progression-free survival in the targeted chemotherapy combination group compared with the chemotherapy alone group.
The funnel plot is asymmetric with more results falling on the left side, suggesting possible publication bias (Figure 15).
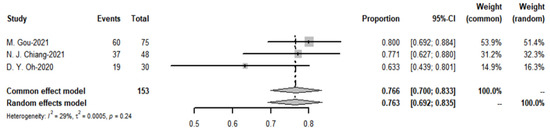
Figure 15.
Funnel plot of progression-free survival in the targeted chemotherapy combination group compared with the chemotherapy alone group.
The pooled PFS for obtaining immune combination chemotherapy drug treatment was 9.89 months, p-value = 0.6 for subgroup analysis, concluding that there was no significant difference in PFS between the two groups (Figure 16).
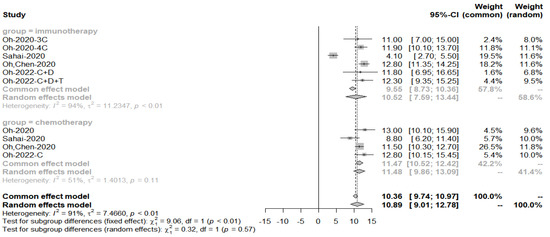
Figure 16.
Progression-free survival in the immuno-chemotherapy combination group compared to the immunotherapy group.
The funnel plot is asymmetric, with more results falling on the right side, suggesting possible publication bias (Figure 17).
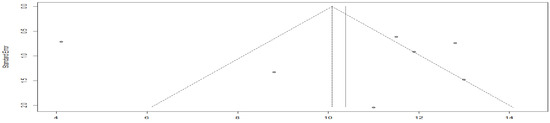
Figure 17.
Funnel plot of progression-free survival in the immuno-chemotherapy combination group compared to the immunotherapy group.
3.7. Meta-Analysis of ORR
The meta-analysis heterogeneity of chemotherapy drugs combined with immune drugs versus chemotherapy drugs alone was 0%, yielding an OR of 1.622 (Figure 18). So, the combination of chemotherapy immune drugs yielded a higher objective response rate than chemotherapy alone.

Figure 18.
Objective response rate in the immuno-chemotherapy combination group compared to the chemotherapy drug alone group.
The pooled objective response rate for the targeted therapy group was 32.1%, I2 = 78%, p-value < 0.01 (Figure 19). The ORR values of the three trials in immunotherapy, which had immunotherapy drugs in combination with chemotherapy drugs to compare with chemotherapy drugs alone, found no significant difference.
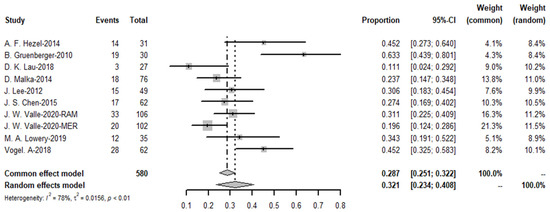
Figure 19.
Objective response rate of targeted therapies.
3.8. Meta-Analysis of DCR
The meta-analysis yielded a pooled DCR of 76.6% (I2 = 29%) for the immune-combination chemotherapy regimen. In contrast, the pooled disease control rate for targeted agents was 79.1% (see Figure 20 and Figure 21).
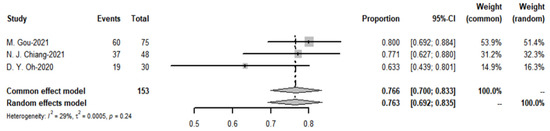
Figure 20.
Disease control rate of targeted therapies and funnel plot.
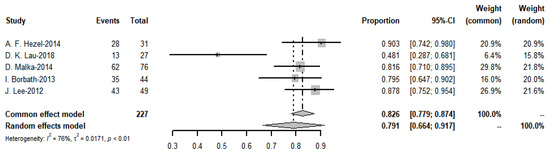
Figure 21.
Disease control rate of immunotherapies and funnel plot.
3.9. Treatment-Related Adverse Events
3.9.1. TRAE of Targeted Therapies
The results of treatment-related adverse events are summarised in Table 7. The top ten of these were neutropenia, thrombocytopenia, anemia, fatigue, diarrhea, leukopenia, neuropathy, rash, hypertension, and hand–foot skin reactions. In the erlotinib group, TRAE was less in the group targeted therapy combined chemotherapy than in the control group with chemotherapy agents alone, especially hand–foot syndrome, which occurred in up to 20 cases in grade 3/4 but not in the combination group. A total of 27 cases of rash occurred in the group with cetuximab and none in the group with chemotherapy alone. Moreover, with cetuximab, the incidence of TRAE (n = 72) was greater than in the placebo group (n = 33). TRAE incidence was higher in both Ramucirumab and Merestinib compared to the placebo group. TRAE was also higher with panitumumab (n = 82) compared to chemotherapy alone (n =33), where skin toxicity was 36 cases. In the trial of panitumumab, the combination was higher than chemotherapy alone. In the trial of Moehler et al. [23], there were 18 cases of grade 3/4 TRAE in the sorafenib group, while there were 30 cases in the chemotherapy alone group, which is more than in the combination group.

