Laparoscopic Cytoreduction Combined with Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Peritoneal Surface Malignancies (PSM): Italian PSM Oncoteam Evidence and Literature Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Literature Search
2.3. Statistical Analysis
3. Results
3.1. Patients
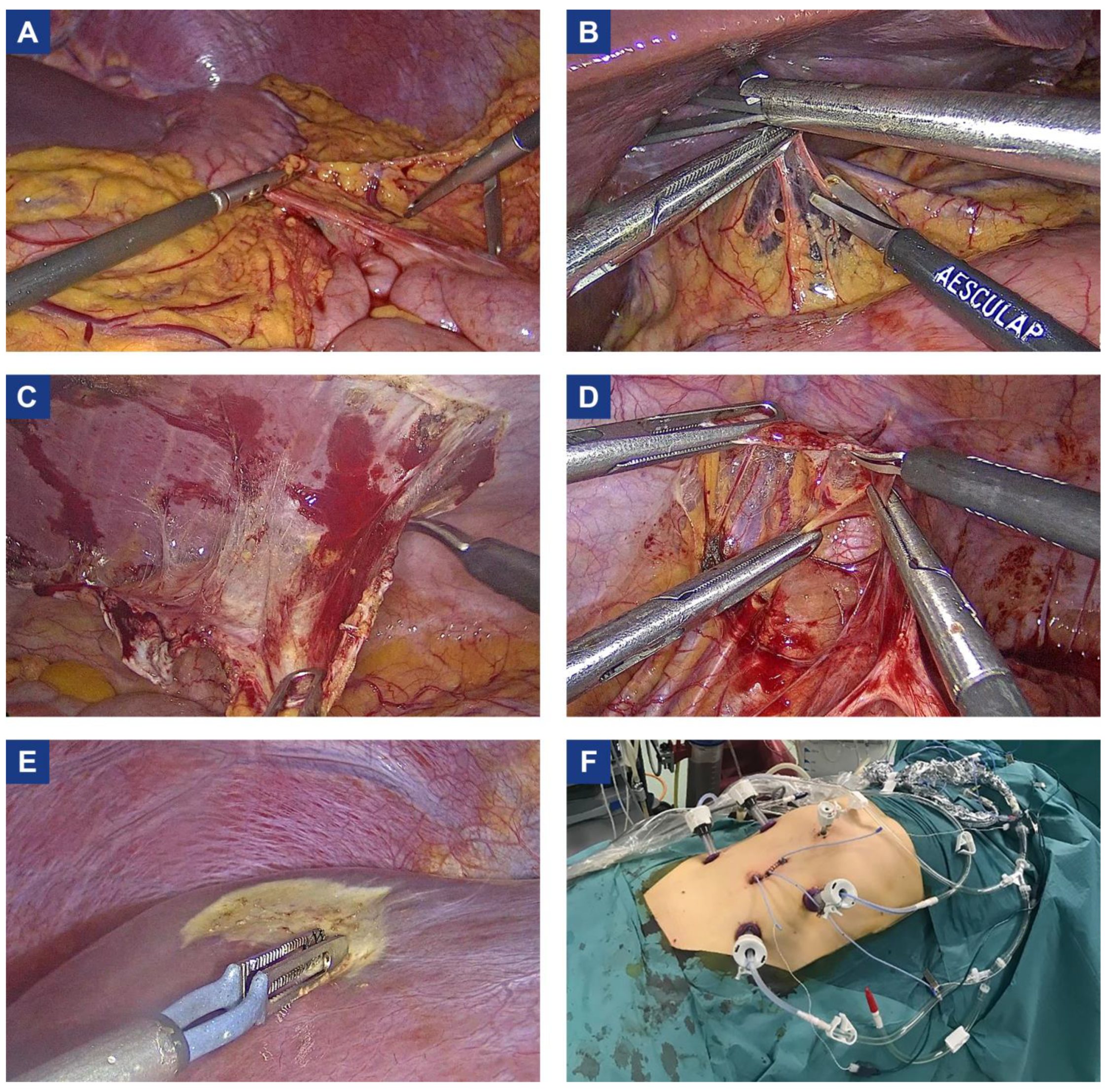
3.2. Surgery
3.3. Postoperative Outcome
3.4. Oncological Outcome
3.5. Review of the Literature
3.5.1. Study Identification
3.5.2. Patients
3.5.3. Intraoperative Data
3.5.4. Postoperative Outcomes
3.5.5. Oncological Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sugarbaker, P.H. Management of Peritoneal-Surface Malignancy: The Surgeon’s Role. Langenbeck’s Arch. Surg. 1999, 384, 576–587. [Google Scholar] [CrossRef] [PubMed]
- Kusamura, S.; Barretta, F.; Yonemura, Y.; Sugarbaker, P.H.; Moran, B.J.; Levine, E.A.; Goere, D.; Baratti, D.; Nizri, E.; Morris, D.L.; et al. The Role of Hyperthermic Intraperitoneal Chemotherapy in Pseudomyxoma Peritonei after Cytoreductive Surgery. JAMA Surg. 2021, 156, e206363. [Google Scholar] [CrossRef]
- Yan, T.D.; Welch, L.; Black, D.; Sugarbaker, P.H. A Systematic Review on the Efficacy of Cytoreductive Surgery Combined with Perioperative Intraperitoneal Chemotherapy for Diffuse Malignancy Peritoneal Mesothelioma. Ann. Oncol. 2007, 18, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Stirrups, R. HIPEC Improves Survival in Stage III Epithelial Ovarian Cancer. Lancet Oncol. 2018, 19, e138. [Google Scholar] [CrossRef] [PubMed]
- van Driel, W.J.; Koole, S.N.; Sikorska, K.; Schagen van Leeuwen, J.H.; Schreuder, H.W.R.; Hermans, R.H.M.; de Hingh, I.H.J.T.; van der Velden, J.; Arts, H.J.; Massuger, L.F.A.G.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef]
- Cao, C.; Yan, T.D.; Black, D.; Morris, D.L. A Systematic Review and Meta-Analysis of Cytoreductive Surgery with Perioperative Intraperitoneal Chemotherapy for Peritoneal Carcinomatosis of Colorectal Origin. Ann. Surg. Oncol. 2009, 16, 2152–2165. [Google Scholar] [CrossRef]
- Granieri, S.; Bonomi, A.; Frassini, S.; Chierici, A.P.; Bruno, F.; Paleino, S.; Kusamura, S.; Germini, A.; Facciorusso, A.; Deraco, M.; et al. Prognostic Impact of Cytoreductive Surgery (CRS) with Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Gastric Cancer Patients: A Meta-Analysis of Randomized Controlled Trials. Eur. J. Surg. Oncol. 2021, 47, 2757–2767. [Google Scholar] [CrossRef] [PubMed]
- Munene, G.; MacK, L.A.; Temple, W.J. Systematic Review on the Efficacy of Multimodal Treatment of Sarcomatosis with Cytoreduction and Intraperitoneal Chemotherapy. Ann. Surg. Oncol. 2011, 18, 207–213. [Google Scholar] [CrossRef]
