The Lymphatic Endothelium in the Context of Radioimmuno-Oncology
Abstract
Simple Summary
Abstract
1. Introduction
2. Lymphatic Vessel Biology
3. Lymphatic Vessel Biology in Cancer
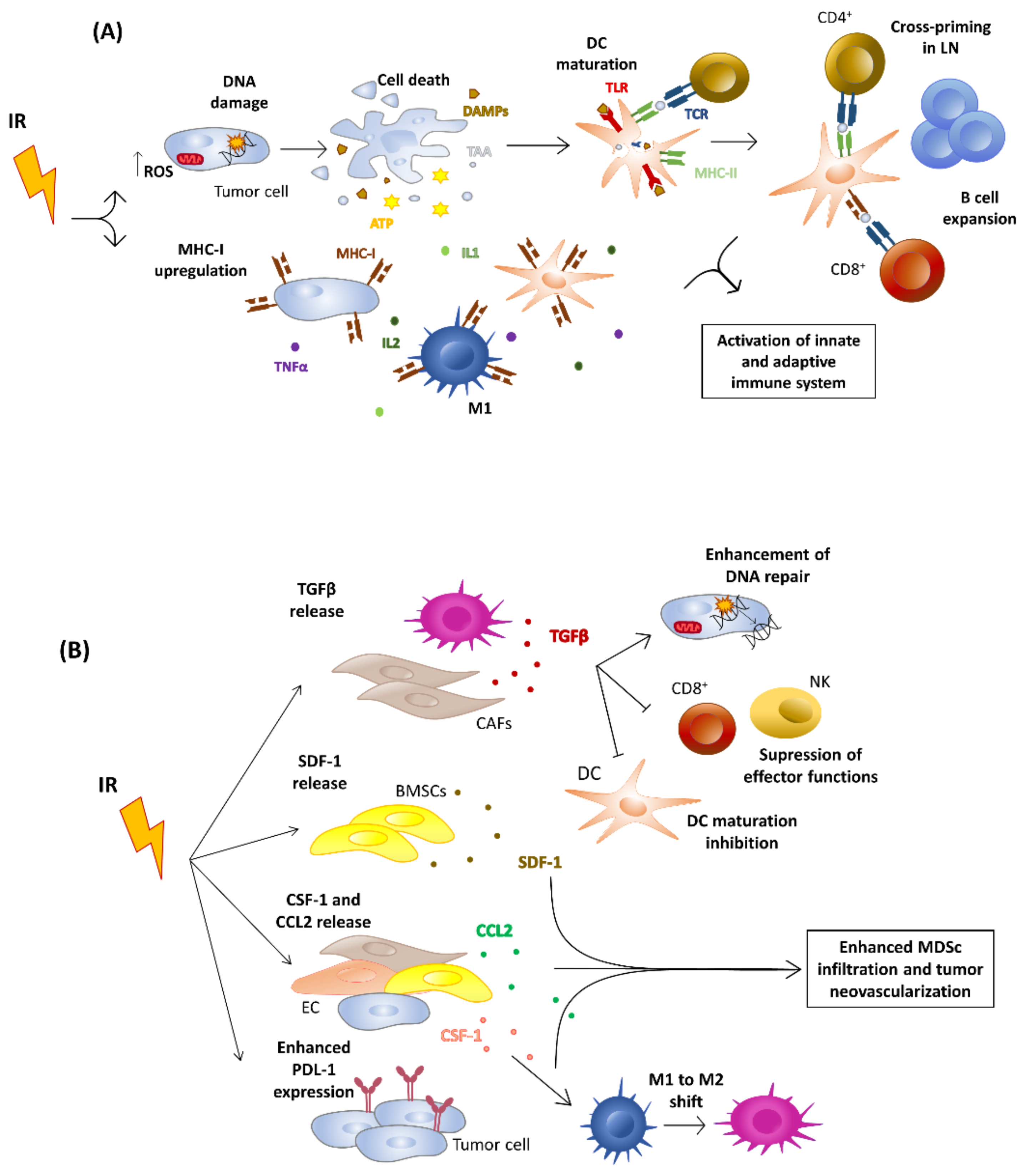
4. Effects of Ionizing Radiation on Tumor Niche
5. Effects of Ionizing Radiation on the Vasculature
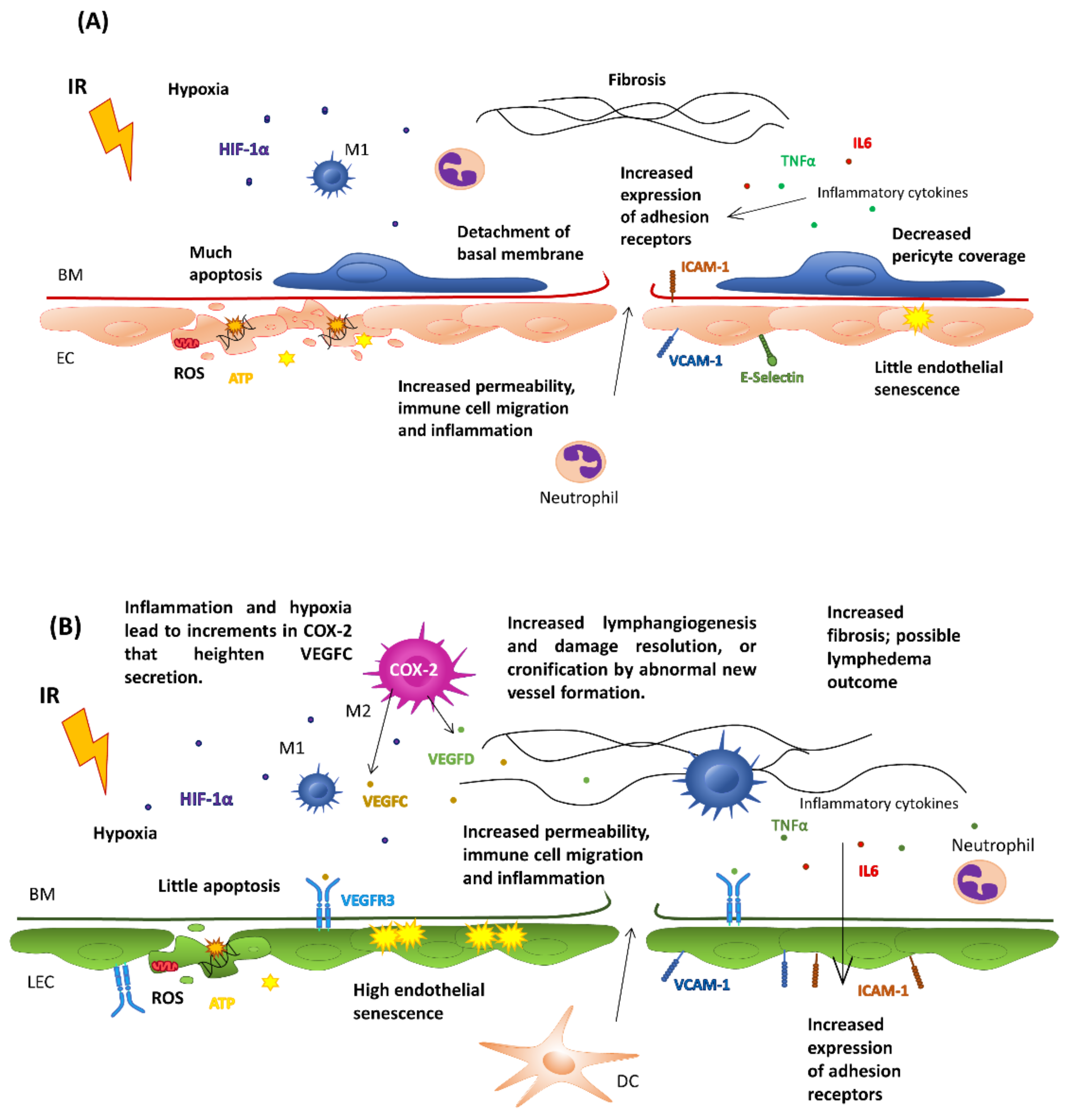
5.1. Effects on Healthy Endothelial Cells
5.2. Effects on Tumor Endothelial Cells
5.3. Effects on the Immune System
6. Effects of Radiotherapy on the Lymphatic Endothelium: Lymphedema
7. Future Perspectives Modeling Lymphatics for Immunotherapy
Author Contributions
Funding
Conflicts of Interest
References
- Applegate, K.E.; Rühm, W.; Wojcik, A.; Bourguignon, M.; Brenner, A.; Hamasaki, K.; Imai, T.; Imaizumi, M.; Imaoka, T.; Kakinuma, S.; et al. Individual response of humans to ionising radiation: Governing factors and importance for radiological protection. Radiat. Environ. Biophys. 2020, 59, 185–209. [Google Scholar] [CrossRef] [PubMed]
- Willers, H.; Azzoli, C.G.; Santivasi, W.; Xia, F. Basic Mechanisms of Therapeutic Resistance to Radiation and Chemotherapy in Lung Cancer. Cancer J. 2013, 19, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Singleton, D.C.; Macann, A.; Wilson, W.R. Therapeutic targeting of the hypoxic tumour microenvironment. Nat. Rev. Clin. Oncol. 2021, 18, 751–772. [Google Scholar] [CrossRef] [PubMed]
- Barker, H.E.; Paget, J.T.E.; Khan, A.; Harrington, K. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat. Rev. Cancer 2015, 15, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Krisnawan, V.E.; Stanley, J.A.; Schwarz, J.K.; DeNardo, D.G. Tumor Microenvironment as a Regulator of Radiation Therapy: New Insights into Stromal-Mediated Radioresistance. Cancers 2020, 12, 2916. [Google Scholar] [CrossRef]
- Liang, H.; Deng, L.; Hou, Y.; Meng, X.; Huang, X.; Rao, E.; Zheng, W.; Mauceri, H.; Mack, M.; Xu, M.; et al. Host STING-dependent MDSC mobilization drives extrinsic radiation resistance. Nat. Commun. 2017, 8, 1736. [Google Scholar] [CrossRef]
- Wennerberg, E.; Lhuillier, C.; Vanpouille-Box, C.; Pilones, K.A.; García-Martínez, E.; Rudqvist, N.P.; Formenti, S.C.; DeMaria, S. Barriers to Radiation-Induced In Situ Tumor Vaccination. Front. Immunol. 2017, 8, 229. [Google Scholar] [CrossRef]
- Walle, T.; Monge, R.M.; Cerwenka, A.; Ajona, D.; Melero, I.; Lecanda, F. Radiation effects on antitumor immune responses: Current perspectives and challenges. Ther. Adv. Med. Oncol. 2018, 10, 1758834017742575. [Google Scholar] [CrossRef]
- Fleischmann, M.; Glatzer, M.; Rödel, C.; Tselis, N. Radioimmunotherapy: Future prospects from the perspective of brachytherapy. J. Contemp. Brachytherapy 2021, 13, 458–467. [Google Scholar] [CrossRef]
- Sanmamed, M.F.; Berraondo, P.; Rodriguez-Ruiz, M.E.; Melero, I. Charting roadmaps towards novel and safe synergistic immunotherapy combinations. Nat. Cancer 2022, 3, 665–680. [Google Scholar] [CrossRef]
- Alcibar, O.L.; Candini, D.; López-Campos, F.; Antequera, M.A.; Macías, V.M.; Conde, A.J.; Pérez, A.R.; Morón, A.H.; Martínez, J.C.; Albiach, C.F.; et al. Time for radioimmunotherapy: An overview to bring improvements in clinical practice. Clin. Transl. Oncol. 2019, 21, 992–1004. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Gilligan, B.M.; Yuan, J.; Li, T. Current status and perspectives in translational biomarker research for PD-1/PD-L1 immune checkpoint blockade therapy. J. Hematol. Oncol. 2016, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef]
- Swamy, K. Vascular normalization and immunotherapy: Spawning a virtuous cycle. Front. Oncol. 2022, 12, 1002957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zarkada, G.; Yi, S.; Eichmann, A. Lymphatic Endothelial Cell Junctions: Molecular Regulation in Physiology and Diseases. Front. Physiol. 2020, 11, 509. [Google Scholar] [CrossRef]
- Karaman, S.; Leppänen, V.-M.; Alitalo, K. Vascular endothelial growth factor signaling in development and disease. Development 2018, 145, dev151019. [Google Scholar] [CrossRef]
- Breslin, J.W.; Yang, Y.; Scallan, J.P.; Sweat, R.S.; Adderley, S.P.; Murfee, W.L. Lymphatic Vessel Network Structure and Physiology. Compr. Physiol. 2018, 9, 207–299. [Google Scholar] [CrossRef]
- Pflicke, H.; Sixt, M. Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels. J. Exp. Med. 2009, 206, 2925–2935. [Google Scholar] [CrossRef]
- Jiang, X. Lymphatic vasculature in tumor metastasis and immunobiology. J. Zhejiang Univ. Sci. B 2019, 21, 3–11. [Google Scholar] [CrossRef]
- Dieterich, L.C.; Detmar, M. Tumor lymphangiogenesis and new drug development. Adv. Drug Deliv. Rev. 2016, 99, 148–160. [Google Scholar] [CrossRef]
- Alitalo, K.; Tammela, T.; Petrova, T.V. Lymphangiogenesis in development and human disease. Nature 2005, 438, 946–953. [Google Scholar] [CrossRef]
- Bhuiyan, M.; Kim, J.-C.; Hwang, S.-N.; Lee, M.-Y.; Kim, S. Ischemic tolerance is associated with VEGF-C and VEGFR-3 signaling in the mouse hippocampus. Neuroscience 2015, 290, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.L.; Volk-Draper, L.D.; Flister, M.J.; Ran, S. New Model of Macrophage Acquisition of the Lymphatic Endothelial Phenotype. PLoS ONE 2012, 7, e31794. [Google Scholar] [CrossRef] [PubMed]
