Cytoreductive Surgery (CRS) and HIPEC for Advanced Ovarian Cancer with Peritoneal Metastases: Italian PSM Oncoteam Evidence and Study Purposes
Abstract
Simple Summary
Abstract
1. Introduction
2. Treatment of Ovarian Cancer Metastatic Peritoneal Surface Malignancies
2.1. CRS in Primary Ovarian Cancer with Peritoneal Metastases
- If an initial visible residual disease after primary CRS goes to complete tumor resection after adjuvant IV or IP chemotherapy, 18% of patients have R0 [106];
- If complete tumor resection was obtained after primary CRS, the probability of having no residual cancer cells after adjuvant chemotherapy is estimated to be 33% for those who receive IV chemotherapy and 50% for those who receive IP chemotherapy.
2.2. HIPEC in Primary Ovarian Cancer with Peritoneal Metastases
- The Dutch trial included patients who had a partial or complete response following neoadjuvant chemotherapy and demonstrated a survival advantage (OS 45.7 vs. 33.9 months, p-value 0.02; PFS 14.2 vs. 10.7 months, p-value 0.003) with the addition of HIPEC (100 mg/m2 of cisplatin for 90 min via the open technique at a temperature of 40 °C); 90% of patients completed a full six cycles of chemotherapy. In terms of toxicity, the two groups showed similar results. However, this study was not stratified about important prognostic factors such as BRCA status, FIGO stage or the histologic type of tumor.
- The Korean trial showed no significant differences in OS and PFS between the HIPEC (75 mg/m2 of cisplatin for 90 min via the closed technique at a temperature of 41.5 °C) and no HIPEC arms (69.5 vs. 61.3 months, p-value 0.43; 19.8 vs. 18.8 months, p-value 0.52). Intra- and post-operative outcomes, such as the extent of surgery, estimated blood loss, residual tumor and length of hospital stay, were not different between both groups. The addition of HIPEC to CRS did not improve survival among patients undergoing primary CRS (p-value 0.29; p-value 0.51, respectively). Interestingly, the neoadjuvant chemotherapy subgroup showed a trend of improved survival in favor of HIPEC (61.8 vs. 48.2, p-value 0.4; 17.4 vs. 15.4 months, p-value 0.04).
3. 1st Evidence−Based Italian Consensus Conference on CRS and HIPEC for Peritoneal Metastases from Ovarian Cancer
4. Timing of CRS and HIPEC in Primary Ovarian Cancer with Peritoneal Metastases
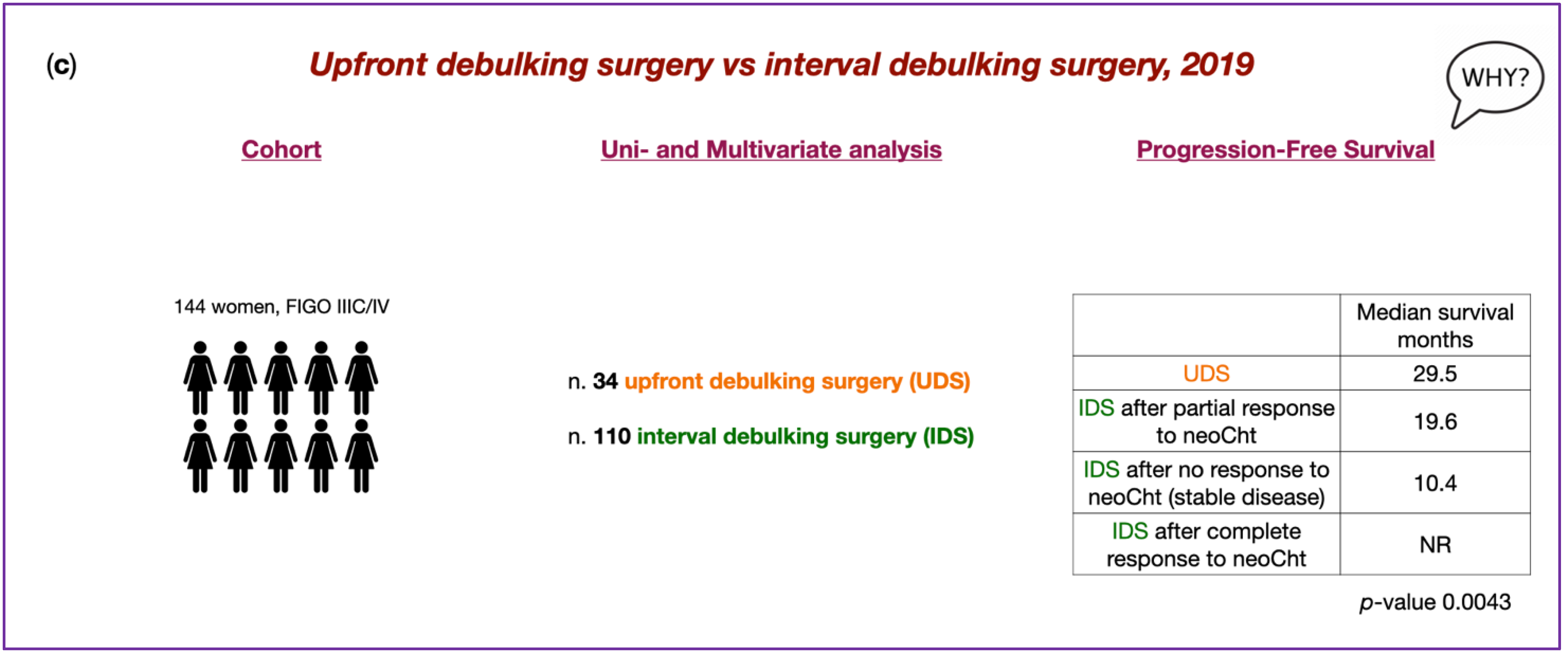
5. Upfront Debulking Surgery versus Interval Debulking Surgery for Advanced Tubo-Ovarian High-Grade Serous Carcinoma and Diffuse Peritoneal Metastases Treated with CRS and HIPEC
6. Comparison of Treatment Protocols Including Six vs. Three Cycles of Neoadjuvant Chemotherapy Followed by CRS and HIPEC in Primary High-Grade Serous Ovarian Cancer with Peritoneal Metastases (OVANAC–HIPEC)
7. CRS and HIPEC in Advanced Ovarian Cancer (CHORINE Study)
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ledermann, J.A.; Raja, F.A.; Fotopoulou, C.; Gonzalez-Martin, A.; Colombo, N.; Sessa, C. Newly Diagnosed and Relapsed Epithelial Ovarian Carcinoma: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013, 24 (Suppl. 6), vi24–vi32. [Google Scholar] [CrossRef]
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of Ovarian Cancer: A Review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar] [CrossRef]
- Mahmood, R.D.; Morgan, R.D.; Edmondson, R.J.; Clamp, A.R.; Jayson, G.C. First-Line Management of Advanced High-Grade Serous Ovarian Cancer. Curr. Oncol. Rep. 2020, 22, 64. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Sopik, V.; Iqbal, J.; Rosen, B.; Narod, S.A. Why Have Ovarian Cancer Mortality Rates Declined? Part I. Incidence. Gynecol. Oncol. 2015, 138, 741–749. [Google Scholar] [CrossRef]
- National Cancer Institute (NIH). Cancer Stat Facts: Ovaria Cancer. Available online: https://seer.cancer.gov/statfacts/html/ovary.html (accessed on 22 June 2022).
- Lheureux, S.; Braunstein, M.; Oza, A.M. Epithelial Ovarian Cancer: Evolution of Management in the Era of Precision Medicine. CA Cancer J. Clin. 2019, 69, 280–304. [Google Scholar] [CrossRef]
- Reid, F. World Ovarian Cancer Coalition Atlas. 2020. Global Trends in Incidence, Mortality, and Survival; World Ovarian Cancer Coalition, 2020; pp. 1–42. [Google Scholar]
- Rosendahl, M.; Høgdall, C.K.; Mosgaard, B.J. Restaging and Survival Analysis of 4036 Ovarian Cancer Patients According to the 2013 FIGO Classification for Ovarian, Fallopian Tube, and Primary Peritoneal Cancer. Int. J. Gynecol. Cancer 2016, 26, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Peres, L.C.; Cushing-Haugen, K.L.; Köbel, M.; Harris, H.R.; Berchuck, A.; Rossing, M.A.; Schildkraut, J.M.; Doherty, J.A. Invasive Epithelial Ovarian Cancer Survival by Histotype and Disease Stage. J. Natl. Cancer Inst. 2019, 111, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian Cancer Statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- SEER Cancer Statistics Review, 1975–2014. Available online: https://seer.cancer.gov/archive/csr/1975_2014/#contents (accessed on 22 June 2022).