- De Mestier, L.; Lardière-Deguelte, S.; Brixi, H.; O’Toole, D.; Ruszniewski, P.; Cadiot, G.; Kianmanesh, R. Updating the Surgical Management of Peritoneal Carcinomatosis in Patients with Neuroendocrine Tumors. Neuroendocrinology 2015, 101, 105–111. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Peritonectomy Procedures. Ann. Surg. 1995, 221, 29–42. [Google Scholar] [CrossRef]
- Foster, J.M.; Sleightholm, R.; Patel, A.; Shostrom, V.; Hall, B.; Neilsen, B.; Bartlett, D.; Smith, L. Morbidity and Mortality Rates Following Cytoreductive Surgery Combined with Hyperthermic Intraperitoneal Chemotherapy Compared with Other High-Risk Surgical Oncology Procedures. JAMA Netw. Open 2019, 2, e186847. [Google Scholar] [CrossRef] [PubMed]
- Sommariva, A.; Zagonel, V.; Rossi, C.R. The Role of Laparoscopy in Peritoneal Surface Malignancies Selected for Hyperthermic Intraperitoneal Chemotherapy (HIPEC). Ann. Surg. Oncol. 2012, 19, 3737–3744. [Google Scholar] [CrossRef] [PubMed]
- Arjona-Sanchez, A.; Aziz, O.; Passot, G.; Salti, G.; Esquivel, J.; Van der Speeten, K.; Piso, P.; Nedelcut, D.S.; Sommariva, A.; Yonemura, Y.; et al. Laparoscopic Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Limited Peritoneal Metastasis. The PSOGI International Collaborative Registry. Eur. J. Surg. Oncol. 2021, 47, 1420–1426. [Google Scholar] [CrossRef] [PubMed]
- Abudeeb, H.; Selvasekar, C.R.; O’Dwyer, S.T.; Chakrabarty, B.; Malcolmson, L.; Renehan, A.G.; Wilson, M.S.; Aziz, O. Laparoscopic Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Perforated Low-Grade Appendiceal Mucinous Neoplasms. Surg. Endosc. 2020, 34, 5516–5521. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of Surgical Complications: A New Proposal with Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLOS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Arjona-Sanchez, A.; Esquivel, J.; Glehen, O.; Passot, G.; Turaga, K.K.; Labow, D.; Rufian-Peña, S.; Morales, R.; van der Speeten, K. A Minimally Invasive Approach for Peritonectomy Procedures and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Limited Peritoneal Carcinomatosis: The American Society of Peritoneal Surface Malignancies (ASPSM) Multi-Institution Analysis. Surg. Endosc. 2019, 33, 854–860. [Google Scholar] [CrossRef]
- Arjona-Sánchez, Á.; Durán, M.; Sánchez-Hidalgo, J.M.; Rufian-Peña, S. Peritoneal Carcinomatosis from Ovarian Carcinoma Treated by Interval Laparoscopic Complete Cytoreduction and HIPEC with Extraction through Natural Orifice. Surg. Oncol. 2019, 31, 14–15. [Google Scholar] [CrossRef]
- Arjona-Sánchez, A.; Cortés-Guiral, D.; Duran-Martínez, M.; Villarejo-Campos, P.; Sánchez-Hidalgo, J.M.; Casado-Adam, A.; Rodriguez-Ortiz, L.; Romero-Ruiz, A.; Rufian-Andujar, B.; Espinosa-Redondo, E.; et al. Complete Laparoscopic Pelvic Peritonectomy plus Hyperthermic Intraperitoneal Chemotherapy. Tech. Coloproctol. 2020, 24, 1083–1088. [Google Scholar] [CrossRef]