Table 7.
Treatment-Related Adverse Events of targeted therapies.
3.9.2. TRAE of Immunotherapies
Grade 3–4 adverse reactions to immunotherapies are summarised in Table 8 below. Sun (2018) [44] showed no significant difference in TRAEs between the monotherapy and combination groups. the TOPAZ-1 trial by D. Y. Oh [50] concluded that the incidence of grade 3/4 adverse reactions was lower in the durvalumab group than in the placebo group. The incidence of grade 3/4 treatment-related adverse events (TRAEs) was 62.7% in the durvalumab-treated group and 64.9% in the placebo-treated group. The rates of TRAEs leading to treatment discontinuation were 8.9% and 11.4%, respectively.

Table 8.
Treatment-related adverse events of immunotherapies.
3.9.3. TRAE of Combined Therapies
In the study by J. Zhou et al. [52] 43% of patients had a TRAE of grade 3 or higher. In the trial of Q. Zhang et al. [53] 84.2% of patients had one TRAE. Fatigue (n = 14), anorexia (n = 8), increased alanine aminotransferase (ALT) (n = 7) or aspartate aminotransferase (AST) (n = 7), rash (n = 6), hypertension (n = 5), and hoarseness (n = 5), were the most common TRAEs of any grade. An amount of 34.2% of patients had grade 3 TRAEs, the most prevalent of which were fatigue (n = 5) and hypertension (n = 3). One patient experienced a grade 4 cerebral hemorrhage as a result of hypertension, while five (13.9%) and one (2.8%) patient experienced dose reductions and treatment suspensions as a result of TRAEs. Due to Lenvatinib-related adverse effects, the dose of four individuals was reduced from 8 mg to 4 mg per day. Due to treatment-related cerebral bleeding, one patient terminated Lenvatinib plus PD-1 inhibitor therapy. There were six postoperative problems among patients who had resection, including two cases of biliary leakage, two cases of pleural effusion, one case of delayed liver function recovery, and one incidence of upper gastrointestinal haemorrhage (Table 9).

Table 9.
Treatment-related adverse event of combined therapies.
4. Discussion
This study provides an up-to-date, evidence-based systematic review of all clinical trials published between 2010 and 2022 that include all types of BTC. A rigorous quality assessment and a detailed description of trial design, inclusion criteria, characteristics, control arms, and outcomes served as the foundation for this work. Out of the total 709 studies identified, we examined 32 phase 2 studies and one phase 3 study.
From 2010 to 2020, 18 of the 33 studies of targeted or immunologic agents as first-line agents in patients with BTC had positive results. According to the meta-analyses, the effect of immunotherapy in combination with chemotherapy on patients’ ORR was statistically significant.
None of the four trials of sorafenib (Khoueiry (2012) [24], Khoueiry (2014) [25], Lee (2012) [33], and Moehler (2014) [23]) showed evidence of efficacy of sorafenib as a first-line agent in combination with chemotherapy. Lowery (2019) [37] showed that binimetinib could be safely combined with gemcitabine and cisplatin in advanced BTC. However, the observed efficacy signal was modest and not superior to using gemcitabine plus cisplatin alone. The combination of panitumumab with chemotherapy did not improve ORR, PFS, or OS in patients with advanced BTC with KRAS WT. According to a meta-analysis by Vogel (2018) [40], EGFR receptor antagonists did not reveal a benefit on sustained patient survival compared to chemotherapy alone only benefit. Therefore, no further studies investigating the combination of chemotherapy with anti-EGFR antibodies are needed. In contrast, Lau (2018) [28] showed that Everolimus showed clinical activity as first-line monotherapy for advanced BTC in unselected patients.
In three phase 2 trials, sorafenib did not improve survival in this setting. Similarly, other EGFRs, such as Vandetanib and Cetuximab, also failed to improve the prognosis of patients. All these trials suggest that adding EGFR-targeting agents to GEMOX is feasible and safe but ineffective. The lack of efficacy may be related to the heterogeneity of the target population for advanced BTC, the suboptimal treatments explored, or the need for alternative endpoints after survival.
From the results, it appears that Malka (2014) [29], Lee (2013) [32], Valle (2020) [20], and Leone (2016) [36] all showed that targeted agents were able to improve PFS compared to chemotherapy alone. Still, only one agent, Merestinib, showed a longer OS, while Oh (2020) [50] achieved median OS of 18.1 and 20.7 months among immunotherapies.
Two studies on the combination of immunotherapy and targeted therapy were included. Preliminary data showed that lenvatinib in combination with PD-1 inhibitors showed some efficacy in patients with advanced ICC. Both pembrolizumab and nivolumab showed antitumour effects when combined with lenvatinib. The effect of this combined regimen on overall survival in individuals with advanced ICC is still being studied in clinical trials.
Despite the low response rate of targeted and immunotherapy in BTC and the scarcity of clinical trial data, more research is needed, and better individualised therapy as well as drug combinations may be the way forward for such promising antitumour agents.
It needs to indicate that, in the included studies, outcome data were not counted separately according to the patient’s site of tumour development. They therefore could not be compared based on differences in the anatomical characteristics of the biliary tract.
