Sulfatase 2 Affects Polarization of M2 Macrophages through the IL-8/JAK2/STAT3 Pathway in Bladder Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Collection
2.2. Cell Lines
2.3. Cell Proliferation Assay
2.4. Western Blot and Co-IP
2.5. RNA Extraction and Real-Time Polymerase Chain Reaction (qPCR)
2.6. Reagents and Enzyme-Linked Immunosorbent Assay (ELISA) Assay
2.7. Animal Models
2.8. Flow Cytometry
2.9. Establishment of Stable Cell Lines
2.10. Cocultivation System
2.11. Colony Formation
2.12. Cell Migration and Invasion
2.13. Immunohistochemistry (IHC)
2.14. Statistical Analysis
3. Results
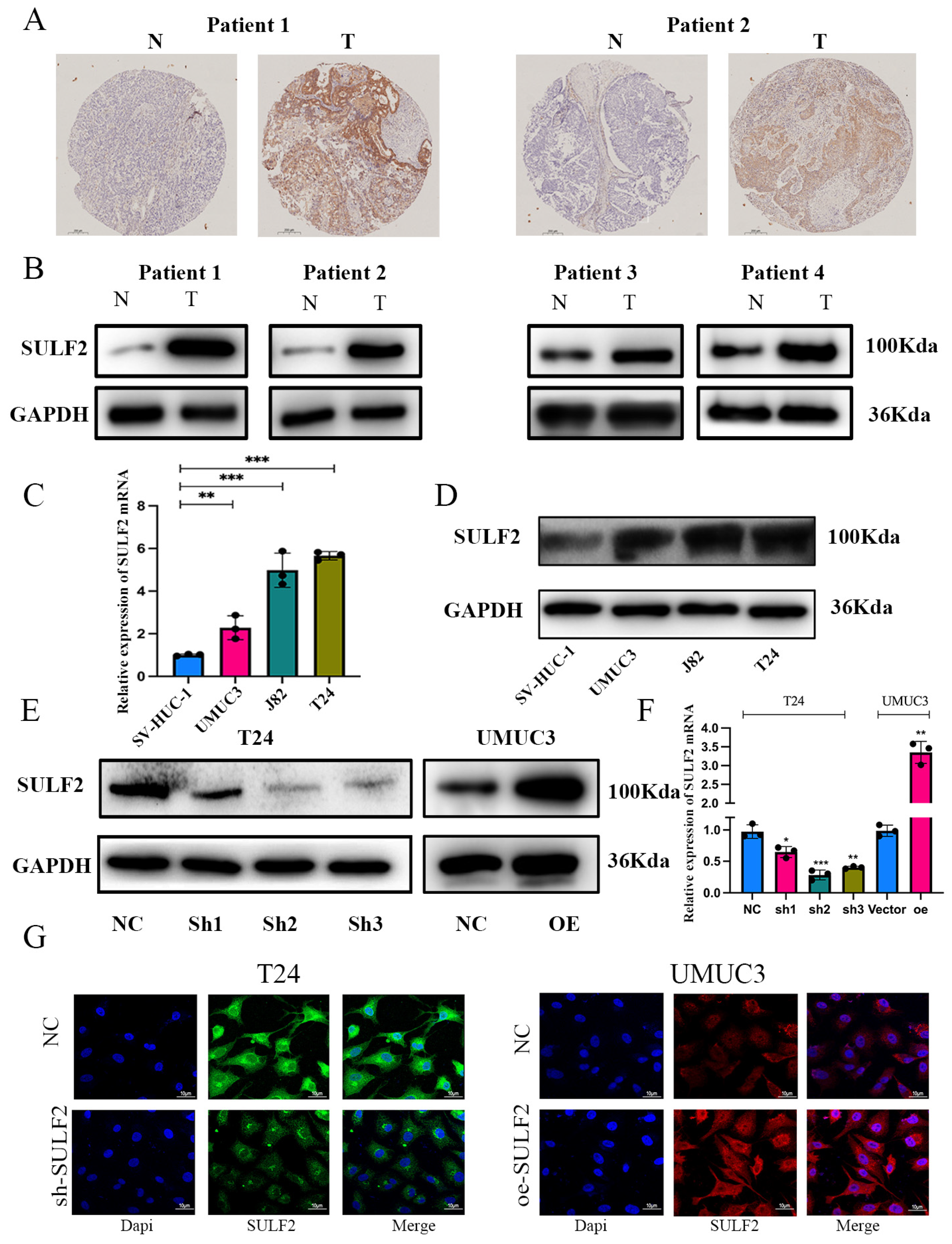
3.1. SULF2 was Highly Expressed in BCa and Associated with Poor Prognosis of Patients
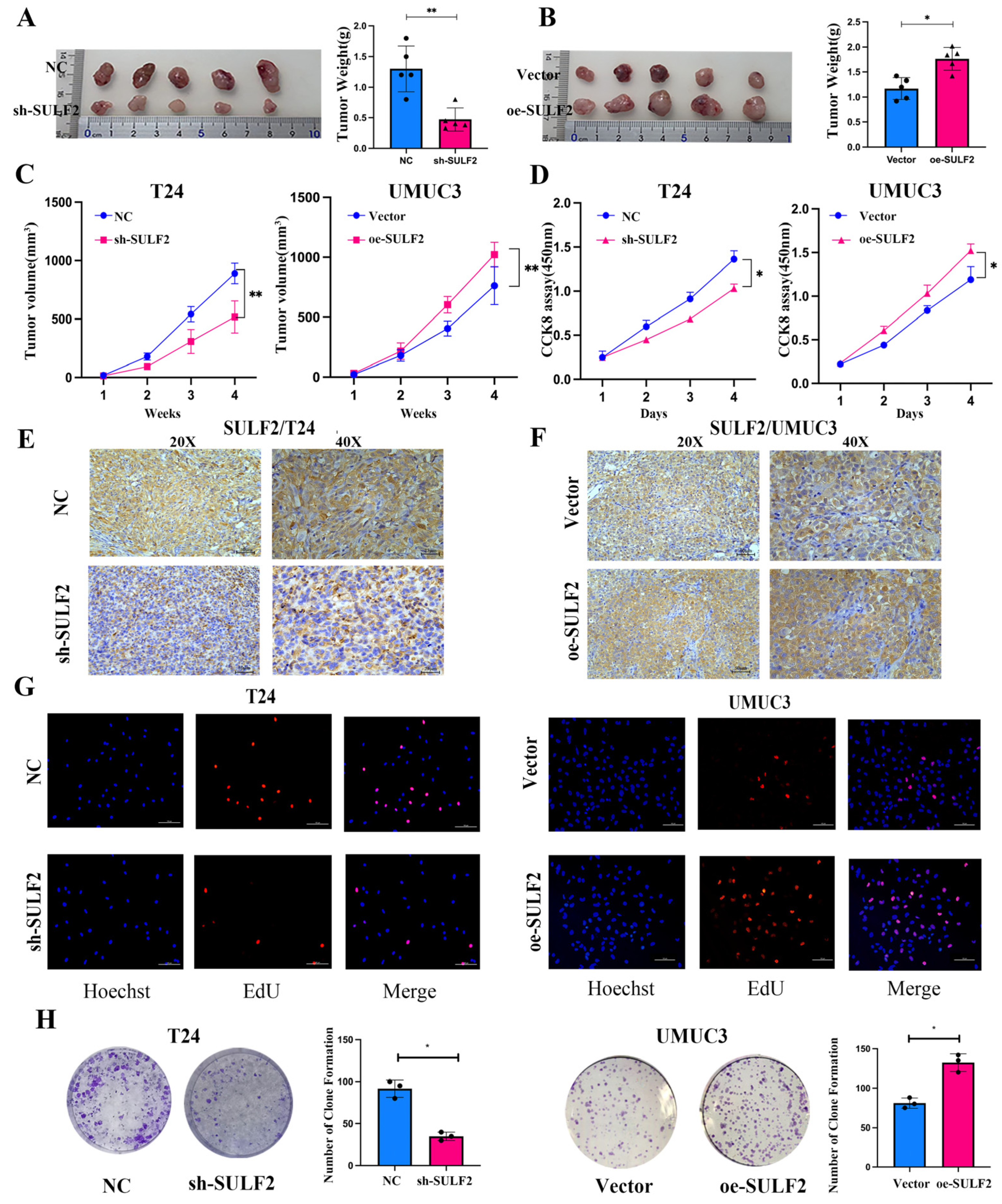
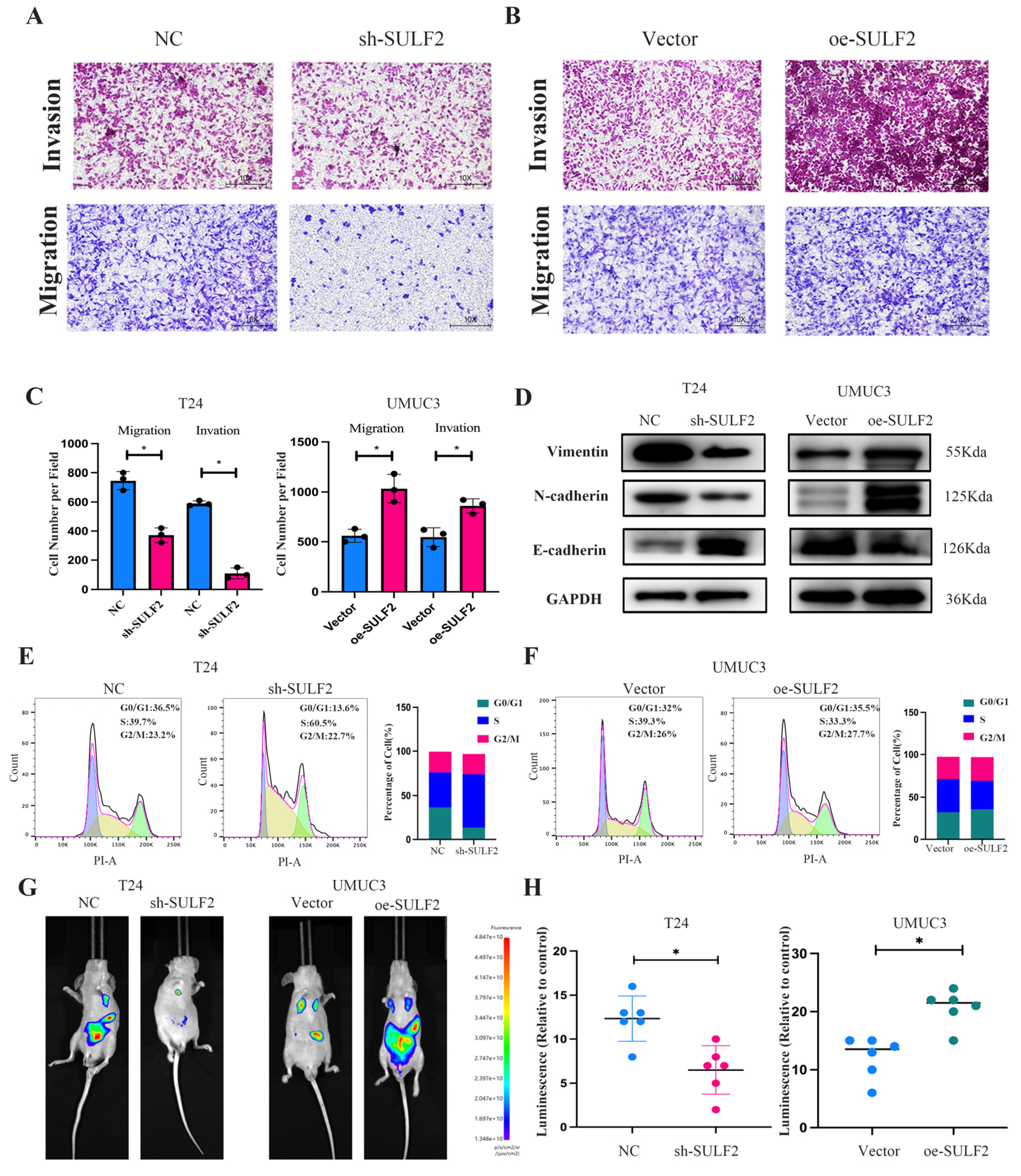
3.2. SULF2 Affects the Proliferation, Migration, and Invasion of BCa Cells
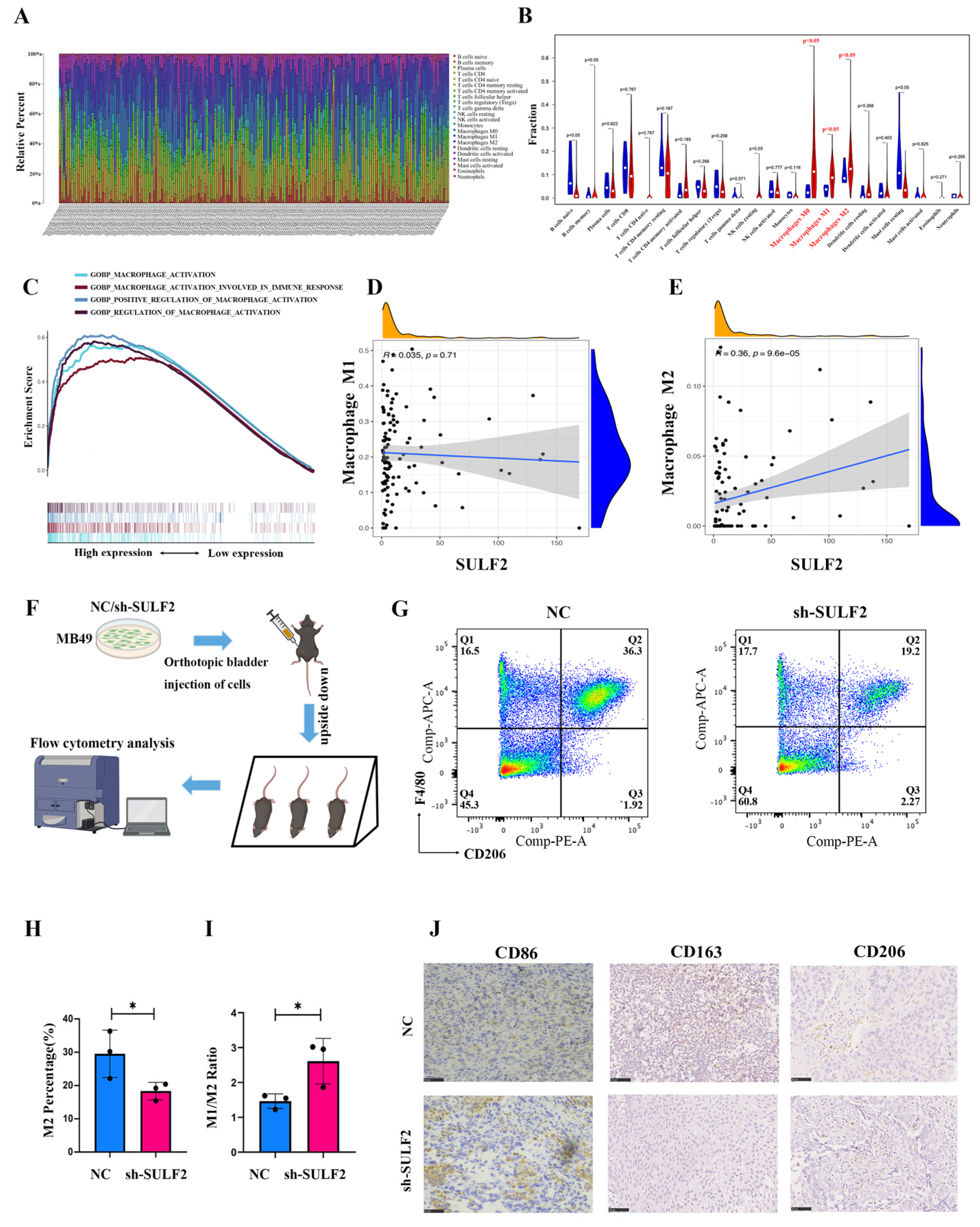
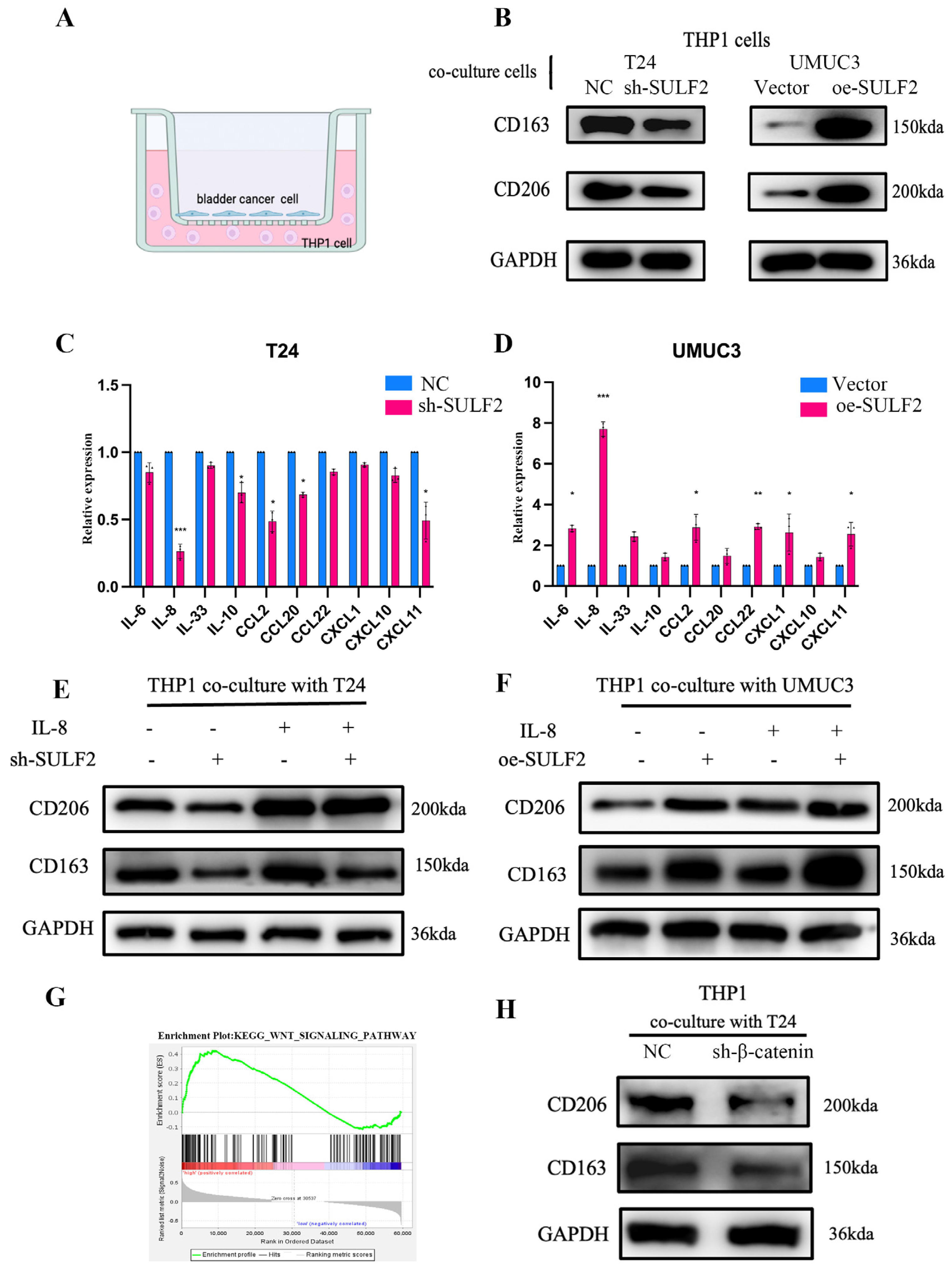
3.3. SULF2 Promotes Differentiation of Macrophages into M2-Type in BCa Microenvironment
3.4. SULF2 Affects the Secretion of IL-8 through β-Catenin
3.5. SULF2 Promotes Macrophage Polarization through the IL-8/JAK2/STAT3 Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.; Xiao, J.F.; Agarwal, N.; Duex, J.E.; Theodorescu, D. Advances in bladder cancer biology and therapy. Nat. Rev. Cancer 2021, 21, 104–121. [Google Scholar] [CrossRef] [PubMed]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Linares Espinós, E.