Immune Response and Effects of COVID-19 Vaccination in Patients with Lung Cancer—COVID Lung Vaccine Study
Abstract
Simple Summary
Abstract
1. Introduction
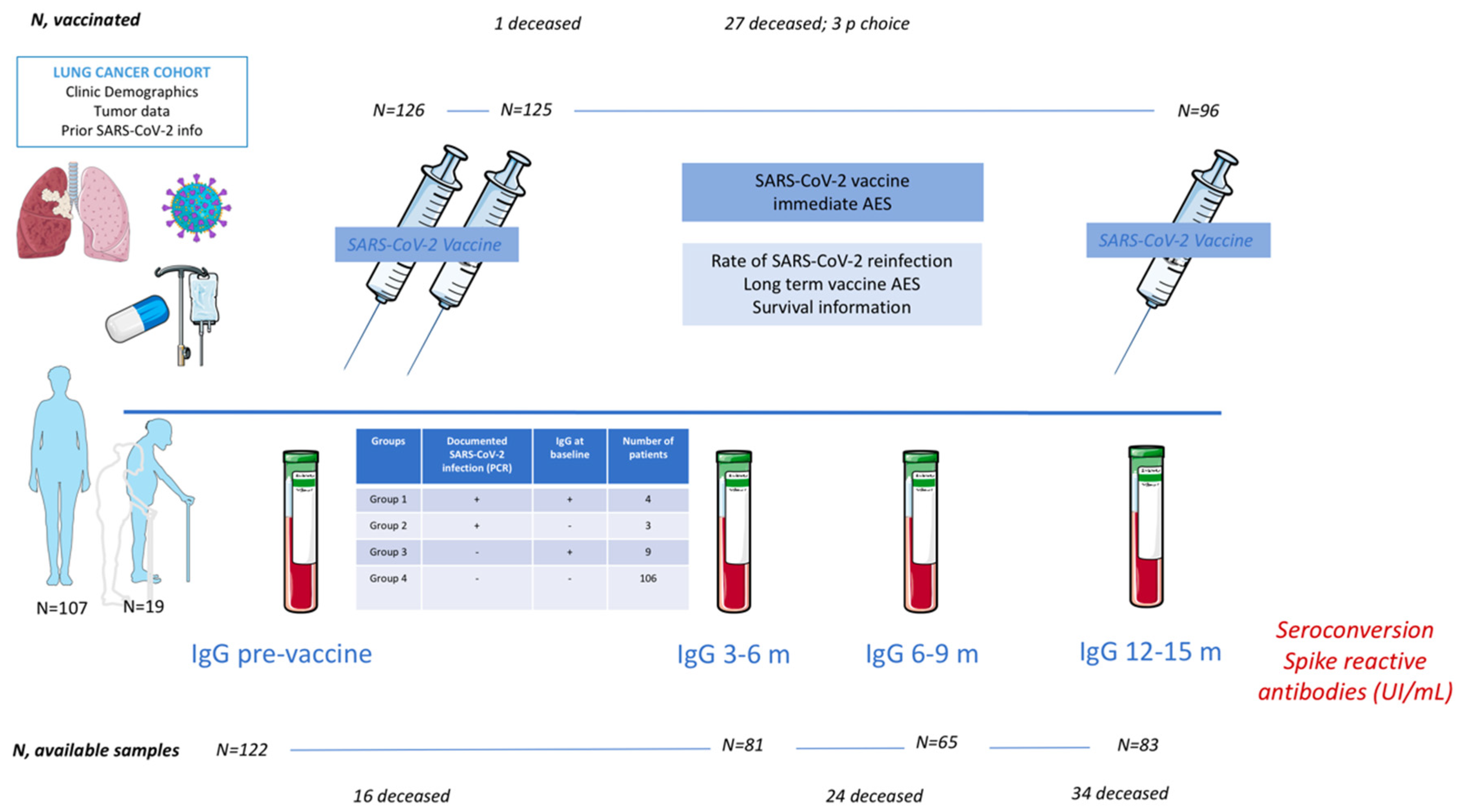
2. Material and Methods
2.1. Serologies
2.2. Statistical Considerations
3. Results
3.1. Cohort Description
3.2. Type of Vaccines
3.3. Prior Serologic Status
3.4. Seroconversion
3.5. Adverse Events to Vaccines
3.6. Protection
3.7. Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Zhu, F.; Xie, L.; Wang, C.; Wang, J.; Chen, R.; Jia, P.; Guan, H.Q.; Peng, L.; Chen, Y.; et al. Clinical characteristics of COVID-19-infected cancer patients: A retrospective case study in three hospitals within Wuhan, China. Ann. Oncol. 2020, 31, 894–901. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0923753420363833 (accessed on 3 December 2022). [CrossRef] [PubMed]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Qu, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. Available online: http://www.nejm.org/doi/10.1056/NEJMoa2002032 (accessed on 3 December 2022). [CrossRef] [PubMed]
- Kuderer, N.M.; Choueiri, T.K.; Shah, D.P.; Shyr, Y.; Rubinstein, S.M.; Rivera, D.R.; Shete, S.; Hsu, C.-Y.; Desai, A.; de Lima Lopes, G., Jr.; et al. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 2020, 395, 1907–1918. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0140673620311879 (accessed on 3 December 2022). [CrossRef]
