Effects of Irradiation on Brain Tumors Using MR-Based Electrical Conductivity Imaging
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tumor Cell Culture
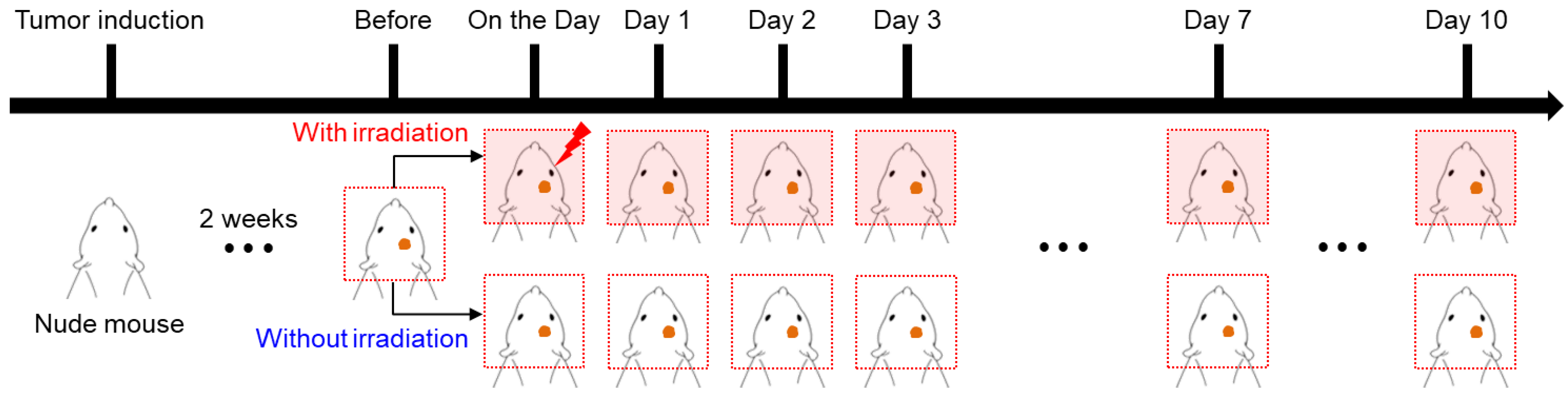
2.2. Animal Preparation for Tumor Model
2.3. Radiation Exposure
2.4. Imaging Experiments
2.5. Conductivity Measurement and Analysis
3. Results
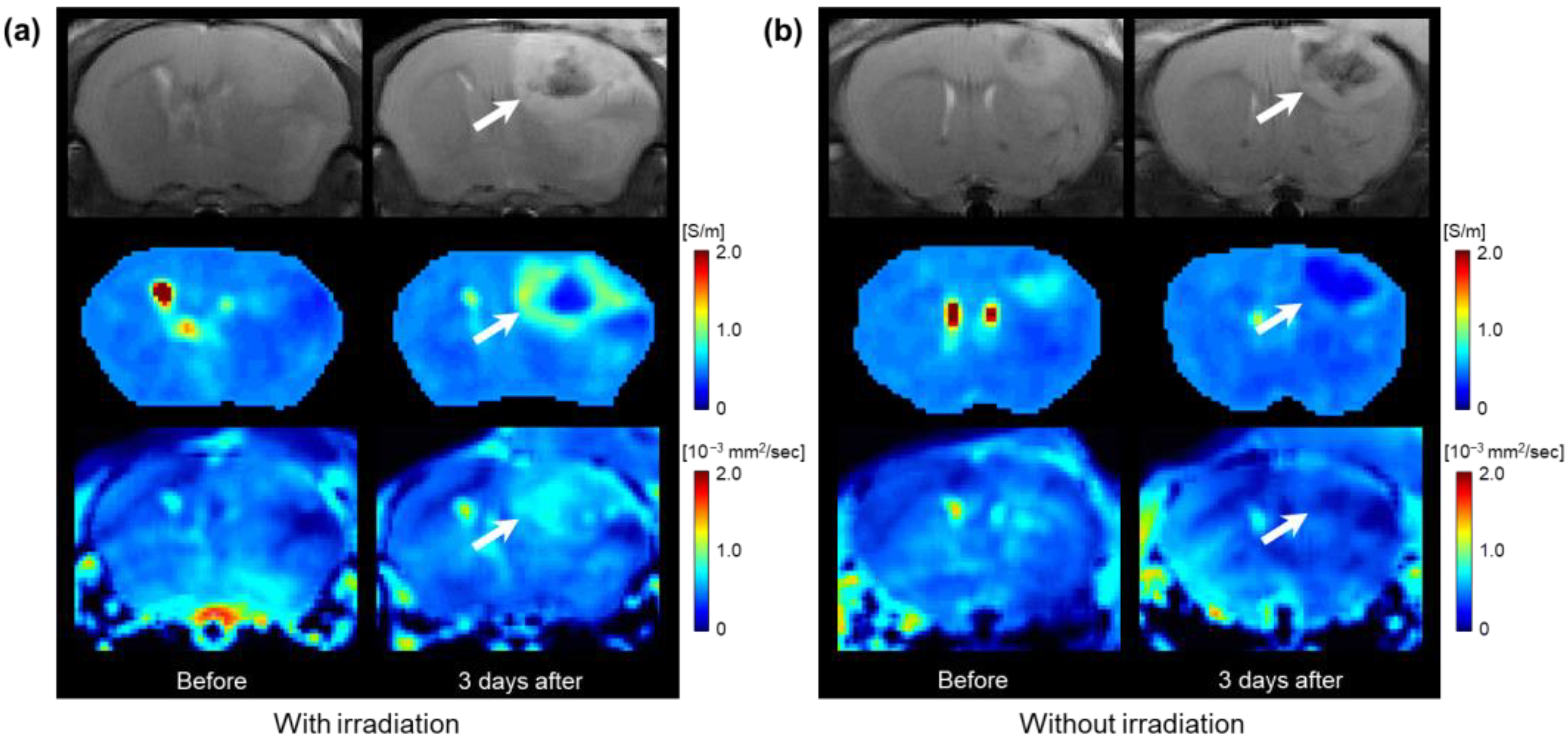
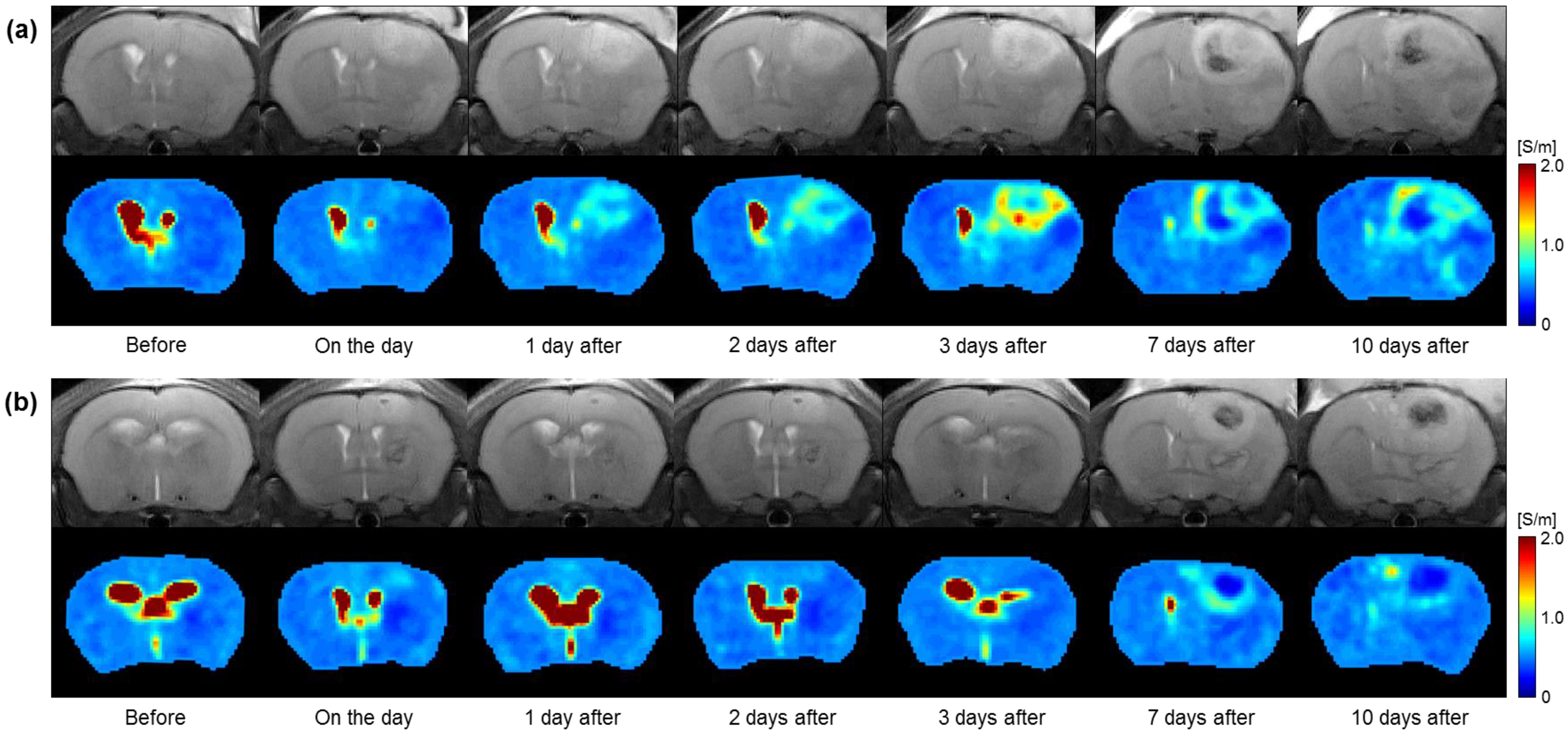
3.1. Conductivity Images of Brain Tumors with and without Irradiation
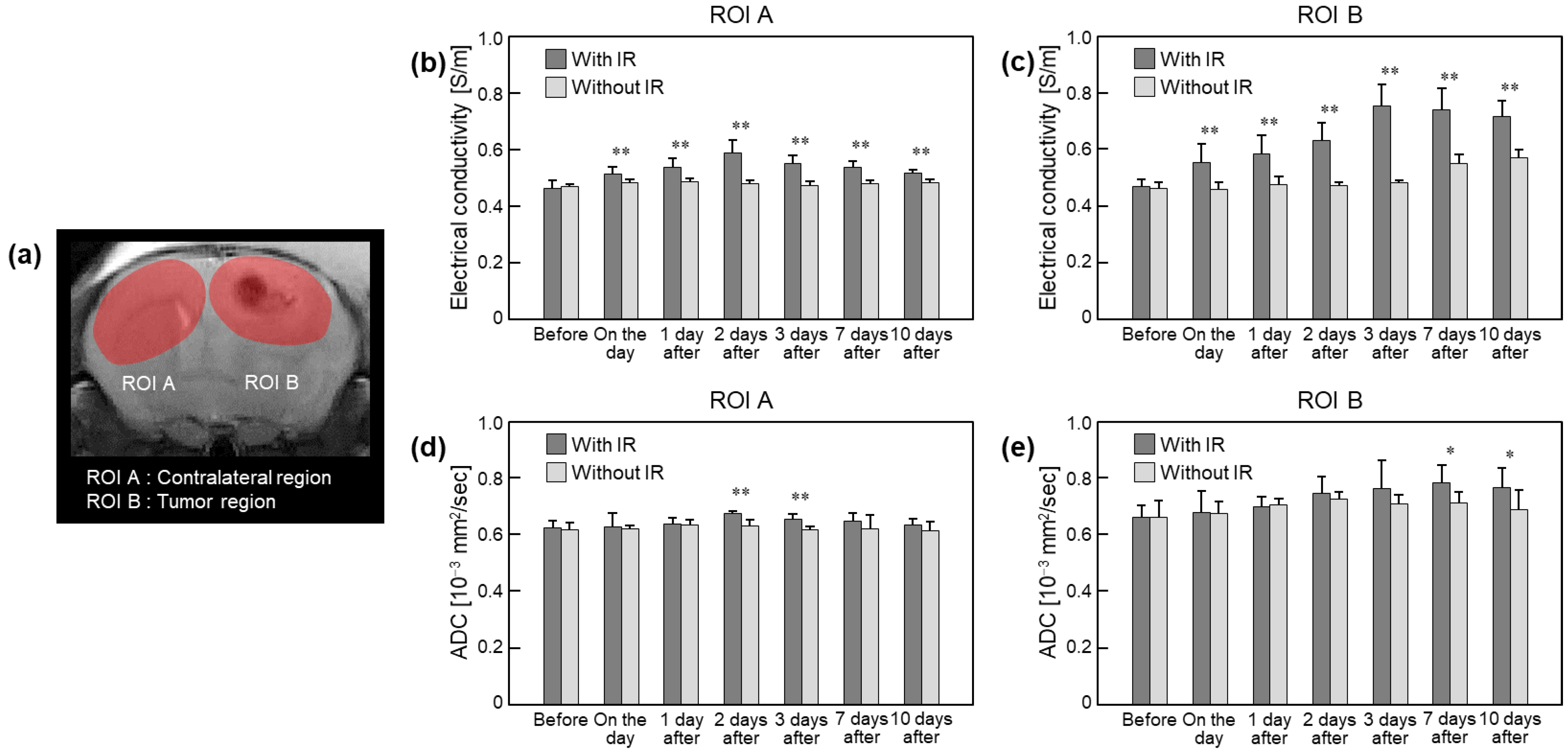
3.2. Quantification of Irradiation Effects in Brain Tumors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garsa, A.; Jang, J.K.; Baxi, S.; Chen, C.; Akinniranye, O.; Hall, O.; Larkin, J.; Motala, A.; Hempel, S. Radiation Therapy for Brain Metastases: A Systematic Review. Pract. Radiat. Oncol. 2021, 11, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Baskar, R.; Dai, J.; Wenlong, N.; Yeo, R.; Yeoh, K.W. Biological response of cancer cells to radiation treatment. Front. Mol. Biosci. 2014, 1, 24. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Feuvret, L.; Simon, J.-M.; Ribeiro, M.; Nichelli, L.; Jenny, C.; Ricard, D.; Psimaras, D.; Hoang-Xuan, K.; Maingon, P. Neurological side effects of radiation therapy. Neurol. Sci. 2022, 43, 2363–2374. [Google Scholar] [CrossRef] [PubMed]
