Intrinsic and Extrinsic Transcriptional Profiles That Affect the Clinical Response to PD-1 Inhibitors in Patients with Non–Small Cell Lung Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients and PD-L1 Test
2.2. Gene Expression Analysis
2.3. Machine Learning Approach and Statistical Analysis
2.4. Statistical Analysis
3. Results
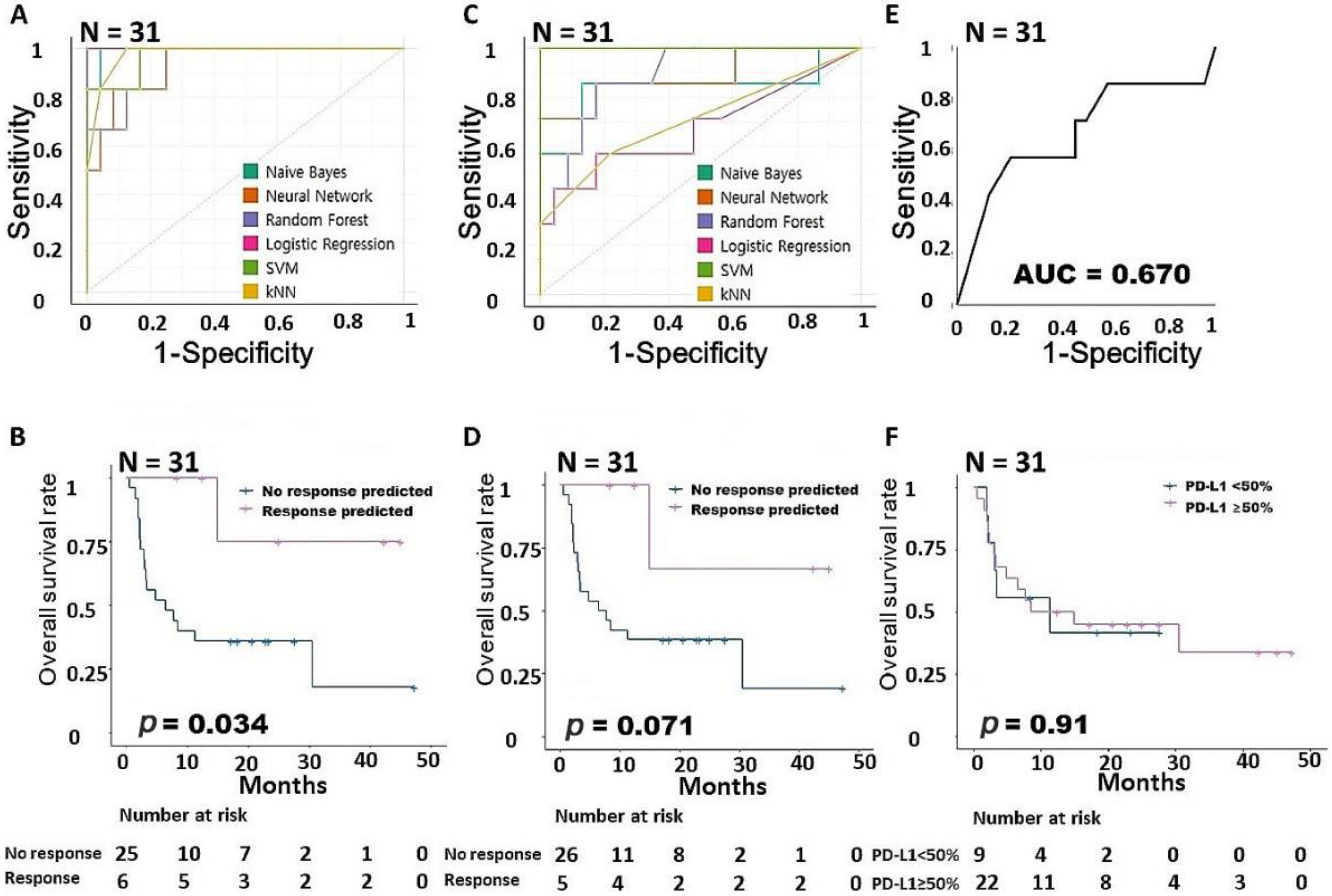
3.1. Feature Selection and Prediction Modeling Using Machine Learning Methods
3.2. Survival Analysis Using TCGA Dataset
3.3. Pathway Analyses
3.4. Differences in Transcriptional Patterns among NSCLC, LUAD, and LUSC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Dempke, W.C.M.; Fenchel, K.; Dale, S.P. Programmed cell death ligand-1 (PD-L1) as a biomarker for non-small cell lung cancer (NSCLC) treatment-are we barking up the wrong tree? Transl. Lung Cancer Res. 2018, 7, S275–S279. [Google Scholar] [CrossRef] [PubMed]
- Havel, J.J.; Chowell, D.; Chan, T.A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 2019, 19, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Ayers, M.; Lunceford, J.; Nebozhyn, M.; Murphy, E.; Loboda, A.; Kaufman, D.R.; Albright, A.; Cheng, J.D.; Kang, S.P.; Shankaran, V.; et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Investig. 2017, 127, 2930–2940. [Google Scholar] [CrossRef] [PubMed]
- Haddad, R.I.; Seiwert, T.Y.; Chow, L.Q.M.; Gupta, S.; Weiss, J.; Gluck, I.; Eder, J.P.; Burtness, B.; Tahara, M.; Keam, B.; et al. Influence of tumor mutational burden, inflammatory gene expression profile, and PD-L1 expression on response to pembrolizumab in head and neck squamous cell carcinoma. J. Immunother. Cancer 2022, 10, e003026. [Google Scholar] [CrossRef] [PubMed]
- Damotte, D.; Warren, S.; Arrondeau, J.; Boudou-Rouquette, P.; Mansuet-Lupo, A.; Biton, J.; Ouakrim, H.; Alifano, M.; Gervais, C.; Bellesoeur, A.; et al. The tumor inflammation signature (TIS) is associated with anti-PD-1 treatment benefit in the CERTIM pan-cancer cohort. J. Transl. Med. 2019, 17, 357. [Google Scholar] [CrossRef] [PubMed]
- Hudson, K.; Cross, N.; Jordan-Mahy, N.; Leyland, R. The Extrinsic and Intrinsic Roles of PD-L1 and Its Receptor PD-1: Implications for Immunotherapy Treatment. Front. Immunol. 2020, 11, 568931. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Zhang, L.; Ren, Y.; Ma, Q. The genomic alterations of lung adenocarcinoma and lung squamous cell carcinoma can explain the differences of their overall survival rates. J. Cell. Physiol. 2019, 234, 10918–10925. [Google Scholar] [CrossRef]
- Mamoshina, P.; Volosnikova, M.; Ozerov, I.V.; Putin, E.; Skibina, E.; Cortese, F.; Zhavoronkov, A. Machine Learning on Human Muscle Transcriptomic Data for Biomarker Discovery and Tissue-Specific Drug Target Identification. Front. Genet. 2018, 9, 242. [Google Scholar] [CrossRef]