- Heinolainen, K.; Karaman, S.; D’Amico, G.; Tammela, T.; Sormunen, R.; Eklund, L.; Alitalo, K.; Zarkada, G. VEGFR3 Modulates Vascular Permeability by Controlling VEGF/VEGFR2 Signaling. Circ. Res. 2017, 120, 1414–1425. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, M.T.; Witte, M.H. Lymphangiogenesis, lymphatic systemomics, and cancer: Context, advances and unanswered questions. Clin. Exp. Metastasis 2018, 35, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Gordon, E.; Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 2016, 17, 611–625. [Google Scholar] [CrossRef]
- Hsu, M.-C.; Pan, M.-R.; Hung, W.-C. Two Birds, One Stone: Double Hits on Tumor Growth and Lymphangiogenesis by Targeting Vascular Endothelial Growth Factor Receptor 3. Cells 2019, 8, 270. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, M.E.; Halford, M.M.; Roufail, S.; Williams, R.A.; Hibbs, M.L.; Grail, D.; Kubo, H.; Stacker, S.A.; Achen, M.G. Vascular Endothelial Growth Factor D Is Dispensable for Development of the Lymphatic System. Mol. Cell. Biol. 2005, 25, 2441–2449. [Google Scholar] [CrossRef]
- Paquet-Fifield, S.; Levy, S.M.; Sato, T.; Shayan, R.; Karnezis, T.; Davydova, N.; Nowell, C.J.; Roufail, S.; Ma, G.Z.-M.; Zhang, Y.-F.; et al. Vascular Endothelial Growth Factor-d Modulates Caliber and Function of Initial Lymphatics in the Dermis. J. Investig. Dermatol. 2013, 133, 2074–2084. [Google Scholar] [CrossRef]
- Wirzenius, M.; Tammela, T.; Uutela, M.; He, Y.; Odorisio, T.; Zambruno, G.; Nagy, J.A.; Dvorak, H.F.; Ylä-Herttuala, S.; Shibuya, M.; et al. Distinct vascular endothelial growth factor signals for lymphatic vessel enlargement and sprouting. J. Exp. Med. 2007, 204, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Nagy, J.A.; Dvorak, A.M.; Dvorak, H.F. Vascular Hyperpermeability, Angiogenesis, and Stroma Generation. Cold Spring Harb. Perspect. Med. 2012, 2, a006544. [Google Scholar] [CrossRef] [PubMed]
- Yoshimatsu, Y.; Miyazaki, H.; Watabe, T. Roles of signaling and transcriptional networks in pathological lymphangiogenesis. Adv. Drug Deliv. Rev. 2016, 99, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Förster, R.; Davalos-Misslitz, A.C.; Rot, A. CCR7 and its ligands: Balancing immunity and tolerance. Nat. Rev. Immunol. 2008, 8, 362–371. [Google Scholar] [CrossRef]
- Morfoisse, F.; Noel, A. Lymphatic and blood systems: Identical or fraternal twins? Int. J. Biochem. Cell Biol. 2019, 114, 105562. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.G. Leucocyte Trafficking via the Lymphatic Vasculature— Mechanisms and Consequences. Front. Immunol. 2019, 10, 471. [Google Scholar] [CrossRef] [PubMed]
- Teijeira, A.; Rouzaut, A.; Melero, I. Initial Afferent Lymphatic Vessels Controlling Outbound Leukocyte Traffic from Skin to Lymph Nodes. Front. Immunol. 2013, 4, 433. [Google Scholar] [CrossRef]
- Hos, D.; Bucher, F.; Regenfuss, B.; Dreisow, M.-L.; Bock, F.; Heindl, L.M.; Eming, S.A.; Cursiefen, C. IL-10 Indirectly Regulates Corneal Lymphangiogenesis and Resolution of Inflammation via Macrophages. Am. J. Pathol. 2016, 186, 159–171. [Google Scholar] [CrossRef]
- Jalkanen, S.; Salmi, M. Lymphatic endothelial cells of the lymph node. Nat. Rev. Immunol. 2020, 20, 566–578. [Google Scholar] [CrossRef]
- Kedl, R.M.; Lindsay, R.S.; Finlon, J.M.; Lucas, E.D.; Friedman, R.S.; Tamburini, B.A.J. Migratory dendritic cells acquire and present lymphatic endothelial cell-archived antigens during lymph node contraction. Nat. Commun. 2017, 8, 2034. [Google Scholar] [CrossRef]
- Fletcher, A.L.; Malhotra, D.; Turley, S.J. Lymph node stroma broaden the peripheral tolerance paradigm. Trends Immunol. 2011, 32, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.N.; Tewalt, E.F.; Rouhani, S.; Buonomo, E.L.; Bruce, A.N.; Xu, X.; Bekiranov, S.; Fu, Y.-X.; Engelhard, V. Tolerogenic Properties of Lymphatic Endothelial Cells Are Controlled by the Lymph Node Microenvironment. PLoS ONE 2014, 9, e87740. [Google Scholar] [CrossRef] [PubMed]
- Hirosue, S.; Vokali, E.; Raghavan, V.R.; Rincon-Restrepo, M.; Lund, A.W.; Corthésy-Henrioud, P.; Capotosti, F.; Winter, C.H.; Hugues, S.; Swartz, M.A. Steady-State Antigen Scavenging, Cross-Presentation, and CD8+T Cell Priming: A New Role for Lymphatic Endothelial Cells. J. Immunol. 2014, 192, 5002–5011. [Google Scholar] [CrossRef]
- Dubrot, J.; Duraes, F.D.V.; Potin, L.; Capotosti, F.; Brighouse, D.; Suter, T.; LeibundGut-Landmann, S.; Garbi, N.; Reith, W.; Swartz, M.A.; et al. Lymph node stromal cells acquire peptide–MHCII complexes from dendritic cells and induce antigen-specific CD4+ T cell tolerance. J. Exp. Med. 2014, 211, 1153–1166. [Google Scholar] [CrossRef]
- Rouhani, S.; Eccles, J.; Riccardi, P.; Peske, J.D.; Tewalt, E.F.; Cohen, J.N.; Liblau, R.; Makinen, T.; Engelhard, V. Roles of lymphatic endothelial cells expressing peripheral tissue antigens in CD4 T-cell tolerance induction. Nat. Commun. 2015, 6, 6771. [Google Scholar] [CrossRef]
- Lukacs-Kornek, V.; Malhotra, D.; Fletcher, A.L.; Acton, S.E.; Elpek, K.G.; Tayalia, P.; Collier, A.-R.; Turley, S.J. Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat. Immunol. 2011, 12, 1096–1104. [Google Scholar] [CrossRef]
- Nörder, M.; Gutierrez, M.G.; Zicari, S.; Cervi, E.; Caruso, A.; Guzmán, C.A. Lymph node-derived lymphatic endothelial cells express functional costimulatory molecules and impair dendritic cell-induced allogenic T-cell proliferation. FASEB J. 2012, 26, 2835–2846. [Google Scholar] [CrossRef]