- Bãlescu, I.; Godoroja, D.; Gongu, M.; Tomulescu, V.; Copãescu, C. Laparoscopic HIPEC for Peritoneal Carcinomatosis from Gastric Cancer—Technique and Early Outcomes of Our First Cases. Chirurgia 2017, 112, 714–725. [Google Scholar] [CrossRef]
- Dumont, F.; Duchalais, E.; Aumont, A.; Thibaudeau, E. Cytoreductive Surgery plus Hyperthermic Intraperitoneal Chemotherapy by Laparoscopy via a Single-Port Approach for Low-Grade Peritoneal Malignancy. Surg. Endosc. 2020, 34, 2789–2795. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, J.; Averbach, A. Combined Laparoscopic Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy in a Patient with Peritoneal Mesothelioma. J. Laparoendosc. Adv. Surg. Tech. 2009, 19, 505–507. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, J.; Averbach, A. Laparoscopic Cytoreductive Surgery and HIPEC in Patients with Limited Pseudomyxoma Peritonei of Appendiceal Origin. Gastroenterol. Res. Pract. 2012, 2012, 981245. [Google Scholar] [CrossRef] [PubMed]
- Fagotti, A.; Petrillo, M.; Costantini, B.; Fanfani, F.; Gallotta, V.; Chiantera, V.; Turco, L.C.; Bottoni, C.; Scambia, G. Minimally Invasive Secondary Cytoreduction plus HIPEC for Recurrent Ovarian Cancer: A Case Series. Gynecol. Oncol. 2014, 132, 303–306. [Google Scholar] [CrossRef]
- Fagotti, A.; Costantini, B.; Gallotta, V.; Cianci, S.; Ronsini, C.; Petrillo, M.; Pacciani, M.; Scambia, G.; Fanfani, F. Minimally Invasive Secondary Cytoreduction Plus HIPEC Versus Open Surgery Plus HIPEC in Isolated Relapse From Ovarian Cancer: A Retrospective Cohort Study on Perioperative Outcomes. J. Minim. Invasive Gynecol. 2015, 22, 428–432. [Google Scholar] [CrossRef]
- Gabriel, E.; Elli, E.; Bagaria, S.; Wasif, N.; Grotz, T.; Stauffer, J.; Kasi, P.M.; Asbun, H. Robotic-Assisted Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy (CRS-HIPEC). J. Robot. Surg. 2019, 13, 175–179. [Google Scholar] [CrossRef]
- Koti, S.; Conte, C.; Kadison, A.B.; Sullivan, J.S.; Wang, J.; Zaidi, R.; Deutsch, G.B. Enhanced Postoperative Recovery with Minimally Invasive Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Surface Malignancies of Gastrointestinal Origin: Minimally Invasive Surgery and HIPEC. Surg. Oncol. 2020, 33, 38–42. [Google Scholar] [CrossRef]
- Mercier, F.; Jeremie, G.; Alyami, M.; Delphine, V.; Vahan, K.; Pascal, R.; Sylvie, I.; Guillaume, P.; Olivier, G. Long-Term Results of Laparoscopic Cytoreductive Surgery and HIPEC for the Curative Treatment of Low-Grade Pseudomyxoma Peritonei and Multicystic Mesothelioma. Surg. Endosc. 2020, 34, 4916–4923. [Google Scholar] [CrossRef]
