Dose Reduction to Motor Structures in Adjuvant Fractionated Stereotactic Radiotherapy of Brain Metastases: nTMS-Derived DTI-Based Motor Fiber Tracking in Treatment Planning
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Navigated Transcranial Magnetic Stimulation
2.3. Diffusion Tensor Imaging Fiber Tracking
2.4. Elastic Image Fusion
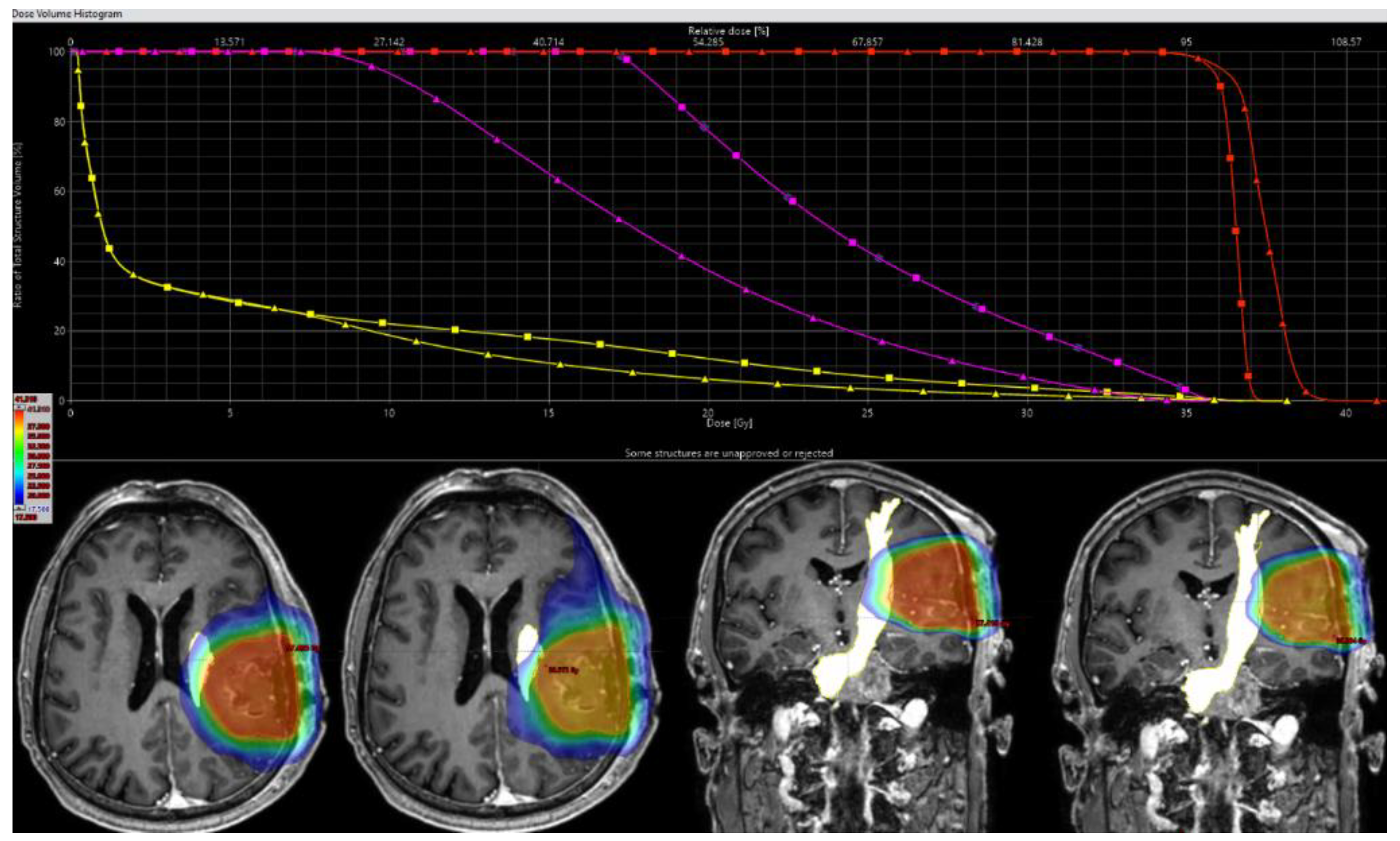
2.5. Target Delineation and Radiotherapy Planning
2.6. Dosimetric Analysis and Comparative Evaluation
2.7. Statistical Analyses
3. Results
3.1. Patient Characteristics
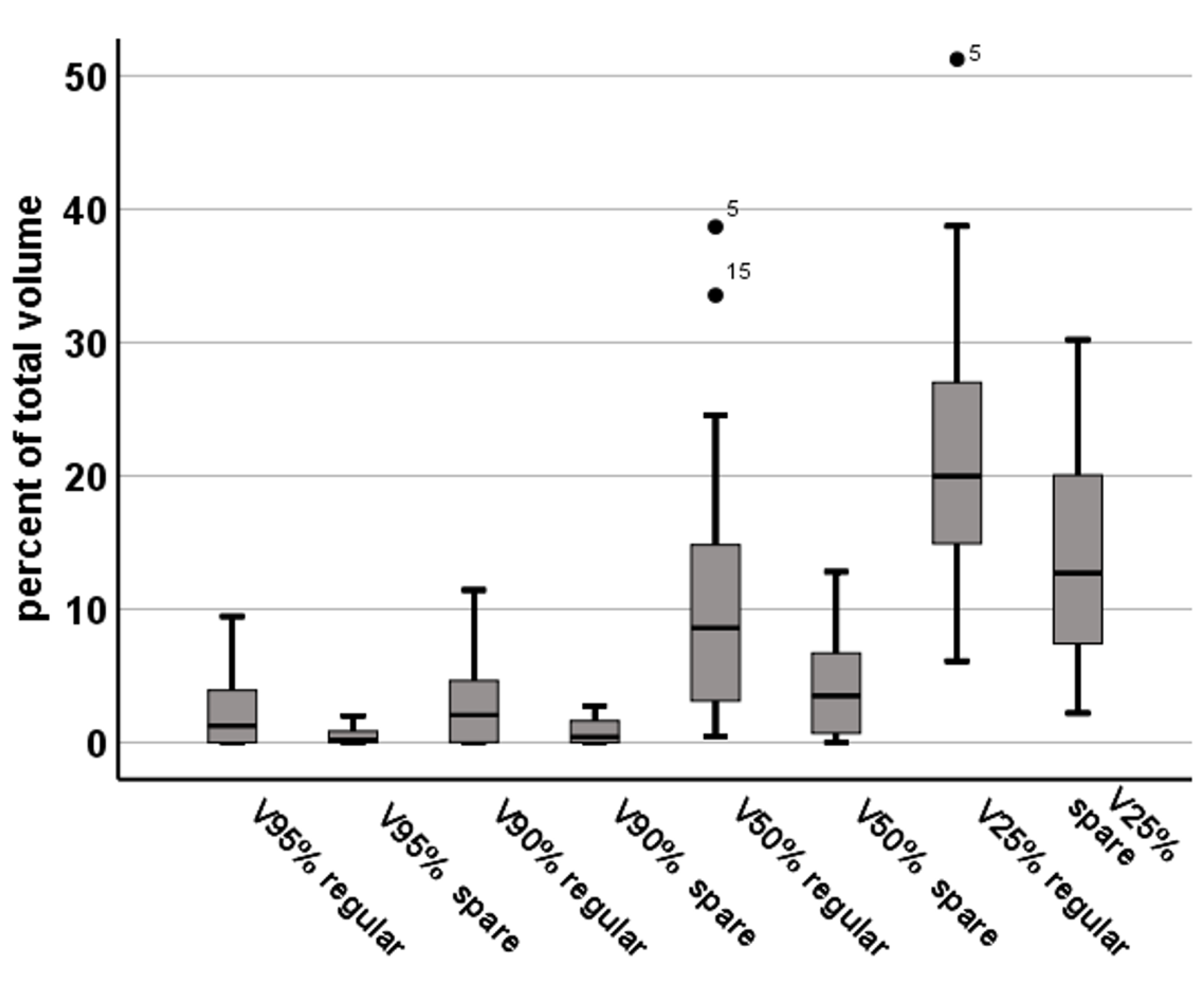
3.2. FTmot.TMS Dose Statistics
3.3. PTV Coverage, Conformity, and Outcome
3.4. Clinical Outcome Data and Follow-Up
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fox, B.D.; Cheung, V.J.; Patel, A.J.; Suki, D.; Rao, G. Epidemiology of metastatic brain tumors. Neurosurg. Clin. N. Am. 2011, 22, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cagney, D.N.; Martin, A.M.; Catalano, P.J.; Redig, A.J.; Lin, N.U.; Lee, E.Q.; Wen, P.Y.; Dunn, I.F.; Bi, W.L.; Weiss, S.E.; et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: A population-based study. Neuro-oncology 2017, 19, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- Lamba, N.; Wen, P.Y.; Aizer, A.A. Epidemiology of brain metastases and leptomeningeal disease. Neuro-oncology 2021, 23, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Sperduto, P.W.; Mesko, S.; Li, J.; Cagney, D.; Aizer, A.; Lin, N.U.; Nesbit, E.; Kruser, T.J.; Chan, J.; Braunstein, S.; et al. Survival in Patients With Brain Metastases: Summary Report on the Updated Diagnosis-Specific Graded Prognostic Assessment and Definition of the Eligibility Quotient. J. Clin. Oncol. 2020, 38, 3773–3784. [Google Scholar] [CrossRef]
- Hart, M.G.; Grant, R.; Walker, M.; Dickinson, H. Surgical resection and whole brain radiation therapy versus whole brain radiation therapy alone for single brain metastases. Cochrane Database Syst. Rev. 2005, 2005, CD003292. [Google Scholar] [CrossRef]
- Siu, T.L.; Jeffree, R.L.; Fuller, J.W. Current strategies in the surgical management of cerebral metastases: An evidence-based review. J. Clin. Neurosci. 2011, 18, 1429–1434. [Google Scholar] [CrossRef]
- Haddad, A.F.; Young, J.S.; Berger, M.S.; Tarapore, P.E. Preoperative Applications of Navigated Transcranial Magnetic Stimulation. Front. Neurol. 2020, 11, 628903. [Google Scholar] [CrossRef]
- Sollmann, N.; Krieg, S.M.; Saisanen, L.; Julkunen, P. Mapping of Motor Function with Neuronavigated Transcranial Magnetic Stimulation: A Review on Clinical Application in Brain Tumors and Methods for Ensuring Feasible Accuracy. Brain Sci. 2021, 11, 897. [Google Scholar] [CrossRef]
- Bulubas, L.; Sollmann, N.; Tanigawa, N.; Zimmer, C.; Meyer, B.; Krieg, S.M. Reorganization of Motor Representations in Patients with Brain Lesions: A Navigated Transcranial Magnetic Stimulation Study. Brain Topogr. 2018, 31, 288–299. [Google Scholar] [CrossRef]
- Conway, N.; Wildschuetz, N.; Moser, T.; Bulubas, L.; Sollmann, N.; Tanigawa, N.; Meyer, B.; Krieg, S.M. Cortical plasticity of motor-eloquent areas measured by navigated transcranial magnetic stimulation in patients with glioma. J. Neurosurg. 2017, 127, 981–991. [Google Scholar] [CrossRef]
- Picht, T.; Mularski, S.; Kuehn, B.; Vajkoczy, P.; Kombos, T.; Suess, O. Navigated transcranial magnetic stimulation for preoperative functional diagnostics in brain tumor surgery. Neurosurgery 2009, 65, 93–98, discussion 98–99. [Google Scholar] [CrossRef]
- Takahashi, S.; Jussen, D.; Vajkoczy, P.; Picht, T. Plastic relocation of motor cortex in a patient with LGG (low grade glioma) confirmed by NBS (navigated brain stimulation). Acta Neurochir. (Wien) 2012, 154, 2003–2008, discussion 2008. [Google Scholar] [CrossRef]
- Frey, D.; Strack, V.; Wiener, E.; Jussen, D.; Vajkoczy, P.; Picht, T. A new approach for corticospinal tract reconstruction based on navigated transcranial stimulation and standardized fractional anisotropy values. Neuroimage 2012, 62, 1600–1609. [Google Scholar] [CrossRef]