- Podgrabinska, S.; Kamalu, O.; Mayer, L.; Shimaoka, M.; Snoeck, H.; Randolph, G.J.; Skobe, M. Inflamed Lymphatic Endothelium Suppresses Dendritic Cell Maturation and Function via Mac-1/ICAM-1-Dependent Mechanism. J. Immunol. 2009, 183, 1767–1779. [Google Scholar] [CrossRef]
- Stacker, S.A.; Williams, S.P.; Karnezis, T.; Shayan, R.; Fox, S.B.; Achen, M.G. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat. Rev. Cancer 2014, 14, 159–172. [Google Scholar] [CrossRef]
- Riedel, A.; Shorthouse, D.; Haas, L.; Hall, B.A.; Shields, J. Tumor-induced stromal reprogramming drives lymph node transformation. Nat. Immunol. 2016, 17, 1118–1127. [Google Scholar] [CrossRef]
- He, M.; He, Q.; Cai, X.; Chen, Z.; Lao, S.; Deng, H.; Liu, X.; Zheng, Y.; Liu, X.; Liu, J.; et al. Role of lymphatic endothelial cells in the tumor microenvironment—A narrative review of recent advances. Transl. Lung Cancer Res. 2021, 10, 2252–2277. [Google Scholar] [CrossRef] [PubMed]
- Morfoisse, F.; Renaud, E.; Hantelys, F.; Prats, A.-C.; Garmy-Susini, B. Role of hypoxia and vascular endothelial growth factors in lymphangiogenesis. Mol. Cell. Oncol. 2014, 1, e29907. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morfoisse, F.; Kuchnio, A.; Frainay, C.; Gomez-Brouchet, A.; Delisle, M.-B.; Marzi, S.; Helfer, A.-C.; Hantelys, F.; Pujol, F.; Guillermet-Guibert, J.; et al. Hypoxia Induces VEGF-C Expression in Metastatic Tumor Cells via a HIF-1α-Independent Translation-Mediated Mechanism. Cell Rep. 2014, 6, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Raju, B.; Haug, S.R.; Ibrahim, S.O.; Heyeraas, K.J. High interstitial fluid pressure in rat tongue cancer is related to increased lymph vessel area, tumor size, invasiveness and decreased body weight. J. Oral Pathol. Med. 2008, 37, 137–144. [Google Scholar] [CrossRef]
- Watari, K.; Shibata, T.; Kawahara, A.; Sata, K.-I.; Nabeshima, H.; Shinoda, A.; Abe, H.; Azuma, K.; Murakami, Y.; Izumi, H.; et al. Tumor-Derived Interleukin-1 Promotes Lymphangiogenesis and Lymph Node Metastasis through M2-Type Macrophages. PLoS ONE 2014, 9, e99568. [Google Scholar] [CrossRef]
- Ran, S.; Volk-Draper, L. Lymphatic Endothelial Cell Progenitors in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1234, 87–105. [Google Scholar] [CrossRef]
- Ji, H.; Cao, R.; Yang, Y.; Zhang, Y.; Iwamoto, H.; Lim, S.; Nakamura, M.; Andersson, P.; Wang, J.; Sun, Y.; et al. TNFR1 mediates TNF-α-induced tumour lymphangiogenesis and metastasis by modulating VEGF-C-VEGFR3 signalling. Nat. Commun. 2014, 5, 4944. [Google Scholar] [CrossRef]
- Wei, R.; Lv, M.; Li, F.; Cheng, T.; Zhang, Z.; Jiang, G.; Zhou, Y.; Gao, R.; Wei, X.; Lou, J.; et al. Human CAFs promote lymphangiogenesis in ovarian cancer via the Hh-VEGF-C signaling axis. Oncotarget 2017, 8, 67315–67328. [Google Scholar] [CrossRef]
- Tacconi, C.; Correale, C.; Gandelli, A.; Spinelli, A.; Dejana, E.; D’Alessio, S.; Danese, S. Vascular Endothelial Growth Factor C Disrupts the Endothelial Lymphatic Barrier to Promote Colorectal Cancer Invasion. Gastroenterology 2015, 148, 1438–1451.e8. [Google Scholar] [CrossRef]
- Jones, D.; Pereira, E.R.; Padera, T.P. Growth and Immune Evasion of Lymph Node Metastasis. Front. Oncol. 2018, 8, 36. [Google Scholar] [CrossRef]
- Cote, B.; Rao, D.; Alany, R.G.; Kwon, G.S.; Alani, A.W. Lymphatic changes in cancer and drug delivery to the lymphatics in solid tumors. Adv. Drug Deliv. Rev. 2019, 144, 16–34. [Google Scholar] [CrossRef]
- Varney, M.L.; Singh, R.K. VEGF-C-VEGFR3/Flt4 axis regulates mammary tumor growth and metastasis in an autocrine manner. Am. J. Cancer Res. 2015, 5, 616–628. [Google Scholar] [PubMed]
- He, Y.; Kozaki, K.-I.; Karpanen, T.; Koshikawa, K.; Yla-Herttuala, S.; Takahashi, T.; Alitalo, K. Suppression of Tumor Lymphangiogenesis and Lymph Node Metastasis by Blocking Vascular Endothelial Growth Factor Receptor 3 Signaling. J. Natl. Cancer Inst. 2002, 94, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, G.; Zhang, J.; Tang, P.; Yang, C.; Wang, G.; Chen, J.; Liu, J.; Zhang, L.; Ouyang, L. Discovery, Synthesis, and Evaluation of Highly Selective Vascular Endothelial Growth Factor Receptor 3 (VEGFR3) Inhibitor for the Potential Treatment of Metastatic Triple-Negative Breast Cancer. J. Med. Chem. 2021, 64, 12022–12048. [Google Scholar] [CrossRef]
- Rizeq, B.; Malki, M.I. The Role of CCL21/CCR7 Chemokine Axis in Breast Cancer Progression. Cancers 2020, 12, 1036. [Google Scholar] [CrossRef] [PubMed]
- Karnezis, T.; Farnsworth, R.H.; Harris, N.C.; Williams, S.P.; Caesar, C.; Byrne, D.J.; Herle, P.; Macheda, M.L.; Shayan, R.; Zhang, Y.-F.; et al. CCL27/CCL28–CCR10 Chemokine Signaling Mediates Migration of Lymphatic Endothelial Cells. Cancer Res. 2019, 79, 1558–1572. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Homey, B.; Soto, H.; Ge, N.; Catron, D.; Buchanan, M.E.; McClanahan, T.; Murphy, E.; Yuan, W.; Wagner, S.N.; et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001, 410, 50–56. [Google Scholar] [CrossRef]
- Cabioglu, N.; Yazici, M.S.; Arun, B.; Broglio, K.R.; Hortobagyi, G.N.; Price, J.E.; Sahin, A. CCR7 and CXCR4 as Novel Biomarkers Predicting Axillary Lymph Node Metastasis in T1 Breast Cancer. Clin. Cancer Res. 2005, 11, 5686–5693. [Google Scholar] [CrossRef]