- Morton, M.; Chambers, L.M.; Costales, A.B.; Chichura, A.; Gruner, M.; Horowitz, M.P.; Rose, P.G.; Yao, M.; Debernardo, R.; Michener, C. Assessing Feasibility and Perioperative Outcomes with Minimally Invasive Surgery Compared with Laparotomy for Interval Debulking Surgery with Hyperthermic Intraperitoneal Chemotherapy for Advanced Epithelial Ovarian Cancer. Gynecol. Oncol. 2021, 160, 45–50. [Google Scholar] [CrossRef]
- Parkin, E.; Selvasekar, C.; Wilson, M.; Renehan, A.; O’Dwyer, S.; Aziz, O. Laparoscopic Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy (L-CRS/HIPEC) for Perforated Low-Grade Appendiceal Mucinous Neoplasm (LAMN II). Ann. Surg. Oncol. 2019, 26, 2285. [Google Scholar] [CrossRef]
- Passot, G.; Bakrin, N.; Isaac, S.; Decullier, E.; Gilly, F.N.; Glehen, O.; Cotte, E. Postoperative Outcomes of Laparoscopic vs Open Cytoreductive Surgery plus Hyperthermic Intraperitoneal Chemotherapy for Treatment of Peritoneal Surface Malignancies. Eur. J. Surg. Oncol. 2014, 40, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ortiz, L.; Arjona-Sánchez, A.; Ibañez-Rubio, M.; Sánchez-Hidalgo, J.; Casado-Adam, A.; Rufián-Peña, S.; Briceño-Delgado, J. Laparoscopic Cytoreductive Surgery and HIPEC: A Comparative Matched Analysis. Surg. Endosc. 2021, 35, 1778–1785. [Google Scholar] [CrossRef] [PubMed]
- Salti, G.I.; Naffouje, S.A. Feasibility of Hand-Assisted Laparoscopic Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Surface Malignancy. Surg. Endosc. 2019, 33, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Sommariva, A.; Tonello, M.; De Simoni, O.; Barina, A.; Riccardo Rossi, C.; Pilati, P. Laparoscopic Hyperthermic Intraperitoneal Chemotherapy for Appendiceal Tumors. Asian J. Endosc. Surg. 2020, 13, 614–617. [Google Scholar] [CrossRef]
- Liang, H.; Guo, H.; Zhang, C.; Zhu, F.L.; Wu, Y.; Zhang, K.; Li, H.; Han, J. Feasibility and Outcome of Primary Laparoscopic Cytoreductive Surgery for Advanced Epithelial Ovarian Cancer: A Comparison to Laparotomic Surgery in Retrospective Cohorts. Oncotarget 2017, 8, 113239–113247. [Google Scholar] [CrossRef]
- Kagawa, Y.; Yamada, D.; Yamasaki, M.; Miyamoto, A.; Mizushima, T.; Yamabe, K.; Imazato, M.; Fukunaga, H.; Kobayashi, S.; Shimizu, J.; et al. The Association between the Increased Performance of Laparoscopic Colon Surgery and a Reduced Risk of Surgical Site Infection. Surg. Today 2019, 49, 474–481. [Google Scholar] [CrossRef]
- Mahner, S.; Eulenburg, C.; Staehle, A.; Wegscheider, K.; Reuss, A.; Pujade-Lauraine, E.; Harter, P.; Ray-Coquard, I.; Pfisterer, J.; Du Bois, A. Prognostic Impact of the Time Interval between Surgery and Chemotherapy in Advanced Ovarian Cancer: Analysis of Prospective Randomised Phase III Trials. Eur. J. Cancer 2013, 49, 142–149. [Google Scholar] [CrossRef]