BTC has an immunogenic profile, implying that immunotherapy is promising. However, current studies show that immune checkpoint inhibitors have limited activity in first-line therapy. With three drugs approved for marketing as second-line therapy, targeted agents have shown some success, but evidence of efficacy as first-line therapy is still lacking. More high-quality RCTs based on patient target genotyping are required to investigate the efficacy of using targeted agents as first-line BTC therapy. This article summarises and analyses current clinical trials in which immune or targeted agents have been added to standard BTC treatment as first-line therapy. Most clinical trials for targeted or immune agents as first-line treatments for BTC are still in the early stages, and future results will provide more evidence for future research. Future multi-institutional clinical trials should allow for large-scale studies that stratify patients based on anatomical subtype and genetic drivers to predict response and prognosis to new treatment regimens.
The drawback of this study is that our conclusions are based on some unadjusted analyses and may be influenced by additional confounding factors, including gender, age, genotypic mutation status, prior systemic medication, and other characteristics. Second, we were unable to conduct further subgroup analyses to assess the efficacy and safety of immunotherapy or targeted therapies in patients with various conditions due to a lack of etiological data.
5. Conclusions
This study demonstrates that immunotherapy combined with chemotherapy as first-line treatment can provide survival benefits by improving the objective response rate for patients with unresectable BTC. The potential for combination therapy to become a new trend in clinical treatment is promising. However, because the research design of existing clinical trials is insufficient, more comparable and high-quality investigations of regimens based on immunotherapies or targeted therapy as first-line treatment are required.
Author Contributions
X.Y. contributed to the study selection and data extraction. X.Y. and H.Z. contributed to data analysis. X.Y., H.Z., Y.L., C.O.L.U. and H.H. participated in study design, drafting and revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the University of Macau (MYRG2020-00230-ICMS).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We would like to thank the suggestions from our colleagues at the University of Macau.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Appendix A
Appendix A.1. Full Search Strategy for PubMed
(biliary tract cancer OR cholangiocarcinoma OR CCA OR BTC OR iCC OR biliary tract cancer) AND (IDH OR isocitrate dehydrogenase OR ivosidenib OR AG-120 OR Tibsovo OR BRAF OR B-RAF OR Erdafitinib OR JNJ-42756493 OR Balversa OR Futibatinib OR TAS-120 OR Infigratinib OR Truseltiq OR NVP-BGJ398 OR Pemigatinib OR Pemazyre OR INCB054828 OR Entrectinib OR Rozlytrek OR RXDX-101 OR NMS-E628 OR Gunagratinib OR ICP-192 OR Lenvatinib OR E 7080 OR monomethanesulfonate OR Lenvima OR ER-203492-00 OR Dabrafenib OR GSK 2118436 OR Trametinib OR JTP 74057 OR GSK 1120212 OR NTRK OR Entrectinib OR Rozlytrek OR RXDX-101 OR NMS-E628 OR Larotrectinib OR BAY2757556 OR LOXO101 OR ARRY470 OR Vitrakvipd-1 OR PD-1 OR pd-l1 OR pd-l2 OR programmed cell death receptor OR programmed cell death 1 receptor OR programmed cell death 2 receptor OR Pembrolizumab OR SCH-900475 OR Keytruda OR MK-3475 OR Lambrolizumab OR Nivolumab OR Opdivo OR ONO 4538 OR MDX 1106 OR BMS 936558 OR Bintrafusp alfa OR M7824) AND (random* OR randomised OR randomised OR prospective OR controlled clinical trial OR random allocation OR randomly OR RCT OR cohort OR double-blinded OR single-blinded OR placebo OR clinical trial).
Appendix A.2. Full Search Strategy for Web of Science
TS = (biliary tract cancer OR cholangiocarcinoma OR CCA OR BTC OR iCC OR biliary tract cancer) AND TS = (IDH OR isocitrate dehydrogenase OR ivosidenib OR AG-120 OR Tibsovo OR BRAF OR B-RAF OR Erdafitinib OR JNJ-42756493 OR Balversa OR Futibatinib OR TAS-120 OR Infigratinib OR Truseltiq OR NVP-BGJ398 OR Pemigatinib OR Pemazyre OR INCB054828 OR Entrectinib OR Rozlytrek OR RXDX-101 OR NMS-E628 OR Gunagratinib OR ICP-192 OR Lenvatinib OR E 7080 OR monomethanesulfonate OR Lenvima OR ER-203492-00 OR Dabrafenib OR GSK 2118436 OR Trametinib OR JTP 74057 OR GSK 1120212 OR NTRK OR Entrectinib OR Rozlytrek OR RXDX-101 OR NMS-E628 OR Larotrectinib OR BAY2757556 OR LOXO101 OR ARRY470 OR Vitrakvipd-1 OR PD-1 OR pd-l1 OR pd-l2 OR programmed cell death receptor OR programmed cell death 1 receptor OR programmed cell death 2 receptor OR Pembrolizumab OR SCH-900475 OR Keytruda OR MK-3475 OR Lambrolizumab OR Nivolumab OR Opdivo OR ONO 4538 OR MDX 1106 OR BMS 936558 OR Bintrafusp alfa OR M7824) AND TS = (random* OR randomised OR randomised OR prospective OR controlled clinical trial OR random allocation OR randomly OR RCT OR cohort study OR double-blinded OR single-blinded OR placebo).