- Kawada, K.; Hosogi, H.; Sonoshita, M.; Sakashita, H.; Manabe, T.; Shimahara, Y.; Sakai, Y.; Takabayashi, A.; Oshima, M.; Taketo, M.M. Chemokine receptor CXCR3 promotes colon cancer metastasis to lymph nodes. Oncogene 2007, 26, 4679–4688. [Google Scholar] [CrossRef]
- Pham, T.H.; Baluk, P.; Xu, Y.; Grigorova, I.; Bankovich, A.; Pappu, R.; Coughlin, S.R.; McDonald, D.M.; Schwab, S.; Cyster, J.G. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J. Exp. Med. 2010, 207, 17–27. [Google Scholar] [CrossRef]
- Ponnusamy, S.; Selvam, S.P.; Mehrotra, S.; Kawamori, T.; Snider, A.J.; Obeid, L.; Shao, Y.; Sabbadini, R.; Ogretmen, B. Communication between host organism and cancer cells is transduced by systemic sphingosine kinase 1/sphingosine 1-phosphate signalling to regulate tumour metastasis. EMBO Mol. Med. 2012, 4, 761–775. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.-Z.; Li, J.; Zhang, H.; Kitamura, T.; Zhang, J.; Campion, L.R.; Kaiser, E.A.; Snyder, L.A.; Pollard, J.W. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011, 475, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, S.; Brown, L.F.; Kodama, S.; Paavonen, K.; Alitalo, K.; Detmar, M. VEGF-C–induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood 2007, 109, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Harrell, M.I.; Iritani, B.M.; Ruddell, A. Tumor-Induced Sentinel Lymph Node Lymphangiogenesis and Increased Lymph Flow Precede Melanoma Metastasis. Am. J. Pathol. 2007, 170, 774–786. [Google Scholar] [CrossRef]
- Olmeda, D.; Cerezo-Wallis, D.; Riveiro-Falkenbach, E.; Pennacchi, P.C.; Contreras-Alcalde, M.; Ibarz, N.; Cifdaloz, M.; Catena, X.; Calvo, T.G.; Cañón, E.; et al. Whole-body imaging of lymphovascular niches identifies pre-metastatic roles of midkine. Nature 2017, 546, 676–680. [Google Scholar] [CrossRef]
- Harris, A.R.; Perez, M.J.; Munson, J.M. Docetaxel facilitates lymphatic-tumor crosstalk to promote lymphangiogenesis and cancer progression. BMC Cancer 2018, 18, 718. [Google Scholar] [CrossRef]
- Alam, A.; Blanc, I.; Gueguen-Dorbes, G.; Duclos, O.; Bonnin, J.; Barron, P.; Laplace, M.-C.; Morin, G.; Gaujarengues, F.; Dol, F.; et al. SAR131675, a Potent and Selective VEGFR-3–TK Inhibitor with Antilymphangiogenic, Antitumoral, and Antimetastatic Activities. Mol. Cancer Ther. 2012, 11, 1637–1649. [Google Scholar] [CrossRef]
- Dufies, M.; Giuliano, S.; Ambrosetti, D.; Claren, A.; Ndiaye, P.D.; Mastri, M.; Moghrabi, W.; Cooley, L.S.; Ettaiche, M.; Chamorey, E.; et al. Sunitinib Stimulates Expression of VEGFC by Tumor Cells and Promotes Lymphangiogenesis in Clear Cell Renal Cell Carcinomas. Cancer Res 2017, 77, 1212–1226. [Google Scholar] [CrossRef]
- Saif, M.W.; Knost, J.A.; Chiorean, E.G.; Kambhampati, S.R.P.; Yu, D.; Pytowski, B.; Qin, A.; Kauh, J.S.; O’Neil, B.H. Phase 1 study of the anti-vascular endothelial growth factor receptor 3 monoclonal antibody LY3022856/IMC-3C5 in patients with advanced and refractory solid tumors and advanced colorectal cancer. Cancer Chemother. Pharmacol. 2016, 78, 815–824. [Google Scholar] [CrossRef]
- Fankhauser, M.; Broggi, M.A.S.; Potin, L.; Bordry, N.; Jeanbart, L.; Lund, A.W.; Da Costa, E.; Hauert, S.; Rincon-Restrepo, M.; Tremblay, C.; et al. Tumor lymphangiogenesis promotes T cell infiltration and potentiates immunotherapy in melanoma. Sci. Transl. Med. 2017, 9, eaal4712. [Google Scholar] [CrossRef]
- Sasso, M.S.; Mitrousis, N.; Wang, Y.; Briquez, P.S.; Hauert, S.; Ishihara, J.; Hubbell, J.A.; Swartz, M.A. Lymphangiogenesis-inducing vaccines elicit potent and long-lasting T cell immunity against melanomas. Sci. Adv. 2021, 7, eabe4362. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Deng, Q.; Ma, L.; Li, Q.; Chen, Y.; Liao, Y.; Zhou, F.; Zhang, C.; Shao, L.; Feng, J.; et al. Meningeal lymphatic vessels regulate brain tumor drainage and immunity. Cell Res. 2020, 30, 229–243. [Google Scholar] [CrossRef]
- Song, E.; Mao, T.; Dong, H.; Boisserand, L.S.B.; Antila, S.; Bosenberg, M.; Alitalo, K.; Thomas, J.-L.; Iwasaki, A. VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature 2020, 577, 689–694. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Hoffman-Censits, J.; Powles, T.; van der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y.; et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016, 387, 1909–1920. [Google Scholar] [CrossRef]
- Williamson, C.W.; Sherer, M.V.; Zamarin, D.; Sharabi, A.B.; Dyer, B.A.; Mell, L.K.; Mayadev, J.S. Immunotherapy and radiation therapy sequencing: State of the data on timing, efficacy, and safety. Cancer 2021, 127, 1553–1567. [Google Scholar] [CrossRef] [PubMed]
- Dar, T.B.; Biteghe, F.A.N.; Kakar-Bhanot, R.; Aniogo, E.C.; Malindi, Z.; Akinrinmade, O.A.; Chalomie, N.E.T.; Kombe, A.J.K.; Angone, S.A.; Ndong, J.M.N. Synergistic effects of radiotherapy and targeted immunotherapy in improving tumor treatment efficacy: A review. Clin. Transl. Oncol. 2022, 24, 2255–2271. [Google Scholar] [CrossRef] [PubMed]
- Vanpouille-Box, C.; Formenti, S.C.; Demaria, S. Toward Precision Radiotherapy for Use with Immune Checkpoint Blockers. Clin. Cancer Res. 2018, 24, 259–265. [Google Scholar] [CrossRef]
- Rodriguez-Ruiz, M.E.; Vitale, I.; Harrington, K.J.; Melero, I.; Galluzzi, L. Immunological impact of cell death signaling driven by radiation on the tumor microenvironment. Nat. Immunol. 2020, 21, 120–134. [Google Scholar] [CrossRef]
