Low-Energy X-Ray Intraoperative Radiation Therapy (Lex-IORT) for Resected Brain Metastases: A Single-Institution Experience
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
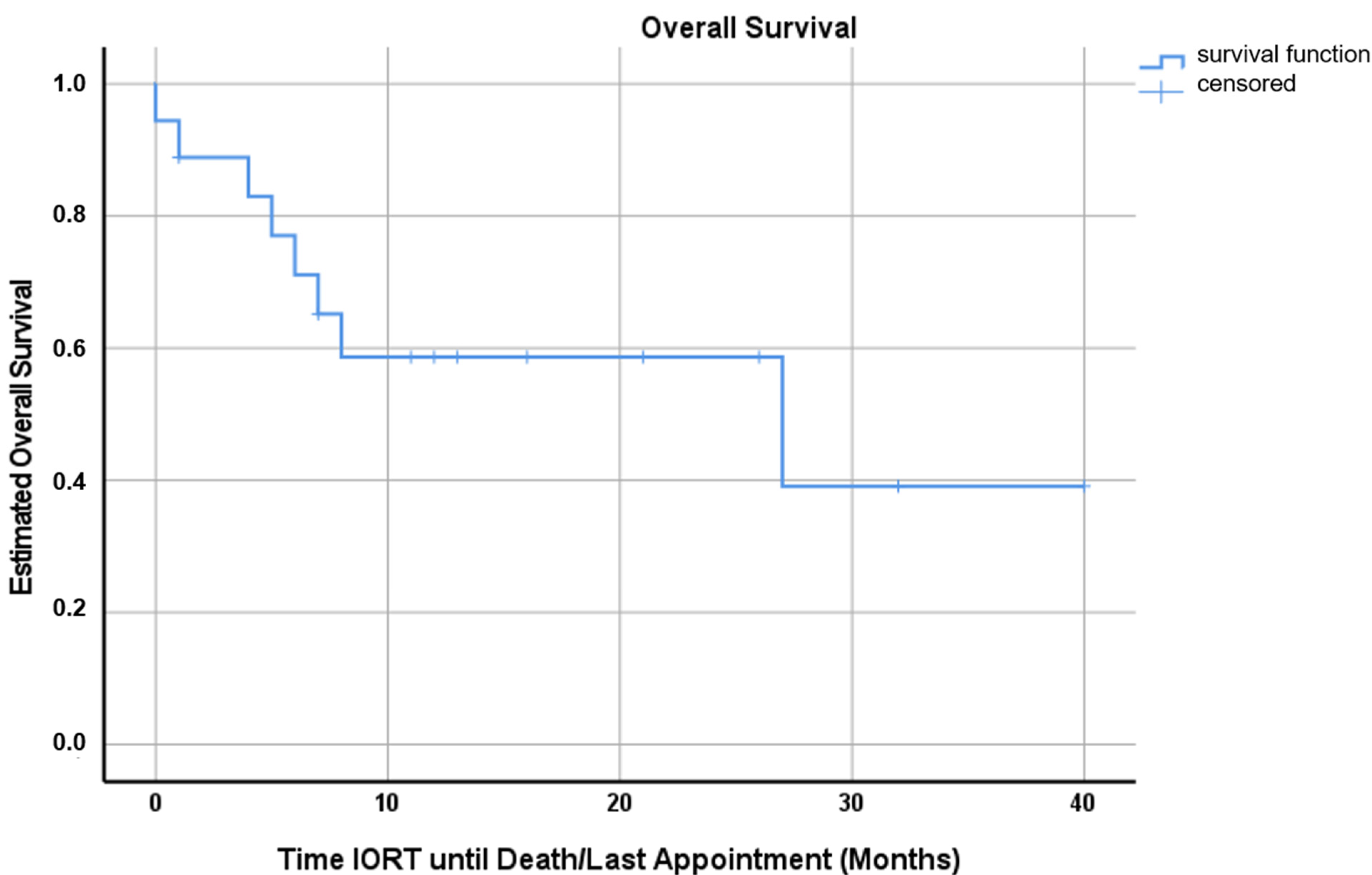
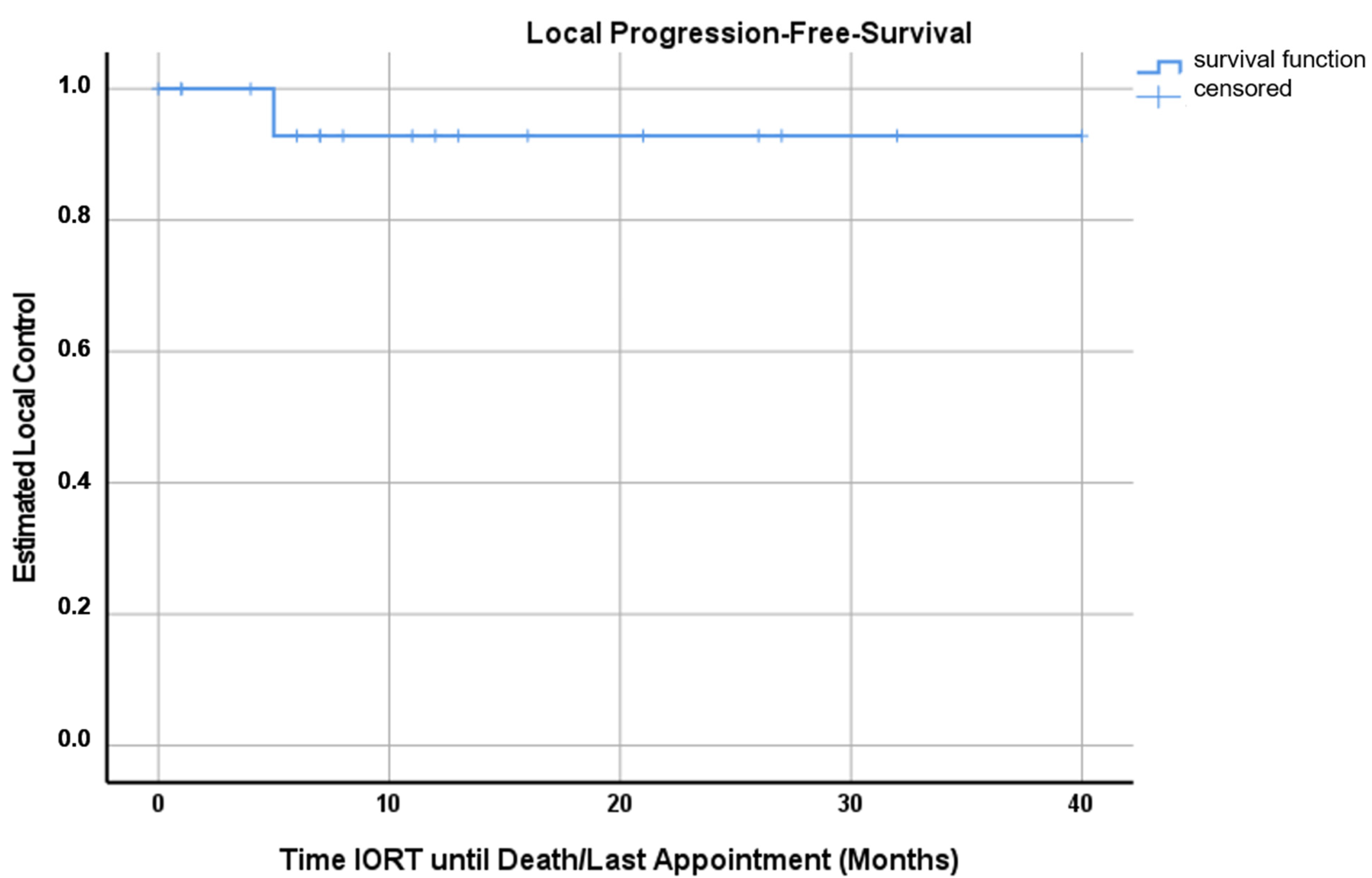
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sperduto, P.W.; Kased, N.; Roberge, D.; Xu, Z.; Shanley, R.; Luo, X.; Sneed, P.K.; Chao, S.T.; Weil, R.J.; Suh, J.; et al. Summary report on the graded prognostic assessment: An accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J. Clin. Oncol. 2012, 30, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Barnholtz-Sloan, J.S.; Yu, C.; Sloan, A.E.; Vengoechea, J.; Wang, M.; Dignam, J.J.; Vogelbaum, M.A.; Sperduto, P.W.; Mehta, M.P.; Machtay, M.; et al. A nomogram for individualized estimation of survival among patients with brain metastasis. Neuro Oncol. 2012, 14, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Schouten, L.J.; Rutten, J.; Huveneers, H.A.; Twijnstra, A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 2002, 94, 2698–2705. [Google Scholar] [CrossRef] [PubMed]
- Scoccianti, S.; Ricardi, U. Treatment of brain metastases: Review of phase III randomized controlled trials. Radiother. Oncol. 2012, 102, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Nugent, J.L.; Bunn, P.A., Jr.; Matthews, M.J.; Ihde, D.C.; Cohen, M.H.; Gazdar, A.; Minna, J.D. CNS metastases in small cell bronchogenic carcinoma: Increasing frequency and changing pattern with lengthening survival. Cancer 1979, 44, 1885–1893. [Google Scholar] [CrossRef]
- Sloan, A.E.; Nock, C.J.; Einstein, D.B. Diagnosis and treatment of melanoma brain metastasis: A literature review. Cancer Control 2009, 16, 248–255. [Google Scholar] [CrossRef]
- Nayak, L.; Lee, E.Q.; Wen, P.Y. Epidemiology of brain metastases. Curr. Oncol. Rep. 2012, 14, 48–54. [Google Scholar] [CrossRef]
- Fox, B.D.; Cheung, V.J.; Patel, A.J.; Suki, D.; Rao, G. Epidemiology of metastatic brain tumors. Neurosurg. Clin. N. Am. 2011, 22, 1–6. [Google Scholar] [CrossRef]
- Hart, M.G.; Grant, R.; Walker, M.; Dickinson, H. Surgical resection and whole brain radiation therapy versus whole brain radiation therapy alone for single brain metastases. Cochrane Database Syst. Rev. 2005, 2005, CD003292. [Google Scholar] [CrossRef]
- Siu, T.L.; Jeffree, R.L.; Fuller, J.W. Current strategies in the surgical management of cerebral metastases: An evidence-based review. J. Clin. Neurosci. 2011, 18, 1429–1434. [Google Scholar] [CrossRef]