- Eaton, D.J.; Lee, J.; Patel, R.; Millin, A.E.; Paddick, I.; Walker, C. Stereotactic radiosurgery for benign brain tumors: Results of multicenter benchmark planning studies. Pract. Radiat. Oncol. 2018, 8, e295–e304. [Google Scholar] [CrossRef]
- Jalali, R.; Gupta, T.; Goda, J.S.; Goswami, S.; Shah, N.; Dutta, D.; Krishna, U.; Deodhar, J.; Menon, P.; Kannan, S.; et al. Efficacy of stereotactic conformal radiotherapy vs conventional radiotherapy on benign and low-grade brain tumors: A randomized clinical trial. JAMA Oncol. 2017, 3, 1368–1376. [Google Scholar] [CrossRef]
- Tai, A.X.; Nayar, V.V. Update on Trigeminal Neuralgia. Curr. Treat. Options Neurol. 2019, 21, 42. [Google Scholar] [CrossRef]
- Chivukula, S.; Kim, W.; Zhuo, X.; Tenn, S.; Kaprealian, T.; DeSalles, A.; Pouratian, N. Radiosurgery for Secondary Trigeminal Neuralgia: Revisiting the Treatment Paradigm. World Neurosurg. 2017, 99, 288–294. [Google Scholar] [CrossRef]
- Ding, D.; Starke, R.M.; Kano, H.; Mathieu, D.; Huang, P.; Kondziolka, D.; Feliciano, C.; Rodriguez-Mercado, R.; Almodovar, L.; Grills, I.S.; et al. Radiosurgery for Cerebral Arteriovenous Malformations in A Randomized Trial of Unruptured Brain Arteriovenous Malformations (ARUBA)-Eligible Patients: A Multicenter Study. Stroke 2016, 47, 342–349. [Google Scholar] [CrossRef]
- Otmani, N. Oral and maxillofacial side effects of radiation therapy on children. J. Can. Dent. Assoc. 2007, 73, 257–261. [Google Scholar]