- Prat, J. Staging Classification for Cancer of the Ovary, Fallopian Tube, and Peritoneum. Int. J. Gynaecol.Obstet. 2014, 124, 1–5. [Google Scholar] [CrossRef]
- Zeppernick, F.; Meinhold-Heerlein, I. The New FIGO Staging System for Ovarian, Fallopian Tube, and Primary Peritoneal Cancer. Arch. Gynecol. Obstet. 2014, 290, 839–842. [Google Scholar] [CrossRef]
- Falzone, L.; Scandurra, G.; Lombardo, V.; Gattuso, G.; Lavoro, A.; Distefano, A.B.; Scibilia, G.; Scollo, P. A Multidisciplinary Approach Remains the Best Strategy to Improve and Strengthen the Management of Ovarian Cancer. Int. J. Oncol. 2021, 59, 53. [Google Scholar] [CrossRef]
- Halkia, E.; Spiliotis, J.; Sugarbaker, P. Diagnosis and Management of Peritoneal Metastases from Ovarian Cancer. Gastroenterol. Res. Pract. 2012, 2012, 541842. [Google Scholar] [CrossRef]
- Heintz, A.; Odicino, F.; Maisonneuve, P.; Quinn, M.; Benedet, J.; Creasman, W.; Ngan, H.; Pecorelli, S.; Beller, U. Carcinoma of the Ovary. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int. J. Gynaecol. Obstet. 2006, 95 (Suppl. 1), S161–S192. [Google Scholar] [CrossRef]
- Friedrich, M.; Friedrich, D.; Kraft, C.; Rogmans, C. Multimodal Treatment of Primary Advanced Ovarian Cancer. Anticancer Res. 2021, 41, 3253–3260. [Google Scholar] [CrossRef] [PubMed]
- Du Bois, A.; Reuss, A.; Pujade-Lauraine, E.; Harter, P.; Ray-Coquard, I.; Pfisterer, J. Role of Surgical Outcome as Prognostic Factor in Advanced Epithelial Ovarian Cancer: A Combined Exploratory Analysis of 3 Prospectively Randomized Phase 3 Multicenter Trials: By the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour Les Etudes Des Cancers de l’Ovaire (GINECO). Cancer 2009, 115, 1234–1244. [Google Scholar] [CrossRef]
- Roviello, F.; Roviello, G.; Petrioli, R.; Marrelli, D. Hyperthermic Intraperitoneal Chemotherapy for the Treatment of Ovarian Cancer: A Brief Overview of Recent Results. Crit. Rev. Oncol. Hematol. 2015, 95, 297–305. [Google Scholar] [CrossRef]
- Berek, J.S.; Renz, M.; Kehoe, S.; Kumar, L.; Friedlander, M. Cancer of the Ovary, Fallopian Tube, and Peritoneum: 2021 Update. Int. J. Gynaecol. Obstet. 2021, 155 (Suppl. 1), 61–85. [Google Scholar] [CrossRef] [PubMed]
- Kurman, R.J. Origin and Molecular Pathogenesis of Ovarian High-Grade Serous Carcinoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013, 24 (Suppl. 10), x16–x21. [Google Scholar] [CrossRef]
- Kurman, R.J.; Shih, I.M. The Dualistic Model of Ovarian Carcinogenesis: Revisited, Revised, and Expanded. Am. J. Pathol. 2016, 186, 733–747. [Google Scholar] [CrossRef] [PubMed]
- Kurman, R.J.; Shih, I.M. Molecular Pathogenesis and Extraovarian Origin of Epithelial Ovarian Cancer—Shifting the Paradigm. Hum. Pathol. 2011, 42, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Makar, A.P.; Tropé, C.G.; Tummers, P.; Denys, H.; Vandecasteele, K. Advanced Ovarian Cancer: Primary or Interval Debulking? Five Categories of Patients in View of the Results of Randomized Trials and Tumor Biology: Primary Debulking Surgery and Interval Debulking Surgery for Advanced Ovarian Cancer. Oncologist 2016, 21, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Braicu, E.I.; Sehouli, J.; Richter, R.; Pietzner, K.; Denkert, C.; Fotopoulou, C. Role of Histological Type on Surgical Outcome and Survival Following Radical Primary Tumour Debulking of Epithelial Ovarian, Fallopian Tube and Peritoneal Cancers. Br. J. Cancer 2011, 105, 1818–1824. [Google Scholar] [CrossRef]
- Meinhold-Heerlein, I.; Fotopoulou, C.; Harter, P.; Kurzeder, C.; Mustea, A.; Wimberger, P.; Hauptmann, S.; Sehouli, J. Statement by the Kommission Ovar of the AGO: The New FIGO and WHO Classifications of Ovarian, Fallopian Tube and Primary Peritoneal Cancer. Geburtshilfe Frauenheilkd. 2015, 75, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Kossaï, M.; Leary, A.; Scoazec, J.Y.; Genestie, C. Ovarian Cancer: A Heterogeneous Disease. Pathobiology 2018, 85, 41–49. [Google Scholar] [CrossRef]
- Meinhold-Heerlein, I.; Fotopoulou, C.; Harter, P.; Kurzeder, C.; Mustea, A.; Wimberger, P.; Hauptmann, S.; Sehouli, J. The New WHO Classification of Ovarian, Fallopian Tube, and Primary Peritoneal Cancer and Its Clinical Implications. Arch. Gynecol. Obstet. 2016, 293, 695–700. [Google Scholar] [CrossRef]
- Duska, L.R.; Kohn, E.C. The New Classifications of Ovarian, Fallopian Tube, and Primary Peritoneal Cancer and Their Clinical Implications. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, viii8–viii12. [Google Scholar] [CrossRef]
- Yates, L.R.; Campbell, P.J. Evolution of the Cancer Genome. Nat. Rev. Genet. 2012, 13, 795–806. [Google Scholar] [CrossRef]
- Klein, C.A. Parallel Progression of Primary Tumours and Metastases. Nat. Rev. Cancer 2009, 9, 302–312. [Google Scholar] [CrossRef]
- Schwarz, R.F.; Ng, C.K.Y.; Cooke, S.L.; Newman, S.; Temple, J.; Piskorz, A.M.; Gale, D.; Sayal, K.; Murtaza, M.; Baldwin, P.J.; et al. Spatial and Temporal Heterogeneity in High-Grade Serous Ovarian Cancer: A Phylogenetic Analysis. PLoS Med. 2015, 12, e1001789. [Google Scholar] [CrossRef]
- Lavoro, A.; Scalisi, A.; Candido, S.; Zanghì, G.; Rizzo, R.; Gattuso, G.; Caruso, G.; Libra, M.; Falzone, L. Identification of the Most Common BRCA Alterations through Analysis of Germline Mutation Databases: Is Droplet Digital PCR an Additional Strategy for the Assessment of Such Alterations in Breast and Ovarian Cancer Families? Int. J. Oncol. 2022, 60, 58. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Integrated Genomic Analyses of Ovarian Carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kim, H.-S.; Rim, J.H.; Lee, J.-Y.; Nam, E.J.; Kim, S.W.; Kim, S.; Kim, Y.T. Germline BRCA, Chemotherapy Response Scores, and Survival in the Neoadjuvant Treatment of Ovarian Cancer. BMC Cancer 2020, 20, 185. [Google Scholar] [CrossRef] [PubMed]
- Iavazzo, C.; Gkegkes, I.D.; Vrachnis, N. Primary Peritoneal Cancer in BRCA Carriers after Prophylactic Bilateral Salpingo-Oophorectomy. J. Turkish Ger. Gynecol. Assoc. 2016, 17, 73–76. [Google Scholar] [CrossRef]
- Caeiro, C.; Leão, I.; Oliveira, I.; Sousa, I.; André, T. Recurrent Ovarian Cancer with BRCAness Phenotype: A Treatment Challenge. Adv. Ther. 2022, 39, 5289–5299. [Google Scholar] [CrossRef] [PubMed]
- Madariaga, A.; Lheureux, S.; Oza, A. Tailoring Ovarian Cancer Treatment: Implications of BRCA1/2 Mutations. Cancers 2019, 11, 416. [Google Scholar] [CrossRef]
- Foo, T.; George, A.; Banerjee, S. PARP Inhibitors in Ovarian Cancer: An Overview of the Practice-changing Trials. Genes Chromosom. Cancer 2021, 60, 385–397. [Google Scholar] [CrossRef]
- Haunschild, C.E.; Tewari, K.S. Bevacizumab Use in the Frontline, Maintenance and Recurrent Settings for Ovarian Cancer. Futur. Oncol. 2020, 16, 225–246. [Google Scholar] [CrossRef]
- Gadducci, A.; Aletti, G.D.; Landoni, F.; Lazzari, R.; Mangili, G.; Olivas, P.; Pignata, S.; Salutari, V.; Sartori, E.; Scambia, G.; et al. Management of Ovarian Cancer: Guidelines of the Italian Medical Oncology Association (AIOM). Tumori 2021, 107, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Cloyd, J.M.; Hays, J.; Patel, S.H. The Role of Hyperthermic Intraperitoneal Chemotherapy for Non-Colorectal Peritoneal Surface Malignancies. J. Gastrointest. Surg. 2021, 25, 303–318. [Google Scholar] [CrossRef]
- Hoppenot, C.; Schuitevoerder, D.; Izquierdo, F.J.; Plana, A.; Pothuri, B.; Diane Yamada, S.; Kim, J.; Lee, N.K.; Abbott, D.E.; Abdel-Misih, S.; et al. The Chicago Consensus on Peritoneal Surface Malignancies: Management of Ovarian Neoplasms. Cancer 2020, 126, 2553–2560. [Google Scholar] [CrossRef]
- Mercado, C.; Zingmond, D.; Karlan, B.Y.; Sekaris, E.; Gross, J.; Maggard-Gibbons, M.; Tomlinson, J.S.; Ko, C.Y. Quality of Care in Advanced Ovarian Cancer: The Importance of Provider Specialty. Gynecol. Oncol. 2010, 117, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Guiral, D.; Hübner, M.; Alyami, M.; Bhatt, A.; Ceelen, W.; Glehen, O.; Lordick, F.; Ramsay, R.; Sgarbura, O.; Van Der Speeten, K.; et al. Primary and Metastatic Peritoneal Surface Malignancies. Nat. Rev. Dis. Prim. 2021, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, B.; Arvieux, C.; Glehen, O.; Beaujard, A.C.; Rivoire, M.; Baulieux, J.; Fontaumard, E.; Brachet, A.; Caillot, J.L.; Faure, J.L.; et al. Peritoneal Carcinomatosis from Non-Gynecologic Malignancies: Results of the EVOCAPE 1 Multicentric Prospective Study. Cancer 2000, 88, 358–363. [Google Scholar] [CrossRef]
- Rubino, M.S.; Abdel-Misih, R.Z.; Bennett, J.J.; Petrelli, N.J. Peritoneal Surface Malignancies and Regional Treatment: A Review of the Literature. Surg. Oncol. 2012, 21, 87–94. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Peritonectomy Procedures. Ann. Surg. 1995, 221, 29–42. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Management of Peritoneal-Surface Malignancy: The Surgeon’s Role. Langenbecks Arch. Surg. 1999, 384, 576–587. [Google Scholar] [CrossRef]
- Spratt, J.S.; Adcock, R.A.; Muskovin, M.; Sherrill, W.; McKeown, J. Clinical Delivery System for Intraperitoneal Hyperthermic Chemotherapy. Cancer Res. 1980, 40, 256–260. [Google Scholar]
- Alyami, M.; Hübner, M.; Grass, F.; Bakrin, N.; Villeneuve, L.; Laplace, N.; Passot, G.; Glehen, O.; Kepenekian, V. Pressurised Intraperitoneal Aerosol Chemotherapy: Rationale, Evidence, and Potential Indications. Lancet Oncol. 2019, 20, e368–e377. [Google Scholar] [CrossRef]