- Aftahy, A.K.; Barz, M.; Lange, N.; Baumgart, L.; Thunstedt, C.; Eller, M.A.; Wiestler, B.; Bernhardt, D.; Combs, S.E.; Jost, P.J.; et al. The Impact of Postoperative Tumor Burden on Patients With Brain Metastases. Front. Oncol. 2022, 12, 869764. [Google Scholar] [CrossRef] [PubMed]
- Patchell, R.A.; Tibbs, P.A.; Regine, W.F.; Dempsey, R.J.; Mohiuddin, M.; Kryscio, R.J.; Markesbery, W.R.; Foon, K.A.; Young, B. Postoperative radiotherapy in the treatment of single metastases to the brain: A randomized trial. JAMA 1998, 280, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Prabhu, R.S.; Kandula, S.; Oliver, D.E.; Kim, S.; Hadjipanayis, C.; Olson, J.J.; Oyesiku, N.; Curran, W.J.; Khan, M.K.; et al. Intracranial control and radiographic changes with adjuvant radiation therapy for resected brain metastases: Whole brain radiotherapy versus stereotactic radiosurgery alone. J. Neurooncol. 2014, 120, 657–663. [Google Scholar] [CrossRef]
- Patel, A.J.; Suki, D.; Hatiboglu, M.A.; Rao, V.Y.; Fox, B.D.; Sawaya, R. Impact of surgical methodology on the complication rate and functional outcome of patients with a single brain metastasis. J. Neurosurg. 2015, 122, 1132–1143. [Google Scholar] [CrossRef] [PubMed]
- DeAngelis, L.M.; Mandell, L.R.; Thaler, H.T.; Kimmel, D.W.; Galicich, J.H.; Fuks, Z.; Posner, J.B. The role of postoperative radiotherapy after resection of single brain metastases. Neurosurgery 1989, 24, 798–805. [Google Scholar] [CrossRef]
- Smalley, S.R.; Schray, M.F.; Laws, E.R., Jr.; O’Fallon, J.R. Adjuvant radiation therapy after surgical resection of solitary brain metastasis: Association with pattern of failure and survival. Int. J. Radiat. Oncol. Biol. Phys. 1987, 13, 1611–1616. [Google Scholar] [CrossRef]
- Brown, P.D.; Ballman, K.V.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Whitton, A.C.; Greenspoon, J.; Parney, I.F.; Laack, N.N.I.; Ashman, J.B.; et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC.3): A multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1049–1060. [Google Scholar] [CrossRef]
- Mahajan, A.; Ahmed, S.; McAleer, M.F.; Weinberg, J.S.; Li, J.; Brown, P.; Settle, S.; Prabhu, S.S.; Lang, F.F.; Levine, N.; et al. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: A single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1040–1048. [Google Scholar] [CrossRef]
- Nieder, C.; Astner, S.T.; Grosu, A.L.; Andratschke, N.H.; Molls, M. The role of postoperative radiotherapy after resection of a single brain metastasis. Combined analysis of 643 patients. Strahlenther Onkol. 2007, 183, 576–580. [Google Scholar] [CrossRef]