- Cruz, J.A.; Wishart, D.S. Applications of machine learning in cancer prediction and prognosis. Cancer Inf. 2007, 2, 59–77. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Geiss, G.K.; Bumgarner, R.E.; Birditt, B.; Dahl, T.; Dowidar, N.; Dunaway, D.L.; Fell, H.P.; Ferree, S.; George, R.D.; Grogan, T.; et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat. Biotechnol. 2008, 26, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Wu, Q.; West, J.; Bai, J. Machine learning-based microarray analyses indicate low-expression genes might collectively influence PAH disease. PLoS Comput. Biol. 2019, 15, e1007264. [Google Scholar] [CrossRef] [PubMed]
- Shamsaei, B.; Gao, C. Comparison of some machine learning and statistical algorithms for classification and prediction of human cancer type. In Proceedings of the 2016 IEEE-EMBS International Conference on Biomedical and Health Informatics (BHI), Las Vegas, NV, USA, 24–27 February 2016; pp. 296–299. [Google Scholar]
- Abdulsalam, S.; Mohammed, A.; Ajao, J.; Babatunde, R.; Ogundokun, R.; Christopher, C.; Arowolo, M. Performance Evaluation of ANOVA and RFE Algorithms for Classifying Microarray Dataset Using SVM. In European, Mediterranean, and Middle Eastern Conference on Information Systems; Springer: Cham, Swizerland, 2020. [Google Scholar] [CrossRef]
- Demšar, J.; Curk, T.; Erjavec, A.; Gorup, Č.; Hočevar, T.; Milutinovič, M.; Možina, M.; Polajnar, M.; Toplak, M.; Starič, A.; et al. Orange: Data mining toolbox in Python. J. Mach. Learn. Res. 2013, 14, 2349–2353. [Google Scholar]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Zhang, J.; Bu, X.; Wang, H.; Zhu, Y.; Geng, Y.; Nihira, N.T.; Tan, Y.; Ci, Y.; Wu, F.; Dai, X.; et al. Cyclin D-CDK4 kinase destabilizes PD-L1 via cullin 3-SPOP to control cancer immune surveillance. Nature 2018, 553, 91–95. [Google Scholar] [CrossRef]
- Schulz, D.; Wetzel, M.; Eichberger, J.; Piendl, G.; Brockhoff, G.; Wege, A.K.; Reichert, T.E.; Ettl, T.; Bauer, R.J. Differential Expression of PD-L1 during Cell Cycle Progression of Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2021, 22, 13087. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, X.; Xu, J.; Li, E.; Lao, M.; Tang, T.; Zhang, G.; Guo, C.; Zhang, X.; Chen, W.; et al. NEK2 inhibition triggers anti-pancreatic cancer immunity by targeting PD-L1. Nat. Commun. 2021, 12, 4536. [Google Scholar] [CrossRef]