- Diller, L.; Chow, E.J.; Gurney, J.G.; Hudson, M.M.; Kadin-Lottick, N.S.; Kawashima, T.I.; Leisenring, W.M.; Meacham, L.R.; Mertens, A.C.; Mulrooney, D.A.; et al. Chronic disease in the Childhood Cancer Survivor Study cohort: A review of published findings. J. Clin. Oncol. 2009, 27, 2339–2355. [Google Scholar] [CrossRef]
- Finger, P.T. Radiation Therapy for Orbital Tumors: Concepts, Current Use, and Ophthalmic Radiation Side Effects. Surv. Ophthalmol. 2009, 54, 545–568. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.R.; Suhag, V.; Semwal, M.; Sharma, N. Radiotherapy: Basic concepts and recent advances. Med. J. Armed Forces India 2010, 66, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Park, J.M.; Choi, C.H.; An, H.J.; Kim, Y.J.; Kim, J.H. Retrospective study comparing MR-guided radiation therapy (MRgRT) setup strategies for prostate treatment: Repositioning vs. replanning. Radiat. Oncol. 2019, 14, 139. [Google Scholar] [CrossRef] [PubMed]
- Grégoire, V.; Guckenberger, M.; Haustermans, K.; Lagendijk, J.J.W.; Ménard, C.; Pötter, R.; Slotman, B.J.; Tanderup, K.; Thorwarth, D.; Van Herk, M.; et al. Image guidance in radiation therapy for better cure of cancer. Mol. Oncol. 2020, 14, 1470–1491. [Google Scholar] [CrossRef]
- Guckenberger, M.; Flentje, M. Intensity-modulated radiotherapy for the treatment of pelvic lymph nodes in prostate cancer. Futur. Oncol. 2007, 3, 43–47. [Google Scholar] [CrossRef]
