Colorectal Cancer with Peritoneal Metastases: The Impact of the Results of PROPHYLOCHIP, COLOPEC, and PRODIGE 7 Trials on Peritoneal Disease Management
Abstract
Simple Summary
Abstract
1. Introduction
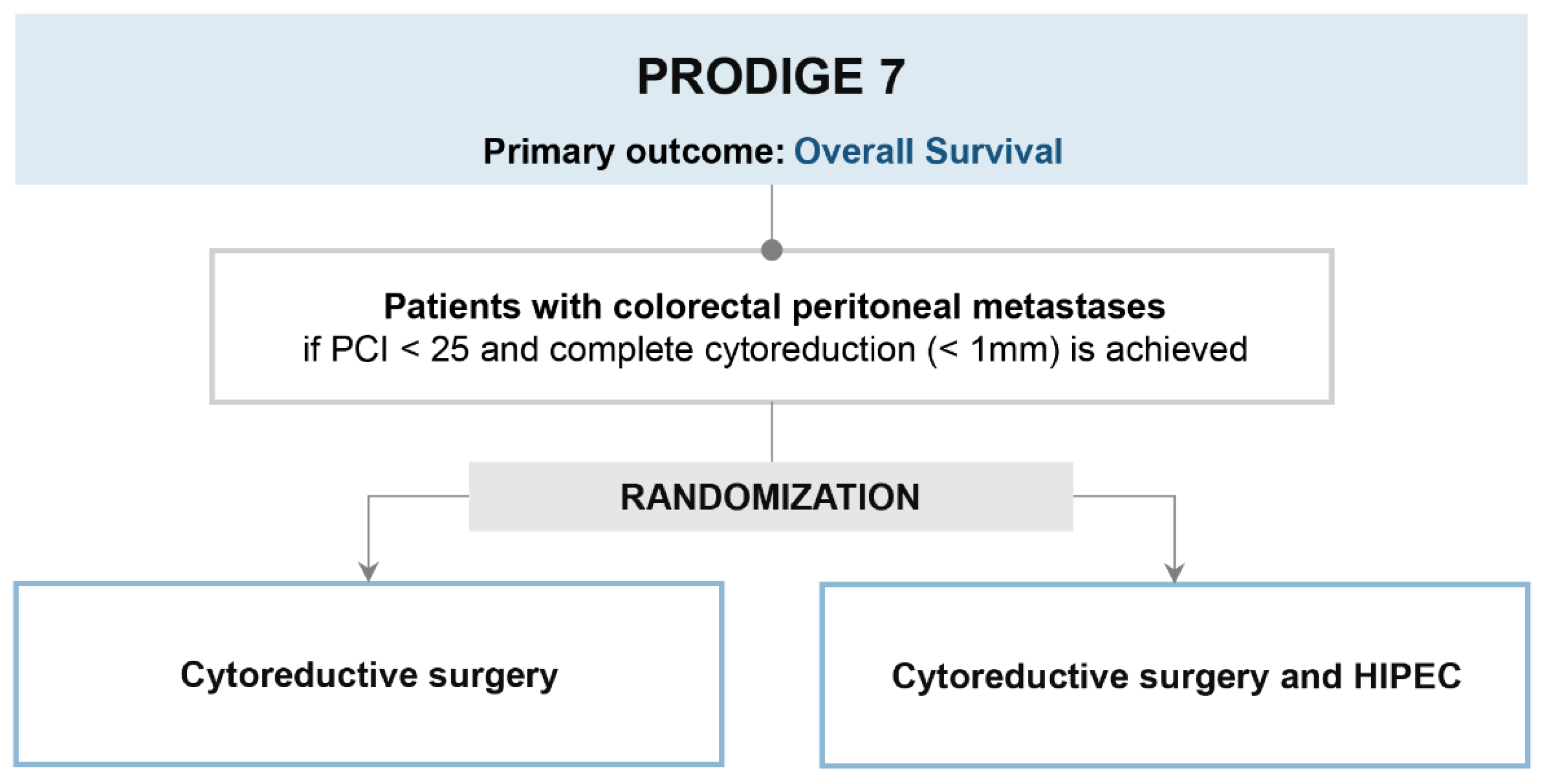
2. PRODIGE 7 Trial (Is HIPEC Useful in Patients Undergoing Complete Cytoreductive Surgery?)
2.1. Trial Design and Results
2.2. Strengths
2.3. Limits
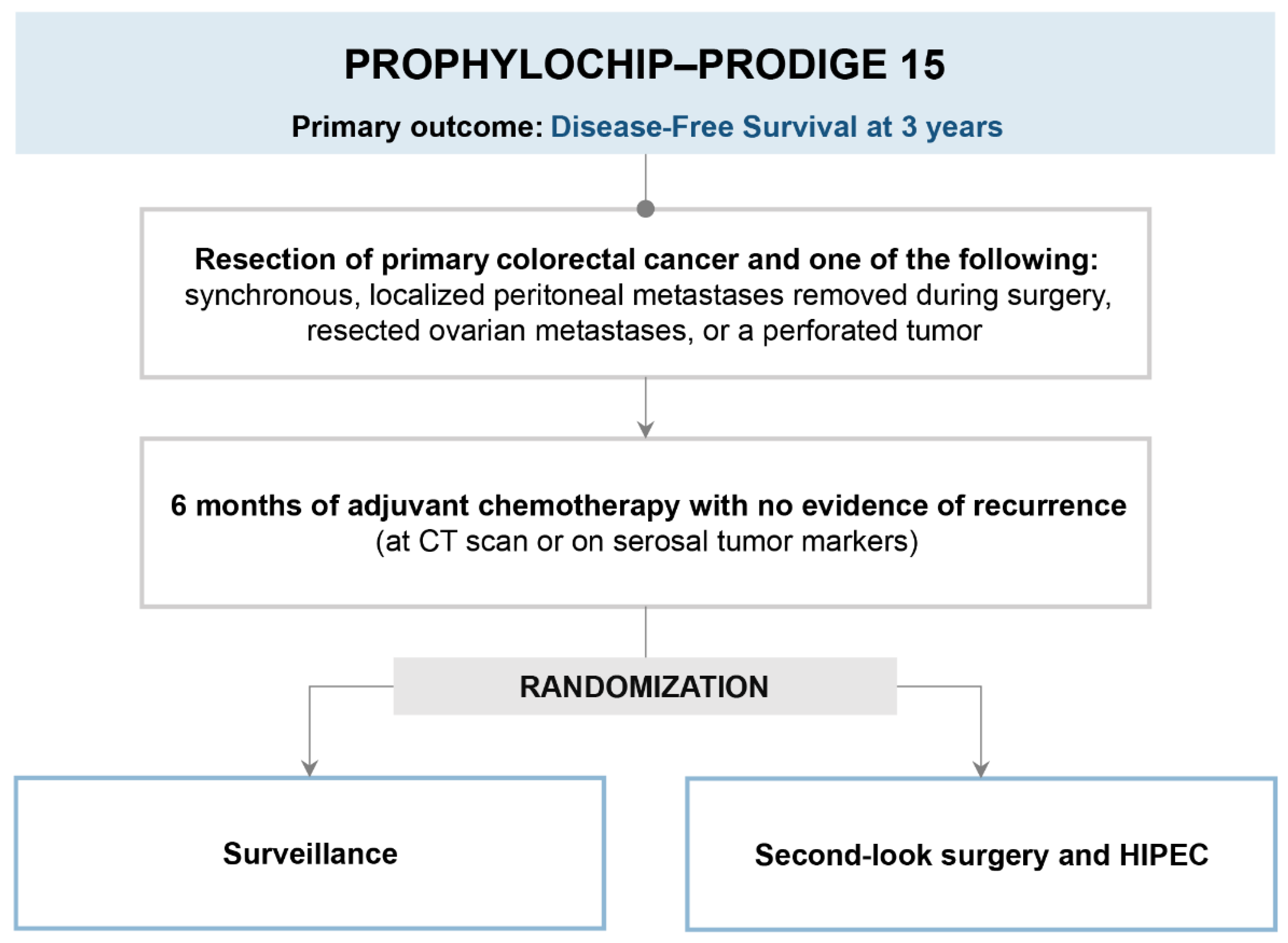
3. PROPHYLOCHIP-PRODIGE 15 (Is Second-Look Surgery and HIPEC Useful in Patients at High Risk of Peritoneal Recurrence?)
3.1. Trial Design and Results
3.2. Strengths
3.3. Limits
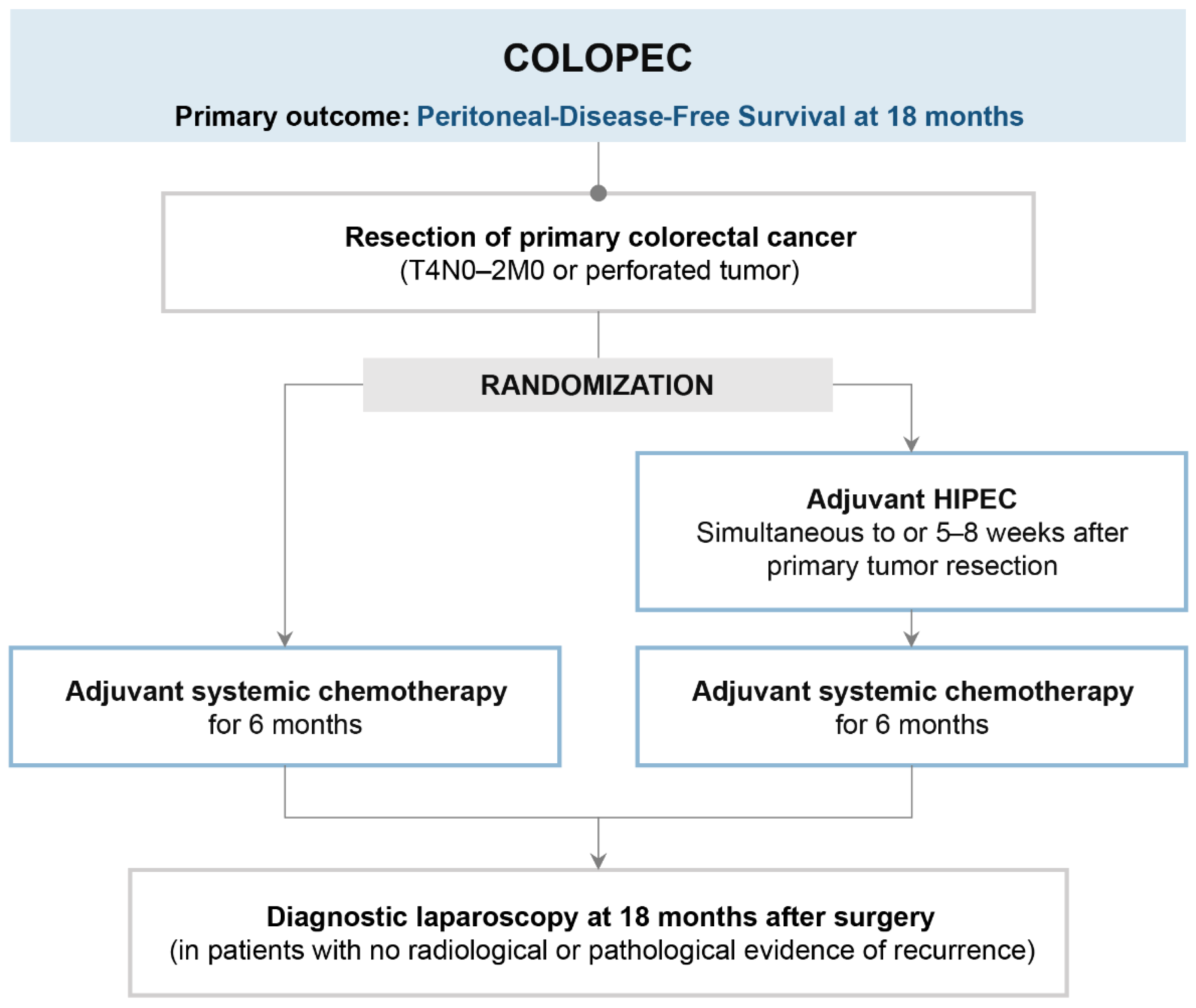
4. COLOPEC (Is Prophylactic HIPEC Useful in Patients at High Risk of Peritoneal Metastases?)
4.1. Trial Design and Results
4.2. Strengths
4.3. Limits
5. The Impact of Trials and Future Directions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Franko, J.; Shi, Q.; Meyers, J.P.; Maughan, T.S.; Adams, R.A.; Seymour, M.T.; Saltz, L.; Punt, C.J.A.; Koopman, M.; Tournigand, C.; et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: An analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016, 17, 1709–1719. [Google Scholar] [CrossRef] [PubMed]
- Segelman, J.; Granath, F.; Holm, T.; Machado, M.; Mahteme, H.; Martling, A. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br. J. Surg. 2012, 99, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Kerscher, A.G.; Chua, T.C.; Gasser, M.; Maeder, U.; Kunzmann, V.; Isbert, C.; Germer, C.T.; Pelz, J.O.W. Impact of peritoneal carcinomatosis in the disease history of colorectal cancer management: A longitudinal experience of 2406 patients over two decades. Br. J. Cancer 2013, 108, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Franko, J.; Shi, Q.; Goldman, C.D.; Pockaj, B.A.; Nelson, G.D.; Goldberg, R.M.; Pitot, H.C.; Grothey, A.; Alberts, S.R.; Sargent, D. Treatment of Colorectal Peritoneal Carcinomatosis with Systemic Chemotherapy: A Pooled Analysis of North Central Cancer Treatment Group Phase III Trials N9741 and N9841. J. Clin. Oncol. 2012, 30, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Verwaal, V.J.; Van Ruth, S.; De Bree, E.; Van Slooten, G.W.; Van Tinteren, H.; Boot, H.; Zoetmulder, F.A. Randomized Trial of Cytoreduction and Hyperthermic Intraperitoneal Chemotherapy Versus Systemic Chemotherapy and Palliative Surgery in Patients with Peritoneal Carcinomatosis of Colorectal Cancer. J. Clin. Oncol. 2003, 21, 3737–3743. [Google Scholar] [CrossRef]