Appendix A.3. Full Search Strategy for Web of Embase
‘biliary tract cancer’:ab,ti OR ‘Cholangiocarcinoma’:ab,ti OR ‘CCA’:ab,ti OR ‘BTC’:ab,ti OR ‘iCC’:ab,ti OR ‘biliary tract cancer’:ab,ti.
IDH’:ab,ti OR ‘isocitrate dehydrogenase’:ab,ti OR ‘Ivosidenib’:ab,ti OR ‘AG-120’:ab,ti OR ‘Tibsovo’:ab,ti OR ‘BRAF’:ab,ti OR ‘Erdafitinib’:ab,ti OR ‘JNJ-42756493’:ab,ti OR ‘Balversa’:ab,ti OR ‘Futibatinib’:ab,ti OR ‘TAS-120’:ab,ti OR ‘Infigratinib’:ab,ti OR ‘Truseltiq’:ab,ti OR ‘NVP-BGJ398’:ab,ti OR ‘Pemigatinib’:ab,ti OR ‘Pemazyre’:ab,ti OR ‘INCB054828’:ab,ti OR ‘Entrectinib’:ab,ti OR ‘Rozlytrek’:ab,ti OR ‘RXDX-101’:ab,ti OR ‘NMS-E628’:ab,ti OR ‘Gunagratinib’:ab,ti OR ‘ICP-192’:ab,ti OR ‘Lenvatinib’:ab,ti OR ‘E-7080’:ab,ti OR ‘Monomethanesulfonate’:ab,ti OR ‘Lenvima’:ab,ti OR ‘ER-203492-00’:ab,ti OR ‘Dabrafenib’:ab,ti OR ‘GSK 2118436’:ab,ti OR ‘Trametinib’:ab,ti OR ‘JTP 74057’:ab,ti OR ‘GSK 1120212’:ab,ti OR ‘NTRK’:ab,ti OR ‘Entrectinib’:ab,ti OR ‘Rozlytrek’:ab,ti OR ‘RXDX-101’:ab,ti OR ‘NMS-E628’:ab,ti OR ‘Larotrectinib’:ab,ti OR ‘BAY2757556’:ab,ti OR ‘LOXO101’:ab,ti OR ‘ARRY470’:ab,ti OR ‘Vitrakvipd-1’:ab,ti OR ‘PD-1’:ab,ti OR ‘pd-l1’:ab,ti OR ‘pd-l2’:ab,ti OR ‘programmed cell death receptor’:ab,ti OR ‘programmed cell death 1 receptor’:ab,ti OR ‘programmed cell death 2 receptor’:ab,ti OR ‘Pembrolizumab’:ab,ti OR ‘SCH-900475’:ab,ti OR ‘Keytruda’:ab,ti OR ‘MK-3475’:ab,ti OR ‘Lambrolizumab’:ab,ti OR ‘Nivolumab’:ab,ti OR ‘Opdivo’:ab,ti OR ‘ONO 4538’:ab,ti OR ‘MDX 1106’:ab,ti OR ‘BMS 936558’:ab,ti OR ‘Bintrafusp alfa’:ab,ti OR ‘M7824’:ab,ti’random*’:ab,ti OR ‘randomised’:ab,ti OR ‘randomized’:ab,ti OR ‘Prospective’:ab,ti OR ‘controlled clinical trial’:ab,ti OR ‘random allocation’:ab,ti OR ‘Randomly’:ab,ti OR ‘RCT’:ab,ti OR ‘Cohort’:ab,ti OR ‘double-blinded’:ab,ti OR ‘single-blinded’:ab,ti OR ‘Placebo’:ab,ti OR ‘clinical trial’:ab,ti.