- Mundt, A.J.; E Lujan, A.; Rotmensch, J.; E Waggoner, S.; Yamada, S.; Fleming, G.; Roeske, J.C. Intensity-modulated whole pelvic radiotherapy in women with gynecologic malignancies. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 1330–1337. [Google Scholar] [CrossRef]
- Feng, F.Y.; Kim, H.M.; Lyden, T.H.; Haxer, M.J.; Feng, M.; Worden, F.P.; Chepeha, D.B.; Eisbruch, A. Intensity-modulated radiotherapy of head and neck cancer aiming to reduce dysphagia: Early dose-effect relationships for the swallowing structures. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 1289–1298. [Google Scholar] [CrossRef]
- Elkind, M.M.; Sutton, H. Radiation response of mammalian cells grown in culture: 1. Repair of X-ray damage in surviving Chinese hamster cells. Radiat. Res. 1960, 13, 556–593. [Google Scholar] [CrossRef]
- Park, J.A.; Kang, K.J.; Ko, I.O.; Lee, K.C.; Choi, B.K.; Katoch, N.; Kim, J.W.; Kim, H.J.; Kwon, O.I.; Woo, E.J. In vivo measurement of brain tissue response after irradiation: Comparison of T2 relaxation, apparent diffusion coefficient, and electrical conductivity. IEEE Trans. Med. Imaging 2019, 38, 2779–2784. [Google Scholar] [CrossRef]
- Kim, J.W.; Park, J.A.; Katoch, N.; Yang, J.; Park, S.; Choi, B.K.; Song, S.G.; Kim, T.H.; Kim, H.J. Image-based evaluation of irradiation effects in brain tissues by measuring absolute electrical conductivity using MRI. Cancers 2021, 13, 5490. [Google Scholar] [CrossRef]