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, T.; Qian, B.Z.; Pollard, J.W. Immune cell promotion of metastasis. Nat. Rev. Immunol. 2015, 15, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, T.D.; Prieto-Dominguez, N.; Gowda, P.S.; Ubil, E. Mechanisms of Macrophage Plasticity in the Tumor Environment: Manipulating Activation State to Improve Outcomes. Front. Immunol. 2021, 12, 642285. [Google Scholar] [CrossRef]
- Qian, B.Z.; Pollard, J.W. Macrophage diversity enhances tumor progression and metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef]
- Ai, X.; Do, A.T.; Kusche-Gullberg, M.; Lindahl, U.; Lu, K.; Emerson, C.P. Substrate specificity and domain functions of extracellular heparan sulfate 6-O-endosulfatases, QSulf1 and QSulf2. J. Biol. Chem. 2006, 281, 4969. [Google Scholar] [CrossRef]
- Kirkpatrick, C.A.; Selleck, S.B. Heparan sulfate proteoglycans at a glance. J. Cell Sci. 2007, 120, 1829–1832. [Google Scholar] [CrossRef]
- Lindahl, U.; Li, J.P. Heparanase—Discovery and Targets. Adv. Exp. Med. Biol. 2020, 1221, 61–69. [Google Scholar] [CrossRef]
- Ai, X.; Kitazawa, T.; Do, A.T.; Kusche-Gullberg, M.; Labosky, P.A.; Emerson, C.P. SULF1 and SULF2 regulate heparan sulfate-mediated GDNF signaling for esophageal innervation. Development 2007, 134, 3327–3338. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Yeh, B.W.; Chan, T.C.; Yang, K.F.; Li, W.M.; Huang, C.N.; Ke, H.L.; Li, C.C.; Yeh, H.C.; Liang, P.I.; et al. Sulfatase-1 overexpression indicates poor prognosis in urothelial carcinoma of the urinary bladder and upper tract. Oncotarget 2017, 8, 47216–47229. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ahn, J.; Edwards, N.J.; Benicky, J.; Rozeboom, A.M.; Davidson, B.; Karamboulas, C.; Nixon, K.C.J.; Ailles, L.; Goldman, R. Extracellular Heparan 6-O-Endosulfatases SULF1 and SULF2 in Head and Neck Squamous Cell Carcinoma and Other Malignancies. Cancers 2022, 14, 5553. [Google Scholar] [CrossRef] [PubMed]
- Wade, A.; Engler, J.R.; Tran, V.M.; Phillips, J.J. Measuring sulfatase expression and invasion in glioblastoma. Methods Mol. Biol. 2015, 1229, 507–516. [Google Scholar] [PubMed]
- Luo, X.; Campbell, N.A.; He, L.; O’Brien, D.R.; Singer, M.S.; Lemjabbar-Alaoui, H.; Ahn, K.S.; Smoot, R.; Torbenson, M.S.; Rosen, S.D.; et al. SULF2 monoclonal antibody 5D5 suppresses human cholangiocarcinoma xenograft growth via regulation of a SULF2-PDGFRβ-YAP signaling axis. Hepatology 2021, 74, 1411. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zheng, Z.; Mao, S.; Zhang, W.; Liu, J.; Li, C.; Liu, S.; Yao, X. Construction and Validation of a Novel Eight-Gene Risk Signature to Predict the Progression and Prognosis of Bladder Cancer. Front. Oncol. 2021, 11, 632459. [Google Scholar] [CrossRef] [PubMed]