- Sollmann, N.; Wildschuetz, N.; Kelm, A.; Conway, N.; Moser, T.; Bulubas, L.; Kirschke, J.S.; Meyer, B.; Krieg, S.M. Associations between clinical outcome and navigated transcranial magnetic stimulation characteristics in patients with motor-eloquent brain lesions: A combined navigated transcranial magnetic stimulation-diffusion tensor imaging fiber tracking approach. J. Neurosurg. 2018, 128, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Sollmann, N.; Zhang, H.; Fratini, A.; Wildschuetz, N.; Ille, S.; Schroder, A.; Zimmer, C.; Meyer, B.; Krieg, S.M. Risk Assessment by Presurgical Tractography Using Navigated TMS Maps in Patients with Highly Motor- or Language-Eloquent Brain Tumors. Cancers (Basel) 2020, 12, 1264. [Google Scholar] [CrossRef] [PubMed]
- Krieg, S.M.; Sollmann, N.; Obermueller, T.; Sabih, J.; Bulubas, L.; Negwer, C.; Moser, T.; Droese, D.; Boeckh-Behrens, T.; Ringel, F.; et al. Changing the clinical course of glioma patients by preoperative motor mapping with navigated transcranial magnetic brain stimulation. BMC Cancer 2015, 15, 231. [Google Scholar] [CrossRef]
- Sollmann, N.; Ille, S.; Hauck, T.; Maurer, S.; Negwer, C.; Zimmer, C.; Ringel, F.; Meyer, B.; Krieg, S.M. The impact of preoperative language mapping by repetitive navigated transcranial magnetic stimulation on the clinical course of brain tumor patients. BMC Cancer 2015, 15, 261. [Google Scholar] [CrossRef]
- Tarapore, P.E.; Tate, M.C.; Findlay, A.M.; Honma, S.M.; Mizuiri, D.; Berger, M.S.; Nagarajan, S.S. Preoperative multimodal motor mapping: A comparison of magnetoencephalography imaging, navigated transcranial magnetic stimulation, and direct cortical stimulation. J. Neurosurg. 2012, 117, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Patchell, R.A.; Tibbs, P.A.; Regine, W.F.; Dempsey, R.J.; Mohiuddin, M.; Kryscio, R.J.; Markesbery, W.R.; Foon, K.A.; Young, B. Postoperative radiotherapy in the treatment of single metastases to the brain: A randomized trial. JAMA 1998, 280, 1485–1489. [Google Scholar] [CrossRef]
- Patel, K.R.; Prabhu, R.S.; Kandula, S.; Oliver, D.E.; Kim, S.; Hadjipanayis, C.; Olson, J.J.; Oyesiku, N.; Curran, W.J.; Khan, M.K.; et al. Intracranial control and radiographic changes with adjuvant radiation therapy for resected brain metastases: Whole brain radiotherapy versus stereotactic radiosurgery alone. J. Neurooncol. 2014, 120, 657–663. [Google Scholar] [CrossRef]
- Lunsford, L.D.; Khan, A.A.; Niranjan, A.; Kano, H.; Flickinger, J.C.; Kondziolka, D. Stereotactic radiosurgery for symptomatic solitary cerebral cavernous malformations considered high risk for resection. J. Neurosurg. 2010, 113, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Minniti, G.; Clarke, E.; Lanzetta, G.; Osti, M.F.; Trasimeni, G.; Bozzao, A.; Romano, A.; Enrici, R.M. Stereotactic radiosurgery for brain metastases: Analysis of outcome and risk of brain radionecrosis. Radiat. Oncol. 2011, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Salans, M.; Tibbs, M.D.; Karunamuni, R.; Yip, A.; Huynh-Le, M.P.; Macari, A.C.; Reyes, A.; Tringale, K.; McDonald, C.R.; Hattangadi-Gluth, J.A. Longitudinal change in fine motor skills after brain radiotherapy and in vivo imaging biomarkers associated with decline. Neuro-oncology 2021, 23, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Scoccianti, S.; Detti, B.; Gadda, D.; Greto, D.; Furfaro, I.; Meacci, F.; Simontacchi, G.; Di Brina, L.; Bonomo, P.; Giacomelli, I.; et al. Organs at risk in the brain and their dose-constraints in adults and in children: A radiation oncologist’s guide for delineation in everyday practice. Radiother. Oncol. 2015, 114, 230–238. [Google Scholar] [CrossRef]
- Kirkpatrick, J.P.; Marks, L.B.; Mayo, C.S.; Lawrence, Y.R.; Bhandare, N.; Ryu, S. Estimating normal tissue toxicity in radiosurgery of the CNS: Application and limitations of QUANTEC. J. Radiosurg. SBRT 2011, 1, 95–107. [Google Scholar]