- Lesbats, C.; Katoch, N.; Minhas, A.S.; Taylor, A.; Kim, H.J.; Woo, E.J.; Poptani, H. High-frequency electrical properties tomography at 9.4T as a novel contrast mechanism for brain tumors. Magn. Reson. Med. 2021, 86, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Tha, K.K.; Katscher, U.; Yamaguchi, S.; Stehning, C.; Terasaka, S.; Fujima, N.; Kudo, K.; Kazumata, K.; Yamamoto, T.; Van Cauteren, M.; et al. Noninvasive electrical conductivity measurement by MRI: A test of its validity and the electrical conductivity characteristics of glioma. Eur. Radiol. 2018, 28, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Katoch, N.; Choi, B.K.; Sajib, S.Z.K.; Lee, E.A.; Kim, H.J.; Kwon, O.I.; Woo, E.J. Conductivity tensor imaging of in vivo human brain and experimental validation using giant vesicle suspension. IEEE Trans. Med. Imaging 2019, 38, 1569–1577. [Google Scholar] [CrossRef]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Comm. 2022, 11, 5120. [Google Scholar] [CrossRef] [PubMed]
- Bortner, C.D.; Cidlowski, J.A. Ion channels and apoptosis in cancer. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130104. [Google Scholar] [CrossRef]
- Stewart, F.A.; Akleyev, A.V.; Hauer-Jensen, M.; Hendry, J.H.; Kleiman, N.J.; MacVittie, T.J.; Aleman, B.M.; Edgar, A.B.; Mabuchi, K.; Muirhead, C.R.; et al. ICRP PUBLICATION 118: ICRP Statement on Tissue Reactions and Early and Late Effects of Radiation in Normal Tissues and Organs—Threshold Doses for Tissue Reactions in a Radiation Protection Context. Ann. ICRP 2012, 41, 1–322. [Google Scholar] [PubMed]