- Saraswat, D.; Shayya, H.J.; Polanco, J.J.; Tripathi, A.; Welliver, R.R.; Pol, S.U.; Seidman, R.A.; Broome, J.E.; O’Bara, M.A.; van Kuppervelt, T.H.; et al. Overcoming the inhibitory microenvironment surrounding oligodendrocyte progenitor cells following experimental demyelination. Nat. Commun. 2021, 12, 1923. [Google Scholar] [CrossRef]
- Niu, X.; Li, J.; Zhao, X.; Wang, Q.; Wang, G.; Hou, R.; Li, X.; An, P.; Yin, G.; Zhang, K. Dermal mesenchymal stem cells: A resource of migration-associated function in psoriasis? Stem Cell Res. Ther. 2019, 10, 54. [Google Scholar] [CrossRef]
- Lai, J.P.; Oseini, A.M.; Moser, C.D.; Yu, C.; Elsawa, S.F.; Hu, C.; Nakamura, I.; Han, T.; Aderca, I.; Isomoto, H.; et al. The oncogenic effect of sulfatase 2 in human hepatocellular carcinoma is mediated in part by glypican 3-dependent Wnt activation. Hepatology 2010, 52, 1680–1689. [Google Scholar] [CrossRef]
- Tian, L.; Zhao, J.L.; Kang, J.Q.; Guo, S.B.; Zhang, N.; Shang, L.; Zhang, Y.L.; Zhang, J.; Jiang, X.; Lin, Y. Astragaloside IV Alleviates the Experimental DSS-Induced Colitis by Remodeling Macrophage Polarization Through STAT Signaling. Front. Immunol. 2021, 12, 740565. [Google Scholar] [CrossRef]
- Irey, E.A.; Lassiter, C.M.; Brady, N.J.; Chuntova, P.; Wang, Y.; Knutson, T.P.; Henzler, C.; Chaffee, T.S.; Vogel, R.I.; Nelson, A.C.; et al. JAK/STAT inhibition in macrophages promotes therapeutic resistance by inducing expression of protumorigenic factors. Proc. Natl. Acad. Sci. USA 2019, 116, 12442–12451. [Google Scholar] [CrossRef] [PubMed]