- Eitz, K.A.; Lo, S.S.; Soliman, H.; Sahgal, A.; Theriault, A.; Pinkham, M.B.; Foote, M.C.; Song, A.J.; Shi, W.; Redmond, K.J.; et al. Multi-institutional Analysis of Prognostic Factors and Outcomes After Hypofractionated Stereotactic Radiotherapy to the Resection Cavity in Patients With Brain Metastases. JAMA Oncol. 2020, 6, 1901–1909. [Google Scholar] [CrossRef]
- Minniti, G.; Esposito, V.; Clarke, E.; Scaringi, C.; Lanzetta, G.; Salvati, M.; Raco, A.; Bozzao, A.; Maurizi Enrici, R. Multidose stereotactic radiosurgery (9 Gy x 3) of the postoperative resection cavity for treatment of large brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Minniti, G.; Scaringi, C.; Paolini, S.; Lanzetta, G.; Romano, A.; Cicone, F.; Osti, M.; Enrici, R.M.; Esposito, V. Single-Fraction Versus Multifraction (3 × 9 Gy) Stereotactic Radiosurgery for Large (>2 cm) Brain Metastases: A Comparative Analysis of Local Control and Risk of Radiation-Induced Brain Necrosis. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Specht, H.M.; Kessel, K.A.; Oechsner, M.; Meyer, B.; Zimmer, C.; Combs, S.E. Hypofractionated stereotactic radiotherapy (H-FSRT) to the resection cavity in patients with brain metastases. Strahlenther Onkol. 2016, in press. [Google Scholar] [CrossRef] [PubMed]
- Atalar, B.; Choi, C.Y.; Harsh, G.R.t.; Chang, S.D.; Gibbs, I.C.; Adler, J.R.; Soltys, S.G. Cavity volume dynamics after resection of brain metastases and timing of postresection cavity stereotactic radiosurgery. Neurosurgery 2013, 72, 180–185; discussion 185. [Google Scholar] [CrossRef]
- Jarvis, L.A.; Simmons, N.E.; Bellerive, M.; Erkmen, K.; Eskey, C.J.; Gladstone, D.J.; Hug, E.B.; Roberts, D.W.; Hartford, A.C. Tumor bed dynamics after surgical resection of brain metastases: Implications for postoperative radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 943–948. [Google Scholar] [CrossRef]
- Scharl, S.; Kirstein, A.; Kessel, K.A.; Diehl, C.; Oechsner, M.; Straube, C.; Meyer, B.; Zimmer, C.; Combs, S.E. Stereotactic irradiation of the resection cavity after surgical resection of brain metastases—When is the right timing? Acta Oncol. 2019, 58, 1714–1719. [Google Scholar] [CrossRef]
- Rogers, S.; Stauffer, A.; Lomax, N.; Alonso, S.; Eberle, B.; Gomez Ordonez, S.; Lazeroms, T.; Kessler, E.; Brendel, M.; Schwyzer, L.; et al. Five fraction stereotactic radiotherapy after brain metastasectomy: A single-institution experience and literature review. J. Neurooncol. 2021, 155, 35–43. [Google Scholar] [CrossRef]