- Elias, D.; Lefevre, J.H.; Chevalier, J.; Brouquet, A.; Marchal, F.; Classe, J.-M.; Ferron, G.; Guilloit, J.-M.; Meeus, P.; Goéré, D.; et al. Complete Cytoreductive Surgery Plus Intraperitoneal Chemohyperthermia with Oxaliplatin for Peritoneal Carcinomatosis of Colorectal Origin. J. Clin. Oncol. 2009, 27, 681–685. [Google Scholar] [CrossRef]
- Goéré, D.; Malka, D.; Tzanis, D.; Gava, V.; Boige, V.; Eveno, C.; Maggiori, L.; Dumont, F.; Ducreux, M.; Elias, D. Is There a Possibility of a Cure in Patients with Colorectal Peritoneal Carcinomatosis Amenable to Complete Cytoreductive Surgery and Intraperitoneal Chemotherapy? Ann. Surg. 2013, 257, 1065–1071. [Google Scholar] [CrossRef]
- Razenberg, L.G.; Lemmens, V.E.; Verwaal, V.J.; Punt, C.J.; Tanis, P.J.; Creemers, G.-J.; de Hingh, I.H. Challenging the dogma of colorectal peritoneal metastases as an untreatable condition: Results of a population-based study. Eur. J. Cancer 2016, 65, 113–120. [Google Scholar] [CrossRef]
- Braam, H.J.; Boerma, D.; Wiezer, M.J.; Van Ramshorst, B. Cytoreductive surgery and HIPEC in treatment of colorectal peritoneal carcinomatosis: Experiment or standard care? A survey among oncologic surgeons and medical oncologists. Int. J. Clin. Oncol. 2015, 20, 928–934. [Google Scholar] [CrossRef]
- Klaver, C.E.L.; Groenen, H.; Morton, D.G.; Laurberg, S.; Bemelman, W.A.; Tanis, P.J. The research committee of the European Society of Coloproctology Recommendations and consensus on the treatment of peritoneal metastases of colorectal origin: A systematic review of national and international guidelines. Color. Dis. 2016, 19, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Quénet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Larentzakis, A.; O’Dwyer, S.T.; Becker, J.; Shuweihdi, F.; Aziz, O.; Selvasekar, C.R.; Fulford, P.; Renehan, A.G.; Wilson, M. Referral pathways and outcome of patients with colorectal peritoneal metastasis (CRPM). Eur. J. Surg. Oncol. (EJSO) 2019, 45, 2310–2315. [Google Scholar] [CrossRef] [PubMed]