- Glehen, O.; Gilly, F.N.; Arvieux, C.; Cotte, E.; Boutitie, F.; Mansvelt, B.; Bereder, J.M.; Lorimier, G.; Quenet, F.; Elias, D. Peritoneal Carcinomatosis from Gastric Cancer: A Multi-Institutional Study of 159 Patients Treated by Cytoreductive Surgery Combined with Perioperative Intraperitoneal Chemotherapy. Ann. Surg. Oncol. 2010, 17, 2370–2377. [Google Scholar] [CrossRef]
- Van Der Speeten, K.; Lemoine, L.; Sugarbaker, P. Overview of the Optimal Perioperative Intraperitoneal Chemotherapy Regimens Used in Current Clinical Practice. Pleura Peritoneum 2017, 2, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Auer, R.C.; Sivajohanathan, D.; Biagi, J.; Conner, J.; Kennedy, E.; May, T. Indications for Hyperthermic Intraperitoneal Chemotherapy with Cytoreductive Surgery: A Systematic Review. Eur. J. Cancer 2020, 127, 76–95. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.K.; Alvarez, R.D.; Bakkum-Gamez, J.N.; Barroilhet, L.; Behbakht, K.; Berchuck, A.; Berek, J.S.; Chen, L.M.; Cristea, M.; DeRosa, M.; et al. NCCN Guidelines Insights: Ovarian Cancer, Version 1.2019. J. Natl. Compr. Cancer Netw. 2019, 17, 896–909. [Google Scholar] [CrossRef]
- Sato, S.; Itamochi, H. Neoadjuvant Chemotherapy in Advanced Ovarian Cancer: Latest Results and Place in Therapy. Ther. Adv. Med. Oncol. 2014, 6, 293–304. [Google Scholar] [CrossRef]
- Ray, M.D.; Dhall, K. Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in the Management of Peritoneal Surface Malignancies—An Evidence-Based Review. Curr. Probl. Cancer 2021, 45, 100737. [Google Scholar] [CrossRef]
- Kim, S.R.; Kotsopoulos, J.; Sun, P.; Bernardini, M.Q.; Laframboise, S.; Ferguson, S.E.; Rosen, B.; Narod, S.A.; May, T. The Impacts of Neoadjuvant Chemotherapy and of Cytoreductive Surgery on 10-Year Survival from Advanced Ovarian Cancer. Int. J. Gynaecol. Obstet. 2021, 153, 417–423. [Google Scholar] [CrossRef]
- Nishio, S.; Ushijima, K. Clinical Significance of Primary Debulking Surgery and Neoadjuvant Chemotherapy-Interval Debulking Surgery in Advanced Ovarian Cancer. Jpn. J. Clin. Oncol. 2020, 50, 379–386. [Google Scholar] [CrossRef]
- Nick, A.M.; Coleman, R.L.; Ramirez, P.T.; Sood, A.K. A Framework for a Personalized Surgical Approach to Ovarian Cancer. Nat. Rev. Clin. Oncol. 2015, 12, 239–245. [Google Scholar] [CrossRef]
- Morgan, R.J.; Alvarez, R.D.; Armstrong, D.K.; Burger, R.A.; Castells, M.; Chen, L.M.; Copeland, L.; Crispens, M.A.; Gershenson, D.; Gray, H.; et al. Ovarian Cancer, Version 3.2012. J. Natl. Compr. Cancer Netw. 2012, 10, 1339–1349. [Google Scholar] [CrossRef]
- Komiyama, S.; Kato, K.; Inokuchi, Y.; Takano, H.; Matsumoto, T.; Hongo, A.; Asai-Sato, M.; Arakawa, A.; Kamiura, S.; Tabata, T.; et al. Bevacizumab Combined with Platinum-Taxane Chemotherapy as First-Line Treatment for Advanced Ovarian Cancer: A Prospective Observational Study of Safety and Efficacy in Japanese Patients (JGOG3022 Trial). Int. J. Clin. Oncol. 2019, 24, 103–114. [Google Scholar] [CrossRef]
- Karam, A.; Ledermann, J.A.; Kim, J.W.; Sehouli, J.; Lu, K.; Gourley, C.; Katsumata, N.; Burger, R.A.; Nam, B.H.; Bacon, M.; et al. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: First-Line Interventions. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Oza, A.M.; Cibula, D.; Benzaquen, A.O.; Poole, C.; Mathijssen, R.H.J.; Sonke, G.S.; Colombo, N.; Špaček, J.; Vuylsteke, P.; Hirte, H.; et al. Olaparib Combined with Chemotherapy for Recurrent Platinum-Sensitive Ovarian Cancer: A Randomised Phase 2 Trial. Lancet Oncol. 2015, 16, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Pujade-Lauraine, E.; Hilpert, F.; Weber, B.; Reuss, A.; Poveda, A.; Kristensen, G.; Sorio, R.; Vergote, I.; Witteveen, P.; Bamias, A.; et al. Bevacizumab Combined with Chemotherapy for Platinum-Resistant Recurrent Ovarian Cancer: The AURELIA Open-Label Randomized Phase III Trial. J. Clin. Oncol. 2014, 32, 1302–1308. [Google Scholar] [CrossRef]
- Perren, T.J.; Swart, A.M.; Pfisterer, J.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; Kurzeder, C.; et al. A Phase 3 Trial of Bevacizumab in Ovarian Cancer. N. Engl. J. Med. 2011, 365, 2484–2496. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, C.; Muzii, L.; Romito, A.; Panici, P.B. First-Line Treatment of Women with Advanced Ovarian Cancer: Focus on Bevacizumab. Onco-Targets Ther. 2019, 12, 1095–1103. [Google Scholar] [CrossRef]
- Rosen, B.; Laframboise, S.; Ferguson, S.; Dodge, J.; Bernardini, M.; Murphy, J.; Segev, Y.; Sun, P.; Narod, S.A. The Impacts of Neoadjuvant Chemotherapy and of Debulking Surgery on Survival from Advanced Ovarian Cancer. Gynecol. Oncol. 2014, 134, 462–467. [Google Scholar] [CrossRef]
- Cummings, M.; Nicolais, O.; Shahin, M. Surgery in Advanced Ovary Cancer: Primary versus Interval Cytoreduction. Diagnostics 2022, 12, 988. [Google Scholar] [CrossRef]
- Vergote, I.; Tropé, C.G.; Amant, F.; Kristensen, G.B.; Ehlen, T.; Johnson, N.; Verheijen, R.H.M.; van der Burg, M.E.L.; Lacave, A.J.; Panici, P.B.; et al. Neoadjuvant Chemotherapy or Primary Surgery in Stage IIIC or IV Ovarian Cancer. N. Engl. J. Med. 2010, 363, 943–953. [Google Scholar] [CrossRef]
- Kehoe, S.; Hook, J.; Nankivell, M.; Jayson, G.C.; Kitchener, H.; Lopes, T.; Luesley, D.; Perren, T.; Bannoo, S.; Mascarenhas, M.; et al. Primary Chemotherapy versus Primary Surgery for Newly Diagnosed Advanced Ovarian Cancer (CHORUS): An Open-Label, Randomised, Controlled, Non-Inferiority Trial. Lancet 2015, 386, 249–257. [Google Scholar] [CrossRef]
- Chi, D.S.; Musa, F.; Dao, F.; Zivanovic, O.; Sonoda, Y.; Leitao, M.M.; Levine, D.A.; Gardner, G.J.; Abu-Rustum, N.R.; Barakat, R.R. An Analysis of Patients with Bulky Advanced Stage Ovarian, Tubal, and Peritoneal Carcinoma Treated with Primary Debulking Surgery (PDS) during an Identical Time Period as the Randomized EORTC-NCIC Trial of PDS vs Neoadjuvant Chemotherapy (NACT). Gynecol. Oncol. 2012, 124, 10–14. [Google Scholar] [CrossRef]
- Onda, T.; Satoh, T.; Ogawa, G.; Saito, T.; Kasamatsu, T.; Nakanishi, T.; Mizutani, T.; Takehara, K.; Okamoto, A.; Ushijima, K.; et al. Comparison of Survival between Primary Debulking Surgery and Neoadjuvant Chemotherapy for Stage III/IV Ovarian, Tubal and Peritoneal Cancers in Phase III Randomised Trial. Eur. J. Cancer 2020, 130, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Fagotti, A.; Ferrandina, M.G.; Vizzielli, G.; Pasciuto, T.; Fanfani, F.; Gallotta, V.; Margariti, P.A.; Chiantera, V.; Costantini, B.; Gueli Alletti, S.; et al. Randomized Trial of Primary Debulking Surgery versus Neoadjuvant Chemotherapy for Advanced Epithelial Ovarian Cancer (SCORPION-NCT01461850). Int. J. Gynecol. Cancer 2020, 30, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Zhu, J.; Kim, J.W.; Liu, J.; Kato, K.; Kim, H.S.; Zhang, Y.; Zhang, P.; Zhu, T.; Aoki, D.; et al. Study of Upfront Surgery versus Neoadjuvant Chemotherapy Followed by Interval Debulking Surgery for Patients with Stage Iiic and Iv Ovarian Cancer, Sgog Sunny (SOC-2) Trial Concept. J. Gynecol. Oncol. 2020, 31, e86. [Google Scholar] [CrossRef] [PubMed]
- Reuss, A.; Du Bois, A.; Harter, P.; Fotopoulou, C.; Sehouli, J.; Aletti, G.; Guyon, F.; Greggi, S.; Mosgaard, B.J.; Reinthaller, A.; et al. TRUST: Trial of Radical Upfront Surgical Therapy in Advanced Ovarian Cancer (ENGOT Ov33/AGO-OVAR OP7). Int. J. Gynecol. Cancer 2019, 29, 1327–1331. [Google Scholar] [CrossRef]
- Classe, J.M.; Ferron, G.; Ouldamer, L.; Gauthier, T.; Emambux, S.; Gladieff, L.; Dupre, P.F.; Anota, A. CHRONO: Randomized Trial of the CHROnology of Surgery after Neoadjuvant Chemotherapy for Ovarian Cancer. Int. J. Gynecol. Cancer 2022, 32, 1071–1075. [Google Scholar] [CrossRef]
- Engel, J.; Eckel, R.; Schubert-Fritschle, G.; Kerr, J.; Kuhn, W.; Diebold, J.; Kimmig, R.; Rehbock, J.; Hölzel, D. Moderate Progress for Ovarian Cancer in the Last 20 Years: Prolongation of Survival, but No Improvement in the Cure Rate. Eur. J. Cancer 2002, 38, 2435–2445. [Google Scholar] [CrossRef]
- Liu, J.F.; Brady, M.F.; Matulonis, U.A.; Miller, A.; Kohn, E.C.; Swisher, E.M.; Cella, D.; Tew, W.P.; Cloven, N.G.; Muller, C.Y.; et al. Olaparib with or without Cediranib versus Platinum-Based Chemotherapy in Recurrent Platinum-Sensitive Ovarian Cancer (NRG-GY004): A Randomized, Open-Label, Phase III Trial. J. Clin. Oncol. 2022, 40, 2138–2147. [Google Scholar] [CrossRef]
- Ergasti, R.; Marchetti, C.; Tudisco, R.; Iervolino, A.; Naldini, A.; Oliva, R.; Inzani, F.; Scambia, G.; Fagotti, A. BRCA Status and Platinum Sensitivity in Advanced Ovarian Cancer According to Chemotherapy Response Score. Int. J. Gynecol. Cancer 2022, 32, 639–645. [Google Scholar] [CrossRef]
- Ghirardi, V.; Ronsini, C.; Trozzi, R.; Di Ilio, C.; Di Giorgio, A.; Cianci, S.; Draisci, G.; Scambia, G.; Fagotti, A. Hyperthermic Intraperitoneal Chemotherapy in Interval Debulking Surgery for Advanced Epithelial Ovarian Cancer: A Single-Center, Real-Life Experience. Cancer 2020, 126, 5256–5262. [Google Scholar] [CrossRef]
- van der Burg, M.E.L.; van Lent, M.; Buyse, M.; Kobierska, A.; Colombo, N.; Favalli, G.; Lacave, A.J.; Nardi, M.; Renard, J.; Pecorelli, S. The Effect of Debulking Surgery after Induction Chemotherapy on the Prognosis in Advanced Epithelial Ovarian Cancer. Gynecological Cancer Cooperative Group of the European Organization for Research and Treatment of Cancer. N. Engl. J. Med. 1995, 332, 629–634. [Google Scholar] [CrossRef]