- Kalapurakal, J.A.; Goldman, S.; Stellpflug, W.; Curran, J.; Sathiaseelan, V.; Marymont, M.H.; Tomita, T. Phase I study of intraoperative radiotherapy with photon radiosurgery system in children with recurrent brain tumors: Preliminary report of first dose level (10 Gy). Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 800–808. [Google Scholar] [CrossRef]
- Cifarelli, C.P.; Brehmer, S.; Vargo, J.A.; Hack, J.D.; Kahl, K.H.; Sarria-Vargas, G.; Giordano, F.A. Intraoperative radiotherapy (IORT) for surgically resected brain metastases: Outcome analysis of an international cooperative study. J. Neurooncol. 2019, 145, 391–397. [Google Scholar] [CrossRef]
- Kahl, K.H.; Balagiannis, N.; Hock, M.; Schill, S.; Roushan, Z.; Shiban, E.; Muller, H.; Grossert, U.; Konietzko, I.; Sommer, B.; et al. Intraoperative radiotherapy with low-energy x-rays after neurosurgical resection of brain metastases-an Augsburg University Medical Center experience. Strahlenther Onkol. 2021, 197, 1124–1130. [Google Scholar] [CrossRef]
- Weil, R.J.; Mavinkurve, G.G.; Chao, S.T.; Vogelbaum, M.A.; Suh, J.H.; Kolar, M.; Toms, S.A. Intraoperative radiotherapy to treat newly diagnosed solitary brain metastasis: Initial experience and long-term outcomes. J. Neurosurg. 2015, 122, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, J.P.; Marks, L.B.; Mayo, C.S.; Lawrence, Y.R.; Bhandare, N.; Ryu, S. Estimating normal tissue toxicity in radiosurgery of the CNS: Application and limitations of QUANTEC. J. Radiosurg. SBRT 2011, 1, 95–107. [Google Scholar] [PubMed]
- Lin, N.U.; Lee, E.Q.; Aoyama, H.; Barani, I.J.; Barboriak, D.P.; Baumert, B.G.; Bendszus, M.; Brown, P.D.; Camidge, D.R.; Chang, S.M.; et al. Response assessment criteria for brain metastases: Proposal from the RANO group. Lancet Oncol. 2015, 16, e270–e278. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.A.; Freilich, J.M.; Abuodeh, Y.; Figura, N.; Patel, N.; Sarangkasiri, S.; Chinnaiyan, P.; Yu, H.H.; Etame, A.B.; Rao, N.G. Fractionated stereotactic radiotherapy to the post-operative cavity for radioresistant and radiosensitive brain metastases. J. Neurooncol. 2014, 118, 179–186. [Google Scholar] [CrossRef]
- Choi, C.Y.; Chang, S.D.; Gibbs, I.C.; Adler, J.R.; Harsh, G.R.t.; Lieberson, R.E.; Soltys, S.G. Stereotactic radiosurgery of the postoperative resection cavity for brain metastases: Prospective evaluation of target margin on tumor control. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 336–342. [Google Scholar] [CrossRef]