- Sommariva, A.; Ansaloni, L.; Baiocchi, G.L.; Cascinu, S.; Cirocchi, R.; Coccolini, F.; Deraco, M.; Fiorentini, G.; Gelmini, R.; Di Giorgio, A.; et al. Diagnostic and therapeutic algorithm for colorectal peritoneal metastases. A consensus of the peritoneal surface malignancies onco-team of the Italian society of surgical oncology. Eur. J. Surg. Oncol. (EJSO) 2020, 47, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Hallam, S.; Tyler, R.; Price, M.; Beggs, A.; Youssef, H. Meta-analysis of prognostic factors for patients with colorectal peritoneal metastasis undergoing cytoreductive surgery and heated intraperitoneal chemotherapy. BJS Open 2019, 3, 585–594. [Google Scholar] [CrossRef] [PubMed]
- van Oudheusden, T.; Braam, H.; Nienhuijs, S.; Wiezer, M.; Van Ramshorst, B.; Luyer, P.; de Hingh, I. Poor outcome after cytoreductive surgery and HIPEC for colorectal peritoneal carcinomatosis with signet ring cell histology. J. Surg. Oncol. 2014, 111, 237–242. [Google Scholar] [CrossRef]
- Schneider, M.A.; Eden, J.; Pache, B.; Laminger, F.; Lopez-Lopez, V.; Steffen, T.; Hübner, M.; Kober, F.; Roka, S.; Campos, P.C.; et al. Mutations of RAS/RAF Proto-oncogenes Impair Survival After Cytoreductive Surgery and HIPEC for Peritoneal Metastasis of Colorectal Origin. Ann. Surg. 2018, 268, 845–853. [Google Scholar] [CrossRef]
- Tonello, M.; Baratti, D.; Sammartino, P.; Di Giorgio, A.; Robella, M.; Sassaroli, C.; Framarini, M.; Valle, M.; Macrì, A.; Graziosi, L.; et al. Microsatellite and RAS/RAF Mutational Status as Prognostic Factors in Colorectal Peritoneal Metastases Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC). Ann. Surg. Oncol. 2021, 29, 3405–3417. [Google Scholar] [CrossRef]
- Kwakman, R.; Schrama, A.M.; van Olmen, J.P.; Otten, R.H.; de Lange-de Klerk, E.S.; de Cuba, E.M.; Kazemier, G.; Te Velde, E.A. Clinicopathological Parameters in Patient Selection for Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Colorectal Cancer Metastases: A Meta-Analysis. Ann. Surg. 2016, 263, 1102–1111. [Google Scholar] [CrossRef]