- Tseng, J.H.; Cowan, R.A.; Zhou, Q.; Iasonos, A.; Byrne, M.; Polcino, T.; Polen-De, C.; Gardner, G.J.; Sonoda, Y.; Zivanovic, O.; et al. Continuous Improvement in Primary Debulking Surgery for Advanced Ovarian Cancer: Do Increased Complete Gross Resection Rates Independently Lead to Increased Progression-Free and Overall Survival? Gynecol. Oncol. 2018, 151, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, N.S.; Miller, A.; Rungruang, B.; Richard, S.D.; Rodriguez, N.; Bookman, M.A.; Hamilton, C.A.; Krivak, T.C.; Maxwell, G.L. Does Aggressive Surgery Improve Outcomes? Interaction between Preoperative Disease Burden and Complex Surgery in Patients with Advanced-Stage Ovarian Cancer: An Analysis of GOG 182. J. Clin. Oncol. 2015, 33, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.J.; Hodeib, M.; Chang, J.; Bristow, R.E. Survival Impact of Complete Cytoreduction to No Gross Residual Disease for Advanced-Stage Ovarian Cancer: A Meta-Analysis. Gynecol. Oncol. 2013, 130, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Riester, M.; Wei, W.; Waldron, L.; Culhane, A.C.; Trippa, L.; Oliva, E.; Kim, S.H.; Michor, F.; Huttenhower, C.; Parmigiani, G.; et al. Risk Prediction for Late-Stage Ovarian Cancer by Meta-Analysis of 1525 Patient Samples. J. Natl. Cancer Inst. 2014, 106, dju048. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Armasu, S.M.; Kalli, K.R.; Maurer, M.J.; Heinzen, E.P.; Keeney, G.L.; Cliby, W.A.; Oberg, A.L.; Kaufmann, S.H.; Goode, E.L. Pooled Clustering of High-Grade Serous Ovarian Cancer Gene Expression Leads to Novel Consensus Subtypes Associated with Survival and Surgical Outcomes. Clin. Cancer Res. 2017, 23, 4077–4085. [Google Scholar] [CrossRef] [PubMed]
- Boccardi, V.; Marano, L.; Rossetti, R.R.A.; Rizzo, M.R.; Di Martino, N.; Paolisso, G. Serum CD26 Levels in Patients with Gastric Cancer: A Novel Potential Diagnostic Marker. BMC Cancer 2015, 15, 703. [Google Scholar] [CrossRef]
- Harter, P.; Sehouli, J.; Lorusso, D.; Reuss, A.; Vergote, I.; Marth, C.; Kim, J.-W.; Raspagliesi, F.; Lampe, B.; Aletti, G.; et al. A Randomized Trial of Lymphadenectomy in Patients with Advanced Ovarian Neoplasms. N. Engl. J. Med. 2019, 380, 822–832. [Google Scholar] [CrossRef]
- Harter, P.; Sehouli, J.; Lorusso, D.; Reuss, A.; Vergote, I.; Marth, C.; Kim, J.W.; Raspagliesi, F.; Lampe, B.; Landoni, F.; et al. LION: Lymphadenectomy in Ovarian Neoplasms—A Prospective Randomized AGO Study Group Led Gynecologic Cancer Intergroup Trial. N. Engl. J. Med. 2017, 35, 5500. [Google Scholar] [CrossRef]
- Sinukumar, S.; Rajan, F.; Mehta, S.; Damodaran, D.; Zaveri, S.; Kammar, P.; Bhatt, A. A Comparison of Outcomes Following Total and Selective Peritonectomy Performed at the Time of Interval Cytoreductive Surgery for Advanced Serous Epithelial Ovarian, Fallopian Tube and Primary Peritoneal Cancer—A Study by INDEPSO. Eur. J. Surg. Oncol. 2021, 47, 75–81. [Google Scholar] [CrossRef]
- Pignata, S.; Scambia, G.; Katsaros, D.; Gallo, C.; Pujade-Lauraine, E.; De Placido, S.; Bologna, A.; Weber, B.; Raspagliesi, F.; Panici, P.B.; et al. Carboplatin plus Paclitaxel Once a Week versus Every 3 Weeks in Patients with Advanced Ovarian Cancer (MITO-7): A Randomised, Multicentre, Open-Label, Phase 3 Trial. Lancet Oncol. 2014, 15, 396–405. [Google Scholar] [CrossRef]
- Clamp, A.R.; James, E.C.; McNeish, I.A.; Dean, A.; Kim, J.-W.; O’Donnell, D.M.; Gallardo-Rincon, D.; Blagden, S.; Brenton, J.; Perren, T.J.; et al. Weekly Dose-Dense Chemotherapy in First-Line Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer Treatment (ICON8): Overall Survival Results from an Open-Label, Randomised, Controlled, Phase 3 Trial. Lancet Oncol 2022. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, N.; Yasuda, M.; Takahashi, F.; Isonishi, S.; Jobo, T.; Aoki, D.; Tsuda, H.; Sugiyama, T.; Kodama, S.; Kimura, E.; et al. Dose-Dense Paclitaxel Once a Week in Combination with Carboplatin Every 3 Weeks for Advanced Ovarian Cancer: A Phase 3, Open-Label, Randomised Controlled Trial. Lancet 2009, 374, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Tewari, K.S.; Java, J.J.; Eskander, R.N.; Monk, B.J.; Burger, R.A. Early Initiation of Chemotherapy Following Complete Resection of Advanced Ovarian Cancer Associated with Improved Survival: NRG Oncology/Gynecologic Oncology Group Study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016, 27, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Orr, B.; Edwards, R.P. Diagnosis and Treatment of Ovarian Cancer. Hematol. Oncol. Clin. N. Am. 2018, 32, 943–964. [Google Scholar] [CrossRef]
- Hofstetter, G.; Concin, N.; Braicu, I.; Chekerov, R.; Sehouli, J.; Cadron, I.; Van Gorp, T.; Trillsch, F.; Mahner, S.; Ulmer, H.; et al. The Time Interval from Surgery to Start of Chemotherapy Significantly Impacts Prognosis in Patients with Advanced Serous Ovarian Carcinoma—Analysis of Patient Data in the Prospective OVCAD Study. Gynecol. Oncol. 2013, 131, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Jayson, G.C.; Kohn, E.C.; Kitchener, H.C.; Ledermann, J.A. Ovarian Cancer. Lancet 2014, 384, 1376–1388. [Google Scholar] [CrossRef]
- Coleridge, S.L.; Bryant, A.; Lyons, T.J.; Goodall, R.J.; Kehoe, S.; Morrison, J. Chemotherapy versus Surgery for Initial Treatment in Advanced Ovarian Epithelial Cancer. Cochrane Database Syst. Rev. 2019, 2019, CD005343. [Google Scholar] [CrossRef]
- Narod, S. Can Advanced-Stage Ovarian Cancer Be Cured? Nat. Rev. Clin. Oncol. 2016, 13, 255–261. [Google Scholar] [CrossRef]
- Cress, R.D.; Chen, Y.S.; Morris, C.R.; Petersen, M.; Leiserowitz, G.S. Characteristics of Long-Term Survivors of Epithelial Ovarian Cancer. Obstet. Gynecol. 2015, 126, 491–497. [Google Scholar] [CrossRef]
- Halsted, W.S.I. The Results of Operations for the Cure of Cancer of the Breast Performed at the Johns Hopkins Hospital from June, 1889, to January, 1894. Ann. Surg. 1894, 20, 497–555. [Google Scholar] [CrossRef]
- Chambers, L.M.; Son, J.; Radeva, M.; Debernardo, R. Evaluation of Non-Completion of Intraperitoneal Chemotherapy in Patients with Advanced Epithelial Ovarian Cancer. J. Gynecol. Oncol. 2019, 30, e93. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-Y.; Koo, Y.-J.; Kim, M.-J.; Kim, T.-J.; Lim, K.-T.; Lee, K.-H. Survival Outcomes and Toxicity of Intraoperative Intraperitoneal Chemotherapy in Advanced Epithelial Ovarian Cancer. Obstet. Gynecol. Sci. 2014, 57, 484. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tewari, D.; Java, J.J.; Salani, R.; Armstrong, D.K.; Markman, M.; Herzog, T.; Monk, B.J.; Chan, J.K. Long-Term Survival Advantage and Prognostic Factors Associated with Intraperitoneal Chemotherapy Treatment in Advanced Ovarian Cancer: A Gynecologic Oncology Group Study. J. Clin. Oncol. 2015, 33, 1460–1466. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, M.; Szlendak, M.; Forma, A.; Baj, J.; Maciejewski, R.; Roviello, G.; Marano, L.; Roviello, F.; Polom, K.; Sitarz, R. Hyperthermic Intraperitoneal Chemotherapy in the Management of Gastric Cancer: A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 681. [Google Scholar] [CrossRef] [PubMed]
- Marano, L.; Marrelli, D.; Sammartino, P.; Biacchi, D.; Graziosi, L.; Marino, E.; Coccolini, F.; Fugazzola, P.; Valle, M.; Federici, O.; et al. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer with Synchronous Peritoneal Metastases: Multicenter Study of “Italian Peritoneal Surface Malignancies Oncoteam-S.I.C.O.”. Ann. Surg. Oncol. 2021, 28, 9060–9070. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H.; Van der Speeten, K.; Stuart, O.A. Pharmacologic Rationale for Treatments of Peritoneal Surface Malignancy from Colorectal Cancer. World J. Gastrointest. Oncol. 2010, 2, 19. [Google Scholar] [CrossRef]
- Riggs, M.J.; Pandalai, P.K.; Kim, J.; Dietrich, C.S. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. Diagnostics 2020, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Medina-Castro, J.M.; Ruiz-DeLeón, A. Role of Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. Chin. Clin. Oncol. 2020, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- González-Moreno, S.; González-Bayón, L.A.; Ortega-Pérez, G.; Macrì, A. Hyperthermic Intraperitoneal Chemotherapy: Rationale and Technique. World J. Gastrointest. Oncol. 2010, 2, 68–75. [Google Scholar] [CrossRef]
- Roviello, F.; Pinto, E.; Corso, G.; Pedrazzani, C.; Caruso, S.; Filippeschi, M.; Petrioli, R.; Marsili, S.; Mazzei, M.A.; Marrelli, D. Safety and Potential Benefit of Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Peritoneal Carcinomatosis from Primary or Recurrent Ovarian Cancer. J. Surg. Oncol. 2010, 102, 663–670. [Google Scholar] [CrossRef]
- Magge, D.; Ramalingam, L.; Shuai, Y.; Edwards, R.P.; Pingpank, J.F.; Ahrendt, S.S.; Holtzman, M.P.; Zeh, H.J.; Bartlett, D.L.; Choudry, H.A. Hyperthermic Intraperitoneal Chemoperfusion as a Component of Multimodality Therapy for Ovarian and Primary Peritoneal Cancer. J. Surg. Oncol. 2017, 116, 320–328. [Google Scholar] [CrossRef]