- Patel, R.B.; Baniel, C.C.; Sriramaneni, R.N.; Bradley, K.; Markovina, S.; Morris, Z.S. Combining brachytherapy and immunotherapy to achieve in situ tumor vaccination: A review of cooperative mechanisms and clinical opportunities. Brachytherapy 2018, 17, 995–1003. [Google Scholar] [CrossRef]
- Sánchez-Paulete, A.R.; Cueto, F.J.; Martínez-López, M.; Labiano, S.; Morales-Kastresana, A.; Rodriguez-Ruiz, M.E.; Jure-Kunkel, M.; Azpilikueta, A.; Aznar, M.A.; Quetglas, J.I.; et al. Cancer Immunotherapy with Immunomodulatory Anti-CD137 and Anti-PD-1 Monoclonal Antibodies Requires BATF3-Dependent Dendritic Cells. Cancer Discov. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Kepp, O.; Tesniere, A.; Zitvogel, L.; Kroemer, G. The immunogenicity of tumor cell death. Curr. Opin. Oncol. 2009, 21, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Reits, E.A.; Hodge, J.W.; Herberts, C.A.; Groothuis, T.A.; Chakraborty, M.; Wansley, E.K.; Camphausen, K.; Luiten, R.M.; De Ru, A.H.; Neijssen, J.; et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med. 2006, 203, 1259–1271. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ruiz, M.E.; Vanpouille-Box, C.; Melero, I.; Formenti, S.C.; Demaria, S. Immunological Mechanisms Responsible for Radiation-Induced Abscopal Effect. Trends Immunol. 2018, 39, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, C.N.; Formenti, S.C.; DeMaria, S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 2017, 8, 15618. [Google Scholar] [CrossRef]
- Willers, H.; Held, K.D. Introduction to Clinical Radiation Biology. Hematol. Clin. N. Am. 2006, 20, 1–24. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, D.; Taniguchi, C.M. Hypoxia inducible factor (HIF) in the tumor microenvironment: Friend or foe? Sci. China Life Sci. 2017, 60, 1114–1124. [Google Scholar] [CrossRef]
- Meijer, T.W.; Kaanders, J.H.; Span, P.N.; Bussink, J. Targeting hypoxia, HIF-1, and tumor glucose metabolism to improve radiotherapy efficacy. Clin. Cancer Res. 2012, 18, 5585–5594. [Google Scholar] [CrossRef]
- Song, C.W.; Kim, H.; Cho, H.; Kim, M.-S.; Paek, S.-H.; Park, H.-J.; Griffin, R.J.; Terezakis, S.; Cho, L.C. HIF-1α Inhibition Improves Anti-Tumor Immunity and Promotes the Efficacy of Stereotactic Ablative Radiotherapy (SABR). Cancers 2022, 14, 3273. [Google Scholar] [CrossRef]
- Ansems, M.; Span, P. The tumor microenvironment and radiotherapy response; a central role for cancer-associated fibroblasts. Clin. Transl. Radiat. Oncol. 2020, 22, 90–97. [Google Scholar] [CrossRef]
- Sanford, D.E.; Belt, B.A.; Panni, R.Z.; Mayer, A.; Deshpande, A.D.; Carpenter, D.; Mitchem, J.B.; Plambeck-Suess, S.M.; Worley, L.A.; Goetz, B.D.; et al. Inflammatory Monocyte Mobilization Decreases Patient Survival in Pancreatic Cancer: A Role for Targeting the CCL2/CCR2 Axis. Clin. Cancer Res. 2013, 19, 3404–3415. [Google Scholar] [CrossRef]
- Ozpiskin, O.M.; Zhang, L.; Li, J.J. Immune targets in the tumor microenvironment treated by radiotherapy. Theranostics 2019, 9, 1215–1231. [Google Scholar] [CrossRef] [PubMed]
- Klug, F.; Prakash, H.; Huber, P.E.; Seibel, T.; Bender, N.; Halama, N.; Pfirschke, C.; Voss, R.H.; Timke, C.; Umansky, L.; et al. Low-Dose Irradiation Programs Macrophage Differentiation to an iNOS+/M1 Phenotype that Orchestrates Effective T Cell Immunotherapy. Cancer Cell 2013, 24, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Dovedi, S.J.; Adlard, A.L.; Lipowska-Bhalla, G.; McKenna, C.; Jones, S.; Cheadle, E.J.; Stratford, I.J.; Poon, E.; Morrow, M.; Stewart, R.; et al. Acquired Resistance to Fractionated Radiotherapy Can Be Overcome by Concurrent PD-L1 Blockade. Cancer Res. 2014, 74, 5458–5468. [Google Scholar] [CrossRef] [PubMed]
- Nesseler, J.P.; Lee, M.-H.; Nguyen, C.; Kalbasi, A.; Sayre, J.W.; Romero, T.; Nickers, P.; McBride, W.H.; Schaue, D. Tumor Size Matters—Understanding Concomitant Tumor Immunity in the Context of Hypofractionated Radiotherapy with Immunotherapy. Cancers 2020, 12, 714. [Google Scholar] [CrossRef] [PubMed]
- Grassberger, C.; Ellsworth, S.G.; Wilks, M.Q.; Keane, F.K.; Loeffler, J.S. Assessing the interactions between radiotherapy and antitumour immunity. Nat. Rev. Clin. Oncol. 2019, 16, 729–745. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.S.; Oduyebo, T.; Cobb, L.P.; Cholakian, D.; Kong, X.; Fader, A.N.; Levinson, K.L.; Tanner, E.J.; Stone, R.L.; Piotrowski, A.; et al. Lymphopenia and its association with survival in patients with locally advanced cervical cancer. Gynecol. Oncol. 2016, 140, 76–82. [Google Scholar] [CrossRef]
- Rafat, M.; Aguilera, T.A.; Vilalta, M.; Bronsart, L.L.; Soto, L.A.; von Eyben, R.; Golla, M.A.; Ahrari, Y.; Melemenidis, S.; Afghahi, A.; et al. Macrophages Promote Circulating Tumor Cell–Mediated Local Recurrence following Radiotherapy in Immunosuppressed Patients. Cancer Res. 2018, 78, 4241–4252. [Google Scholar] [CrossRef]
- Jackowski, S.; Janusch, M.; Fiedler, E.; Marsch, W.C.; Ulbrich, E.J.; Gaisbauer, G.; Dunst, J.; Kerjaschki, D.; Helmbold, P. Radiogenic Lymphangiogenesis in the Skin. Am. J. Pathol. 2007, 171, 338–348. [Google Scholar] [CrossRef]
- Stewart, J.A.M.T.P.F.A. Radiation Nephropathy&The Link Between Functional Damage and Vascular Mediated Inflammatory and Thrombotic Changes. Acta Oncol. 2001, 40, 952–957. [Google Scholar] [CrossRef]
- Cui, Y.; Wilder, J.; Rietz, C.; Gigliotti, A.; Tang, X.; Shi, Y.; Guilmette, R.; Wang, H.; George, G.; De Magaldi, E.N.; et al. Radiation-Induced Impairment in Lung Lymphatic Vasculature. Lymphat. Res. Biol. 2014, 12, 238–250. [Google Scholar] [CrossRef]