- Milano, M.T.; Grimm, J.; Niemierko, A.; Soltys, S.G.; Moiseenko, V.; Redmond, K.J.; Yorke, E.; Sahgal, A.; Xue, J.; Mahadevan, A.; et al. Single- and Multifraction Stereotactic Radiosurgery Dose/Volume Tolerances of the Brain. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 68–86. [Google Scholar] [CrossRef]
- Niyazi, M.; Brada, M.; Chalmers, A.J.; Combs, S.E.; Erridge, S.C.; Fiorentino, A.; Grosu, A.L.; Lagerwaard, F.J.; Minniti, G.; Mirimanoff, R.O.; et al. ESTRO-ACROP guideline "target delineation of glioblastomas". Radiother. Oncol. 2016, 118, 35–42. [Google Scholar] [CrossRef]
- Soliman, H.; Ruschin, M.; Angelov, L.; Brown, P.D.; Chiang, V.L.S.; Kirkpatrick, J.P.; Lo, S.S.; Mahajan, A.; Oh, K.S.; Sheehan, J.P.; et al. Consensus Contouring Guidelines for Postoperative Completely Resected Cavity Stereotactic Radiosurgery for Brain Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 436–442. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Berkey, B.; Gaspar, L.E.; Mehta, M.; Curran, W. A new prognostic index and comparison to three other indices for patients with brain metastases: An analysis of 1,960 patients in the RTOG database. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 510–514. [Google Scholar] [CrossRef]
- Krieg, S.M.; Lioumis, P.; Makela, J.P.; Wilenius, J.; Karhu, J.; Hannula, H.; Savolainen, P.; Lucas, C.W.; Seidel, K.; Laakso, A.; et al. Protocol for motor and language mapping by navigated TMS in patients and healthy volunteers; workshop report. Acta Neurochir. (Wien) 2017, 159, 1187–1195. [Google Scholar] [CrossRef]
- Saisanen, L.; Julkunen, P.; Niskanen, E.; Danner, N.; Hukkanen, T.; Lohioja, T.; Nurkkala, J.; Mervaala, E.; Karhu, J.; Kononen, M. Motor potentials evoked by navigated transcranial magnetic stimulation in healthy subjects. J. Clin. Neurophysiol. 2008, 25, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Sollmann, N.; Bulubas, L.; Tanigawa, N.; Zimmer, C.; Meyer, B.; Krieg, S.M. The variability of motor evoked potential latencies in neurosurgical motor mapping by preoperative navigated transcranial magnetic stimulation. BMC Neurosci. 2017, 18, 5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Krieg, S.M.; Buchmann, N.H.; Gempt, J.; Shiban, E.; Meyer, B.; Ringel, F. Diffusion tensor imaging fiber tracking using navigated brain stimulation--a feasibility study. Acta Neurochir. (Wien) 2012, 154, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Sollmann, N.; Meyer, B.; Krieg, S.M. Implementing Functional Preoperative Mapping in the Clinical Routine of a Neurosurgical Department: Technical Note. World Neurosurg. 2017, 103, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, J.; Sollmann, N.; Hiepe, P.; Kirschke, J.S.; Meyer, B.; Krieg, S.M.; Ringel, F. Retrospective distortion correction of diffusion tensor imaging data by semi-elastic image fusion—Evaluation by means of anatomical landmarks. Clin. Neurol. Neurosurg. 2019, 183, 105387. [Google Scholar] [CrossRef]
- Ille, S.; Schroeder, A.; Wagner, A.; Negwer, C.; Kreiser, K.; Meyer, B.; Krieg, S.M. Intraoperative MRI-based elastic fusion for anatomically accurate tractography of the corticospinal tract: Correlation with intraoperative neuromonitoring and clinical status. Neurosurg. Focus 2021, 50, E9. [Google Scholar] [CrossRef]
- Ille, S.; Schwendner, M.; Zhang, W.; Schroeder, A.; Meyer, B.; Krieg, S.M. Tractography for Subcortical Resection of Gliomas Is Highly Accurate for Motor and Language Function: ioMRI-Based Elastic Fusion Disproves the Severity of Brain Shift. Cancers (Basel) 2021, 13, 1787. [Google Scholar] [CrossRef]
- Rosenstock, T.; Grittner, U.; Acker, G.; Schwarzer, V.; Kulchytska, N.; Vajkoczy, P.; Picht, T. Risk stratification in motor area-related glioma surgery based on navigated transcranial magnetic stimulation data. J. Neurosurg. 2017, 126, 1227–1237. [Google Scholar] [CrossRef]