- Garassino, M.C.; Whisenant, J.G.; Huang, L.-C.; Trama, A.; Torri, V.; Agustoni, F.; Baena, J.; Banna, G.; Berardi, R.; Bettini, A.C.; et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): First results of an international, registry-based, cohort study. Lancet Oncol. 2020, 21, 914–922. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1470204520303144 (accessed on 3 December 2022). [CrossRef] [PubMed]
- Singh, A.P.; Berman, A.T.; Marmarelis, M.E.; Haas, A.R.; Feigenberg, S.J.; Braun, J.; Ciunci, C.A.; Bauml, J.M.; Cohen, R.B.; Kucharczuk, J.C.; et al. Management of Lung Cancer During the COVID-19 Pandemic. JCO Oncol. Pract. 2020, 16, 579–586. Available online: https://ascopubs.org/doi/10.1200/OP.20.00286 (accessed on 3 December 2022). [CrossRef]
- Posicionamiento y Recomendaciones de SEOM en Relación Con la Campaña de Vacunación Frente al COVID-19 en Pacientes Con Cáncer. Available online: https://seom.org/images/Posicionamiento_SEOM_vacunacion_COVID19_pacientes_con_cancer (accessed on 3 December 2022).
- ESMO Statements on Vaccination against COVID-19 in People with Cancer. Available online: https://www.esmo.org/covid-19-and-cancer/covid-19-vaccination (accessed on 3 December 2022).
- Recomendaciones FACME Para la Vacunación Frente a COVID-19 en Grupos de Especial Interés. Available online: https://www.sen.es/pdf/2021/facme/Cancer_y_vacunas_frente_a_COVID-19.pdf (accessed on 3 December 2022).