- Muñoz-Casares, F.C.; Medina-Fernández, F.J.; Arjona-Sánchez, A.; Casado-Adam, A.; Sánchez-Hidalgo, J.M.; Rubio, M.J.; Ortega-Salas, R.; Muñoz-Villanueva, M.C.; Rufián-Peña, S.; Briceño, F.J. Peritonectomy Procedures and HIPEC in the Treatment of Peritoneal Carcinomatosis from Ovarian Cancer: Long-Term Outcomes and Perspectives from a High-Volume Center. Eur. J. Surg. Oncol. 2016, 42, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhu, Y.; Liu, C.; Chao, G.; Cui, R.; Zhang, Z. The Prognosis Impact of Hyperthermic Intraperitoneal Chemotherapy (HIPEC) plus Cytoreductive Surgery (CRS) in Advanced Ovarian Cancer: The Meta-Analysis. J. Ovarian Res. 2019, 12, 33. [Google Scholar] [CrossRef]
- Polom, K.; Roviello, G.; Generali, D.; Marano, L.; Petrioli, R.; Marsili, S.; Caputo, E.; Marrelli, D.; Roviello, F. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Treatment of Ovarian Cancer. Int. J. Hyperthermia 2016, 32, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.R.; Richards, A.; Liauw, W.; Morris, D.L. Hyperthermic Intraperitoneal Chemotherapy (HIPEC) and Cytoreductive Surgery (CRS) in Ovarian Cancer: A Systematic Review and Meta-Analysis. Eur. J. Surg. Oncol. 2015, 41, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Jaaback, K.; Johnson, N.; Lawrie, T.A. Intraperitoneal Chemotherapy for the Initial Management of Primary Epithelial Ovarian Cancer. Cochrane Database Syst. Rev. 2016, 2016, CD005340. [Google Scholar] [CrossRef]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and Response Criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Boerner, T.; Zivanovic, O.; Chi, D.S. Narrative Review of Cytoreductive Surgery and Intraperitoneal Chemotherapy for Peritoneal Metastases in Ovarian Cancer. J. Gastrointest. Oncol. 2021, 12, S137–S143. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Wang, Y.; Wang, J.; Wang, K.; Tian, J.; Zhao, Y.; Chen, L.; Wang, J.; Luo, J.; Jia, M.; et al. Evaluation of Cytoreductive Surgery with or without Hyperthermic Intraperitoneal Chemotherapy for Stage III Epithelial Ovarian Cancer. JAMA Netw. Open 2020, 3, e2013940. [Google Scholar] [CrossRef]
- Antonio, C.C.P.; Alida, G.G.; Elena, G.G.; Rocío, G.S.; Jerónimo, M.G.; Luis, A.R.J.; Aníbal, N.D.; Francisco, B.V.; Jesús, G.R.Á.; Pablo, R.R.; et al. Cytoreductive Surgery with or without HIPEC after Neoadjuvant Chemotherapy in Ovarian Cancer: A Phase 3 Clinical Trial. Ann. Surg. Oncol. 2022, 29, 2617–2625. [Google Scholar] [CrossRef]
- Bakrin, N.; Bereder, J.M.; Decullier, E.; Classe, J.M.; Msika, S.; Lorimier, G.; Abboud, K.; Meeus, P.; Ferron, G.; Quenet, F.; et al. Peritoneal Carcinomatosis Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Advanced Ovarian Carcinoma: A French Multicentre Retrospective Cohort Study of 566 Patients. Eur. J. Surg. Oncol. 2013, 39, 1435–1443. [Google Scholar] [CrossRef]
- Ozols, R.F.; Bundy, B.N.; Greer, B.E.; Fowler, J.M.; Clarke-Pearson, D.; Burger, R.A.; Mannel, R.S.; DeGeest, K.; Hartenbach, E.M.; Baergen, R.; et al. Phase III Trial of Carboplatin and Paclitaxel Compared with Cisplatin and Paclitaxel in Patients with Optimally Resected Stage III Ovarian Cancer: A Gynecologic Oncology Group Study. J. Clin. Oncol. 2003, 21, 3194–3200. [Google Scholar] [CrossRef]
- McGuire, W.P.; Hoskins, W.J.; Brady, M.F.; Kucera, P.R.; Partridge, E.E.; Look, K.Y.; Clarke-Pearson, D.L.; Davidson, M. Cyclophosphamide and Cisplatin Compared with Paclitaxel and Cisplatin in Patients with Stage III and Stage IV Ovarian Cancer. N. Engl. J. Med. 1996, 334, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Roviello, F.; Caruso, S.; Marrelli, D.; Pedrazzani, C.; Neri, A.; De Stefano, A.; Pinto, E. Treatment of Peritoneal Carcinomatosis with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: State of the Art and Future Developments. Surg. Oncol. 2011, 20, e38–e54. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Cytoreductive Surgery Using Peritonectomy and Visceral Resections for Peritoneal Surface Malignancy. Transl. Gastrointest. Cancer 2013, 2, 54–74. [Google Scholar]
- Jones, N.L.; Chen, L.; Chatterjee, S.; Tergas, A.I.; Burke, W.M.; Hou, J.Y.; Ananth, C.V.; Neugut, A.I.; Hershman, D.L.; Wright, J.D. National Trends in Extended Procedures for Ovarian Cancer Debulking Surgery. Int. J. Gynecol. Cancer 2018, 28, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Helm, C.W.; Richard, S.D.; Pan, J.; Bartlett, D.; Goodman, M.D.; Hoefer, R.; Lentz, S.S.; Levine, E.A.; Loggie, B.W.; Metzinger, D.S.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer: First Report of the HYPER-O Registry. Int. J. Gynecol. Cancer 2010, 20, 61–69. [Google Scholar] [CrossRef]
- Batista, T.P.; Carneiro, V.C.G.; Tancredi, R.; Filho, L.B.; Rangel, R.L.C.; Lopes, A.; Sarmento, B.J.Q.; Leão, C.S. A Phase 2 Trial of Short-Course Hyperthermic IntraPeritoneal Chemotherapy (HIPEC) at Interval Cytoreductive Surgery (ICRS) for Advanced Ovarian Cancer. Rev. Col. Bras. Cir. 2022, 49, e20223135. [Google Scholar] [CrossRef] [PubMed]
- Batista, T.P.; Carneiro, V.C.G.; Tancredi, R.; Teles, A.L.B.; Badiglian-Filho, L.; Leão, C.S. Neoadjuvant Chemotherapy Followed by Fast-Track Cytoreductive Surgery plus Short-Course Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Advanced Ovarian Cancer: Preliminary Results of a Promising All-in-One Approach. Cancer Manag. Res. 2017, 9, 869–878. [Google Scholar] [CrossRef]
- Markman, M.; Bundy, B.N.; Alberts, D.S.; Fowler, J.M.; Clark-Pearson, D.L.; Carson, L.F.; Wadler, S.; Sickel, J. Phase III Trial of Standard-Dose Intravenous Cisplatin plus Paclitaxel versus Moderately High-Dose Carboplatin Followed by Intravenous Paclitaxel and Intraperitoneal Cisplatin in Small-Volume Stage III Ovarian Carcinoma: An Intergroup Study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J. Clin. Oncol. 2001, 19, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Aotani, E.; Hamano, T.; Nagao, S.; Yoshikawa, H.; Sugiyama, T.; Kigawa, J.; Aoki, D.; Katsumata, N.; Takeuchi, M.; et al. A Randomized Phase II/III Trial of 3 Weekly Intraperitoneal versus Intravenous Carboplatin in Combination with Intravenous Weekly Dose-Dense Paclitaxel for Newly Diagnosed Ovarian, Fallopian Tube and Primary Peritoneal Cancer. Jpn. J. Clin. Oncol. 2011, 41, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Lee, J.Y.; Cho, M.S.; Nam, E.J.; Kim, S.W.; Kim, S.; Kim, Y.T. Incorporation of Paclitaxel-Based Hyperthermic Intraperitoneal Chemotherapy in Patients with Advanced-Stage Ovarian Cancer Treated with Neoadjuvant Chemotherapy Followed by Interval Debulking Surgery: A Protocol-Based Pilot Study. J. Gynecol. Oncol. 2019, 30, e3. [Google Scholar] [CrossRef] [PubMed]
- Manzanedo, I.; Pereira, F.; Serrano, A.; Pérez-Viejo, E.; Martínez-Torres, B.; Carrión, L.; Calzas, J. The Use of Cisplatin plus Doxorubicin or Paclitaxel in Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Stage IIIC or IV Epithelial Ovarian Cancer: A Comparative Study. Clin. Transl. Oncol. 2019, 21, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Coccolini, F.; Fugazzola, P.; Montori, G.; Ansaloni, L.; Chiarugi, M. Intraperitoneal Chemotherapy for Ovarian Cancer with Peritoneal Metastases, Systematic Review of the Literature and Focused Personal Experience. J. Gastrointest. Oncol. 2021, 12 (Suppl. 1), S144. [Google Scholar] [CrossRef]
- Walker, J.L.; Brady, M.F.; Wenzel, L.; Fleming, G.F.; Huang, H.Q.; DiSilvestro, P.A.; Fujiwara, K.; Alberts, D.S.; Zheng, W.; Tewari, K.S.; et al. Randomized Trial of Intravenous versus Intraperitoneal Chemotherapy Plus Bevacizumab in Advanced Ovarian Carcinoma: An NRG Oncology/Gynecologic Oncology Group Study. J. Clin. Oncol. 2019, 37, 1380–1390. [Google Scholar] [CrossRef]
- Kim, S.I.; Kim, J.-W. Role of Surgery and Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. ESMO Open 2021, 6, 100149. [Google Scholar] [CrossRef]
- van Driel, W.J.; Koole, S.N.; Sikorska, K.; Schagen van Leeuwen, J.H.; Schreuder, H.W.R.; Hermans, R.H.M.; de Hingh, I.H.J.T.; van der Velden, J.; Arts, H.J.; Massuger, L.F.A.G.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef]
- Lim, M.C.; Chang, S.-J.; Park, B.; Yoo, H.J.; Yoo, C.W.; Nam, B.H.; Park, S.-Y.; Seo, S.-S.; Kang, S.; Yun, J.Y.; et al. Survival after Hyperthermic Intraperitoneal Chemotherapy and Primary or Interval Cytoreductive Surgery in Ovarian Cancer. JAMA Surg. 2022, 157, 374. [Google Scholar] [CrossRef]
- Marrelli, D.; Petrioli, R.; Cassetti, D.; D’Ignazio, A.; Marsili, S.; Mazzei, M.A.; Lazzi, S.; Roviello, F. A Novel Treatment Protocol with 6 Cycles of Neoadjuvant Chemotherapy Followed by Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Stage III Primary Ovarian Cancer. Surg. Oncol. 2021, 37, 101523. [Google Scholar] [CrossRef]
- Bogani, G.; Matteucci, L.; Tamberi, S.; Ditto, A.; Sabatucci, I.; Murgia, F.; Arcangeli, V.; Maltese, G.; Comerci, G.; Stefanetti, M.; et al. RECIST 1.1 Criteria Predict Recurrence-Free Survival in Advanced Ovarian Cancer Submitted to Neoadjuvant Chemotherapy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 237, 93–99. [Google Scholar] [CrossRef]
- Koole, S.; Van Stein, R.; Sikorska, K.; Barton, D.; Perrin, L.; Brennan, D.; Zivanovic, O.; Mosgaard, B.J.; Fagotti, A.; Colombo, P.E.; et al. Primary Cytoreductive Surgery with or without Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for FIGO Stage III Epithelial Ovarian Cancer: OVHIPEC-2, a Phase III Randomized Clinical Trial. Int. J. Gynecol. Cancer 2020, 30, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Lin, Z. Efficacy of HIPEC in the Treatment of Advanced-Stage Epithelial Ovarian Cancer after Cytoreductive Surgery. Available online: ClinicalTrials.Gov (accessed on 14 December 2017).