- Elias, D.; Delpero, J.R.; Sideris, L.; Benhamou, E.; Pocard, M.; Baton, O.; Giovannini, M.; Lasser, P. Treatment of Peritoneal Carcinomatosis From Colorectal Cancer: Impact of Complete Cytoreductive Surgery and Difficulties in Conducting Randomized Trials. Ann. Surg. Oncol. 2004, 11, 518–521. [Google Scholar] [CrossRef]
- Prabhu, A.; Brandl, A.; Wakama, S.; Sako, S.; Ishibashi, H.; Mizumoto, A.; Takao, N.; Ichinose, M.; Motoi, S.; Liu, Y.; et al. Effect of oxaliplatin-based chemotherapy on chemosensitivity in patients with peritoneal metastasis from colorectal cancer treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: Proof-of-concept study. BJS Open 2021, 5, zraa075. [Google Scholar] [CrossRef] [PubMed]
- Nagourney, R.A.; Evans, S.; Tran, P.H.; Nagourney, A.J.; Sugarbaker, P.H. Colorectal cancer cells from patients treated with FOLFOX or CAPOX are resistant to oxaliplatin. Eur. J. Surg. Oncol. (EJSO) 2020, 47, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Ubink, I.; Bolhaqueiro, A.C.F.; Elias, S.G.; Raats, D.A.E.; Constantinides, A.; Peters, N.A.; Wassenaar, E.C.E.; de Hingh, I.H.J.T.; Rovers, K.P.; van Grevenstein, W.M.U.; et al. Organoids from colorectal peritoneal metastases as a platform for improving hyperthermic intraperitoneal chemotherapy. Br. J. Surg. 2019, 106, 1404–1414. [Google Scholar] [CrossRef]
- Wisselink, D.D.; Braakhuis, L.L.; Gallo, G.; van Grevenstein, W.M.; van Dieren, S.; Kok, N.F.; de Reuver, P.R.; Tanis, P.J.; de Hingh, I.H. Systematic review of published literature on oxaliplatin and mitomycin C as chemotherapeutic agents for hyperthermic intraperitoneal chemotherapy in patients with peritoneal metastases from colorectal cancer. Crit. Rev. Oncol. 2019, 142, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, S.D.; Sasikumar, S.; Moaven, O.; Sivakumar, H.; Shen, P.; Levine, E.A.; Soker, S.; Skardal, A.; Votanopoulos, K.I. Personalized Identification of Optimal HIPEC Perfusion Protocol in Patient-Derived Tumor Organoid Platform. Ann. Surg. Oncol. 2020, 27, 4950–4960. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, Q.; Wei, M.; Deng, X.; Gu, C.; Wang, Z. Oxaliplatin versus mitomycin C in HIPEC for peritoneal metastasis from colorectal cancer: A systematic review and meta-analysis of comparative studies. Int. J. Color. Dis. 2020, 35, 1831–1839. [Google Scholar] [CrossRef]