- Ribas, A.; Sengupta, R.; Locke, T.; Zaidi, S.K.; Campbell, K.M.; Carethers, J.M.; Jaffee, E.M.; Wherry, E.J.; Soria, J.-C.; D’Souza, G. Priority COVID-19 Vaccination for Patients with Cancer while Vaccine Supply Is Limited. Cancer Discov. 2021, 11, 233–236. Available online: https://aacrjournals.org/cancerdiscovery/article/11/2/233/2781/Priority-COVID-19-Vaccination-for-Patients-with (accessed on 3 December 2022). [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. Available online: http://www.nejm.org/doi/10.1056/NEJMoa2034577 (accessed on 3 December 2022). [CrossRef]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2020, 396, 1979–1993. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0140673620324661 (accessed on 3 December 2022). [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. Available online: http://www.nejm.org/doi/10.1056/NEJMoa2035389 (accessed on 3 December 2022). [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. Available online: http://www.nejm.org/doi/10.1056/NEJMoa2101544 (accessed on 3 December 2022). [CrossRef]
- Trinité, B.; Tarrés-Freixas, F.; Rodon, J.; Pradenas, E.; Urrea, V.; Marfil, S.; de la Concepción, M.L.R.; Ávila-Nieto, C.; Aguilar-Gurrieri, C.; Barajas, A.; et al. SARS-CoV-2 infection elicits a rapid neutralizing antibody response that correlates with disease severity. Sci. Rep. 2021, 11, 2608. Available online: http://www.nature.com/articles/s41598-021-81862-9 (accessed on 3 December 2022). [CrossRef]
- Riva, L.; Yuan, S.; Yin, X.; Martin-Sancho, L.; Matsunaga, N.; Pache, L.; Burgstaller-Muehlbacher, S.; De Jesus, P.D.; Teriete, P.; Hull, M.V.; et al. Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature 2020, 586, 113–119. Available online: http://www.nature.com/articles/s41586-020-2577-1 (accessed on 3 December 2022). [CrossRef] [PubMed]
- Wang, Z.; Yang, L.; Song, X. Oral GS-441524 derivatives: Next-generation inhibitors of SARS-CoV-2 RNA-dependent RNA polymerase. Front. Immunol. 2022, 13, 1015355. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1015355/full (accessed on 3 December 2022). [CrossRef] [PubMed]
- Jiaranaikulwanitch, J.; Yooin, W.; Chutiwitoonchai, N.; Thitikornpong, W.; Sritularak, B.; Rojsitthisak, P.; Vajragupta, O. Discovery of Natural Lead Compound from Dendrobium sp. against SARS-CoV-2 Infection. Pharmaceuticals 2022, 15, 620. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, Z. Natural Products, Alone or in Combination with FDA-Approved Drugs, to Treat COVID-19 and Lung Cancer. Biomedicines 2021, 9, 689. Available online: https://www.mdpi.com/2227-9059/9/6/689 (accessed on 3 December 2022). [CrossRef]
- Lee, J.-G.; Huang, W.; Lee, H.; van de Leemput, J.; Kane, M.A.; Han, Z. Characterization of SARS-CoV-2 proteins reveals Orf6 pathogenicity, subcellular localization, host interactions and attenuation by Selinexor. Cell Biosci. 2021, 11, 58. [Google Scholar] [CrossRef]
- Burki, T. The future of Paxlovid for COVID-19. Lancet Respir. Med. 2022, 10, e68. [Google Scholar] [CrossRef]
- Hottinger, A.F.; George, A.-C.C.; Bel, M.; Favet, L.; Combescure, C.; Meier, S.; Grillet, S.; Posfay-Barbe, K.; Kaiser, L.; Siegrist, C.-A.; et al. A Prospective Study of the Factors Shaping Antibody Responses to the AS03-Adjuvanted Influenza A/H1N1 Vaccine in Cancer Outpatients. Oncologist 2012, 17, 436–445. Available online: https://academic.oup.com/oncolo/article/17/3/436/6403295 (accessed on 3 December 2022). [CrossRef]