- Wachter, S.; Gerstner, N.; Goldner, G.; Pötzi, R.; Wambersie, A.; Pötter, R. Endoscopic scoring of late rectal mucosal damage after conformal radiotherapy for prostatic carcinoma. Radiother. Oncol. 2000, 54, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Venkatesulu, B.P.; Mahadevan, L.S.; Aliru, M.L.; Yang, X.; Bodd, M.H.; Singh, P.K.; Yusuf, S.W.; Abe, J.-I.; Krishnan, S. Radiation-Induced Endothelial Vascular Injury: A Review of Possible Mechanisms. JACC Basic Transl. Sci. 2018, 3, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Guipaud, O.; Jaillet, C.; Clement-Colmou, K.; François, A.; Supiot, S.; Milliat, F. The importance of the vascular endothelial barrier in the immune-inflammatory response induced by radiotherapy. Br. J. Radiol. 2018, 91, 20170762. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Griffin, R.J.; Hui, S.; Levitt, S.H.; Song, C.W. Radiation-induced vascular damage in tumors: Implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS). Radiat. Res. 2012, 177, 311–327. [Google Scholar] [CrossRef]
- Russell, N.S.; Floot, B.; van Werkhoven, E.; Schriemer, M.; de Jong-Korlaar, R.; Woerdeman, L.A.; Stewart, F.A.; Scharpfenecker, M. Blood and lymphatic microvessel damage in irradiated human skin: The role of TGF-β, endoglin and macrophages. Radiother. Oncol. 2015, 116, 455–461. [Google Scholar] [CrossRef]
- Jani, A.; Shaikh, F.; Barton, S.; Willis, C.; Banerjee, D.; Mitchell, J.; Hernandez, S.L.; Hei, T.; Kadenhe-Chiweshe, A.; Yamashiro, D.J.; et al. High-Dose, Single-Fraction Irradiation Rapidly Reduces Tumor Vasculature and Perfusion in a Xenograft Model of Neuroblastoma. Int. J. Radiat. Oncol. 2015, 94, 1173–1180. [Google Scholar] [CrossRef]
- Wang, Y.; Boerma, M.; Zhou, D. Ionizing Radiation-Induced Endothelial Cell Senescence and Cardiovascular Diseases. Radiat. Res. 2016, 186, 153–161. [Google Scholar] [CrossRef]
- Fernández-Alvarez, V.; López, F.; Suárez, C.; Strojan, P.; Eisbruch, A.; Silver, C.E.; Mendenhall, W.M.; Langendijk, J.A.; Rinaldo, A.; Lee, A.W.M.; et al. Radiation-induced carotid artery lesions. Strahlenther. Onkol. 2018, 194, 699–710. [Google Scholar] [CrossRef]
- Hoving, S.; Heeneman, S.; Gijbels, M.J.; Poele, J.A.T.; Russell, N.S.; Daemen, M.; Stewart, F.A. Single-Dose and Fractionated Irradiation Promote Initiation and Progression of Atherosclerosis and Induce an Inflammatory Plaque Phenotype in ApoE−/− Mice. Int. J. Radiat. Oncol. 2008, 71, 848–857. [Google Scholar] [CrossRef]
- Zou, B.; Schuster, J.P.; Niu, K.; Huang, Q.; Rühle, A.; Huber, P.E. Radiotherapy-induced heart disease: A review of the literature. Precis. Clin. Med. 2019, 2, 270–282. [Google Scholar] [CrossRef]
- Korpela, E.; Liu, S.K. Endothelial perturbations and therapeutic strategies in normal tissue radiation damage. Radiat. Oncol. 2014, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ruiz, M.E.; Garasa, S.; Rodriguez, I.; Solorzano, J.L.; Barbes, B.; Yanguas, A.; Teijeira, A.; Etxeberria, I.; Aristu, J.J.; Halin, C.; et al. Intercellular Adhesion Molecule-1 and Vascular Cell Adhesion Molecule Are Induced by Ionizing Radiation on Lymphatic Endothelium. Int. J. Radiat. Oncol. 2017, 97, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Jaillet, C.; Morelle, W.; Slomianny, M.-C.; Paget, V.; Tarlet, G.; Buard, V.; Selbonne, S.; Caffin, F.; Rannou, E.; Martinez, P.; et al. Radiation-induced changes in the glycome of endothelial cells with functional consequences. Sci. Rep. 2017, 7, 5290. [Google Scholar] [CrossRef] [PubMed]
- Hallahan, D.E.; Virudachalam, S. Intercellular adhesion molecule 1 knockout abrogates radiation induced pulmonary inflammation. Proc. Natl. Acad. Sci. USA 1997, 94, 6432–6437. [Google Scholar] [CrossRef] [PubMed]
- Gabryś, D.; Greco, O.; Patel, G.; Prise, K.M.; Tozer, G.M.; Kanthou, C. Radiation Effects on the Cytoskeleton of Endothelial Cells and Endothelial Monolayer Permeability. Int. J. Radiat. Oncol. 2007, 69, 1553–1562. [Google Scholar] [CrossRef]
- Cervelli, T.; Panetta, D.; Navarra, T.; Andreassi, M.G.; Basta, G.; Galli, A.; Salvadori, P.A.; Picano, E.; Del Turco, S. Effects of single and fractionated low-dose irradiation on vascular endothelial cells. Atherosclerosis 2014, 235, 510–518. [Google Scholar] [CrossRef]
- Nam, J.-K.; Kim, J.-H.; Park, M.-S.; Kim, E.H.; Kim, J.; Lee, Y.-J. Radiation-Induced Fibrotic Tumor Microenvironment Regulates Anti-Tumor Immune Response. Cancers 2021, 13, 5232. [Google Scholar] [CrossRef]
- Moding, E.J.; Lee, C.-L.; Castle, K.D.; Oh, P.; Mao, L.; Zha, S.; Min, H.D.; Ma, Y.; Das, S.; Kirsch, D.G. Atm deletion with dual recombinase technology preferentially radiosensitizes tumor endothelium. J. Clin. Investig. 2014, 124, 3325–3338. [Google Scholar] [CrossRef]
- Naito, H.; Iba, T.; Takakura, N. Mechanisms of new blood-vessel formation and proliferative heterogeneity of endothelial cells. Int. Immunol. 2020, 32, 295–305. [Google Scholar] [CrossRef]
- Bristow, R.G.; Hill, R.P. Hypoxia, DNA repair and genetic instability. Nat. Rev. Cancer 2008, 8, 180–192. [Google Scholar] [CrossRef]
- Hill, R.P. The changing paradigm of tumour response to irradiation. Br. J. Radiol. 2017, 90, 20160474. [Google Scholar] [CrossRef] [PubMed]