- Xu, Q.; Long, Q.; Zhu, D.; Fu, D.; Zhang, B.; Han, L.; Qian, M.; Guo, J.; Xu, J.; Cao, L.; et al. Targeting amphiregulin (AREG) derived from senescent stromal cells diminishes cancer resistance and averts programmed cell death 1 ligand (PD-L1)-mediated immunosuppression. Aging Cell 2019, 18, e13027. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Zhao, B.; Zhou, W.; Liu, H.; Fukumoto, T.; Gabrilovich, D.; Zhang, R. Sensitization of ovarian tumor to immune checkpoint blockade by boosting senescence-associated secretory phenotype. iScience 2021, 24, 102016. [Google Scholar] [CrossRef] [PubMed]
- Jerby-Arnon, L.; Shah, P.; Cuoco, M.S.; Rodman, C.; Su, M.J.; Melms, J.C.; Leeson, R.; Kanodia, A.; Mei, S.; Lin, J.R.; et al. A Cancer Cell Program Promotes T Cell Exclusion and Resistance to Checkpoint Blockade. Cell 2018, 175, 984–997.e924. [Google Scholar] [CrossRef] [PubMed]
- Ruscetti, M.; Morris, J.P.T.; Mezzadra, R.; Russell, J.; Leibold, J.; Romesser, P.B.; Simon, J.; Kulick, A.; Ho, Y.J.; Fennell, M.; et al. Senescence-Induced Vascular Remodeling Creates Therapeutic Vulnerabilities in Pancreas Cancer. Cell 2020, 181, 424–441.e421. [Google Scholar] [CrossRef] [PubMed]
- Casey, S.C.; Tong, L.; Li, Y.; Do, R.; Walz, S.; Fitzgerald, K.N.; Gouw, A.M.; Baylot, V.; Gütgemann, I.; Eilers, M.; et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science 2016, 352, 227–231. [Google Scholar] [CrossRef]
- Xiong, W.; Zhang, B.; Yu, H.; Zhu, L.; Yi, L.; Jin, X. RRM2 Regulates Sensitivity to Sunitinib and PD-1 Blockade in Renal Cancer by Stabilizing ANXA1 and Activating the AKT Pathway. Adv. Sci. 2021, 8, e2100881. [Google Scholar] [CrossRef]
- Chen, X.; Lu, Y.; Yu, H.; Du, K.; Zhang, Y.; Nan, Y.; Huang, Q. Pan-cancer analysis indicates that MYBL2 is associated with the prognosis and immunotherapy of multiple cancers as an oncogene. Cell Cycle 2021, 20, 2291–2308. [Google Scholar] [CrossRef]
- Tsukita, Y.; Fujino, N.; Miyauchi, E.; Saito, R.; Fujishima, F.; Itakura, K.; Kyogoku, Y.; Okutomo, K.; Yamada, M.; Okazaki, T.; et al. Axl kinase drives immune checkpoint and chemokine signalling pathways in lung adenocarcinomas. Mol. Cancer 2019, 18, 24. [Google Scholar] [CrossRef]
- Shen, W.; Tong, D.; Chen, J.; Li, H.; Hu, Z.; Xu, S.; He, S.; Ge, Z.; Zhang, J.; Mao, Q.; et al. Silencing oncogene cell division cycle associated 5 induces apoptosis and G1 phase arrest of non-small cell lung cancer cells via p53-p21 signaling pathway. J. Clin. Lab. Anal. 2022, 36, e24396. [Google Scholar] [CrossRef]
- Lee, Y.; Wu, Z.; Yang, S.; Schreiner, S.M.; Gonzalez-Smith, L.D.; Rhie, S.K. Characterizing and Targeting Genes Regulated by Transcription Factor MYBL2 in Lung Adenocarcinoma Cells. Cancers 2022, 14, 4979. [Google Scholar] [CrossRef]