- Connor, M.; Karunamuni, R.; McDonald, C.; White, N.; Pettersson, N.; Moiseenko, V.; Seibert, T.; Marshall, D.; Cervino, L.; Bartsch, H.; et al. Dose-dependent white matter damage after brain radiotherapy. Radiother. Oncol. 2016, 121, 209–216. [Google Scholar] [CrossRef]
- Karunamuni, R.; Bartsch, H.; White, N.S.; Moiseenko, V.; Carmona, R.; Marshall, D.C.; Seibert, T.M.; McDonald, C.R.; Farid, N.; Krishnan, A.; et al. Dose-Dependent Cortical Thinning After Partial Brain Irradiation in High-Grade Glioma. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 297–304. [Google Scholar] [CrossRef]
- El Shafie, R.A.; Tonndorf-Martini, E.; Schmitt, D.; Weber, D.; Celik, A.; Dresel, T.; Bernhardt, D.; Lang, K.; Hoegen, P.; Adeberg, S.; et al. Pre-Operative Versus Post-Operative Radiosurgery of Brain Metastases-Volumetric and Dosimetric Impact of Treatment Sequence and Margin Concept. Cancers (Basel) 2019, 11, 294. [Google Scholar] [CrossRef] [PubMed]
- Lomax, N.J.; Scheib, S.G. Quantifying the degree of conformity in radiosurgery treatment planning. Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 1409–1419. [Google Scholar] [CrossRef] [PubMed]
- Paddick, I. A simple scoring ratio to index the conformity of radiosurgical treatment plans. Technical note. J. Neurosurg. 2000, 93 (Suppl. 3), 219–222. [Google Scholar] [CrossRef] [PubMed]
- Krieg, S.M.; Shiban, E.; Buchmann, N.; Gempt, J.; Foerschler, A.; Meyer, B.; Ringel, F. Utility of presurgical navigated transcranial magnetic brain stimulation for the resection of tumors in eloquent motor areas. J. Neurosurg. 2012, 116, 994–1001. [Google Scholar] [CrossRef]
- Lin, N.U.; Lee, E.Q.; Aoyama, H.; Barani, I.J.; Barboriak, D.P.; Baumert, B.G.; Bendszus, M.; Brown, P.D.; Camidge, D.R.; Chang, S.M.; et al. Response assessment criteria for brain metastases: Proposal from the RANO group. Lancet Oncol. 2015, 16, e270–e278. [Google Scholar] [CrossRef]
- Ferini, G.; Viola, A.; Valenti, V.; Tripoli, A.; Molino, L.; Marchese, V.A.; Illari, S.I.; Rita Borzi, G.; Prestifilippo, A.; Umana, G.E.; et al. Whole Brain Irradiation or Stereotactic RadioSurgery for five or more brain metastases (WHOBI-STER): A prospective comparative study of neurocognitive outcomes, level of autonomy in daily activities and quality of life. Clin. Transl. Radiat. Oncol. 2022, 32, 52–58. [Google Scholar] [CrossRef]
- Gagliano, A.; Prestifilippo, A.; Cantale, O.; Ferini, G.; Fisichella, G.; Fontana, P.; Sciacca, D.; Giuffrida, D. Role of the Combination of Cyclin-Dependent Kinase Inhibitors (CDKI) and Radiotherapy (RT) in the Treatment of Metastatic Breast Cancer (MBC): Advantages and Risks in Clinical Practice. Front. Oncol. 2021, 11, 643155. [Google Scholar] [CrossRef]
- Le Rhun, E.; Guckenberger, M.; Smits, M.; Dummer, R.; Bachelot, T.; Sahm, F.; Galldiks, N.; de Azambuja, E.; Berghoff, A.S.; Metellus, P.; et al. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with brain metastasis from solid tumours. Ann. Oncol. 2021, 32, 1332–1347. [Google Scholar] [CrossRef]
- Noordijk, E.M.; Vecht, C.J.; Haaxma-Reiche, H.; Padberg, G.W.; Voormolen, J.H.; Hoekstra, F.H.; Tans, J.T.; Lambooij, N.; Metsaars, J.A.; Wattendorff, A.R.; et al. The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. Int. J. Radiat. Oncol. Biol. Phys. 1994, 29, 711–717. [Google Scholar] [CrossRef]
- Patchell, R.A.; Tibbs, P.A.; Walsh, J.W.; Dempsey, R.J.; Maruyama, Y.; Kryscio, R.J.; Markesbery, W.R.; Macdonald, J.S.; Young, B. A randomized trial of surgery in the treatment of single metastases to the brain. N. Engl. J. Med. 1990, 322, 494–500. [Google Scholar] [CrossRef]