- Chevallay, M.; Jung, M.; Berlth, F.; Seung-Hun, C.; Morel, P.; Mönig, S. Laparoscopic Surgery for Gastric Cancer: The European Point of View. J. Oncol. 2019, 2019, 8738502. [Google Scholar] [CrossRef]
- Song, X.J.; Liu, Z.L.; Zeng, R.; Ye, W.; Liu, C.W. A Meta-Analysis of Laparoscopic Surgery versus Conventional Open Surgery in the Treatment of Colorectal Cancer. Medicine 2019, 98, e15347. [Google Scholar] [CrossRef]
- Gesson-Paute, A.; Ferron, G.; Thomas, F.; De Lara, E.C.; Chatelut, E.; Querleu, D. Pharmacokinetics of Oxaliplatin during Open versus Laparoscopically Assisted Heated Intraoperative Intraperitoneal Chemotherapy (HIPEC): An Experimental Study. Ann. Surg. Oncol. 2008, 15, 339–344. [Google Scholar] [CrossRef]
- Sánchez-García, S.; Villarejo-Campos, P.; Padilla-Valverde, D.; Amo-Salas, M.; Martín-Fernández, J. Intraperitoneal Chemotherapy Hyperthermia (HIPEC) for Peritoneal Carcinomatosis of Ovarian Cancer Origin by Fluid and CO2 Recirculation Using the Closed Abdomen Technique (PRS-1.0 Combat): A Clinical Pilot Study. Int. J. Hyperth. 2016, 32, 488–495. [Google Scholar] [CrossRef]
- Gao, T.; Huang, X.X.; Wang, W.Y.; Wu, M.F.; Lin, Z.Q.; Li, J. Feasibility and Safety of Neoadjuvant Laparoscopic Hyperthermic Intraperitoneal Chemotherapy in Patients with Advanced Stage Ovarian Cancer: A Single-Center Experience. Cancer Manag. Res. 2019, 11, 6931–6940. [Google Scholar] [CrossRef] [PubMed]
- Newhook, T.E.; Agnes, A.; Blum, M.; Estrella, J.S.; Das, P.; Ho, L.; Ajani, J.A.; Minsky, B.D.; Mansfield, P.; Badgwell, B.D. Laparoscopic Hyperthermic Intraperitoneal Chemotherapy Is Safe for Patients with Peritoneal Metastases from Gastric Cancer and May Lead to Gastrectomy. Ann. Surg. Oncol. 2019, 26, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Valle, S.J.; Alzahrani, N.A.; Alzahrani, S.E.; Liauw, W.; Morris, D.L. Laparoscopic Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Refractory Malignant Ascites in Patients Unsuitable for Cytoreductive Surgery. Int. J. Surg. 2015, 23, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.; de Hingh, I.; Van Der Speeten, K.; Hubner, M.; Deraco, M.; Bakrin, N.; Villeneuve, L.; Kusamura, S.; Glehen, O. HIPEC Methodology and Regimens: The Need for an Expert Consensus. Ann. Surg. Oncol. 2021, 28, 9098–9113. [Google Scholar] [CrossRef] [PubMed]

| Patients Characteristics (n = 14) | |
|---|---|
| Age, years (median, IQR) | 61.0 (51.75–73.50) |
| Gender (n,%) | |
| Male Female | 5 (35.7) 9 (64.3) |
| Primary tumor (n,%) | |
| Pseudomyxoma Colorectal Ovarian NET Appendix | 6 (42.9) 3 (21.4) 2 (14.3) 1 (7.1) 2 (14.3) |