- Beck, C.R.; McKenzie, B.C.; Hashim, A.B.; Harris, R.C.; Nguyen-Van-Tam, J.S. Influenza Vaccination for Immunocompromised Patients: Systematic Review and Meta-analysis by Etiology. J. Infect. Dis. 2012, 206, 1250–1259. Available online: https://academic.oup.com/jid/article-lookup/doi/10.1093/infdis/jis487 (accessed on 3 December 2022). [CrossRef]
- Rondy, M.; Larrauri, A.; Casado, I.; Alfonsi, V.; Pitigoi, D.; Launay, O.; Syrjänen, R.K.; Gefenaite, G.; Machado, A.; Vučina, V.V.; et al. 2015/16 seasonal vaccine effectiveness against hospitalisation with influenza A(H1N1)pdm09 and B among elderly people in Europe: Results from the I-MOVE+ project. Eurosurveillance 2017, 22, 30580. Available online: https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2017.22.30.30580 (accessed on 3 December 2022). [CrossRef]
- Gounant, V.; Ferré, V.M.; Soussi, G.; Charpentier, C.; Flament, H.; Fidouh, N.; Collin, G.; Namour, C.; Assoun, S.; Bizot, A.; et al. Efficacy of Severe Acute Respiratory Syndrome Coronavirus-2 Vaccine in Patients With Thoracic Cancer: A Prospective Study Supporting a Third Dose in Patients With Minimal Serologic Response After Two Vaccine Doses. J. Thorac. Oncol. 2021, 17, 239–251. Available online: https://linkinghub.elsevier.com/retrieve/pii/S155608642103286X (accessed on 3 December 2022). [CrossRef] [PubMed]
- Fendler, A.; Shepherd, S.T.C.; Au, L.; Wilkinson, K.A.; Wu, M.; Byrne, F.; Cerrone, M.; Schmitt, A.M.; Joharatnam-Hogan, N.; Shum, B.; et al. Adaptive immunity and neutralizing antibodies against SARS-CoV-2 variants of concern following vaccination in patients with cancer: The CAPTURE study. Nat. Cancer 2021, 2, 1305–1320. Available online: https://www.nature.com/articles/s43018-021-00274-w (accessed on 3 December 2022). [CrossRef] [PubMed]
- Zheng, J.; Deng, Y.; Zhao, Z.; Mao, B.; Lu, M.; Lin, Y.; Huang, A. Characterization of SARS-CoV-2-specific humoral immunity and its potential applications and therapeutic prospects. Cell Mol. Immunol. 2022, 19, 150–157. Available online: https://www.nature.com/articles/s41423-021-00774-w (accessed on 3 December 2022). [CrossRef]
- Sadarangani, M.; Marchant, A.; Kollmann, T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat. Rev. Immunol. 2021, 21, 475–484. Available online: http://www.nature.com/articles/s41577-021-00578-z (accessed on 3 December 2022). [CrossRef] [PubMed]
- Zeng, C.; Evans, J.P.; Reisinger, S.; Woyach, J.; Liscynesky, C.; El Boghdadly, Z.; Rubinstein, M.P.; Chakravarthy, K.; Saif, L.; Oltz, E.M.; et al. Impaired neutralizing antibody response to COVID-19 mRNA vaccines in cancer patients. Cell Biosci. 2021, 11, 197. Available online: https://cellandbioscience.biomedcentral.com/articles/10.1186/s13578-021-00713-2 (accessed on 3 December 2022). [CrossRef] [PubMed]
- Mostaghimi, D.; Valdez, C.N.; Larson, H.T.; Kalinich, C.C.; Iwasaki, A. Prevention of host-to-host transmission by SARS-CoV-2 vaccines. Lancet Infect. Dis. 2022, 22, e52–e58. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1473309921004722 (accessed on 3 December 2022). [CrossRef] [PubMed]