- Goéré, D.; Glehen, O.; Quenet, F.; Guilloit, J.-M.; Bereder, J.-M.; Lorimier, G.; Thibaudeau, E.; Ghouti, L.; Pinto, A.; Tuech, J.-J.; et al. Second-look surgery plus hyperthermic intraperitoneal chemotherapy versus surveillance in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP–PRODIGE 15): A randomised, phase 3 study. Lancet Oncol. 2020, 21, 1147–1154. [Google Scholar] [CrossRef]
- Klaver, C.E.L.; Wisselink, D.D.; Punt, C.J.A.; Snaebjornsson, P.; Crezee, J.; Aalbers, A.G.J.; Brandt, A.; Bremers, A.J.A.; Burger, J.W.A.; Fabry, H.F.J.; et al. Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): A multicentre, open-label, randomised trial. Lancet Gastroenterol. Hepatol. 2019, 4, 761–770. [Google Scholar] [CrossRef]
- Evrard, S. Autopsy of an expert consensus: End of hyperthermic intraperitoneal chemotherapy in colorectal carcinomatosis. Eur. J. Surg. Oncol. (EJSO) 2018, 44, 1845–1846. [Google Scholar] [CrossRef]
- Königsrainer, A.; Rau, B. Cytoreductive Surgery (CRS) and Hyperthermic IntraPeritoneal Chemotherapy (HIPEC): Don’t throw the baby out with the bathwater. Pleura Peritoneum 2018, 3, 20180131. [Google Scholar] [CrossRef]
- Bushati, M.; Rovers, K.; Sommariva, A.; Sugarbaker, P.; Morris, D.; Yonemura, Y.; Quadros, C.; Somashekhar, S.; Ceelen, W.; Dubé, P.; et al. The current practice of cytoreductive surgery and HIPEC for colorectal peritoneal metastases: Results of a worldwide web-based survey of the Peritoneal Surface Oncology Group International (PSOGI). Eur. J. Surg. Oncol. (EJSO) 2018, 44, 1942–1948. [Google Scholar] [CrossRef] [PubMed]
- van de Vlasakker, V.C.; Lurvink, R.J.; Cashin, P.H.; Ceelen, W.; Deraco, M.; Goéré, D.; González-Moreno, S.; Lehmann, K.; Li, Y.; Moran, B.; et al. The impact of PRODIGE 7 on the current worldwide practice of CRS-HIPEC for colorectal peritoneal metastases: A web-based survey and 2021 statement by Peritoneal Surface Oncology Group International (PSOGI). Eur. J. Surg. Oncol. (EJSO) 2021, 47, 2888–2892. [Google Scholar] [CrossRef] [PubMed]