- Kaeppler, J.R.; Chen, J.; Buono, M.; Vermeer, J.; Kannan, P.; Cheng, W.; Voukantsis, D.; Thompson, J.M.; Hill, M.A.; Allen, D.; et al. Endothelial cell death after ionizing radiation does not impair vascular structure in mouse tumor models. EMBO Rep. 2022, 23, e53221. [Google Scholar] [CrossRef] [PubMed]
- Shaked, Y. The pro-tumorigenic host response to cancer therapies. Nat. Rev. Cancer 2019, 19, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Kozin, S.V.; Kamoun, W.S.; Huang, Y.; Dawson, M.R.; Jain, R.K.; Duda, D.G. Recruitment of Myeloid but not Endothelial Precursor Cells Facilitates Tumor Regrowth after Local Irradiation. Cancer Res. 2010, 70, 5679–5685. [Google Scholar] [CrossRef] [PubMed]
- Kioi, M.; Vogel, H.; Schultz, G.; Hoffman, R.M.; Harsh, G.R.; Brown, J.M. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J. Clin. Investig. 2010, 120, 694–705. [Google Scholar] [CrossRef]
- Herrera, F.G.; Ronet, C.; de Olza, M.O.; Barras, D.; Crespo, I.; Andreatta, M.; Corria-Osorio, J.; Spill, A.; Benedetti, F.; Genolet, R.; et al. Low-Dose Radiotherapy Reverses Tumor Immune Desertification and Resistance to Immunotherapy. Cancer Discov. 2022, 12, 108–133. [Google Scholar] [CrossRef]
- Ochoa-De-Olza, M.; Bourhis, J.; Coukos, G.; Herrera, F.G. Low-dose irradiation for reversing immunotherapy resistance: How to translate? J. Immunother. Cancer 2022, 10, e004939. [Google Scholar] [CrossRef]
- Emauge, L.; Eterme, M.; Etartour, E.; Ehelley, D. Control of the Adaptive Immune Response by Tumor Vasculature. Front. Oncol. 2014, 4, 61. [Google Scholar] [CrossRef]
- Hendry, S.A.; Farnsworth, R.H.; Solomon, B.; Achen, M.G.; Stacker, S.A.; Fox, S.B. The Role of the Tumor Vasculature in the Host Immune Response: Implications for Therapeutic Strategies Targeting the Tumor Microenvironment. Front. Immunol. 2016, 7, 621. [Google Scholar] [CrossRef]
- Frey, B.; Rückert, M.; Weber, J.; Mayr, X.; Derer, A.; Lotter, M.; Bert, C.; Rödel, F.; Fietkau, R.; Gaipl, U.S. Hypofractionated Irradiation Has Immune Stimulatory Potential and Induces a Timely Restricted Infiltration of Immune Cells in Colon Cancer Tumors. Front. Immunol. 2017, 8, 231. [Google Scholar] [CrossRef]
- Herrera, F.G.; Bourhis, J.; Coukos, G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA A Cancer J. Clin. 2017, 67, 65–85. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, S.; Wang, B.; Kawashima, N.; Braunstein, S.; Badura, M.; Cameron, T.O.; Babb, J.; Schneider, R.; Formenti, S.C.; Dustin, M.; et al. Radiation-Induced CXCL16 Release by Breast Cancer Cells Attracts Effector T Cells. J. Immunol. 2008, 181, 3099–3107. [Google Scholar] [CrossRef]
- Krombach, J.; Hennel, R.; Brix, N.; Orth, M.; Schoetz, U.; Ernst, A.; Schuster, J.; Zuchtriegel, G.; Reichel, C.A.; Bierschenk, S.; et al. Priming anti-tumor immunity by radiotherapy: Dying tumor cell-derived DAMPs trigger endothelial cell activation and recruitment of myeloid cells. Oncoimmunology 2018, 8, e1523097. [Google Scholar] [CrossRef]
- Miller, M.; Zheng, Y.; Gadde, S.; Pfirschke, C.; Zope, H.; Engblom, C.; Kohler, R.H.; Iwamoto, Y.; Yang, K.S.; Askevold, B.; et al. Tumour-associated macrophages act as a slow-release reservoir of nano-therapeutic Pt(IV) pro-drug. Nat. Commun. 2015, 6, 8692. [Google Scholar] [CrossRef] [PubMed]
- Shiao, S.L.; Ruffell, B.; DeNardo, D.G.; Faddegon, B.A.; Park, C.C.; Coussens, L.M. TH2-Polarized CD4+ T Cells and Macrophages Limit Efficacy of Radiotherapy. Cancer Immunol. Res. 2015, 3, 518–525. [Google Scholar] [CrossRef]
- Sung, H.K.; Morisada, T.; Cho, C.-H.; Oike, Y.; Lee, J.; Sung, E.K.; Chung, J.H.; Suda, T.; Koh, G.Y. Intestinal and peri-tumoral lymphatic endothelial cells are resistant to radiation-induced apoptosis. Biochem. Biophys. Res. Commun. 2006, 345, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Pan, S.-L.; Wang, J.-C.; Kuo, S.-H.; Cheng, J.C.-H.; Teng, C.-M. Radiation-induced VEGF-C expression and endothelial cell proliferation in lung cancer. Strahlenther. und Onkol. 2014, 190, 1154–1162. [Google Scholar] [CrossRef]
- Kesler, C.T.; Kuo, A.H.; Wong, H.-K.; Masuck, D.J.; Shah, J.L.; Kozak, K.R.; Held, K.D.; Padera, T.P. Vascular endothelial growth factor-C enhances radiosensitivity of lymphatic endothelial cells. Angiogenesis 2014, 17, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Avraham, T.; Yan, A.; Zampell, J.C.; Daluvoy, S.V.; Haimovitz-Friedman, A.; Cordeiro, A.P.; Mehrara, B.J. Radiation therapy causes loss of dermal lymphatic vessels and interferes with lymphatic function by TGF-β1-mediated tissue fibrosis. Am. J. Physiol. Physiol. 2010, 299, C589–C605. [Google Scholar] [CrossRef]
- Kirkby, N.S.; Chan, M.V.; Zaiss, A.K.; Garcia-Vaz, E.; Jiao, J.; Berglund, L.M.; Verdu, E.F.; Ahmetaj-Shala, B.; Wallace, J.L.; Herschman, H.R.; et al. Systematic study of constitutive cyclooxygenase-2 expression: Role of NF-κB and NFAT transcriptional pathways. Proc. Natl. Acad. Sci. USA 2016, 113, 434–439. [Google Scholar] [CrossRef]
- Björndahl, M.A.; Cao, R.; Burton, J.B.; Brakenhielm, E.; Religa, P.; Galter, D.; Wu, L.; Cao, Y. Vascular Endothelial Growth Factor-A Promotes Peritumoral Lymphangiogenesis and Lymphatic Metastasis. Cancer Res. 2005, 65, 9261–9268. [Google Scholar] [CrossRef] [PubMed]