- Ali, Y.M.; Ferrari, M.; Lynch, N.J.; Yaseen, S.; Dudler, T.; Gragerov, S.; Demopulos, G.; Heeney, G.L.; Schwaeble, W.J. Lectin Pathway Mediates Complement Activation by SARS-CoV-2 Proteins. Front. Immunol. 2021, 12, 714511. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2021.714511/full (accessed on 3 December 2022). [CrossRef]
- Bahnan, W.; Wrighton, S.; Sundwall, M.; Bläckberg, A.; Larsson, O.; Höglund, U.; Khakzad, H.; Godzwon, M.; Walle, M.; Elder, E.; et al. Spike-Dependent Opsonization Indicates Both Dose-Dependent Inhibition of Phagocytosis and That Non-Neutralizing Antibodies Can Confer Protection to SARS-CoV-2. Front. Immunol. 2022, 12, 808932. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2021.808932/full (accessed on 3 December 2022). [CrossRef]
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernán, M.A.; Lipsitch, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2021, 385, 1078–1090. Available online: http://www.nejm.org/doi/10.1056/NEJMoa2110475 (accessed on 3 December 2022). [CrossRef]
- Ozonoff, A.; Nanishi, E.; Levy, O. Bell’s palsy and SARS-CoV-2 vaccines. Lancet Infect. Dis. 2021, 21, 450–452. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1473309921000761 (accessed on 3 December 2022). [CrossRef]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. Available online: http://www.nejm.org/doi/10.1056/NEJMoa2104840 (accessed on 3 December 2022). [CrossRef] [PubMed]
- Perry, J.; Osman, S.; Wright, J.; Richard-Greenblatt, M.; Buchan, S.A.; Sadarangani, M.; Bolotin, S. Does a humoral correlate of protection exist for SARS-CoV-2? A systematic review. PLoS ONE 2022, 17, e0266852. Available online: https://dx.plos.org/10.1371/journal.pone.0266852 (accessed on 3 December 2022). [CrossRef] [PubMed]
- Abbasi, J. Homing In On a SARS-CoV-2 Correlate of Protection. JAMA 2022, 327, 115. Available online: https://jamanetwork.com/journals/jama/fullarticle/2787928 (accessed on 3 December 2022). [CrossRef]
- Gilbert, P.B.; Montefiori, D.C.; McDermott, A.B.; Fong, Y.; Benkeser, D.; Deng, W.; Zhou, H.; Houchens, C.R.; Martins, K.; Jayashankar, L.; et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 2022, 375, 43–50. Available online: https://www.science.org/doi/10.1126/science.abm3425 (accessed on 3 December 2022). [CrossRef]
- Variantes de SARS-CoV-2 en España: Linajes BA.2.12.1, BA.4 y BA.5 de Ómicron. Available online: https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/20220628-ERR.pdf (accessed on 3 December 2022).
- Goldblatt, D.; Fiore-Gartland, A.; Johnson, M.; Hunt, A.; Bengt, C.; Zavadska, D.; Snipe, H.D.; Brown, J.S.; Workman, L.; Zar, H.J.; et al. Towards a population-based threshold of protection for COVID-19 vaccines. Vaccine 2021, 40, 306–315. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0264410X21016157 (accessed on 3 December 2022). [CrossRef]
- Alkhatib, M.; Salpini, R.; Carioti, L.; Ambrosio, F.A.; D’Anna, S.; Duca, L.; Costa, G.; Bellocchi, M.C.; Piermatteo, L.; Artese, A.; et al. Update on SARS-CoV-2 Omicron Variant of Concern and Its Peculiar Mutational Profile. Microbiol. Spectr. 2022, 10, e02732-21. Available online: https://journals.asm.org/doi/10.1128/spectrum.02732-21 (accessed on 3 December 2022). [CrossRef]