| ECOG 0 (n,%) | 14 (100.0) |
| Neoadjuvant chemotherapy (n,%) | 3 (21.4) |
| Status of PM | |
| Synchronous Metachronous | 0 (0.0) 16 (100.0) |
| Conversion to open (n,%) | - |
| CC 0 (n,%) | 14 (100.0) |
| PCI (median, IQR) | 3 (2–4.25) |
| Surgery time, minutes (median, IQR) | 487.5 (433.8–567.5) |
| Peritonectomy Procedures (n,%) | 8 (57.1) |
| Visceral Resections (n,%) | 6 (42.9) |
| Blood transfusion (n,%) | 2 (14.3) |
| Length of stay, days (median, IQR) | 6 (5–10.25) |
| Minor complications, grade I–II (n,%) | 2 (14.3) |
| Major complications, grade III–IV (n,%) | 2 (14.3) |
| 90-days mortality (n,%) | - |
| Author, Year | Patients | Histopathology | Previous Surgery | Neoadjuvant Chemotherapy |
|---|---|---|---|---|
| Lap/Open | Lap/Open | Lap/Open | ||
| n | n | n | ||
| Abudeeb, 2020 [14] | 55/29 | LAMN-II | 55/29 | 0/0 |
| Arjona-Sánchez, 2019 [17] | 90 | Multiple histologies | 81 | NA |
| Arjona-Sánchez, 2019 [18] | 6 | Ovarian | NA | 6 |
| Arjona-Sánchez, 2020 [19] | 12 | Multiple histologies | NA | 6 |
| Bãlescu, 2017 [20] | 2 | Gastric | 2 | 2 |
| Dumont, 2020 [21] | 12 | Pseudomyxoma, mesothelioma | 2 | 0 |
| Esquivel, 2009 [22] | 1 | Mesothelioma | 1 | NA |
| Esquivel, 2012 [23] | 19 | Pseudomyxoma | 19 | 0 |
| Fagotti, 2014 [24] | 10 | Ovarian | 10 | 10 |
| Fagotti, 2015 [25] | 11/11 | Ovarian | 11/11 | 11/11 |
| Gabriel, 2019 [26] | 1 | Pseudomyxoma | 1 | 0 |
| Koti, 2020 [27] | 7/38 | Multiple histologies | NA | NA |
| Mercier, 2020 [28] | 32/11 | Pseudomyxoma, mesothelioma, ovarian | NA | 0 |
| Morton, 2021 [29] | 10/40 | Ovarian | NA | 10/40 |
| Parkin, 2019 [30] | 1 | LAMN-II | NA | NA |
| Passot, 2014 [31] | 8/41 | Pseudomyxoma, mesothelioma | 49 | NA |
| Rodríguez-Ortiz, 2020 [32] | 18/42 | Multiple histologies | 12/24 | 8/30 |
| Salti, 2018 [33] | 11/11 | Pseudomyxoma, colorectal | NA | 3/3 |
| Sommariva, 2019 [34] | 3 | LAMN-II | 3 | 0 |
| Author, Year | Patients | CC 0 | Peritoneal Cancer Index | Surgery Duration | Morbidity (Grade ≥ III) * within 90 Days from Surgery | Mortality within 30 Days from Surgery | Length of Hospital Stay | Time to Chemotherapy |
|---|---|---|---|---|---|---|---|---|
| Lap/Open | Lap/Open | Lap/Open | Lap/Open | Lap/Open | Lap/Open | Lap/Open | Lap/Open | |
| n | n | Median PCI | Minutes | n | n | Days | Weeks | |
| Abudeeb, 2020 [14] | 55/29 | 55/29 | 1/2 | 528/438 | 5/9 | 0/0 | 6/10 | NA |
| Arjona-Sánchez, 2019 [17] | 90 | 90 | 4.1 | 282 | 0/9 | 0 | 7.4 | NA |
| Arjona-Sánchez, 2019 [18] | 6 | 6 | <10 | 480 | 0 | 0 | 5 | NA |
| Arjona-Sánchez, 2020 [19] | 12 | 12 | 4.5 | 468 | 2 | 0 | 5.5 | 3.5 |
| Bãlescu, 2017 [20] | 2 | 2 | 4.5 | 336 | 0 | 0 | NA | NA |
| Dumont, 2020 [21] | 12 | 12 | 2 | 240 | 2 | 0 | 8.5 | NA |