- Jewell, A.; Mcmahon, M.; Khabele, D. Cancers Heated Intraperitoneal Chemotherapy in the Management of Advanced Ovarian Cancer. Cancers 2018, 10, 296. [Google Scholar] [CrossRef] [PubMed]
- Cascales-Campos, P.A.; Gil, J.; Gil, E.; Feliciangeli, E.; González-Gil, A.; Parrilla, J.J.; Parrilla, P. Treatment of Microscopic Disease with Hyperthermic Intraoperative Intraperitoneal Chemotherapy after Complete Cytoreduction Improves Disease-Free Survival in Patients with Stage IIIC/IV Ovarian Cancer. Ann. Surg. Oncol. 2014, 21, 2383–2389. [Google Scholar] [CrossRef] [PubMed]
- Lesnock, J.L.; Darcy, K.M.; Tian, C.; Deloia, J.A.; Thrall, M.M.; Zahn, C.; Armstrong, D.K.; Birrer, M.J.; Krivak, T.C. BRCA1 Expression and Improved Survival in Ovarian Cancer Patients Treated with Intraperitoneal Cisplatin and Paclitaxel: A Gynecologic Oncology Group Study. Br. J. Cancer 2013, 108, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- McGee, J.; Bookman, M.; Harter, P.; Marth, C.; McNeish, I.; Moore, K.N.; Poveda, A.; Hilpert, F.; Hasegawa, K.; Bacon, M.; et al. Fifth Ovarian Cancer Consensus Conference: Individualized Therapy and Patient Factors. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Steffen, T.; Häller, L.; Bijelic, L.; Glatzer, M.; Glehen, O.; Goéré, D.; De Hingh, I.; Li, Y.; Moran, B.J.; Morris, D.L.; et al. Decision-Making Analysis for Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer: A Survey by the Executive Committee of the Peritoneal Surface Oncology Group International (PSOGI). Oncology 2021, 99, 41–48. [Google Scholar] [CrossRef]
- Marano, L.; Carbone, L.; Poto, G.E.; Gambelli, M.; Nguefack Noudem, L.L.; Grassi, G.; Manasci, F.; Curreri, G.; Giuliani, A.; Piagnerelli, R.; et al. Handgrip Strength Predicts Length of Hospital Stay in an Abdominal Surgical Setting: The Role of Frailty beyond Age. Aging Clin. Exp. Res. 2022, 34, 811–817. [Google Scholar] [CrossRef]
- Macrì, A.; Accarpio, F.; Arcoraci, V.; Casella, F.; De Cian, F.; De Iaco, P.; Orsenigo, E.; Roviello, F.; Scambia, G.; Saladino, E.; et al. Predictors of Morbidity and Mortality in Patients Submitted to Cytoreductive Surgery plus Hyperthermic Intraperitoneal Chemotherapy for Ovarian Carcinomatosis: A Multicenter Study. Pleura Peritoneum 2020, 6, 21–30. [Google Scholar] [CrossRef]
- Boccardi, V.; Marano, L. The Geriatric Surgery: The Importance of Frailty Identification beyond Chronological Age. Geriatrics 2020, 5, 12. [Google Scholar] [CrossRef]
- Verwaal, V.J.; van Ruth, S.; de Bree, E.; van Slooten, G.W.; van Tinteren, H.; Boot, H.; Zoetmulder, F.A.N. Randomized Trial of Cytoreduction and Hyperthermic Intraperitoneal Chemotherapy versus Systemic Chemotherapy and Palliative Surgery in Patients with Peritoneal Carcinomatosis of Colorectal Cancer. J. Clin. Oncol. 2003, 21, 3737–3743. [Google Scholar] [CrossRef]
- Murphy, E.M.; Sexton, R.; Moran, B.J. Early Results of Surgery in 123 Patients with Pseudomyxoma Peritonei from a Perforated Appendiceal Neoplasm. Dis. Colon Rectum 2007, 50, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Gilly, F.; Quenet, F.; Bereder, J.M.; Sidéris, L.; Mansvelt, B.; Lorimier, G.; Glehen, O. Pseudomyxoma Peritonei: A French Multicentric Study of 301 Patients Treated with Cytoreductive Surgery and Intraperitoneal Chemotherapy. Eur. J. Surg. Oncol. 2010, 36, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.D.; Welch, L.; Black, D.; Sugarbaker, P.H. A Systematic Review on the Efficacy of Cytoreductive Surgery Combined with Perioperative Intraperitoneal Chemotherapy for Diffuse Malignancy Peritoneal Mesothelioma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2007, 18, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.D.; Deraco, M.; Baratti, D.; Kusamura, S.; Elias, D.; Glehen, O.; Gilly, F.N.; Levine, E.A.; Shen, P.; Mohamed, F.; et al. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Malignant Peritoneal Mesothelioma: Multi-Institutional Experience. J. Clin. Oncol. 2009, 27, 6237–6242. [Google Scholar] [CrossRef]
- Charo, L.M.; Jou, J.; Binder, P.; Hohmann, S.F.; Saenz, C.; McHale, M.; Eskander, R.N.; Plaxe, S. Current Status of Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Ovarian Cancer in the United States. Gynecol. Oncol. 2020, 159, 681–686. [Google Scholar] [CrossRef]
- Colombo, N.; Sessa, C.; Du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO-ESGO Consensus Conference Recommendations on Ovarian Cancer: Pathology and Molecular Biology, Early and Advanced Stages, Borderline Tumours and Recurrent Disease†. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef]
- Spiliotis, J.; Prodromidou, A. Narrative Review of Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Patients with Advanced Ovarian Cancer: A Critical Reappraisal of the Current Evidence. J. Gastrointest. Oncol. 2021, 12, S182–S188. [Google Scholar] [CrossRef]
- Fotopoulou, C.; Sehouli, J.; Mahner, S.; Harter, P.; Van Nieuwenhuysen, E.; Gonzalez-Martin, A.; Vergote, I.; Chiva, L.; Du Bois, A. HIPEC: HOPE or HYPE in the Fight against Advanced Ovarian Cancer? Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, 1610–1613. [Google Scholar] [CrossRef]
- Cavaliere, D.; Cirocchi, R.; Coccolini, F.; Fagotti, A.; Fambrini, M.; Federici, O.; Lorusso, D.; Vaira, M.; Ceresoli, M.; Delrio, P.; et al. 1st Evidence-Based Italian Consensus Conference on Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Carcinosis from Ovarian Cancer. Tumori 2017, 103, 525–536. [Google Scholar] [CrossRef]
- Chi, D.S.; Eisenhauer, E.L.; Zivanovic, O.; Sonoda, Y.; Abu-Rustum, N.R.; Levine, D.A.; Guile, M.W.; Bristow, R.E.; Aghajanian, C.; Barakat, R.R. Improved Progression-Free and Overall Survival in Advanced Ovarian Cancer as a Result of a Change in Surgical Paradigm. Gynecol. Oncol. 2009, 114, 26–31. [Google Scholar] [CrossRef]