- Kamp, M.A.; Rapp, M.; Slotty, P.J.; Turowski, B.; Sadat, H.; Smuga, M.; Dibue-Adjei, M.; Steiger, H.J.; Szelenyi, A.; Sabel, M. Incidence of local in-brain progression after supramarginal resection of cerebral metastases. Acta Neurochir. (Wien) 2015, 157, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Kim, Y.Z.; Nam, B.H.; Shin, S.H.; Yang, H.S.; Lee, J.S.; Zo, J.I.; Lee, S.H. Reduced local recurrence of a single brain metastasis through microscopic total resection. J. Neurosurg. 2009, 110, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.J.; Suki, D.; Hatiboglu, M.A.; Rao, V.Y.; Fox, B.D.; Sawaya, R. Impact of surgical methodology on the complication rate and functional outcome of patients with a single brain metastasis. J. Neurosurg. 2015, 122, 1132–1143. [Google Scholar] [CrossRef] [PubMed]
- Bassaganyas-Vancells, C.; Roldan, P.; Gonzalez, J.J.; Ferres, A.; Garcia, S.; Culebras, D.; Hoyos, J.; Reyes, L.; Torales, J.; Ensenat, J. Combined Use of 5-Aminolevulinic Acid and Intraoperative Low-Field Magnetic Resonance Imaging in High-Grade Glioma Surgery. World Neurosurg. 2019, 130, e206–e212. [Google Scholar] [CrossRef]
- Carl, B.; Bopp, M.; Sass, B.; Pojskic, M.; Gjorgjevski, M.; Voellger, B.; Nimsky, C. Reliable navigation registration in cranial and spine surgery based on intraoperative computed tomography. Neurosurg. Focus 2019, 47, E11. [Google Scholar] [CrossRef] [PubMed]
- Giammalva, G.R.; Ferini, G.; Musso, S.; Salvaggio, G.; Pino, M.A.; Gerardi, R.M.; Brunasso, L.; Costanzo, R.; Paolini, F.; Di Bonaventura, R.; et al. Intraoperative Ultrasound: Emerging Technology and Novel Applications in Brain Tumor Surgery. Front. Oncol. 2022, 12, 818446. [Google Scholar] [CrossRef]
- Krieg, S.M.; Shiban, E.; Buchmann, N.; Meyer, B.; Ringel, F. Presurgical navigated transcranial magnetic brain stimulation for recurrent gliomas in motor eloquent areas. Clin. Neurophysiol. 2013, 124, 522–527. [Google Scholar] [CrossRef]
- Giampiccolo, D.; Howells, H.; Bahrend, I.; Schneider, H.; Raffa, G.; Rosenstock, T.; Vergani, F.; Vajkoczy, P.; Picht, T. Preoperative transcranial magnetic stimulation for picture naming is reliable in mapping segments of the arcuate fasciculus. Brain Commun. 2020, 2, fcaa158. [Google Scholar] [CrossRef]
- Umana, G.E.; Scalia, G.; Graziano, F.; Maugeri, R.; Alberio, N.; Barone, F.; Crea, A.; Fagone, S.; Giammalva, G.R.; Brunasso, L.; et al. Navigated Transcranial Magnetic Stimulation Motor Mapping Usefulness in the Surgical Management of Patients Affected by Brain Tumors in Eloquent Areas: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12, 644198. [Google Scholar] [CrossRef]
- Nahed, B.V.; Alvarez-Breckenridge, C.; Brastianos, P.K.; Shih, H.; Sloan, A.; Ammirati, M.; Kuo, J.S.; Ryken, T.C.; Kalkanis, S.N.; Olson, J.J. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guidelines on the Role of Surgery in the Management of Adults With Metastatic Brain Tumors. Neurosurgery 2019, 84, E152–E155. [Google Scholar] [CrossRef]
- Suki, D.; Abouassi, H.; Patel, A.J.; Sawaya, R.; Weinberg, J.S.; Groves, M.D. Comparative risk of leptomeningeal disease after resection or stereotactic radiosurgery for solid tumor metastasis to the posterior fossa. J. Neurosurg. 2008, 108, 248–257. [Google Scholar] [CrossRef]
- Suki, D.; Hatiboglu, M.A.; Patel, A.J.; Weinberg, J.S.; Groves, M.D.; Mahajan, A.; Sawaya, R. Comparative risk of leptomeningeal dissemination of cancer after surgery or stereotactic radiosurgery for a single supratentorial solid tumor metastasis. Neurosurgery 2009, 64, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.A.; Freilich, J.M.; Abuodeh, Y.; Figura, N.; Patel, N.; Sarangkasiri, S.; Chinnaiyan, P.; Yu, H.H.; Etame, A.B.; Rao, N.G. Fractionated stereotactic radiotherapy to the post-operative cavity for radioresistant and radiosensitive brain metastases. J. Neurooncol. 2014, 118, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Eitz, K.A.; Lo, S.S.; Soliman, H.; Sahgal, A.; Theriault, A.; Pinkham, M.B.; Foote, M.C.; Song, A.J.; Shi, W.; Redmond, K.J.; et al. Multi-institutional Analysis of Prognostic Factors and Outcomes After Hypofractionated Stereotactic Radiotherapy to the Resection Cavity in Patients With Brain Metastases. JAMA Oncol. 2020, 6, 1901–1909. [Google Scholar] [CrossRef]