- Han, X.; Xue, X.; Zhou, H.; Zhang, G. A molecular view of the radioresistance of gliomas. Oncotarget 2017, 8, 100931–100941. [Google Scholar] [CrossRef]
- Kelley, K.; Knisely, J.; Symons, M.; Ruggieri, R. Radioresistance of Brain Tumors. Cancers 2016, 8, 42. [Google Scholar] [CrossRef]
- Walker, C.; Baborie, A.; Crooks, D.; Wilkins, S.; Jenkinson, M.D. Biology, genetics and imaging of glial cell tumours. Br. J. Radiol. 2011, 84, S90–S106. [Google Scholar] [CrossRef]
- Liu, Z.G.; Jiao, D. Necroptosis, tumor necrosis and tumorigenesis. Cell Stress 2020, 4, 1–8. [Google Scholar] [CrossRef]
- Szabó, C. Mechanisms of cell necrosis. Crit. Care Med. 2005, 33, S530–S534. [Google Scholar] [CrossRef] [PubMed]
- Proskuryakov, S.Y.; Konoplyannikov, A.G.; Gabai, V.V. Necrosis: A specific form of programmed cell death? Exp. Cell. Res. 2003, 283, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.S.; Ko, I.O.; Park, H.; Jeong, Y.J.; Park, J.A.; Kim, K.S.; Park, M.J.; Lee, H.J. Radiation-induced changes in tumor vessels and microenvironment contribute to therapeutic resistance in glioblastoma. Front. Oncol. 2019, 9, 1259. [Google Scholar] [CrossRef] [PubMed]
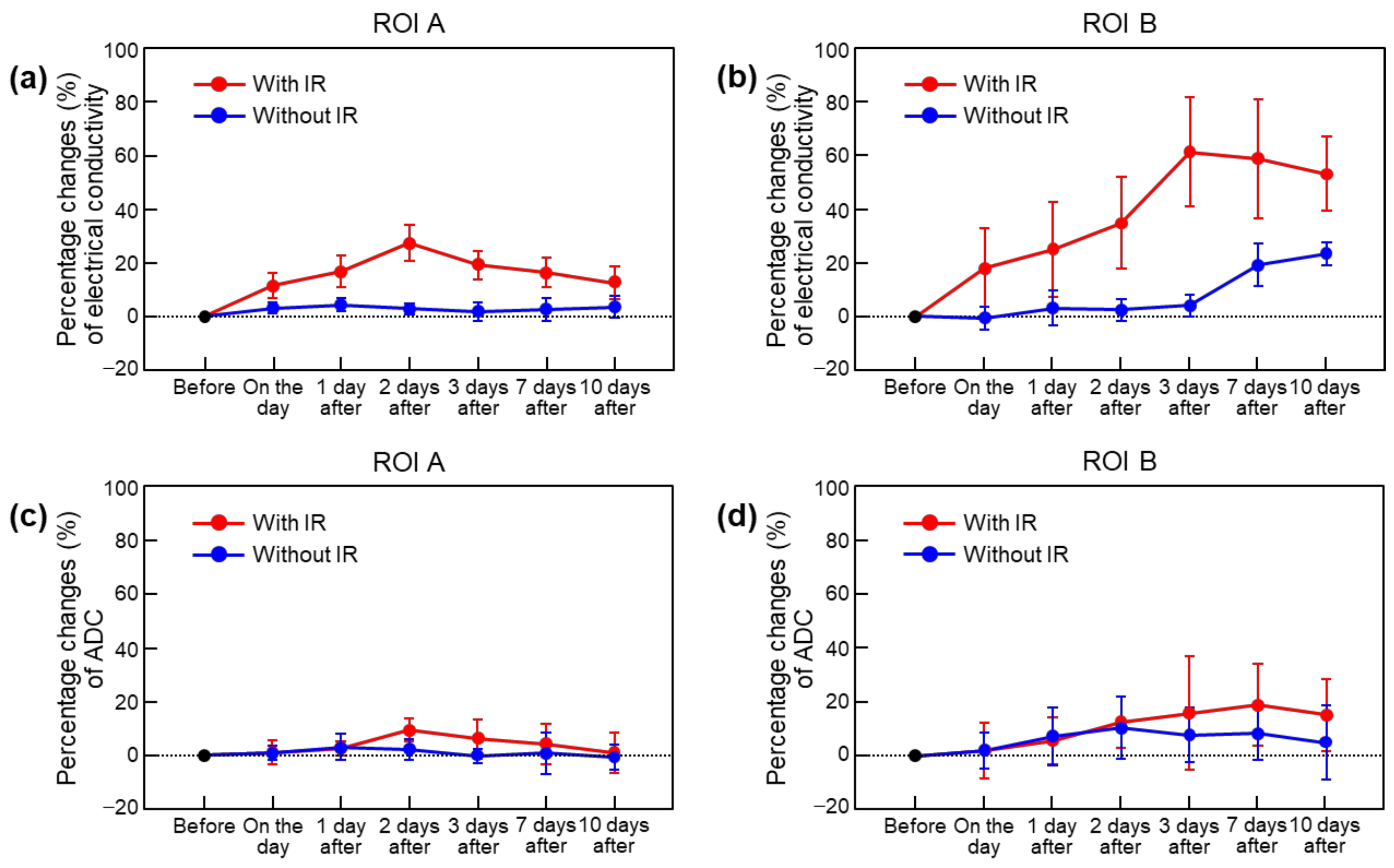
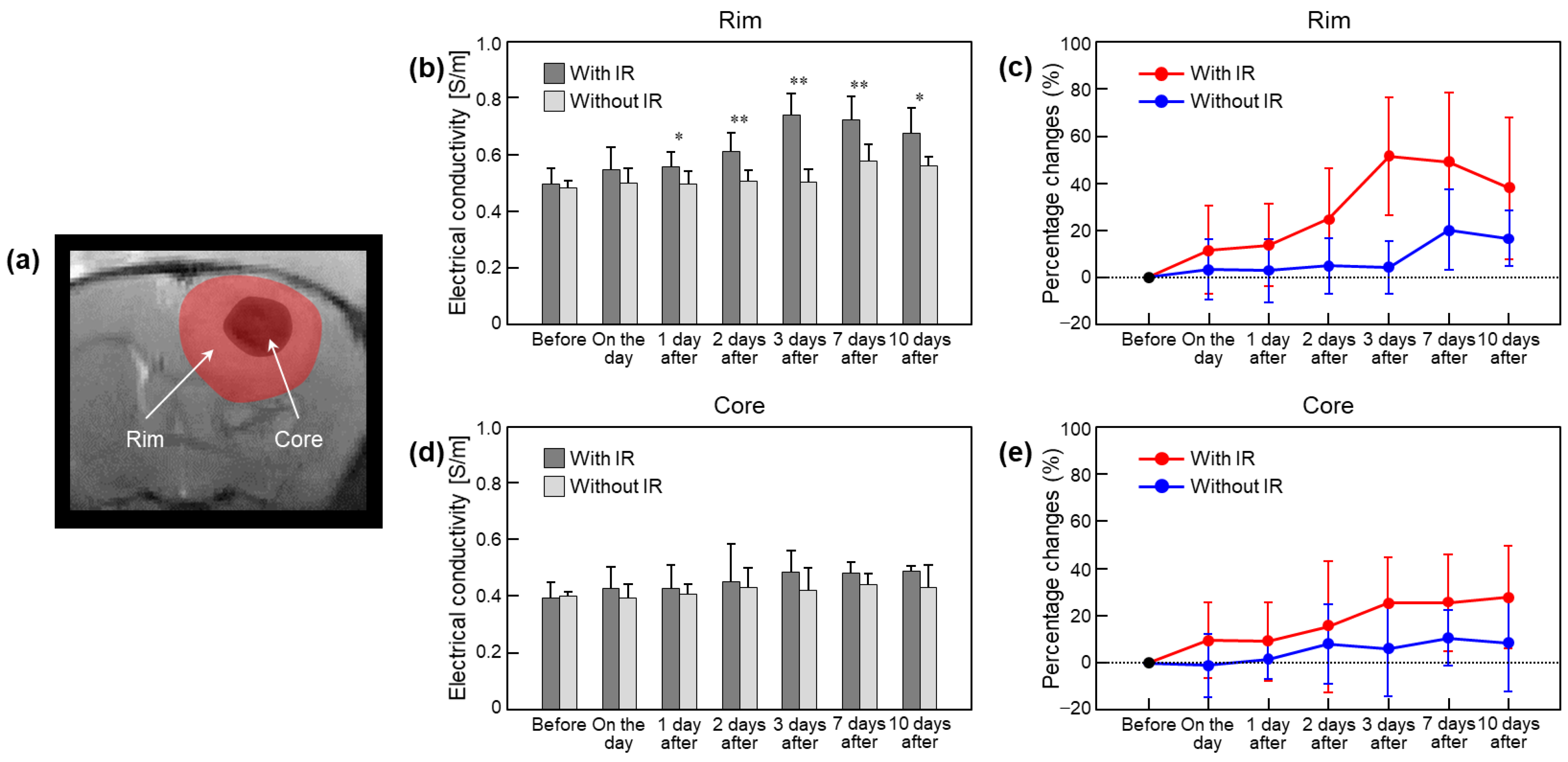
| In Vivo Brain | Electrical Conductivity (S/m) (Percentage Change, %) | |||||||
|---|---|---|---|---|---|---|---|---|
| Before | On the Day | 1 Day After | 2 Days After | 3 Days After | 7 Days After | 10 Days After | ||