- Garcia-Beltran, W.F.; St Denis, K.J.; Hoelzemer, A.; Lam, E.C.; Nitido, A.D.; Sheehan, M.L.; Berrios, C.; Ofoman, O.; Chang, C.C.; Hauser, B.M.; et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 2022, 185, 457–466.e4. Available online: http://www.ncbi.nlm.nih.gov/pubmed/34931201 (accessed on 3 December 2022). [CrossRef]
- Lièvre, A.; Turpin, A.; Ray-Coquard, I.; Le Malicot, K.; Thariat, J.; Ahle, G.; Neuzillet, C.; Paoletti, X.; Bouché, O.; Aldabbagh, K.; et al. Risk factors for Coronavirus Disease 2019 (COVID-19) severity and mortality among solid cancer patients and impact of the disease on anticancer treatment: A French nationwide cohort study (GCO-002 CACOVID-19). Eur. J. Cancer 2020, 141, 62–81. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0959804920310431 (accessed on 3 December 2022). [CrossRef]
- Passaro, A.; Bestvina, C.; Velez, M.V.; Garassino, M.C.; Garon, E.; Peters, S. Severity of COVID-19 in patients with lung cancer: Evidence and challenges. J. Immunother. Cancer 2021, 9, e002266. Available online: https://jitc.bmj.com/lookup/doi/10.1136/jitc-2020-002266 (accessed on 3 December 2022). [CrossRef]
- Mack, P.C.; Gomez, J.E.; Rodilla, A.M.; Carreño, J.M.; Hsu, C.-Y.; Rolfo, C.; Meshulami, N.; Moore, A.; Brody, R.I.; King, J.C.; et al. Longitudinal COVID-19-vaccination-induced antibody responses and Omicron neutralization in patients with lung cancer. Cancer Cell 2022, 40, 575–577. Available online: https://linkinghub.elsevier.com/retrieve/pii/S153561082200174X (accessed on 3 December 2022). [CrossRef]
- Canaday, D.H.; Oyebanji, O.A.; White, E.; Keresztesy, D.; Payne, M.; Wilk, D.; Carias, L.; Aung, H.; Denis, K.S.; Sheehan, M.L.; et al. COVID-19 vaccine booster dose needed to achieve Omicron-specific neutralisation in nursing home residents. Ebiomedicine 2022, 80, 104066. Available online: https://linkinghub.elsevier.com/retrieve/pii/S235239642200247X (accessed on 3 December 2022). [CrossRef] [PubMed]
- Akkaya, M.; Kwak, K.; Pierce, S.K. B cell memory: Building two walls of protection against pathogens. Nat. Rev. Immunol. 2020, 20, 229–238. Available online: http://www.nature.com/articles/s41577-019-0244-2 (accessed on 3 December 2022). [CrossRef] [PubMed]
- Gudbjartsson, D.F.; Norddahl, G.L.; Melsted, P.; Gunnarsdottir, K.; Holm, H.; Eythorsson, E.; Arnthorsson, A.O.; Helgason, D.; Bjarnadottir, K.; Ingvarsson, R.F.; et al. Humoral Immune Response to SARS-CoV-2 in Iceland. N. Engl. J. Med. 2020, 383, 1724–1734. Available online: http://www.nejm.org/doi/10.1056/NEJMoa2026116 (accessed on 3 December 2022). [CrossRef] [PubMed]
- Tretyn, A.; Szczepanek, J.; Skorupa, M.; Jarkiewicz-Tretyn, J.; Sandomierz, D.; Dejewska, J.; Ciechanowska, K.; Jarkiewicz-Tretyn, A.; Koper, W.; Pałgan, K. Differences in the Concentration of Anti-SARS-CoV-2 IgG Antibodies Post-COVID-19 Recovery or Post-Vaccination. Cells 2021, 10, 1952. Available online: https://www.mdpi.com/2073-4409/10/8/1952 (accessed on 3 December 2022). [CrossRef]


| Overall Population | N = 126 |
|---|---|
| Age [median, (range)] | 66 (46–83) |
| Variable | N (%) |
| Sex Male Female | 78 (61.9%) 48 (38.1%) |
| PS PS0 PS1 PS2 | 39 (30.9%) 75 (59.5%) 12 (9.5%) |
| Histology NSCLC LCNEC Carcinoid SCLC | 111 (88.1%) 2 (1.6%) 1 (0.8%) 12 (9.5%) |