- Leung, V.; Huo, Y.; Liauw, W.; Morris, D. Oxaliplatin versus Mitomycin C for HIPEC in colorectal cancer peritoneal carcinomatosis. Eur. J. Surg. Oncol. (EJSO) 2016, 43, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Bakkers, C.; van Erning, F.; Rovers, K.; Nienhuijs, S.; Burger, J.; Lemmens, V.; Aalbers, A.; Kok, N.; Boerma, D.; Brandt, A.; et al. Long-term survival after hyperthermic intraperitoneal chemotherapy using mitomycin C or oxaliplatin in colorectal cancer patients with synchronous peritoneal metastases: A nationwide comparative study. Eur. J. Surg. Oncol. (EJSO) 2020, 46, 1902–1907. [Google Scholar] [CrossRef] [PubMed]
- Prada-Villaverde, A.; Esquivel, J.; Lowy, A.M.; Markman, M.; Chua, T.; Pelz, J.; Baratti, D.; Baumgartner, J.M.; Berri, R.; Bretcha-Boix, P.; et al. The American Society of Peritoneal Surface Malignancies evaluation of HIPEC with Mitomycin C versus Oxaliplatin in 539 patients with colon cancer undergoing a complete cytoreductive surgery. J. Surg. Oncol. 2014, 110, 779–785. [Google Scholar] [CrossRef]
- Frühling, P.; Ghanipour, L.; Dranichnikov, P.; Enblad, M.; Birgisson, H.; Cashin, P.H. Oxaliplatin-based hyperthermic intraperitoneal chemotherapy with single drug versus multiple drug treatment for colorectal cancer with peritoneal metastases: An observational cohort study. J. Gastrointest. Oncol. 2021, 12, 516–526. [Google Scholar] [CrossRef]
- Seyfried, N.; Yurttas, C.; Burkard, M.; Oswald, B.; Tolios, A.; Herster, F.; Kauer, J.; Jäger, T.; Königsrainer, I.; Thiel, K.; et al. Prolonged Exposure to Oxaliplatin during HIPEC Improves Effectiveness in a Preclinical Micrometastasis Model. Cancers 2022, 14, 1158. [Google Scholar] [CrossRef]
- Cashin, P.; Sugarbaker, P.H. Hyperthermic intraperitoneal chemotherapy (HIPEC) for colorectal and appendiceal peritoneal metastases: Lessons learned from PRODIGE 7. J. Gastrointest. Oncol. 2021, 12, S120–S128. [Google Scholar] [CrossRef]
- Pinto, A.; Pocard, M. Hyperthermic intraperitoneal chemotherapy with cisplatin and mitomycin C for colorectal cancer peritoneal metastases: A systematic review of the literature. Pleura Peritoneum 2019, 4, 20190006. [Google Scholar] [CrossRef]