| ROI A | With IR | 0.462 ± 0.029 (-) | 0.514 ± 0.022 (11.4 ± 4.9) | 0.538 ± 0.030 (16.6 ± 5.9) | 0.588 ± 0.044 (27.2 ± 6.7) | 0.550 ± 0.030 (19.1 ± 5.3) | 0.536 ± 0.021 (16.3 ± 5.6) | 0.515 ± 0.013 (12.4 ± 6.1) |
| Without IR | 0.467 ± 0.010 (-) | 0.482 ± 0.014 (3.0 ± 1.9) | 0.487 ± 0.010 (4.2 ± 2.4) | 0.480 ± 0.009 (2.8 ± 1.9) | 0.473 ± 0.013 (1.7 ± 3.4) | 0.479 ± 0.012 (2.6 ± 4.3) | 0.483 ± 0.012 (3.5 ± 4.0) | |
| ROI B | With IR | 0.469 ± 0.024 (-) | 0.552 ± 0.066 (18.0 ± 14.6) | 0.582 ± 0.067 (24.8 ± 17.6) | 0.629 ± 0.064 (34.7 ± 17.1) | 0.752 ± 0.075 (61.1 ± 20.4) | 0.739 ± 0.075 (58.4 ± 22.2) | 0.714 ± 0.056 (52.9 ± 13.8) |
| Without IR | 0.463 ± 0.020 (-) | 0.459 ± 0.024 (−0.7 ± 4.3) | 0.476 ± 0.026 (3.0 ± 6.5) | 0.473 ± 0.012 (2.3 ± 4.2) | 0.482 ± 0.008 (4.0 ± 4.1) | 0.550 ± 0.030 (19.1 ± 8.0) | 0.570 ± 0.029 (23.2 ± 4.4) | |
| Rim | With IR | 0.496 ± 0.057 (-) | 0.547 ± 0.077 (11.6 ± 18.6) | 0.556 ± 0.053 (13.7 ± 17.3) | 0.610 ± 0.067 (24.9 ± 21.2) | 0.738 ± 0.077 (51.2 ± 25.0) | 0.723 ± 0.080 (48.8 ± 29.3) | 0.674 ± 0.091 (37.6 ± 29.9) |
| Without IR | 0.483 ± 0.024 (-) | 0.498 ± 0.053 (3.4 ± 12.6) | 0.494 ± 0.048 (2.9 ± 13.4) | 0.504 ± 0.042 (4.9 ± 11.6) | 0.501 ± 0.046 (4.1 ± 11.2) | 0.576 ± 0.058 (20.1 ± 16.9) | 0.560 ± 0.034 (16.5 ± 11.8) | |
| Core | With IR | 0.392 ± 0.058 (-) | 0.427 ± 0.075 (9.7 ± 15.9) | 0.426 ± 0.084 (9.2 ± 16.5) | 0.452 ± 0.131 (15.4 ± 27.7) | 0.485 ± 0.076 (25.4 ± 19.5) | 0.481 ± 0.039 (25.5 ± 20.4) | 0.488 ± 0.020 (27.8 ± 21.6) |