- Overmeire, E.V.; Laoui, D.; Keirsse, J.; Ginderachter, J.V.; Sarukhan, A. Mechanisms Driving Macrophage Diversity and Specialization in Distinct Tumor Microenvironments and Parallelisms with Other Tissues. Front. Immunol. 2014, 5, 127. [Google Scholar] [CrossRef] [PubMed]
- Karnevi, E.; Andersson, R.; Rosendahl, A.H. Tumour-educated macrophages display a mixed polarisation and enhance pancreatic cancer cell invasion. Immunol. Cell Biol. 2014, 92, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.K.; Sayegh, N.; Agarwal, N. Seeing the forest for the trees—Single-cell atlases link CD8+ T cells and macrophages to disease progression and treatment response in kidney cancer—ScienceDirect. Cancer Cell 2021, 39, 594–596. [Google Scholar] [CrossRef]
- Munir, M.T.; Kay, M.K.; Kang, M.H.; Rahman, M.M.; Al-Harrasi, A.; Choudhury, M.; Moustaid-Moussa, N.; Hussain, F.; Rahman, S.M. Tumor-Associated Macrophages as Multifaceted Regulators of Breast Tumor Growth. Int. J. Mol. Sci. 2021, 22, 6526. [Google Scholar] [CrossRef]
- Xue, Y.; Tong, L.; LiuAnwei Liu, F.; Liu, A.; Zeng, S.; Xiong, Q.; Yang, Z.; He, X.; Sun, Y.; Xu, C. Tumorinfiltrating M2 macrophages driven by specific genomic alterations are associated with prognosis in bladder cancer. Oncol. Rep. 2019, 42, 581–594. [Google Scholar] [CrossRef]
- Al-Gayyar, M.M.; Abbas, A.; Hamdan, A.M. Chemopreventive and hepatoprotective roles of adiponectin (SULF2 inhibitor) in hepatocelluar carcinoma. Biol. Chem. 2016, 397, 257–267. [Google Scholar] [CrossRef]