| Stage I–II IIIA IIIB IV | 6 (4.8%) 4 (3.2%) 20 (15.9%) 96 (76.2%) |
| Molecular alterations EGFR ALK BRAF ROS1 MET RET | 16 (12.7%) 5 (3.9%) 2 (1.6%) 1 (0.8%) 1 (0.8%) 1 (0.8%) |
| Treatment 1L ChT 2L ChT Adjuvant ChT 1L ChTIT Adjuvant ChTIT Neoadjuvant ChTIT 1L IT 2L IT Consolidation IT Other IT lines ChTRT 1L TKI 2L ITK Other * Active surveillance | 17 (13.5%) 5 (3.9%) 3 (2.4%) 16 (12.7%) 1 (0.8%) 1 (0.8%) 17 (13.5%) 20 (15.9%) 6 (4.8%) 1 (0.8%) 4 (3.2%) 20 (15.9%) 5 (3.9%) 1 (0.8%) 9 (7.2%) |
| Population ≥75 year old | N = 19 |
| Age [median, (range)] | 77 (75–83) |
| Variable | N (%) |
| Sex Male Female | 13 (68.4%) 6 (31.6%) |
| PS PS0 PS1 PS2 | 4 (21.1%) 13 (68.4%) 2 (10.5%) |
| Histology NSCLC Carcinoid SCLC | 14 (73.7%) 1 (5.3%) 4 (21.1%) |
| Stage IIIA IIIB IV | 2 (10.5%) 4 (20.1%) 13 (68.4%) |
| Molecular alterations EGFR ALK KRAS | 4 (21.1%) 1 (5.3%) 1 (5.3%) |
| Treatment 1L ChT 2L ChT 1L ChTIT 1L IT Consolidation IT 1L TKI 2L TKI Other * Active surveillance | 3 (15.8%) 2 (10.5%) 4 (21.1%) 1 (5.3%) 1 (5.3%) 4 (21.1%) 1 (5.3%) 1 (5.3%) 2 (10.5%) |
| Overall Population | N = 126 |
|---|---|
| Variable | N (%) |
| Prior infection Group 1 (infection + IgG+) Group 2 (infection + IgG−) Group 3 (UK infection IgG+) Group 4 (UK infection IgG−) | 4 (3.2%) 3 (2.3%) 9 (7.1%) 110 (87.3%) |
| Type of SARS-CoV-2 vaccine (1st/2nd doses) Moderna® Others | 120 (95.2%) 6 (4.8%) |
| Local adverse events after 1st dose Local pain at the vaccine administration site (grade 1) Local inflammation at the administration site (grade 1/grade 2) | 44 (34.9%) 2 (1.6%)/1(0.8%) |
| General adverse events after 1st dose Fever (grade 1) Muscle pain (grade 1) Asthenia (grade 1/grade 2) Headache (grade 1) | 8 (6.4%) 5 (3.9%) 6 (4.8%)/1 (0.8%) 1 (0.8%) |
| Local adverse events after 2nd dose Local pain at the vaccine administration site (grade 1) Local inflammation at the administration site (grade 1) | 46 (35%) 2 (1.6%) |
| General adverse events after 2nd dose Fever (grade 1) Muscle pain (grade 1) Articular pain (grade 1) Arthomialgias (grade 1) Asthenia (grade1) Headache (grade 1) Regional lymph node enlargement General rash (grade 2) | 21(16.6%) 13 (18.3%) 1 (0.8%) 2 (1.6%) 11 (8.7%) 3 (2.4%) 1 (0.8%) 1 (0.8%) |
| Type of SARS-CoV-2 vaccine (3rd dose was administered to 96 patients) Moderna® Others | 93 (96.8%) 3 (3.2%) |
| Local adverse events after third dose Local pain at the vaccine administration site (grade 1) Local inflammation at the administration site (grade 1) | 16 (16.6%) 1 (1.05%) |
| General adverse events after third dose Fever (grade 1) Muscle pain (grade 1) Asthenia (grade1) Headache (grade 1) Diarrhea (grade 1) | 9 (9.4%) 6 (6.25%) 5 (5.2%) 2 (2.1%) 1 (1.05%) |
| Infections after vaccination Rate of infection Patients infected after 1st dose Patient infected after 2nd dose Patients infected after 3rd dose Rate of reinfection Patient with prior COVID-19 infection who were re-infected after vaccination Group 1 (infection + IgG+) Group 2 (infection + IgG−) Group 3 (UK infection IgG+) Severity of infection Asymptomatic (nosocomial) Mild symptoms Severe symptoms | 1 (0.8%) 5 (3.9%) in 4 p 2nd dose was the last dose 10 (7.9%) 2 p reinfection 0 1 (0.8%) 1 (0.8%) 3 12 1 |