- Eisenhauer, E.L.; Abu-Rustum, N.R.; Sonoda, Y.; Aghajanian, C.; Barakat, R.R.; Chi, D.S. The Effect of Maximal Surgical Cytoreduction on Sensitivity to Platinum-Taxane Chemotherapy and Subsequent Survival in Patients with Advanced Ovarian Cancer. Gynecol. Oncol. 2008, 108, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Stuart, G.C.E.; Kitchener, H.; Bacon, M.; DuBois, A.; Friedlander, M.; Ledermann, J.; Marth, C.; Thigpen, T.; Trimble, E. 2010 Gynecologic Cancer InterGroup (GCIG) Consensus Statement on Clinical Trials in Ovarian Cancer: Report from the Fourth Ovarian Cancer Consensus Conference. Int. J. Gynecol. Cancer 2011, 21, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Van de Laar, R.; Zusterzeel, P.L.M.; Van Gorp, T.; Buist, M.R.; Van Driel, W.J.; Gaarenstroom, K.N.; Arts, H.J.G.; Van Huisseling, J.C.M.; Hermans, R.H.M.; Pijnenborg, J.M.A.; et al. Cytoreductive Surgery Followed by Chemotherapy versus Chemotherapy Alone for Recurrent Platinum-Sensitive Epithelial Ovarian Cancer (SOCceR Trial): A Multicenter Randomised Controlled Study. BMC Cancer 2014, 14, 22. [Google Scholar] [CrossRef]
- Bristow, R.E.; Tomacruz, R.S.; Armstrong, D.K.; Trimble, E.L.; Montz, F.J. Survival Effect of Maximal Cytoreductive Surgery for Advanced Ovarian Carcinoma during the Platinum Era: A Meta-Analysis. J. Clin. Oncol. 2002, 20, 1248–1259. [Google Scholar] [CrossRef]
- Di Giorgio, A.; Cardi, M.; Biacchi, D.; Sibio, S.; Accarpio, F.; Ciardi, A.; Cornali, T.; Framarino, M.; Sammartino, P. Depth of Colorectal-Wall Invasion and Lymph-Node Involvement as Major Outcome Factors Influencing Surgical Strategy in Patients with Advanced and Recurrent Ovarian Cancer with Diffuse Peritoneal Metastases. World J. Surg. Oncol. 2013, 11, 64. [Google Scholar] [CrossRef]
- Scarabelli, C.; Gallo, A.; Franceschi, S.; Campagnutta, E.; De Piero, G.; Giorda, G.; Visentin, M.C.; Carbone, A. Primary Cytoreductive Surgery with Rectosigmoid Colon Resection for Patients with Advanced Epithelial Ovarian Carcinoma. Cancer 2000, 88, 389–397. [Google Scholar] [CrossRef]
- Chi, D.S.; Eisenhauer, E.L.; Lang, J.; Huh, J.; Haddad, L.; Abu-Rustum, N.R.; Sonoda, Y.; Levine, D.A.; Hensley, M.; Barakat, R.R. What Is the Optimal Goal of Primary Cytoreductive Surgery for Bulky Stage IIIC Epithelial Ovarian Carcinoma (EOC)? Gynecol. Oncol. 2006, 103, 559–564. [Google Scholar] [CrossRef]
- Hoskins, W.J.; McGuire, W.P.; Brady, M.F.; Homesley, H.D.; Creasman, W.T.; Berman, M.; Ball, H.; Berek, J.S. The Effect of Diameter of Largest Residual Disease on Survival after Primary Cytoreductive Surgery in Patients with Suboptimal Residual Epithelial Ovarian Carcinoma. Am. J. Obstet. Gynecol. 1994, 170, 974–980. [Google Scholar] [CrossRef]
- Eisenkop, S.M.; Spirtos, N.M. Procedures Required to Accomplish Complete Cytoreduction of Ovarian Cancer: Is There a Correlation with “Biological Aggressiveness” and Survival? Gynecol. Oncol. 2001, 82, 435–441. [Google Scholar] [CrossRef]
- Mulier, S.; Claes, J.-P.; Dierieck, V.; Amiel, J.-O.; Pahaut, J.-P.; Marcelis, L.; Bastin, F.; Vanderbeeken, D.; Finet, C.; Cran, S.; et al. Survival Benefit of Adding Hyperthermic IntraPEritoneal Chemotherapy (HIPEC) at the Different Time-Points of Treatment of Ovarian Cancer: Review of Evidence. Curr. Pharm. Des. 2012, 18, 3793–3803. [Google Scholar] [CrossRef][Green Version]
- Di Giorgio, A.; De Iaco, P.; De Simone, M.; Garofalo, A.; Scambia, G.; Pinna, A.D.; Verdecchia, G.M.; Ansaloni, L.; Macrì, A.; Cappellini, P.; et al. Cytoreduction (Peritonectomy Procedures) Combined with Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Advanced Ovarian Cancer: Retrospective Italian Multicenter Observational Study of 511 Cases. Ann. Surg. Oncol. 2017, 24, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Ferron, J.G.; Uzan, C.; Rey, A.; Gouy, S.; Pautier, P.; Lhommé, C.; Duvillard, P.; Morice, P. Histological Response Is Not a Prognostic Factor after Neoadjuvant Chemotherapy in Advanced-Stage Ovarian Cancer with No Residual Disease. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 147, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, M.; Zannoni, G.F.; Beltrame, L.; Martinelli, E.; DiFeo, A.; Paracchini, L.; Craparotta, I.; Mannarino, L.; Vizzielli, G.; Scambia, G.; et al. Identification of High-Grade Serous Ovarian Cancer MiRNA Species Associated with Survival and Drug Response in Patients Receiving Neoadjuvant Chemotherapy: A Retrospective Longitudinal Analysis Using Matched Tumor Biopsies. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016, 27, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Da Costa Miranda, V.; De Souza Fêde, Â.B.; Dos Anjos, C.H.; Da Silva, J.R.; Sanchez, F.B.; Da Silva Bessa, L.R.; De Paula Carvalho, J.; Filho, E.A.; De Freitas, D.; Del Pilar Estevez Diz, M. Neoadjuvant Chemotherapy with Six Cycles of Carboplatin and Paclitaxel in Advanced Ovarian Cancer Patients Unsuitable for Primary Surgery: Safety and Effectiveness. Gynecol. Oncol. 2014, 132, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Mueller, J.J.; Zhou, Q.C.; Iasonos, A.; O’Cearbhaill, R.E.; Alvi, F.A.; El Haraki, A.; Eriksson, A.G.Z.; Gardner, G.J.; Sonoda, Y.; Levine, D.A.; et al. Neoadjuvant Chemotherapy and Primary Debulking Surgery Utilization for Advanced-Stage Ovarian Cancer at a Comprehensive Cancer Center. Gynecol. Oncol. 2016, 140, 436–442. [Google Scholar] [CrossRef]
- Biacchi, D.; Accarpio, F.; Ansaloni, L.; Macrì, A.; Ciardi, A.; Federici, O.; Spagnoli, A.; Cavaliere, D.; Vaira, M.; Sapienza, P.; et al. Upfront Debulking Surgery versus Interval Debulking Surgery for Advanced Tubo-Ovarian High-Grade Serous Carcinoma and Diffuse Peritoneal Metastases Treated with Peritonectomy Procedures plus HIPEC. J. Surg. Oncol. 2019, 120, 1208–1219. [Google Scholar] [CrossRef]
- Ansaloni, L.; De Iaco, P.; Frigerio, L. Re: “Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy as Upfront Therapy for Advanced Epithelial Ovarian Cancer: Multi-Institutional Phase II Trial.”—Proposal of a Clinical Trial of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy in Advanced Ovarian Cancer, the CHORINE Study. Gynecol. Oncol. 2012, 125, 279–281. [Google Scholar] [CrossRef]