- Hammond, E.; Khurana, A.; Shridhar, V.; Dredge, K. The Role of Heparanase and Sulfatases in the Modification of Heparan Sulfate Proteoglycans within the Tumor Microenvironment and Opportunities for Novel Cancer Therapeutics. Front. Oncol. 2014, 4, 195. [Google Scholar] [CrossRef]
- Pasquale, Z.; Roberto, D.; Manuela, P.; Chiara, C.; Erica, S.; Angela, R.; Carmen, D.; Eleonora, P.; Azzurra, A.C.; Marcella, M. TRF2 positively regulates SULF2 expression increasing VEGF-A release and activity in tumor microenvironment. Nucleic Acids Res. 2019, 7, 3365–3382. [Google Scholar]
- Costa, F.H.B.D.; Lewis, M.S.; Truong, A.; Carson, D.D.; Farach-Carson, M.C. SULF1 suppresses Wnt3A-driven growth of bone metastatic prostate cancer in perlecan-modified 3D cancer-stroma-macrophage triculture models. PLoS ONE 2020, 15, e0230354. [Google Scholar]
- Kumagai, S.; Ishibashi, K.; Kataoka, M.; Oguro, T.; Kiko, Y.; Yanagida, T.; Aikawa, K.; Kojima, Y. Impact of Sulfatase-2 on cancer progression and prognosis in patients with renal cell carcinoma. Cancer Sci. 2016, 107, 1632–1641. [Google Scholar] [CrossRef] [PubMed]
- Vicente, C.M.; Lima, M.A.; Nader, H.B.; Toma, L. SULF2 overexpression positively regulates tumorigenicity of human prostate cancer cells. J. Exp. Clin. Cancer Res. 2015, 34, 25. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, K.; Baldwin, E.T.; Mukaida, N. Interleukin-8 and MCAF: Novel leukocyte recruitment and activating cytokines. Chem. Immunol. 1992, 51, 236–265. [Google Scholar] [PubMed]