Appendix A.4. Full Search Strategy for Cochrane
(biliary tract cancer):ab,ti,kw OR (Cholangiocarcinoma):ab,ti,kw OR (CCA):ab,ti,kw OR (BTC):ab,ti,kw OR (iCC):ab,ti,kw OR (biliary tract cancer):ab,ti,kw (IDH):ab,ti,kw OR (isocitrate dehydrogenase):ab,ti,kw OR (Ivosidenib):ab,ti,kw OR (AG-120):ab,ti,kw OR (Tibsovo):ab,ti,kw OR (BRAF):ab,ti,kw OR (Erdafitinib):ab,ti,kw OR (JNJ-42756493):ab,ti,kw OR (Balversa):ab,ti,kw OR (Futibatinib):ab,ti,kw OR (TAS-120):ab,ti,kw OR (Infigratinib):ab,ti,kw OR (Truseltiq):ab,ti,kw OR (NVP-BGJ398):ab,ti,kw OR (Pemigatinib):ab,ti,kw OR (Pemazyre):ab,ti,kw OR (INCB054828):ab,ti,kw OR (Entrectinib):ab,ti,kw OR (Rozlytrek):ab,ti,kw OR (RXDX-101):ab,ti,kw OR (NMS-E628):ab,ti,kw OR (Gunagratinib):ab,ti,kw OR (ICP-192):ab,ti,kw OR (Lenvatinib):ab,ti,kw OR (E-7080):ab,ti,kw OR (Monomethanesulfonate):ab,ti,kw OR (Lenvima):ab,ti,kw OR (Dabrafenib):ab,ti,kw OR (GSK 2118436):ab,ti,kw OR (Trametinib):ab,ti,kw OR (JTP 74057):ab,ti,kw OR (GSK 1120212):ab,ti,kw OR (NTRK):ab,ti,kw OR (Entrectinib):ab,ti,kw OR (Rozlytrek):ab,ti,kw OR (RXDX-101):ab,ti,kw OR (NMS-E628):ab,ti,kw OR (Larotrectinib):ab,ti,kw OR (BAY2757556):ab,ti,kw OR (LOXO101):ab,ti,kw OR (ARRY470):ab,ti,kw OR (Vitrakvipd-1):ab,ti,kw OR (PD-1):ab,ti,kw OR (pd-l1):ab,ti,kw OR (pd-l2):ab,ti,kw OR (programmed cell death receptor):ab,ti,kw OR (programmed cell death 1 receptor):ab,ti,kw OR (programmed cell death 2 receptor):ab,ti,kw OR (Pembrolizumab):ab,ti,kw OR (SCH-900475):ab,ti,kw OR (Keytruda):ab,ti,kw OR (MK-3475):ab,ti,kw OR (Lambrolizumab):ab,ti,kw OR (Nivolumab):ab,ti,kw OR (Opdivo):ab,ti,kw OR (ONO 4538):ab,ti,kw OR (MDX 1106):ab,ti,kw OR (BMS 936558):ab,ti,kw OR (Bintrafusp alfa):ab,ti,kw OR (M7824):ab,ti,kw (random*):ab,ti,kw OR (randomised):ab,ti,kw OR (randomized):ab,ti,kw OR (Prospective):ab,ti,kw OR (controlled clinical trial):ab,ti,kw OR (random allocation):ab,ti,kw OR (Randomly):ab,ti,kw OR (RCT):ab,ti,kw OR (Cohort):ab,ti,kw OR (double-blinded):ab,ti,kw OR (single-blinded):ab,ti,kw OR (Placebo):ab,ti,kw OR (clinical trial):ab,ti,kw.
References
- Altekruse, S.F.; Devesa, S.S.; Dickie, L.A.; McGlynn, K.A.; Kleiner, D.E. Histological classification of liver and intrahepatic bile duct cancers in SEER registries. J. Regist. Manag. 2011, 38, 201–205. [Google Scholar]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.J.; Jabbour, S.; Parekh, N.; Lin, Y.; Moss, R.A. Increasing mortality in the United States from cholangiocarcinoma: An analysis of the National Center for Health Statistics Database. BMC Gastroenterol. 2016, 16, 117. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Bathon, M.; Saborowski, A. Immunotherapies in clinical development for biliary tract cancer. Expert Opin. Investig. Drugs 2020, 30, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.W.; Lamarca, A.; Goyal, L.; Barriuso, J.; Zhu, A.X. New Horizons for Precision Medicine in Biliary Tract CancersPrecision Medicine in BTC. Cancer Discov. 2017, 7, 943–962. [Google Scholar] [CrossRef] [PubMed]
- Ahn, D.H.; Bekaii-Saab, T. Biliary cancer: Intrahepatic cholangiocarcinoma vs. extrahepatic cholangiocarcinoma vs. gallbladder cancers: Classification and therapeutic implications. J. Gastrointest. Oncol. 2017, 8, 293–301. [Google Scholar] [CrossRef]
- Kang, S.; El-Rayes, B.F.; Akce, M. Evolving Role of Immunotherapy in Advanced Biliary Tract Cancers. Cancers 2022, 14, 1748. [Google Scholar] [CrossRef]
- Jiang, Y.; Zeng, Z.; Zeng, J.; Liu, C.; Qiu, J.; Li, Y.; Tang, J.; Mo, N.; Du, L.; Ma, J. Efficacy and Safety of First-Line Chemotherapies for Patients With Advanced Biliary Tract Carcinoma: A Systematic Review and Network Meta-Analysis. Front. Oncol. 2021, 11, 3739. [Google Scholar] [CrossRef]
- Rizzo, A.; Frega, G.; Ricci, A.D.; Palloni, A.; Abbati, F.; De Lorenzo, S.; Deserti, M.; Tavolari, S.; Brandi, G. Anti-EGFR Monoclonal Antibodies in Advanced Biliary Tract Cancer: A Systematic Review and Meta-analysis. Vivo 2020, 34, 479–488. [Google Scholar] [CrossRef]
- Mavros, M.N.; Economopoulos, K.P.; Alexiou, V.G.; Pawlik, T.M. Treatment and Prognosis for Patients With Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg. 2014, 149, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 89, 105906. [Google Scholar]