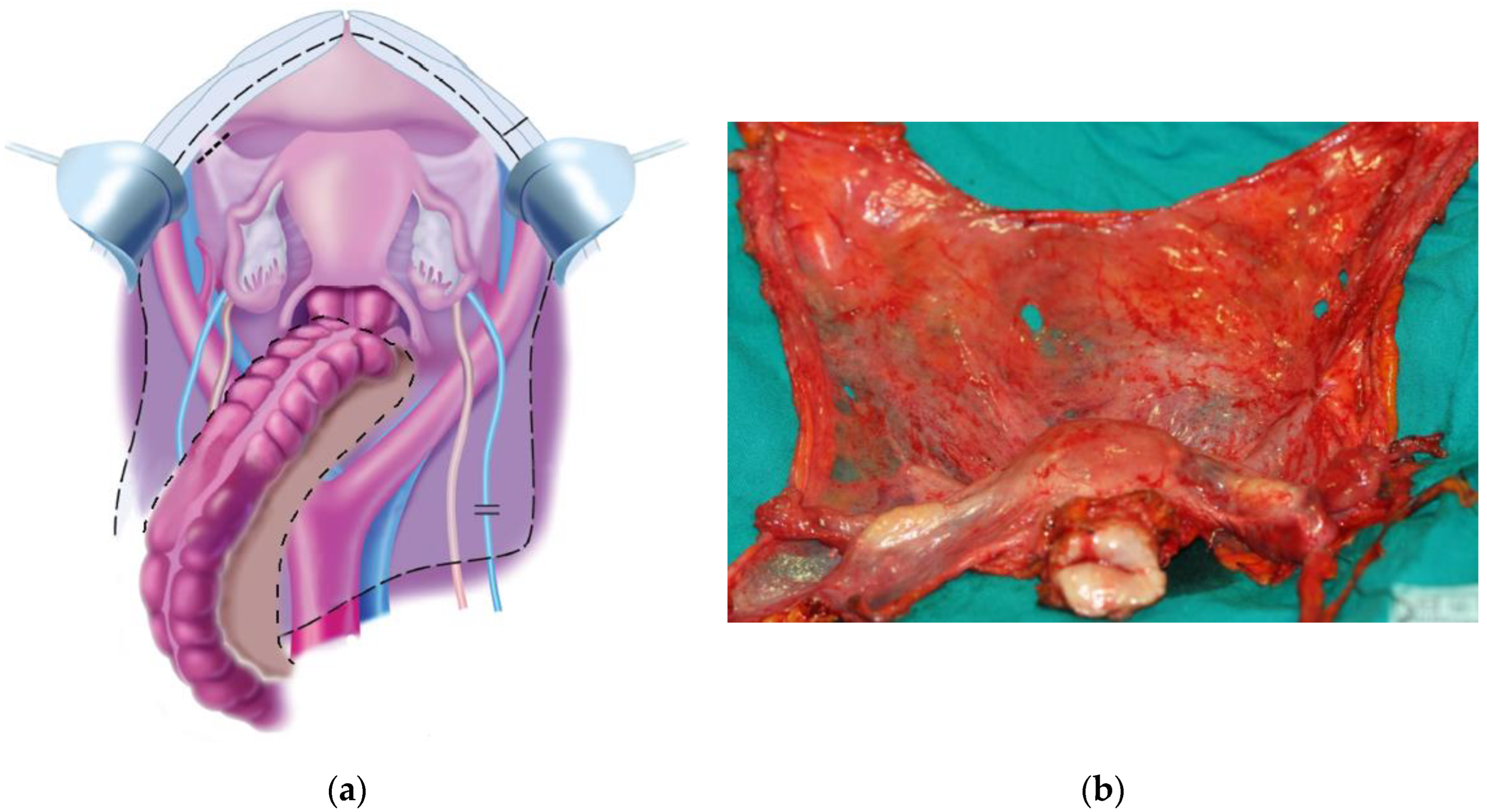
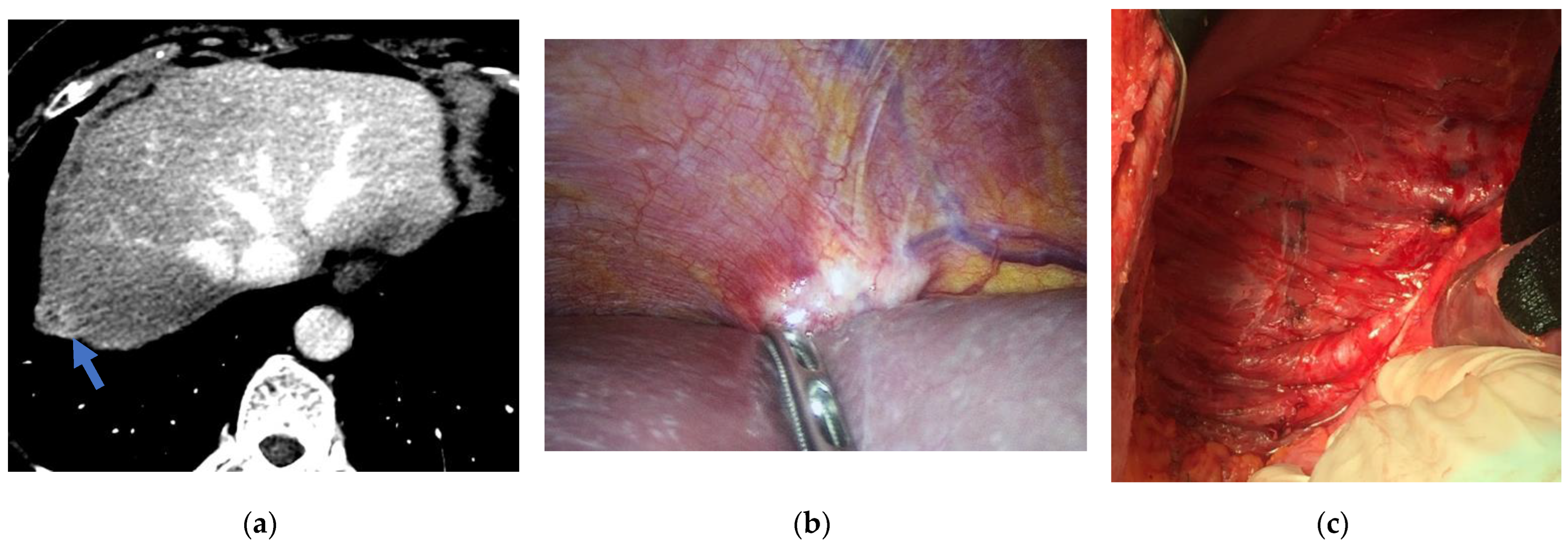
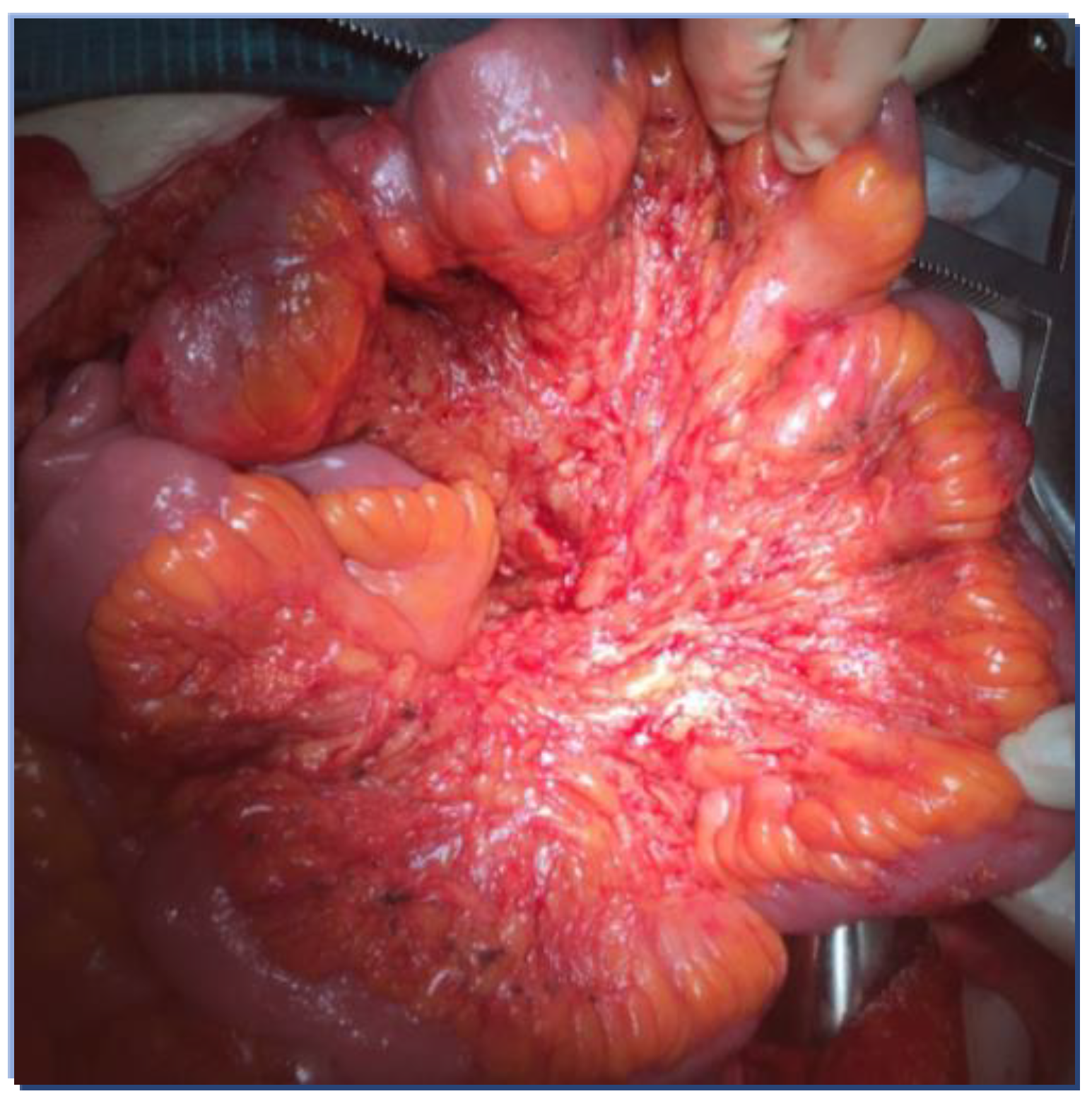


| Stage | TNM | |
|---|---|---|
| I | Tumor confined to ovaries or fallopian tube(s). | T1 N0 M0 |
| IA | Tumor limited to one ovary (capsule intact) or fallopian tube; no tumor on ovarian or fallopian tube surface; no malignant cells in the ascites or peritoneal washings. | T1a N0 M0 |
| IB | Tumor limited to both ovaries (capsules intact) or fallopian tubes; no tumor on ovarian or fallopian tube surface; no malignant cells in the ascites or peritoneal washings. | T1b N0 M0 |
| IC | Tumor limited to one or both ovaries or fallopian tubes, with any of the following: | |
| IC1 | Surgical spill, | T1c1 N0 M0 |
| IC2 | Capsule ruptured before surgery or tumor on ovarian or fallopian tube surface, | T1c2 N0 M0 |
| IC3 | Malignant cells in the ascites or peritoneal washings. | T1c3 N0 M0 |
| II | Tumor involves one or both ovaries or fallopian tubes with pelvic extension (below pelvic brim) or peritoneal cancer. | T2 N0 M0 |
| IIA | Extension and/or implants on uterus and/or fallopian tubes and/or ovaries. | T2a N0 M0 |
| IIB | Extension to other pelvic intraperitoneal tissues. | T2b N0 M0 |
| III | Tumor involves one or both ovaries or fallopian tubes, or peritoneal cancer, with cytologically or histologically confirmed spread to the peritoneum outside the pelvis and/or metastasis to the retroperitoneal lymph nodes. | T1-3 N0-1 M0 |
| IIIA1 | Positive retroperitoneal lymph nodes only (cytologically or histologically proven): | T1-2 N1 M0 |
| IIIA1(i) | Metastasis up to 10 mm in greatest dimension, | |
| IIIA1(i) | Metastasis more than 10 mm in greatest dimension, | |
| IIIA2 | Microscopic extrapelvic (above the pelvic brim) peritoneal involvement with or without positive retroperitoneal lymph nodes. | T3a2 N0-1 M0 |
| IIIB | Macroscopic peritoneal metastasis beyond the pelvis up to 2 cm in greatest dimension, with or without metastasis to the retroperitoneal lymph nodes. | T3b N0-1 M0 |
| IIIC | Macroscopic peritoneal metastasis beyond the pelvis more than 2 cm in greatest dimension, with or without metastasis to the retroperitoneal lymph nodes (includes extension of tumor to capsule of liver and spleen without parenchymal involvement of either organ). | T3c N0-1 M0 |
| IV | Distant metastasis excluding peritoneal metastases. | AnyT anyN M1 |
| IVA | Pleural effusion with positive cytology. | |
| IVB | Parenchymal metastases and metastases to extra-abdominal organs (including inguinal lymph nodes and lymph nodes outside of the abdominal cavity). |
| Trial Code | Center | Treatment | Results |
|---|---|---|---|
| EORTC55971 [71] | Belgium | CRS + AC vs. NAC + CRS + AC in stage IIIC or IV | NAC followed by interval CRS is non-inferior to primary CRS followed by AC regarding OS and PFS. |
| CHORUS [72] | UK | CRS + 6 cycles of AC vs. 3 cycles of NAC + CRS + 3 cycles of AC in stage III or IV | Primary chemotherapy followed by interval CRS is non-inferior to primary CRS regarding OS. |
| JCOG0602 [74] | Japan | CRS + 8 cycles of AC vs. 4 cycles of NAC + CRS + 4 cycles of AC in stage III or IV | The non-inferiority of NAC was not confirmed. |
| SCORPION [75] | Italy | CRS + AC vs. NAC + CRS + AC in stage IIIC or IV | Primary CRS is non-inferior to NAC followed by interval CRS regarding OS and PFS, with different post-operative complications. |
| MSKCC [73] | USA | CRS + AC vs. NAC + CRS + AC in stage IIIC or IV | NAC should be reserved for patients who cannot tolerate primary CRS and/or for whom optimal CRS (≤1 cm residual) is not feasible. |
| CARCINOHIPEC [123] | Spain | NAC + CRS vs. NAC + CRS + HIPEC in FIGO IIIB/C | The addition of HIPEC to CRS improves OS and PFS. |
| OVHIPEC−1 [140] | Netherlands | NAC + CRS + AC vs. NAC + CRS + HIPEC + AC in stage III | HIPEC, combined with CRS, improves OS and PFS, and does not have higher rates of side effects. |
| Lim C.M. et al. [141] | South Korea | (NAC +) CRS vs. (NAC +) CRS + HIPEC in FIGO III or IV | The addition of HIPEC to CRS does not improve OS and PFS. Anyway, NAC subgroup has a trend of improved survival in favor of HIPEC. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marrelli, D.; Ansaloni, L.; Federici, O.; Asero, S.; Carbone, L.; Marano, L.; Baiocchi, G.; Vaira, M.; Coccolini, F.; Di Giorgio, A.; et al. Cytoreductive Surgery (CRS) and HIPEC for Advanced Ovarian Cancer with Peritoneal Metastases: Italian PSM Oncoteam Evidence and Study Purposes. Cancers 2022, 14, 6010. https://doi.org/10.3390/cancers14236010
Marrelli D, Ansaloni L, Federici O, Asero S, Carbone L, Marano L, Baiocchi G, Vaira M, Coccolini F, Di Giorgio A, et al. Cytoreductive Surgery (CRS) and HIPEC for Advanced Ovarian Cancer with Peritoneal Metastases: Italian PSM Oncoteam Evidence and Study Purposes. Cancers. 2022; 14(23):6010. https://doi.org/10.3390/cancers14236010
Chicago/Turabian StyleMarrelli, Daniele, Luca Ansaloni, Orietta Federici, Salvatore Asero, Ludovico Carbone, Luigi Marano, Gianluca Baiocchi, Marco Vaira, Federico Coccolini, Andrea Di Giorgio, and et al. 2022. "Cytoreductive Surgery (CRS) and HIPEC for Advanced Ovarian Cancer with Peritoneal Metastases: Italian PSM Oncoteam Evidence and Study Purposes" Cancers 14, no. 23: 6010. https://doi.org/10.3390/cancers14236010
APA StyleMarrelli, D., Ansaloni, L., Federici, O., Asero, S., Carbone, L., Marano, L., Baiocchi, G., Vaira, M., Coccolini, F., Di Giorgio, A., Framarini, M., Gelmini, R., Palopoli, C., Accarpio, F., & Fagotti, A. (2022). Cytoreductive Surgery (CRS) and HIPEC for Advanced Ovarian Cancer with Peritoneal Metastases: Italian PSM Oncoteam Evidence and Study Purposes. Cancers, 14(23), 6010. https://doi.org/10.3390/cancers14236010