| Esquivel, 2009 [22] | 1 | 1 | 4 | NA | 0 | 0 | 5 | NA |
| Esquivel, 2012 [23] | 19 | 19 | 4.2 | 270 | 1 | 0 | 5.3 | NA |
| Fagotti, 2014 [24] | 10 | 10 | 2 | 180 | 0 | 0 | 4 | 3 |
| Fagotti, 2015 [25] | 11/11 | 11/11 | 2/2 | 180 | 0 | 0 | 4/8.5 | NA |
| Gabriel, 2019 [26] | 1 | 1 | 1 | 426 | 0 | 0 | 4 | NA |
| Koti, 2020 [27] | 7/38 | 7/38 | 5.56/9.61 | 594/468 | 0/3 | 0 | NA | NA |
| Mercier, 2020 [28] | 32/11 | 32/10 | 2.5/7 | 210/240 | 0/1 | 0 | 11/13 | NA |
| Morton, 2021 [29] | 10/40 | 10/40 | NA | 324/336 | 0/4 | 0 | 3/4 | 26/30 |
| Parkin, 2019 [30] | 1 | 0 | 5 | 510 | 0 | 0 | 5 | NA |
| Passot, 2014 [31] | 8/41 | 8 | 2.5 | 210/240 | 1/4 | 0 | 12/19 | NA |
| Rodríguez-Ortiz, 2020 [32] | 18/42 | 18/42 | 3/5 | 420/306 | 1/9 | 0/0 | 4.5/8 | 4/7 |
| Salti, 2018 [33] | 11/11 | 11/11 | 4.1 | 312/288 | 0/0 | 0/0 | 6.5/9.1 | NA |
| Sommariva, 2019 [34] | 3 | 3 | 1 | NA | 0 | 0 | 10 | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sommariva, A.; Valle, M.; Gelmini, R.; Tonello, M.; Carboni, F.; De Manzoni, G.; Sorrentino, L.; Pasqual, E.M.; Bacchetti, S.; Sassaroli, C.; et al. Laparoscopic Cytoreduction Combined with Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Peritoneal Surface Malignancies (PSM): Italian PSM Oncoteam Evidence and Literature Review. Cancers 2023, 15, 279. https://doi.org/10.3390/cancers15010279
Sommariva A, Valle M, Gelmini R, Tonello M, Carboni F, De Manzoni G, Sorrentino L, Pasqual EM, Bacchetti S, Sassaroli C, et al. Laparoscopic Cytoreduction Combined with Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Peritoneal Surface Malignancies (PSM): Italian PSM Oncoteam Evidence and Literature Review. Cancers. 2023; 15(1):279. https://doi.org/10.3390/cancers15010279
Chicago/Turabian StyleSommariva, Antonio, Mario Valle, Roberta Gelmini, Marco Tonello, Fabio Carboni, Giovanni De Manzoni, Lorena Sorrentino, Enrico Maria Pasqual, Stefano Bacchetti, Cinzia Sassaroli, and et al. 2023. "Laparoscopic Cytoreduction Combined with Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Peritoneal Surface Malignancies (PSM): Italian PSM Oncoteam Evidence and Literature Review" Cancers 15, no. 1: 279. https://doi.org/10.3390/cancers15010279
APA StyleSommariva, A., Valle, M., Gelmini, R., Tonello, M., Carboni, F., De Manzoni, G., Sorrentino, L., Pasqual, E. M., Bacchetti, S., Sassaroli, C., Di Giorgio, A., Framarini, M., Marrelli, D., Casella, F., & Federici, O. (2023). Laparoscopic Cytoreduction Combined with Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Peritoneal Surface Malignancies (PSM): Italian PSM Oncoteam Evidence and Literature Review. Cancers, 15(1), 279. https://doi.org/10.3390/cancers15010279