- Methley, A.M.; Campbell, S.; Chew-Graham, C.; McNally, R.; Cheraghi-Sohi, S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv. Res. 2014, 14, 579. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.B.; Hoogstraten, B.; Staquet, M.; Winkler, A. Reporting results of cancer treatment. Cancer 1981, 47, 207–214. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: New York, NY, USA, 2019. [Google Scholar]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomised studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Évid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef]
- Valle, J.; Kelley, R.; Furuse, J.; Edeline, J.; Finn, R.; Ren, Z.; Su, S.-C.; Malhotra, U.; Siegel, A.; Vogel, A. 78TiP KEYNOTE-966 trial in progress: Pembrolizumab plus gemcitabine and cisplatin for advanced biliary tract cancer. Ann. Oncol. 2020, 31, S270–S271. [Google Scholar] [CrossRef]
- Santoro, A.; Gebbia, V.; Pressiani, T.; Testa, A.; Personeni, N.; Bajardi, E.A.; Foa, P.; Buonadonna, A.; Bencardino, K.; Barone, C.; et al. A randomized, multicenter, phase II study of vandetanib monotherapy versus vandetanib in combination with gemcitabine versus gemcitabine plus placebo in subjects with advanced biliary tract cancer: The VanGogh study. Ann. Oncol. 2014, 26, 542–547. [Google Scholar] [CrossRef]
- Valle, J.W.; Wasan, H.; Lopes, A.; Backen, A.C.; Palmer, D.H.; Morris, K.; Duggan, M.; Cunningham, D.; Anthoney, D.A.; Corrie, P.; et al. Cediranib or placebo in combination with cisplatin and gemcitabine chemotherapy for patients with advanced biliary tract cancer (ABC-03): A randomised phase 2 trial. Lancet Oncol. 2015, 16, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Moehler, M.; Maderer, A.; Schimanski, C.; Kanzler, S.; Denzer, U.; Kolligs, F.; Ebert, M.; Distelrath, A.; Geissler, M.; Trojan, J.; et al. Gemcitabine plus sorafenib versus gemcitabine alone in advanced biliary tract cancer: A double-blind placebo-controlled multicentre phase II AIO study with biomarker and serum programme. Eur. J. Cancer 2014, 50, 3125–3135. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Rankin, C.J.; Ben-Josef, E.; Lenz, H.-J.; Gold, P.J.; Hamilton, R.D.; Govindarajan, R.; Eng, C.; Blanke, C.D. SWOG 0514: A phase II study of sorafenib in patients with unresectable or metastatic gallbladder carcinoma and cholangiocarcinoma. Investig. New Drugs 2011, 30, 1646–1651. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Rankin, C.; Siegel, A.B.; Iqbal, S.; Gong, I.-Y.; Micetich, K.; Kayaleh, O.R.; Lenz, H.-J.; Blanke, C.D. S0941: A phase 2 SWOG study of sorafenib and erlotinib in patients with advanced gallbladder carcinoma or cholangiocarcinoma. Br. J. Cancer 2014, 110, 882–887. [Google Scholar] [CrossRef]
- Hezel, A.F.; Noel, M.S.; Allen, J.N.; A Abrams, T.; Yurgelun, M.B.; E Faris, J.; Goyal, L.K.; Clark, J.W.; Blaszkowsky, L.S.; E Murphy, J.; et al. Phase II study of gemcitabine, oxaliplatin in combination with panitumumab in KRAS wild-type unresectable or metastatic biliary tract and gallbladder cancer. Br. J. Cancer 2014, 111, 430–436. [Google Scholar] [CrossRef]
- Gruenberger, B.; Schueller, J.; Heubrandtner, U.; Wrba, F.; Tamandl, D.; Kaczirek, K.; Roka, R.; Freimann-Pircher, S.; Gruenberger, T. Cetuximab, gemcitabine, and oxaliplatin in patients with unresectable advanced or metastatic biliary tract cancer: A phase 2 study. Lancet Oncol. 2010, 11, 1142–1148. [Google Scholar] [CrossRef]
- Lau, D.K.; Tay, R.Y.; Yeung, Y.H.; Chionh, F.; Mooi, J.; Murone, C.; Skrinos, E.; Price, T.J.; Mariadason, J.M.; Tebbutt, N.C. Phase II study of everolimus (RAD001) monotherapy as first-line treatment in advanced biliary tract cancer with biomarker exploration: The RADiChol Study. Br. J. Cancer 2018, 118, 966–971. [Google Scholar] [CrossRef]
- Malka, D.; Cervera, P.; Foulon, S.; Trarbach, T.; de la Fouchardière, C.; Boucher, E.; Fartoux, L.; Faivre, S.; Blanc, J.-F.; Viret, F.; et al. Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): A randomised, open-label, non-comparative phase 2 trial. Lancet Oncol. 2014, 15, 819–828. [Google Scholar] [CrossRef]