- Combs, S.E.; Bilger, A.; Diehl, C.; Bretzinger, E.; Lorenz, H.; Oehlke, O.; Specht, H.M.; Kirstein, A.; Grosu, A.L. Multicenter analysis of stereotactic radiotherapy of the resection cavity in patients with brain metastases. Cancer Med. 2018, 7, 2319–2327. [Google Scholar] [CrossRef]
- Eaton, B.R.; Gebhardt, B.; Prabhu, R.; Shu, H.K.; Curran, W.J., Jr.; Crocker, I. Hypofractionated radiosurgery for intact or resected brain metastases: Defining the optimal dose and fractionation. Radiat. Oncol. 2013, 8, 135. [Google Scholar] [CrossRef]
- Steinmann, D.; Maertens, B.; Janssen, S.; Werner, M.; Fruhauf, J.; Nakamura, M.; Christiansen, H.; Bremer, M. Hypofractionated stereotactic radiotherapy (hfSRT) after tumour resection of a single brain metastasis: Report of a single-centre individualized treatment approach. J. Cancer Res. Clin. Oncol. 2012, 138, 1523–1529. [Google Scholar] [CrossRef]
- Gallo, J.; Garimall, S.; Shanker, M.; Castelli, J.; Watkins, T.; Olson, S.; Huo, M.; Foote, M.C.; Pinkham, M.B. Outcomes Following Hypofractionated Stereotactic Radiotherapy to the Cavity After Surgery for Melanoma Brain Metastases. Clin. Oncol. 2022, 34, 179–186. [Google Scholar] [CrossRef]
- Lehrer, E.J.; Peterson, J.L.; Zaorsky, N.G.; Brown, P.D.; Sahgal, A.; Chiang, V.L.; Chao, S.T.; Sheehan, J.P.; Trifiletti, D.M. Single versus Multifraction Stereotactic Radiosurgery for Large Brain Metastases: An International Meta-analysis of 24 Trials. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 618–630. [Google Scholar] [CrossRef]
- Milano, M.T.; Grimm, J.; Niemierko, A.; Soltys, S.G.; Moiseenko, V.; Redmond, K.J.; Yorke, E.; Sahgal, A.; Xue, J.; Mahadevan, A.; et al. Single- and Multifraction Stereotactic Radiosurgery Dose/Volume Tolerances of the Brain. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 68–86. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.; Ojerholm, E.; Hollander, A.; Briola, C.; Mooij, R.; Bieda, M.; Kolker, J.; Nagda, S.; Geiger, G.; Dorsey, J.; et al. Intracranial control after Cyberknife radiosurgery to the resection bed for large brain metastases. Radiat. Oncol. 2015, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- Scharl, S.; Kirstein, A.; Kessel, K.A.; Duma, M.N.; Oechsner, M.; Straube, C.; Combs, S.E. Cavity volume changes after surgery of a brain metastasis-consequences for stereotactic radiation therapy. Strahlenther Onkol. 2019, 195, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Dagnew, E.; Kanski, J.; McDermott, M.W.; Sneed, P.K.; McPherson, C.; Breneman, J.C.; Warnick, R.E. Management of newly diagnosed single brain metastasis using resection and permanent iodine-125 seeds without initial whole-brain radiotherapy: A two institution experience. Neurosurg. Focus 2007, 22, E3. [Google Scholar] [CrossRef]