- Lehrer, E.J.; Peterson, J.L.; Zaorsky, N.G.; Brown, P.D.; Sahgal, A.; Chiang, V.L.; Chao, S.T.; Sheehan, J.P.; Trifiletti, D.M. Single versus Multifraction Stereotactic Radiosurgery for Large Brain Metastases: An International Meta-analysis of 24 Trials. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Minniti, G.; Esposito, V.; Clarke, E.; Scaringi, C.; Lanzetta, G.; Salvati, M.; Raco, A.; Bozzao, A.; Maurizi Enrici, R. Multidose stereotactic radiosurgery (9 Gy x 3) of the postoperative resection cavity for treatment of large brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 623–629. [Google Scholar] [CrossRef]
- Gorbunov, N.V.; Kiang, J.G. Brain Damage and Patterns of Neurovascular Disorder after Ionizing Irradiation. Complications in Radiotherapy and Radiation Combined Injury. Radiat. Res. 2021, 196, 1–16. [Google Scholar] [CrossRef]
- Assaf, Y.; Pasternak, O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: A review. J. Mol. Neurosci. 2008, 34, 51–61. [Google Scholar] [CrossRef]
- Basser, P.J.; Mattiello, J.; LeBihan, D. MR diffusion tensor spectroscopy and imaging. Biophys. J. 1994, 66, 259–267. [Google Scholar] [CrossRef]
- Huynh-Le, M.P.; Tibbs, M.D.; Karunamuni, R.; Salans, M.; Tringale, K.R.; Yip, A.; Connor, M.; Simon, A.B.; Vitzthum, L.K.; Reyes, A.; et al. Microstructural Injury to Corpus Callosum and Intrahemispheric White Matter Tracts Correlate With Attention and Processing Speed Decline After Brain Radiation. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 337–347. [Google Scholar] [CrossRef]
- Feuvret, L.; Noel, G.; Mazeron, J.J.; Bey, P. Conformity index: A review. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Papp, J.; Csiki, E.; Kovacs, A. Plan Quality Assessment of Fractionated Stereotactic Radiotherapy Treatment Plans in Patients With Brain Metastases. Front. Oncol. 2022, 12, 846609. [Google Scholar] [CrossRef] [PubMed]
- Schwendner, M.J.; Sollmann, N.; Diehl, C.D.; Oechsner, M.; Meyer, B.; Krieg, S.M.; Combs, S.E. The Role of Navigated Transcranial Magnetic Stimulation Motor Mapping in Adjuvant Radiotherapy Planning in Patients With Supratentorial Brain Metastases. Front. Oncol. 2018, 8, 424. [Google Scholar] [CrossRef] [PubMed]
- Dzierma, Y.; Schuermann, M.; Melchior, P.; Nuesken, F.; Oertel, J.; Rube, C.; Hendrix, P. Optimizing Adjuvant Stereotactic Radiotherapy of Motor-Eloquent Brain Metastases: Sparing the nTMS-Defined Motor Cortex and the Hippocampus. Front. Oncol. 2021, 11, 628007. [Google Scholar] [CrossRef] [PubMed]
- Diehl, C.D.; Rosenkranz, E.; Misslbeck, M.; Schwendner, M.; Sollmann, N.; Ille, S.; Meyer, B.; Combs, S.E.; Bernhardt, D.; Krieg, S.M. nTMS-derived DTI-based motor fiber tracking in radiotherapy treatment planning of high-grade gliomas for avoidance of motor structures. Radiother. Oncol. 2022, 171, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Floyd, S.R.; Chang, C.H.; Warnke, P.C.; Chio, C.C.; Kasper, E.M.; Mahadevan, A.; Wong, E.T.; Chen, C.C. Cyberknife hypofractionated stereotactic radiosurgery (HSRS) of resection cavity after excision of large cerebral metastasis: Efficacy and safety of an 800 cGy x 3 daily fractions regimen. J. Neurooncol. 2012, 106, 601–610. [Google Scholar] [CrossRef]
- Maruyama, K.; Kamada, K.; Ota, T.; Koga, T.; Itoh, D.; Ino, K.; Aoki, S.; Tago, M.; Masutani, Y.; Shin, M.; et al. Tolerance of pyramidal tract to gamma knife radiosurgery based on diffusion-tensor tractography. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 1330–1335. [Google Scholar] [CrossRef]
- Blonigen, B.J.; Steinmetz, R.D.; Levin, L.; Lamba, M.A.; Warnick, R.E.; Breneman, J.C. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 996–1001. [Google Scholar] [CrossRef]
- Ruben, J.D.; Dally, M.; Bailey, M.; Smith, R.; McLean, C.A.; Fedele, P. Cerebral radiation necrosis: Incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 499–508. [Google Scholar] [CrossRef]