- Sohal, D.; Mykulowycz, K.; Uehara, T.; Teitelbaum, U.; Damjanov, N.; Giantonio, B.; Carberry, M.; Wissel, P.; Jacobs-Small, M.; O’Dwyer, P.; et al. A phase II trial of gemcitabine, irinotecan and panitumumab in advanced cholangiocarcinoma. Ann. Oncol. 2013, 24, 3061–3065. [Google Scholar] [CrossRef]
- Borbath, I.; Ceratti, A.; Verslype, C.; Demols, A.; Delaunoit, T.; Laurent, S.; Deleporte, A.; Vergauwe, P.; Van Maanen, A.; Sempoux, C.; et al. Combination of gemcitabine and cetuximab in patients with advanced cholangiocarcinoma: A phase II study of the Belgian Group of Digestive Oncology. Ann. Oncol. 2013, 24, 2824–2829. [Google Scholar] [CrossRef]
- Lee, J.K.; Capanu, M.; O’Reilly, E.M.; Ma, J.; Chou, J.F.; Shia, J.; Katz, S.; Gansukh, B.; Reidylagunes, D.; Segal, N.H.; et al. A phase II study of gemcitabine and cisplatin plus sorafenib in patients with advanced biliary adenocarcinomas. Br. J. Cancer 2013, 109, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, S.H.; Chang, H.M.; Kim, J.S.; Choi, H.J.; Lee, M.A.; Jang, J.S.; Jeung, H.C.; Kang, J.H.; Lee, H.W.; et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2012, 13, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Hsu, C.; Chiang, N.J.; Tsai, C.S.; Tsou, H.H.; Huang, S.F.; Bai, L.Y.; Chang, I.C.; Shiah, H.S.; Ho, C.L.; et al. A KRAS mutation status-stratified randomized phase II trial of gemcitabine and oxaliplatin alone or in combination with cetuximab in advanced biliary tract cancer. Ann. Oncol. 2015, 26, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.; Lindebjerg, J.; Ploen, J.; Hansen, T.; Jakobsen, A. Phase II marker-driven trial of panitumumab and chemotherapy in KRAS wild-type biliary tract cancer. Ann. Oncol. 2012, 23, 2341–2346. [Google Scholar] [CrossRef] [PubMed]
- Leone, F.; Marino, D.; Cereda, S.; Filippi, R.; Belli, C.; Spadi, R.; Nasti, G.; Montano, M.; Amatu, A.; Aprile, G.; et al. Panitumumab in combination with gemcitabine and oxaliplatin does not prolong survival in wild-type KRAS advanced biliary tract cancer: A randomized phase 2 trial (V ecti-BIL study). Cancer 2016, 122, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Lowery, M.A.; Bradley, M.; Chou, J.F.; Capanu, M.; Gerst, S.; Harding, J.J.; El Dika, I.; Berger, M.; Zehir, A.; Ptashkin, R.; et al. Binimetinib plus Gemcitabine and Cisplatin Phase I/II Trial in Patients with Advanced Biliary Cancers. Clin. Cancer Res. 2019, 25, 937–945. [Google Scholar] [CrossRef]
- Iyer, R.V.; Pokuri, V.K.; Groman, A.; Ma, W.W.; Malhotra, U.; Iancu, D.M.; Grande, C.; Saab, T.B. A Multicenter Phase II Study of Gemcitabine, Capecitabine, and Bevacizumab for Locally Advanced or Metastatic Biliary Tract Cancer. Am. J. Clin. Oncol. 2018, 41, 649–655. [Google Scholar] [CrossRef]
- Lubner, S.J.; Mahoney, M.R.; Kolesar, J.L.; LoConte, N.K.; Kim, G.P.; Pitot, H.C.; Philip, P.A.; Picus, J.; Yong, W.-P.; Horvath, L.; et al. Report of a Multicenter Phase II Trial Testing a Combination of Biweekly Bevacizumab and Daily Erlotinib in Patients With Unresectable Biliary Cancer: A Phase II Consortium Study. J. Clin. Oncol. 2010, 28, 3491–3497. [Google Scholar] [CrossRef]
- Vogel, A.; Kasper, S.; Bitzer, M.; Block, A.; Sinn, M.; Schulze-Bergkamen, H.; Moehler, M.; Pfarr, N.; Endris, V.; Goeppert, B.; et al. PICCA study: Panitumumab in combination with cisplatin/gemcitabine chemotherapy in KRAS wild-type patients with biliary cancer—a randomised biomarker-driven clinical phase II AIO study. Eur. J. Cancer 2018, 92, 11–19. [Google Scholar] [CrossRef]