- Im, J.H.; Buzzelli, J.N.; Jones, K.; Franchini, F.; Gordon-Weeks, A.; Markelc, B.; Chen, J.; Kim, J.; Cao, Y.; Muschel, R.J. FGF2 alters macrophage polarization, tumour immunity and growth and can be targeted during radiotherapy. Nat. Commun. 2020, 11, 4064. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Jendrossek, V.; Belka, C. The role of PDGF in radiation oncology. Radiat. Oncol. 2007, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, Z.; Wang, Z.; Du, G.; Lun, L. TGF-beta signaling in cancer radiotherapy. Cytokine 2021, 148, 155709. [Google Scholar] [CrossRef] [PubMed]
- Rockson, S.G. Advances in Lymphedema. Circ. Res. 2021, 128, 2003–2016. [Google Scholar] [CrossRef]
- Jia, W.; Hitchcock-Szilagyi, H.; He, W.; Goldman, J.; Zhao, F. Engineering the Lymphatic Network: A Solution to Lymphedema. Adv. Healthc. Mater. 2021, 10, e2001537. [Google Scholar] [CrossRef]
- Lenzi, M.; Bassani, G. The Effect of Radiation on the Lymph and on the Lymph Vessels. Radiology 1963, 80, 814–817. [Google Scholar] [CrossRef]
- Gross, J.P.; Sachdev, S.; Helenowski, I.B.; Lipps, D.; Hayes, J.P.; Donnelly, E.D.; Strauss, J.B. Radiation Therapy Field Design and Lymphedema Risk After Regional Nodal Irradiation for Breast Cancer. Int. J. Radiat. Oncol. 2018, 102, 71–78. [Google Scholar] [CrossRef]
- McLaughlin, S.A.; Wright, M.J.; Morris, K.T.; Giron, G.L.; Sampson, M.R.; Brockway, J.P.; Hurley, K.E.; Riedel, E.R.; Van Zee, K. Prevalence of Lymphedema in Women With Breast Cancer 5 Years After Sentinel Lymph Node Biopsy or Axillary Dissection: Objective Measurements. J. Clin. Oncol. 2008, 26, 5213–5219. [Google Scholar] [CrossRef]
- Allam, O.; Park, K.E.; Chandler, L.; Mozaffari, M.A.; Ahmad, M.; Lu, X.; Alperovich, M. The impact of radiation on lymphedema: A review of the literature. Gland Surg. 2020, 9, 596–602. [Google Scholar] [CrossRef]
- Newman, B.; Lose, F.; Kedda, M.-A.; Francois, M.; Ferguson, K.; Janda, M.; Yates, P.; Spurdle, A.B.; Hayes, S.C. Possible Genetic Predisposition to Lymphedema after Breast Cancer. Lymphat. Res. Biol. 2012, 10, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.; Van Geel, M.; Cornelissen, A.J.; Van Der Hulst, R.R.; Qiu, S.S. Breast Cancer-Related Lymphedema and Genetic Predisposition: A Systematic Review of the Literature. Lymphat. Res. Biol. 2019, 17, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, T.C.; Sayegh, H.E.; Brunelle, C.L.; Daniell, K.M.; Taghian, A.G. Breast cancer-related lymphedema: Risk factors, precautionary measures, and treatments. Gland Surg. 2018, 7, 379–403. [Google Scholar] [CrossRef] [PubMed]
- Dacre, J.E.; Scott, D.L.; Huskisson, E.C. Lymphoedema of the limbs as an extra-articular feature of rheumatoid arthritis. Ann. Rheum. Dis. 1990, 49, 722–724. [Google Scholar] [CrossRef]
- Shallwani, S.M.; Hodgson, P.; Towers, A. Examining Obesity in Lymphedema: A Retrospective Study of 178 New Patients with Suspected Lymphedema at a Canadian Hospital-Based Clinic. Physiother. Can. 2020, 72, 18–25. [Google Scholar] [CrossRef]
- Kwon, S.; Janssen, C.F.; Velasquez, F.C.; Zhang, S.; Aldrich, M.B.; Shaitelman, S.F.; DeSnyder, S.M.; Sevick-Muraca, E.M. Radiation Dose-Dependent Changes in Lymphatic Remodeling. Int. J. Radiat. Oncol. 2019, 105, 852–860. [Google Scholar] [CrossRef]
- Baker, A.; Semple, J.L.; Moore, S.; Johnston, M. Lymphatic Function Is Impaired Following Irradiation of a Single Lymph Node. Lymphat. Res. Biol. 2014, 12, 76–88. [Google Scholar] [CrossRef]
- Yuan, Y.; Arcucci, V.; Levy, S.; Achen, M.G. Modulation of Immunity by Lymphatic Dysfunction in Lymphedema. Front. Immunol. 2019, 10, 76. [Google Scholar] [CrossRef]
- Straub, J.M.; New, J.; Hamilton, C.D.; Lominska, C.; Shnayder, Y.; Thomas, S.M. Radiation-induced fibrosis: Mechanisms and implications for therapy. J. Cancer Res. Clin. Oncol. 2015, 141, 1985–1994. [Google Scholar] [CrossRef]
- Marciscano, A.E.; Ghasemzadeh, A.; Nirschl, T.R.; Theodros, D.; Kochel, C.M.; Francica, B.J.; Muroyama, Y.; Anders, R.A.; Sharabi, A.B.; Velarde, E.; et al. Elective Nodal Irradiation Attenuates the Combinatorial Efficacy of Stereotactic Radiation Therapy and Immunotherapy. Clin. Cancer Res. 2018, 24, 5058–5071. [Google Scholar] [CrossRef]
- Zhou, C.; Ma, L.; Xu, H.; Huo, Y.; Luo, J. Meningeal lymphatics regulate radiotherapy efficacy through modulating anti-tumor immunity. Cell Res. 2022, 32, 543–554. [Google Scholar] [CrossRef] [PubMed]
- O’Melia, M.J.; Manspeaker, M.P.; Thomas, S.N. Tumor-draining lymph nodes are survival niches that support T cell priming against lymphatic transported tumor antigen and effects of immune checkpoint blockade in TNBC. Cancer Immunol. Immunother. 2021, 70, 2179–2195. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suárez, L.; Rodríguez-Ruiz, M.E.; Rouzaut, A. The Lymphatic Endothelium in the Context of Radioimmuno-Oncology. Cancers 2023, 15, 21. https://doi.org/10.3390/cancers15010021
Suárez L, Rodríguez-Ruiz ME, Rouzaut A. The Lymphatic Endothelium in the Context of Radioimmuno-Oncology. Cancers. 2023; 15(1):21. https://doi.org/10.3390/cancers15010021
Chicago/Turabian StyleSuárez, Lucía, María E. Rodríguez-Ruiz, and Ana Rouzaut. 2023. "The Lymphatic Endothelium in the Context of Radioimmuno-Oncology" Cancers 15, no. 1: 21. https://doi.org/10.3390/cancers15010021
APA StyleSuárez, L., Rodríguez-Ruiz, M. E., & Rouzaut, A. (2023). The Lymphatic Endothelium in the Context of Radioimmuno-Oncology. Cancers, 15(1), 21. https://doi.org/10.3390/cancers15010021