- Han, J.H.; Kim, D.G.; Kim, C.Y.; Chung, H.T.; Jung, H.W. Stereotactic radiosurgery for large brain metastases. Prog. Neurol. Surg. 2012, 25, 248–260. [Google Scholar] [CrossRef]
- Gallo, J.; Garimall, S.; Shanker, M.; Castelli, J.; Watkins, T.; Olson, S.; Huo, M.; Foote, M.C.; Pinkham, M.B. Outcomes Following Hypofractionated Stereotactic Radiotherapy to the Cavity After Surgery for Melanoma Brain Metastases. Clin. Oncol. (R. Coll. Radiol.) 2022, 34, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, E.J.; Peterson, J.; Brown, P.D.; Sheehan, J.P.; Quinones-Hinojosa, A.; Zaorsky, N.G.; Trifiletti, D.M. Treatment of brain metastases with stereotactic radiosurgery and immune checkpoint inhibitors: An international meta-analysis of individual patient data. Radiother. Oncol. 2019, 130, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, R.S.; Press, R.H.; Patel, K.R.; Boselli, D.M.; Symanowski, J.T.; Lankford, S.P.; McCammon, R.J.; Moeller, B.J.; Heinzerling, J.H.; Fasola, C.E.; et al. Single-Fraction Stereotactic Radiosurgery (SRS) Alone Versus Surgical Resection and SRS for Large Brain Metastases: A Multi-institutional Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.; Ojerholm, E.; Hollander, A.; Briola, C.; Mooij, R.; Bieda, M.; Kolker, J.; Nagda, S.; Geiger, G.; Dorsey, J.; et al. Intracranial control after Cyberknife radiosurgery to the resection bed for large brain metastases. Radiat. Oncol. 2015, 10, 221. [Google Scholar] [CrossRef]
- Atalar, B.; Choi, C.Y.; Harsh, G.R.T.; Chang, S.D.; Gibbs, I.C.; Adler, J.R.; Soltys, S.G. Cavity volume dynamics after resection of brain metastases and timing of postresection cavity stereotactic radiosurgery. Neurosurgery 2013, 72, 180–185, discussion 185. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, L.A.; Simmons, N.E.; Bellerive, M.; Erkmen, K.; Eskey, C.J.; Gladstone, D.J.; Hug, E.B.; Roberts, D.W.; Hartford, A.C. Tumor bed dynamics after surgical resection of brain metastases: Implications for postoperative radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 943–948. [Google Scholar] [CrossRef]
- Scharl, S.; Kirstein, A.; Kessel, K.A.; Duma, M.N.; Oechsner, M.; Straube, C.; Combs, S.E. Cavity volume changes after surgery of a brain metastasis-consequences for stereotactic radiation therapy. Strahlenther. Onkol. 2019, 195, 207–217. [Google Scholar] [CrossRef]

| Total Number of Patients | 24 | ||
| Gender | Female | 17 | |
| Male | 7 | ||
| Age at primary treatment (Mean and range) | 60.4 years (32–78 years) | ||
| Primary Tumor (Number of patients) | Non-small cell lung cancer | 7 | |
| Breast cancer | 5 | ||
| Colorectal carcinoma | 2 | ||
| Ovarian cancer | 2 | ||
| Ewing sarcoma | 1 | ||
| Malignant melanoma | 1 | ||
| Hidradenocarcinoma | 1 | ||
| Urothelial carcinoma | 1 | ||
| Vulvar cancer | 1 | ||
| Hepatocellular carcinoma | 1 | ||
| Gastric carcinoma | 1 | ||
| Adenocarcinoma-CUP | 1 | ||
| Tumor-affected hemisphere | L | 16 | |
| R | 8 | ||
| Tumor localization | Frontal | 13 | |
| Temporal | 2 | ||
| Parietal | 9 | ||
| Extent of resection | >95% = GTR | 21 | |
| 80–95% = STR | 3 | ||
| Preoperative systemic therapy (number of patients) | Systemic therapy (total) | 16 | |
| Chemotherapy | 14 | ||
| Immunotherapy | 7 | ||
| Postoperative systemic therapy (number of patients) | Systemic therapy (total) | 18 | |
| Chemotherapy | 15 | ||
| Immunotherapy | 12 | ||
| unknown | 3 | ||
| Maximum tumor volume (mean and range) | 11.4 cm3 (0.4–57.1 cm3) | ||
| Distance tumor—nTMS fibre tract (mean and range) | 0.4 cm (0.0–1.9 cm) | ||
| Preoperative motor deficits | BMRC | 5/5 | 14 |
| 4/5 | 7 | ||
| <3/5 | 3 | ||
| Postoperative motor deficits | BMRC | 5/5 | 13 |
| 4/5 | 8 | ||
| <3/5 | 3 | ||
| Follow-up motor deficits (6 months after surgery) | BMRC | 5/5 | 17 |
| 4/5 | 4 | ||
| <3/5 | 1 | ||
| unknown | 2 | ||
| Motor deficits at follow-up before tumor progression (number of patients) | n = 2 | ||
| BMRC | 5/5 | 2 | |
| <3/5 | 1 | ||
| Motor deficits at maximum follow-up (number of patients) | BMRC | 5/5 | 20 |