- Arjona-Sanchez, A.; Cano-Osuna, M.; Gutierrez, A.; Segura, J.; Perez, E.; Concepcion, V.; Sanchez, S.; Garcia, A.; Prieto, I.; Sanchez, P.B.; et al. 314O Adjuvant hyperthermic intraperitoneal chemotherapy in locally advanced colon cancer (HIPECT4): A randomized phase III study. Ann. Oncol. 2022, 33, S680. [Google Scholar] [CrossRef]
| Trials | Type | Setting | Patients | Arms | Primary End Point | Results |
|---|---|---|---|---|---|---|
| PRODIGE7 (France) | Multicenter | Curative | PM with PCI < 20 | CRS alone versus CRS-HIPEC | OS (months) | 41.2 vs. 41.7 p = NS |
| PROPHYLOCHIP-PRODIGE 15 (France) | Multicenter | Prophylactic | High risk (peritoneal/ ovarian metastases radically resected/perforated tumor) after systemic CHT | Follow-up versus CRS-HIPEC | 3-years DFS (Peritoneal or not) | 53% vs. 44% p = 0.82 |
| COLOPEC (The Netherlands) | Multicenter | Adjuvant | High risk (T4/perforated tumor) | Systemic CHT alone versus Systemic CHT + HIPEC | Peritoneal-Disease -Free Survival (at 18 months) | 76.2% vs. 80.9% p = 0.28 |
| Sponsor | Type | Setting | ClinicalTrials.gov Identifier: | Population | Experimental Arm | Primary Outcome |
|---|---|---|---|---|---|---|
| Cordoba (Spain) | Multicenter | Prophylactic | NCT02614534 | Primary cT4 colorectal cancer undergoing curative resection | Proactive cytoreductive surgery + HIPEC (mitomycin C 30 mg/m2) | DFS |
| Zhejiang University (China) | Multicenter | II^look | NCT02179489 | Resected Minimal Synchronous PC or Ovarian Metastases, tumor rupture or identified cT4 after 6 months of systemic chemotherapy | II^ look surgery and HIPEC with MMC (mitomycin C 30 mg/m2) | DFS |
| Policlinico Umberto I, Sapienza University, Rome (Italy) | Multicenter | Prophylactic | NCT02974556 | Primary cT3/4 colorectal cancer undergoing curative resection | Proactive cytoreductive surgery + HIPEC (oxaliplatin 260 mg/m2 + 5-FU 400 mg/m2 and leucovorin 20 mg/m2 i.v.) | DFS |
| Guangzhou Medical University, Beijing (China) | Multicenter | Prophylactic | NCT04370925 | Primary cT4 colorectal cancer | Proactive mitomycin c (30 mg/m2) | DFS |