- Xiao, P.; Long, X.; Zhang, L.; Ye, Y.; Guo, J.; Liu, P.; Zhang, R.; Ning, J.; Yu, W.; Wei, F. Neurotensin/IL-8 pathway orchestrates local inflammatory response and tumor invasion by inducing M2 polarization of Tumor-Associated macrophages and epithelial-mesenchymal transition of hepatocellular carcinoma cells. OncoImmunology 2018, 7, e1440166. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Okamoto, M.; Goda, H.; Tano, T.; Nakashiro, K.I.; Sugita, A.; Fujita, T.; Koido, S.; Homma, S.; Kawakami, Y. Prognostic Significance of Interleukin-8 and CD163-Positive Cell-Infiltration in Tumor Tissues in Patients with Oral Squamous Cell Carcinoma. PLoS ONE 2014, 9, e110378. [Google Scholar] [CrossRef]
- Fang, W.; Lei, Y.; Shen, L.; Cai, J.; Huang, F.; Wei, Q.; Fei, X.; Xi, C.; Guan, H.; Wang, W. Tumor-associated macrophages promote the metastatic potential of thyroid papillary cancer by releasing CXCL8. Carcinogenesis 2014, 35, 1780–1787. [Google Scholar] [CrossRef]
- Huang, S.P.; Guan, X.; Kai, G.Y.; Xu, Y.Z.; Xu, Y.; Wang, H.J.; Pang, T.; Zhang, L.Y.; Liu, Y. Broussonin E suppresses LPS-induced inflammatory response in macrophages via inhibiting MAPK pathway and enhancing JAK2-STAT3 pathway. Chin. J. Nat. Med. 2019, 17, 372–380. [Google Scholar] [CrossRef]