- Huang, K.; Sneed, P.K.; Kunwar, S.; Kragten, A.; Larson, D.A.; Berger, M.S.; Chan, A.; Pouliot, J.; McDermott, M.W. Surgical resection and permanent iodine-125 brachytherapy for brain metastases. J. Neurooncol. 2009, 91, 83–93. [Google Scholar] [CrossRef]
- Petr, M.J.; McPherson, C.M.; Breneman, J.C.; Warnick, R.E. Management of newly diagnosed single brain metastasis with surgical resection and permanent I-125 seeds without upfront whole brain radiotherapy. J. Neurooncol. 2009, 92, 393–400. [Google Scholar] [CrossRef]
- Raleigh, D.R.; Seymour, Z.A.; Tomlin, B.; Theodosopoulos, P.V.; Berger, M.S.; Aghi, M.K.; Geneser, S.E.; Krishnamurthy, D.; Fogh, S.E.; Sneed, P.K.; et al. Resection and brain brachytherapy with permanent iodine-125 sources for brain metastasis. J. Neurosurg. 2017, 126, 1749–1755. [Google Scholar] [CrossRef]
- Wernicke, A.G.; Hirschfeld, C.B.; Smith, A.W.; Taube, S.; Yondorf, M.Z.; Parashar, B.; Nedialkova, L.; Kulidzhanov, F.; Trichter, S.; Sabbas, A.; et al. Clinical Outcomes of Large Brain Metastases Treated With Neurosurgical Resection and Intraoperative Cesium-131 Brachytherapy: Results of a Prospective Trial. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 1059–1068. [Google Scholar] [CrossRef]
- Goodhead, D.T.; Thacker, J.; Cox, R. Weiss Lecture. Effects of radiations of different qualities on cells: Molecular mechanisms of damage and repair. Int. J. Radiat. Biol. 1993, 63, 543–556. [Google Scholar] [CrossRef]
- Herskind, C.; Steil, V.; Kraus-Tiefenbacher, U.; Wenz, F. Radiobiological aspects of intraoperative radiotherapy (IORT) with isotropic low-energy X rays for early-stage breast cancer. Radiat. Res. 2005, 163, 208–215. [Google Scholar] [CrossRef]
- Liu, Q.; Schneider, F.; Ma, L.; Wenz, F.; Herskind, C. Relative Biologic Effectiveness (RBE) of 50 kV X-rays measured in a phantom for intraoperative tumor-bed irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Bander, E.D.; Yuan, M.; Reiner, A.S.; Panageas, K.S.; Ballangrud, A.M.; Brennan, C.W.; Beal, K.; Tabar, V.; Moss, N.S. Durable 5-year local control for resected brain metastases with early adjuvant SRS: The effect of timing on intended-field control. Neuro Oncol. Pract. 2021, 8, 278–289. [Google Scholar] [CrossRef] [PubMed]