| Without IR | 0.399 ± 0.015 (-) | 0.394 ± 0.049 (−1.0 ± 13.5) | 0.405 ± 0.038 (1.6 ± 8.4) | 0.432 ± 0.069 (8.1 ± 16.7) | 0.422 ± 0.076 (6.1 ± 20.1) | 0.440 ± 0.040 (10.6 ± 11.7) | 0.432 ± 0.079 (8.4 ± 20.3) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.A.; Kim, Y.; Yang, J.; Choi, B.K.; Katoch, N.; Park, S.; Hur, Y.H.; Kim, J.W.; Kim, H.J.; Kim, H.C. Effects of Irradiation on Brain Tumors Using MR-Based Electrical Conductivity Imaging. Cancers 2023, 15, 22. https://doi.org/10.3390/cancers15010022
Park JA, Kim Y, Yang J, Choi BK, Katoch N, Park S, Hur YH, Kim JW, Kim HJ, Kim HC. Effects of Irradiation on Brain Tumors Using MR-Based Electrical Conductivity Imaging. Cancers. 2023; 15(1):22. https://doi.org/10.3390/cancers15010022
Chicago/Turabian StylePark, Ji Ae, Youngsung Kim, Jiung Yang, Bup Kyung Choi, Nitish Katoch, Seungwoo Park, Young Hoe Hur, Jin Woong Kim, Hyung Joong Kim, and Hyun Chul Kim. 2023. "Effects of Irradiation on Brain Tumors Using MR-Based Electrical Conductivity Imaging" Cancers 15, no. 1: 22. https://doi.org/10.3390/cancers15010022
APA StylePark, J. A., Kim, Y., Yang, J., Choi, B. K., Katoch, N., Park, S., Hur, Y. H., Kim, J. W., Kim, H. J., & Kim, H. C. (2023). Effects of Irradiation on Brain Tumors Using MR-Based Electrical Conductivity Imaging. Cancers, 15(1), 22. https://doi.org/10.3390/cancers15010022