- Oh, D.-Y.; de Braud, F.; Bridgewater, J.; Furuse, J.; Hsu, C.-H.; Ikeda, M.; Javle, M.; Moehler, M.; Park, J.; Shen, L.; et al. 79TiP A phase II/III, randomized, placebo-controlled study of bintrafusp alfa with gemcitabine plus cisplatin as first-line treatment of biliary tract cancer. Ann. Oncol. 2020, 31, S271–S272. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Lee, K.-H.; Lee, D.-W.; Yoon, J.; Kim, T.-Y.; Bang, J.-H.; Nam, A.-R.; Oh, K.-S.; Kim, J.-M.; Lee, Y.; et al. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: An open-label, single-centre, phase 2 study. Lancet Gastroenterol. Hepatol. 2022, 7, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Huang, S.; Chen, J.; Yu, F.; Zhang, L.; Xiang, X.; Deng, J.; Fang, Z.; Li, J.; Xiong, J. An Assessment of Combination of the Camrelizumab With Chemotherapy in Metastatic Biliary Tract Cancers. Cancer Control 2021, 28, 10732748211017165. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Ma, J.; Han, C.; Wang, J.; Qian, Y.; Chen, G.; Li, X.; Zhang, J.; Song, J.; Zhao, X.; et al. Anti-PD-1 therapy combined with chemotherapy or target therapy in patients with advanced biliary tract cancer in real-world clinical setting. Ann. Oncol. 2018, 29, viii437. [Google Scholar] [CrossRef]
- Gou, M.; Zhang, Y.; Liu, T.; Si, H.; Wang, Z.; Yan, H.; Qian, N.; Dai, G. PD-1 Inhibitors Could Improve the Efficacy of Chemotherapy as First-Line Treatment in Biliary Tract Cancers: A Propensity Score Matching Based Analysis. Front. Oncol. 2021, 11, 2202. [Google Scholar] [CrossRef]
- Oh, D.Y.; Lee, K.H.; Lee, D.W.; Kim, T.Y.; Bang, J.H.; Nam, A.R.; Lee, Y.; Zhang, Q.; Rebelatto, M.; Li, W.; et al. Phase II study assessing tolerability, efficacy, and biomarkers for durvalumab (D) ± tremelimumab (T) and gemcitabine/cisplatin (GemCis) in chemo-naïve advanced biliary tract cancer (aBTC). J. Clin. Oncol. 2020, 38, 4520. [Google Scholar] [CrossRef]
- Chen, X.; Wu, X.; Wu, H.; Gu, Y.; Shao, Y.; Shao, Q.; Zhu, F.; Li, X.; Qian, X.; Hu, J.; et al. Camrelizumab plus gemcitabine and oxaliplatin (GEMOX) in patients with advanced biliary tract cancer: A single-arm, open-label, phase II trial. J. Immunother. Cancer 2020, 8, e001240. [Google Scholar] [CrossRef]
- Chiang, N.-J.; Bai, L.-Y.; Huang, C.-J.; Chen, S.-C.; Hsiao, C.-F.; Shan, Y.-S.; Su, Y.-Y.; Chen, L.; Chen, M.-H. 49P A phase II trial of nivolumab and gemcitabine and S-1 as the first-line treatment in patients with advanced biliary tract cancer. Ann. Oncol. 2021, 32, S377. [Google Scholar] [CrossRef]
- Sahai, V.; Griffith, K.A.; Beg, M.S.; Shaib, W.L.; Mahalingam, D.; Zhen, D.B.; Deming, D.A.; Dey, S.; Mendiratta-Lala, M.; Zalupski, M. A multicenter randomised phase II study of nivolumab in combination with gemcitabine/cisplatin or ipilimumab as first-line therapy for patients with advanced unresectable biliary tract cancer (BilT-01). J. Clin. Oncol. 2020, 38, 4582. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Chen, L.-T.; He, A.; Okusaka, T.; Qin, S.; Chin, S.; Rokutanda, N.; Uchinda, H.; Vogel, A.; Valle, J.; et al. A phase III, randomized, double-blind, placebo-controlled, international study of durvalumab in combination with gemcitabine plus cisplatin for patients with advanced biliary tract cancers: TOPAZ-1. Ann. Oncol. 2019, 30, v319. [Google Scholar] [CrossRef]
- Li, W.; Yu, Y.; Xu, X.; Guo, X.; Wang, Y.; Li, Q.; Wang, Y.; Cui, Y.; Liu, H.; Hao, Q.; et al. Toripalimab with chemotherapy as first-line treatment for advanced biliary tract tumors: Update analytic results of an open-label phase II clinical study (JS001-ZS-BC001). J. Clin. Oncol. 2021, 39, e16170. [Google Scholar] [CrossRef]
- Zhu, A.X.; Meyerhardt, J.A.; Blaszkowsky, L.S.; Kambadakone, A.R.; Muzikansky, A.; Zheng, H.; Clark, J.W.; Abrams, T.A.; Chan, J.A.; Enzinger, P.C.; et al. Efficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary-tract cancers and correlation of changes in 18-fluorodeoxyglucose PET with clinical outcome: A phase 2 study. Lancet Oncol. 2010, 11, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Fan, J.; Shi, G.; Huang, X.; Wu, D.; Yang, G.; Ge, N.; Hou, Y.; Sun, H.; He, Y.; et al. 56P Anti-PD1 antibody toripalimab, lenvatinib and gemox chemotherapy as first-line treatment of advanced and unresectable intrahepatic cholangiocarcinoma: A phase II clinical trial. Ann. Oncol. 2020, 31, S262–S263. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, X.; Wei, S.; Zhang, L.; Tian, Y.; Gao, Z.; Jin, M.; Yan, S. Lenvatinib Plus PD-1 Inhibitors as First-Line Treatment in Patients With Unresectable Biliary Tract Cancer: A Single-Arm, Open-Label, Phase II Study. Front. Oncol. 2021, 11, 751391. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).