| Policlinico Universitario Gemelli, Rome (Italy) | Multicenter | Prophylactic | NCT03914820 | Primary cT4 colorectal cancer with or without perforation, minimal and limited peritoneal metastasis in close proximity to the primary tumor, ovarian metastases undergoing radical resection | HIPEC (mitomycin C 35 mg/m2) | LDFS |
| Hospital Universitario de Fuenlabrada (Madrid, Spain) | Multicenter | Curative | NCT05250648 | PM with PCI less than 20 completely resected (no macroscopic residual disease) | cytoreductive surgery + HIPEC (mitomycin C 35 mg/m2) | PRFS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sommariva, A.; Tonello, M.; Coccolini, F.; De Manzoni, G.; Delrio, P.; Pizzolato, E.; Gelmini, R.; Serra, F.; Rreka, E.; Pasqual, E.M.; et al. Colorectal Cancer with Peritoneal Metastases: The Impact of the Results of PROPHYLOCHIP, COLOPEC, and PRODIGE 7 Trials on Peritoneal Disease Management. Cancers 2023, 15, 165. https://doi.org/10.3390/cancers15010165
Sommariva A, Tonello M, Coccolini F, De Manzoni G, Delrio P, Pizzolato E, Gelmini R, Serra F, Rreka E, Pasqual EM, et al. Colorectal Cancer with Peritoneal Metastases: The Impact of the Results of PROPHYLOCHIP, COLOPEC, and PRODIGE 7 Trials on Peritoneal Disease Management. Cancers. 2023; 15(1):165. https://doi.org/10.3390/cancers15010165
Chicago/Turabian StyleSommariva, Antonio, Marco Tonello, Federico Coccolini, Giovanni De Manzoni, Paolo Delrio, Elisa Pizzolato, Roberta Gelmini, Francesco Serra, Erion Rreka, Enrico Maria Pasqual, and et al. 2023. "Colorectal Cancer with Peritoneal Metastases: The Impact of the Results of PROPHYLOCHIP, COLOPEC, and PRODIGE 7 Trials on Peritoneal Disease Management" Cancers 15, no. 1: 165. https://doi.org/10.3390/cancers15010165
APA StyleSommariva, A., Tonello, M., Coccolini, F., De Manzoni, G., Delrio, P., Pizzolato, E., Gelmini, R., Serra, F., Rreka, E., Pasqual, E. M., Marano, L., Biacchi, D., Carboni, F., Kusamura, S., & Sammartino, P. (2023). Colorectal Cancer with Peritoneal Metastases: The Impact of the Results of PROPHYLOCHIP, COLOPEC, and PRODIGE 7 Trials on Peritoneal Disease Management. Cancers, 15(1), 165. https://doi.org/10.3390/cancers15010165