- Mca, B.; Yu, S.A.; Tz, A.; Sh, A.; Kd, A.; Ar, A.; Xj, A.; Sc, A.; Jie, W.A.; Sl, A. Mammary epithelial cell derived exosomal MiR-221 mediates M1 macrophage polarization via SOCS1/STATs to promote inflammatory response—ScienceDirect. Int. Immunopharmacol. 2020, 83, 106493. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Yang, F.; Zheng, Z.; Li, C.; Mao, S.; Wu, Y.; Wang, R.; Zhang, J.; Zhang, Y.; Wang, H.; et al. Sulfatase 2 Affects Polarization of M2 Macrophages through the IL-8/JAK2/STAT3 Pathway in Bladder Cancer. Cancers 2023, 15, 131. https://doi.org/10.3390/cancers15010131
Zhang W, Yang F, Zheng Z, Li C, Mao S, Wu Y, Wang R, Zhang J, Zhang Y, Wang H, et al. Sulfatase 2 Affects Polarization of M2 Macrophages through the IL-8/JAK2/STAT3 Pathway in Bladder Cancer. Cancers. 2023; 15(1):131. https://doi.org/10.3390/cancers15010131
Chicago/Turabian StyleZhang, Wentao, Fuhan Yang, Zongtai Zheng, Cheng Li, Shiyu Mao, Yuan Wu, Ruiliang Wang, Junfeng Zhang, Yue Zhang, Hong Wang, and et al. 2023. "Sulfatase 2 Affects Polarization of M2 Macrophages through the IL-8/JAK2/STAT3 Pathway in Bladder Cancer" Cancers 15, no. 1: 131. https://doi.org/10.3390/cancers15010131
APA StyleZhang, W., Yang, F., Zheng, Z., Li, C., Mao, S., Wu, Y., Wang, R., Zhang, J., Zhang, Y., Wang, H., Li, W., Huang, J., & Yao, X. (2023). Sulfatase 2 Affects Polarization of M2 Macrophages through the IL-8/JAK2/STAT3 Pathway in Bladder Cancer. Cancers, 15(1), 131. https://doi.org/10.3390/cancers15010131