- McDermott, D.M.; Hack, J.D.; Cifarelli, C.P.; Vargo, J.A. Tumor Cavity Recurrence after Stereotactic Radiosurgery of Surgically Resected Brain Metastases: Implication of Deviations from Contouring Guidelines. Ster. Funct. Neurosurg. 2019, 97, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.B.; Amsbaugh, M.J.; Burton, E.; Nelson, M.; Williams, B.; Koutourousiou, M.; Nauta, H.; Woo, S. Increasing time to postoperative stereotactic radiation therapy for patients with resected brain metastases: Investigating clinical outcomes and identifying predictors associated with time to initiation. J. Neurooncol. 2018, 136, 545–553. [Google Scholar] [CrossRef] [PubMed]

| Sex | Age | Primary | KPS | No of BMs | Extracranial Metastases | GPA | Location | Hemisphere |
Largest Diameter (cm) | Dural Attachment | GTR | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ax | cor | sag | ||||||||||||
| 1 | F | 37 | TNBC | 90 | 8 | yes | 2 | occ | R | 5.1 | 4.7 | 5.5 | yes | yes |
| 2 | M | 67 | Melanoma | 80 | 2 | yes | 1 | occ | R | 5.0 | 4.6 | 4.7 | no | yes |
| 3 | F | 55 | NSCLC | 90 | 7 | yes | 1 | par | L | 3.7 | 3.3 | 4.3 | yes | yes |
| 4 | F | 58 | BC | 70 | 15 | yes | 0.5 | front | R | 2.8 | 2.3 | 2.6 | yes | yes |
| 5 | M | 19 | Osteosarcoma | 80 | 1 | yes | 2.5 | par | L | 2.5 | 2.6 | 2.7 | yes | yes |
| 6 | F | 72 | NSCLC | 70 | 1 | no | 2.5 | par | L | 1.3 | 1.2 | 1.1 | no | yes |
| 7 | F | 74 | RCC | 80 | 1 | yes | 1.5 | front | L | 2.9 | 2.4 | 2.5 | no | yes |
| 8 | F | 51 | Pancreatic Cancer | 100 | 1 | yes | 3 | par | R | 2.5 | 2.4 | 2.4 | yes | yes |
| 9 | F | 54 | NSCLC | 100 | 1 | no | 4 | par | R | 3.7 | 3.3 | 3.8 | no | yes |
| 10 | F | 43 | Melanoma | 90 | 1 | yes | 3 | occ | L | 3 | 2.8 | 3.3 | yes | yes |
| 11 | M | 72 | NSCLC | 70 | 4 | yes | 0.5 | front | L | 2.6 | 2.2 | 2.5 | no | yes |
| 12 | F | 68 | NSCLC | 70 | 2 | yes | 1 | par | L | 3.3 | 2.9 | 3.6 | yes | yes |
| 13 | M | 55 | Melanoma | 100 | 1 | no | 4 | temp | R | 4.5 | 3.5 | 4.1 | yes | yes |
| 14 | M | 55 | Urothelial Cancer | 90 | 1 | yes | 4 | front | L | 2.5 | 3.1 | 3.1 | no | yes |
| 15 | F | 45 | Melanoma | 50 | 5 | no | 2 | front | L | 1.3 | 1.7 | 1.6 | yes | yes |
| 16 | M | 71 | Rectal Cancer | 90 | 2 | no | 2.5 | temp | L | 2.9 | 2.3 | 2.0 | no | yes |
| 17 | M | 55 | RCC | 100 | 1 | yes | 3 | par | L | 2.4 | 2.9 | 2.7 | yes | yes |
| 18 | M | 58 | NSCLC | 80 | 8 | yes | 2.5 | front | L | 2.6 | 2.9 | 2.6 | yes | yes |