| Survival outcome Cancer-progression-related deaths COVID-19-related deaths Other disease or COVID-19-unrelated deaths Alive | 35 (27.8%) 0 3 (2.4%) 88 (69.8%) |
| Population ≥75 years old | N = 19 |
| Variable | N (%) |
| Documented prior infection Yes No | 1 (5.3%) 18 (94.7%) |
| Type of SARS-CoV-2 vaccine (1st/2nd doses) Moderna® Others | 17 (89.5%) 2 (10.5%) |
| Local adverse events after first dose Local pain at the vaccine administration site (grade 1) | 5 (26.3%) |
| General adverse events after first dose Fever (grade 1) Muscle pain (grade 1) Asthenia (grade1) | 1 (5.3%) 1 (5.3%) 1 (5.3%) |
| Local adverse events after second dose Local pain at the vaccine administration site (grade 1) Local inflammation at the administration site (grade 1) | 8 (42.1%) 1 (5.3%) |
| General adverse events after second dose Fever (grade 1) Artromialgias (grade 1) Asthenia (grade1) | 2 (10.5%) 8 (42.1%) 3 (15.8%) |
| Type of SARS-CoV-2 vaccine (3rd dose was administered to 14 patients) Moderna® Others | 13 (92.8%) 1 (7.1%) |
| Local adverse events after third dose Local pain at the vaccine administration site (grade 1) Local inflammation at the administration site (grade 1) | 4 (28.6%) 1 (7.1%) |
| General adverse events after third dose Fever (grade 1) Muscle pain (grade 1) Asthenia (grade 1) Headache (grade 1) | 1 (7.1%) 1 (7.1%) 1 (7.1%) 1 (7.1%) |
| Infections after vaccination Rate of infection Patients infected after 1st dose Patient infected after 2nd dose Patients infected after 3rd dose Rate of reinfection Patient with prior COVID-19 infection who were re-infected after vaccination Group 1 (infection + IgG+) Group 2 (infection + IgG−) Group 3 (UK infection IgG+) Severity of infection Asymptomatic (nosocomial) Mild symptoms | 0 0 2 (14.3%) 0 1 1 |
| Survival outcome Cancer0progression-related deaths COVID-19-related deaths Other disease or COVID-19-unrelated deaths Alive | 5 (26.3%) 0 2 (10.5%) 12 (63.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez, A.; Boigues, M.; Felip, E.; Cucurull, M.; Notario, L.; Pous, A.; Torres, P.; Benitez, M.; Rodriguez, M.; Quirant, B.; et al. Immune Response and Effects of COVID-19 Vaccination in Patients with Lung Cancer—COVID Lung Vaccine Study. Cancers 2023, 15, 137. https://doi.org/10.3390/cancers15010137
Hernandez A, Boigues M, Felip E, Cucurull M, Notario L, Pous A, Torres P, Benitez M, Rodriguez M, Quirant B, et al. Immune Response and Effects of COVID-19 Vaccination in Patients with Lung Cancer—COVID Lung Vaccine Study. Cancers. 2023; 15(1):137. https://doi.org/10.3390/cancers15010137
Chicago/Turabian StyleHernandez, Ainhoa, Marc Boigues, Eudald Felip, Marc Cucurull, Lucia Notario, Anna Pous, Pere Torres, Marta Benitez, Marina Rodriguez, Bibiana Quirant, and et al. 2023. "Immune Response and Effects of COVID-19 Vaccination in Patients with Lung Cancer—COVID Lung Vaccine Study" Cancers 15, no. 1: 137. https://doi.org/10.3390/cancers15010137
APA StyleHernandez, A., Boigues, M., Felip, E., Cucurull, M., Notario, L., Pous, A., Torres, P., Benitez, M., Rodriguez, M., Quirant, B., Romeo, M., Fuster, D., & Moran, T. (2023). Immune Response and Effects of COVID-19 Vaccination in Patients with Lung Cancer—COVID Lung Vaccine Study. Cancers, 15(1), 137. https://doi.org/10.3390/cancers15010137