| 4/5 | 3 | ||
| <3/5 | 1 | ||
| Structure | Unit | Regular | Spare | p-Value |
|---|---|---|---|---|
| nTMS.motor | Dmean (Gy) | 5.2 ± 2.4 | 3.8 ± 1.8 | p < 0.001 |
| exPTV | Dmean (Gy) | 4.8 ± 2.1 | 3.4 ± 1.4 | p < 0.001 |
| FTmotexPTV50% | Dmean (Gy) | 23.4 ± 3.3 | 15.9 ± 4.7 | p < 0.001 |
| FTmotexPTV25% | Dmean (Gy) | 17.6 ± 4.2 | 12.5 ± 3.6 | p < 0.001 |
| V100%35Gy | (in %) | 1.7 ± 2.3 | 0.2 ± 0.4 | p < 0.001 |
| V95%33.23Gy | (in %) | 2.4 ± 3.1 | 0.5 ± 0.7 | p < 0.001 |
| V90%31.5Gy | (in %) | 3.1 ± 3.7 | 0.8 ± 1.0 | p < 0.001 |
| V50%17.5Gy | (in %) | 10.9 ± 9.8 | 4.3 ± 4.0 | p < 0.001 |
| V25%8.78Gy | (in %) | 22.0 ± 10.5 | 14.2 ± 7.9 | p < 0.001 |
| PTV | Regulare | DTI-FT Spared | p-Value |
|---|---|---|---|
| Dmax (in Gy) | 37.2 ± 0.9 | 38.6 ± 1.6 | p = 0.00 |
| Dmean (in Gy) | 35.6 ± 0.9 | 36.0 ± 1.2 | p = 0.06 |
| Dmin (in Gy) | 32.8 ± 1.3 | 31.9 ± 1.6 | p = 0.34 |
| Coverage | 1.0 ± 0.0 | 1.0 ± 0.0 | p = 0.08 |
| Selectivitiy | 0.9 ± 0.1 | 0.8 ± 0.1 | p = 0.00 |
| Paddick CI | 0.9 ± 0.1 | 0.8 ± 0.1 | p = 0.00 |
| OAR | Side | DVH Value | Regular | DTI-FT Spared | p-Value |
|---|---|---|---|---|---|
| Brain | Dmean (Gy) | 3.9 ± 1.9 | 3.5 ± 1.6 | p = 0.51 | |
| Healthy Brain (Brain minus Cavity) | V28Gy (cc) | 25.3 ± 14.9 | 25.9 ± 15.9 | p = 0.40 | |
| V24Gy (cc) | 33.5 ± 19.7 | 35.0 ± 21.9 | p = 0.22 | ||
| Hippocampus | ipsilateral | Dmean (Gy) | 1.8 ± 3.6 | 1.9 ± 3.7 | p = 0.84 |
| contralateral | Dmean(Gy) | 0.9 ± 1.8 | 0.9 ± 1.8 | p = 0.91 | |
| Brainstem | Dmax (Gy) | 1.7 ± 3.0 | 1.8 ± 3.2 | p = 0.59 | |
| Chiasm | Dmax (Gy) | 1.3 ± 2.0 | 1.2 ± 1.8 | p = 0.19 | |
| Optic Nerve | ipsilateral | Dmax (Gy) | 0.9 ± 1.4 | 0.9 ± 1.3 | p = 0.75 |
| contralateral | Dmax (Gy) | 0.7 ± 1.1 | 0.7 ± 1.1 | p = 0.81 | |
| Pituitary | Dmax (Gy) | 0.6 ± 1.0 | 0.6 ± 0.9 | p = 0.41 | |
| Lacrimalis Gland | ipsilateral | Dmax (Gy) | 1.3 ± 2.3 | 1.6 ± 3.0 | p = 0.13 |
| contralateral | Dmax (Gy) | 0.9 ± 1.5 | 0.9 ± 1.6 | p = 0.99 | |
| Cochlea | ipsilateral | Dmax (Gy) | 0.4 ± 0.6 | 0.4 ± 0.7 | p = 0.10 |
| contralateral | Dmax (Gy) | 0.1 ± 0.1 | 0.2 ± 0.1 | p = 0.61 | |
| Bulbus | ipsilateral | Dmax (Gy) | 1.3 ± 2.1 | 1.8 ± 3.1 | p = 0.15 |
| contralateral | Dmax (Gy) | 1.1 ± 1.7 | 1.0 ± 1.5 | p = 0.29 | |
| Lens | ipsilateral | Dmax (Gy) | 0.7 ± 1.3 | 0.8 ± 1.5 | p = 0.85 |
| contralateral | Dmax (Gy) | 0.5 ± 0.7 | 0.5 ± 0.7 | p = 0.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diehl, C.D.; Rosenkranz, E.; Schwendner, M.; Mißlbeck, M.; Sollmann, N.; Ille, S.; Meyer, B.; Combs, S.E.; Krieg, S.M. Dose Reduction to Motor Structures in Adjuvant Fractionated Stereotactic Radiotherapy of Brain Metastases: nTMS-Derived DTI-Based Motor Fiber Tracking in Treatment Planning. Cancers 2023, 15, 282. https://doi.org/10.3390/cancers15010282
Diehl CD, Rosenkranz E, Schwendner M, Mißlbeck M, Sollmann N, Ille S, Meyer B, Combs SE, Krieg SM. Dose Reduction to Motor Structures in Adjuvant Fractionated Stereotactic Radiotherapy of Brain Metastases: nTMS-Derived DTI-Based Motor Fiber Tracking in Treatment Planning. Cancers. 2023; 15(1):282. https://doi.org/10.3390/cancers15010282
Chicago/Turabian StyleDiehl, Christian D., Enrike Rosenkranz, Maximilian Schwendner, Martin Mißlbeck, Nico Sollmann, Sebastian Ille, Bernhard Meyer, Stephanie E. Combs, and Sandro M. Krieg. 2023. "Dose Reduction to Motor Structures in Adjuvant Fractionated Stereotactic Radiotherapy of Brain Metastases: nTMS-Derived DTI-Based Motor Fiber Tracking in Treatment Planning" Cancers 15, no. 1: 282. https://doi.org/10.3390/cancers15010282
APA StyleDiehl, C. D., Rosenkranz, E., Schwendner, M., Mißlbeck, M., Sollmann, N., Ille, S., Meyer, B., Combs, S. E., & Krieg, S. M. (2023). Dose Reduction to Motor Structures in Adjuvant Fractionated Stereotactic Radiotherapy of Brain Metastases: nTMS-Derived DTI-Based Motor Fiber Tracking in Treatment Planning. Cancers, 15(1), 282. https://doi.org/10.3390/cancers15010282