| Patient | Applicator Size (cm) | Dose (Gy) | Time (min:s) | postOP RTx | Second Course RTx | ||
|---|---|---|---|---|---|---|---|
| WBRT | SRS | hFSRT (Cavity) | |||||
| 1 | 4 | 20 | 24:10 | 10 × 3 Gy | - | - | SRS (2x) |
| 2 | 3.5 | 20 | 17:59 | - | - | - | - |
| 3 | 2.5 | 20 | 18:10 | 10 × 3 Gy | - | - | WBRT |
| 4 | 2 | 20 | 12:00 | 10 × 3 Gy | - | - | - |
| 5 | 2 | 20 | 11:28 | - | - | - | - |
| 6 | 1.5 | 25 | 07:14 | - | 20 Gy | - | - |
| 7 | 2.5 | 25 | 22:14 | - | - | - | SRS |
| 8 | 2 | 20 | 12:11 | - | - | - | - |
| 9 | 2.5 | 25 | 22:19 | - | - | - | - |
| 10 | 2 | 20 | 11:55 | - | - | - | SRS |
| 11 | 2 | 25 | 14:14 | - | 20 Gy (3x) | - | - |
| 12 | 2.5 | 25 | 22:41 | 10 × 3 Gy | - | - | - |
| 13 | 2.5 | 20 | 08:17 | - | - | - | - |
| 14 | 1.5 | 30 | 10:41 | - | - | - | - |
| 15 | 1.5 | 20 | 05:32 | 10 × 3 Gy | 20 Gy (2x) | - | - |
| 16 | 2.5 | 16 | 18:02 | - | 20 Gy | 8 × 3 Gy | SRS (2x) |
| 17 | 2 | 16 | 09:35 | - | - | 10 × 3 Gy | - |
| 18 | 2 | 16 | 09:17 | - | - | 6 × 5 Gy | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diehl, C.D.; Pigorsch, S.U.; Gempt, J.; Krieg, S.M.; Reitz, S.; Waltenberger, M.; Barz, M.; Meyer, H.S.; Wagner, A.; Wilkens, J.; et al. Low-Energy X-Ray Intraoperative Radiation Therapy (Lex-IORT) for Resected Brain Metastases: A Single-Institution Experience. Cancers 2023, 15, 14. https://doi.org/10.3390/cancers15010014
Diehl CD, Pigorsch SU, Gempt J, Krieg SM, Reitz S, Waltenberger M, Barz M, Meyer HS, Wagner A, Wilkens J, et al. Low-Energy X-Ray Intraoperative Radiation Therapy (Lex-IORT) for Resected Brain Metastases: A Single-Institution Experience. Cancers. 2023; 15(1):14. https://doi.org/10.3390/cancers15010014
Chicago/Turabian StyleDiehl, Christian D., Steffi U. Pigorsch, Jens Gempt, Sandro M. Krieg, Silvia Reitz, Maria Waltenberger, Melanie Barz, Hanno S. Meyer, Arthur Wagner, Jan Wilkens, and et al. 2023. "Low-Energy X-Ray Intraoperative Radiation Therapy (Lex-IORT) for Resected Brain Metastases: A Single-Institution Experience" Cancers 15, no. 1: 14. https://doi.org/10.3390/cancers15010014
APA StyleDiehl, C. D., Pigorsch, S. U., Gempt, J., Krieg, S. M., Reitz, S., Waltenberger, M., Barz, M., Meyer, H. S., Wagner, A., Wilkens, J., Wiestler, B., Zimmer, C., Meyer, B., & Combs, S. E. (2023). Low-Energy X-Ray Intraoperative Radiation Therapy (Lex-IORT) for Resected Brain Metastases: A Single-Institution Experience. Cancers, 15(1), 14. https://doi.org/10.3390/cancers15010014