- Holland, S.J.; Pan, A.; Franci, C.; Hu, Y.; Chang, B.; Li, W.; Duan, M.; Torneros, A.; Yu, J.; Heckrodt, T.J.; et al. R428, a selective small molecule inhibitor of Axl kinase, blocks tumor spread and prolongs survival in models of metastatic breast cancer. Cancer Res. 2010, 70, 1544–1554. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-W.; Park, Y.S.; Ahn, J.B.; Rha, S.Y.; Kim, H.K.; Lee, P.Y.; Ryu, M.-H.; Lee, J.; Lee, J.K.; Hwang, S. Safety and anti-tumor activity of the transforming growth factor beta receptor I kinase inhibitor, vactosertib, in combination with pembrolizumab in patients with metastatic colorectal or gastric cancer. J. Immunother. Cancer 2019, 7 (Suppl. 1), P377. [Google Scholar]




| 30 intrinsic pathway related genes in a total NSCLC |
| GREM1, AURKA, AXL, RRM2, CDC25A, PRIM1, CCNB1, ATP7A, PCLAF, LMNB1, ERBB2, NSD1, KAT6B, CDCA8, KIF2C, SRM, GPX1, PIK3CB, MYBL2, RUVBL1, UBE2C, RBBP5, FASLG, ARID4B, NCAPG, PIK3R5, CAV1, BAX, MYC, ITGA5. |
| 5 extrinsic pathway related genes in a total NSCLC |
| IL15RA, CCR1, CCL2, CYBB, FCER1G |
| 15 intrinsic pathway related genes in lung adenocarcinoma |
| MYC, RRM2, MAPK14, MYBL2, AXL, CDCA5, AKT1S1, TGFB1, CDK12, PCLAF, BAX, HMOX1, ARID4A, SLC1A5, ARID4B. |
| 15 extrinsic pathway related genes in lung adenocarcinoma |
| CD209, CCL13, LAG3, BCL2L1, HLA-C, IL15RA, MSLN, TAP1, CD8A, NKG7, TAP2, MST1R, CCL5, CCL2, CYBB. |
| 5 intrinsic pathway related genes in lung squamous cell carcinoma |
| SERPINE1, PIK3R5, PIK3CB, RPA3, KIF2C |
| 10 extrinsic pathway related genes in lung squamous cell carcinoma |
| CCR1, FCER1G, CD38, GNLY, CYBB, IL15RA, CCL2, VAV1, CD274, GZMB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byeon, H.E.; Haam, S.; Han, J.H.; Lee, H.W.; Koh, Y.W. Intrinsic and Extrinsic Transcriptional Profiles That Affect the Clinical Response to PD-1 Inhibitors in Patients with Non–Small Cell Lung Cancer. Cancers 2023, 15, 197. https://doi.org/10.3390/cancers15010197
Byeon HE, Haam S, Han JH, Lee HW, Koh YW. Intrinsic and Extrinsic Transcriptional Profiles That Affect the Clinical Response to PD-1 Inhibitors in Patients with Non–Small Cell Lung Cancer. Cancers. 2023; 15(1):197. https://doi.org/10.3390/cancers15010197
Chicago/Turabian StyleByeon, Hye Eun, Seokjin Haam, Jae Ho Han, Hyun Woo Lee, and Young Wha Koh. 2023. "Intrinsic and Extrinsic Transcriptional Profiles That Affect the Clinical Response to PD-1 Inhibitors in Patients with Non–Small Cell Lung Cancer" Cancers 15, no. 1: 197. https://doi.org/10.3390/cancers15010197
APA StyleByeon, H. E., Haam, S., Han, J. H., Lee, H. W., & Koh, Y. W. (2023). Intrinsic and Extrinsic Transcriptional Profiles That Affect the Clinical Response to PD-1 Inhibitors in Patients with Non–Small Cell Lung Cancer. Cancers, 15(1), 197. https://doi.org/10.3390/cancers15010197






