Differentiation of Perilesional Edema in Glioblastomas and Brain Metastases: Comparison of Diffusion Tensor Imaging, Neurite Orientation Dispersion and Density Imaging and Diffusion Microstructure Imaging
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Imaging
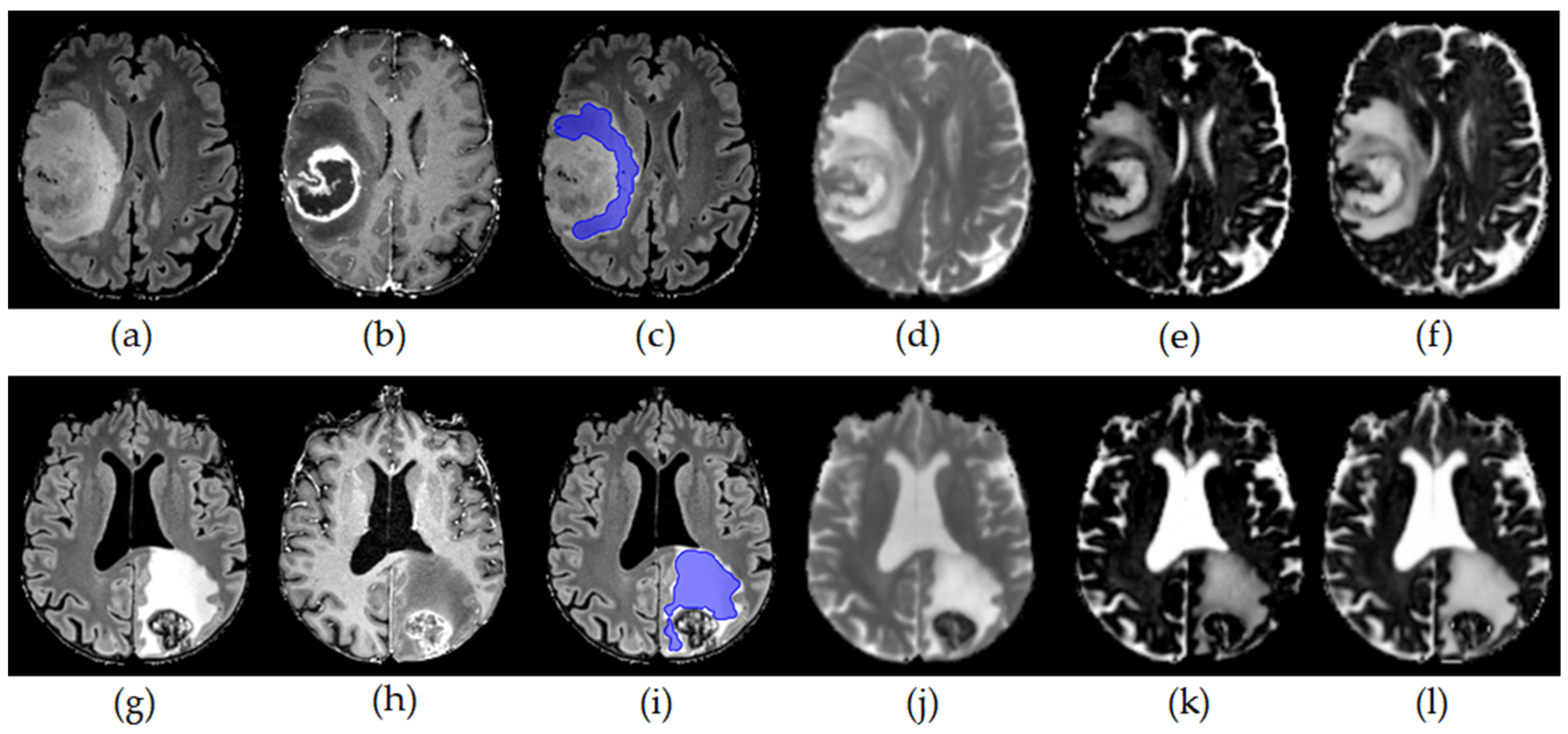
2.2. Image Postprocessing and ROI Based Analysis
2.3. Statistical Analysis
3. Results
3.1. Study Population
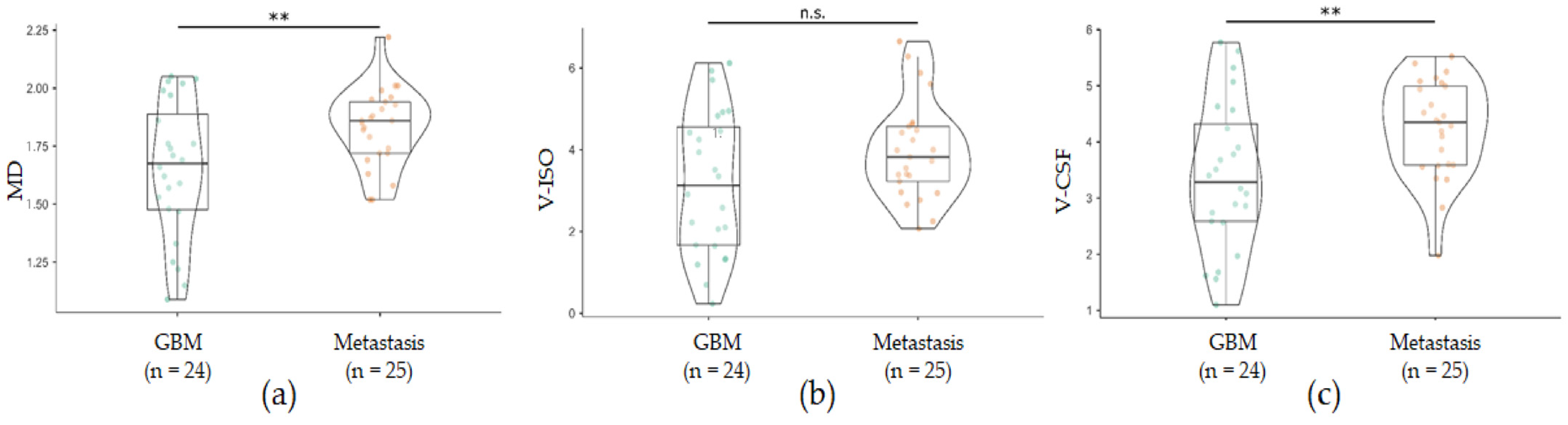
3.2. Increased Free Water in Perilesional T2w Hyperintensities in GBM Compared with Brain Metastases
3.3. Increased Free Fluid in Perilesional T2w Hyperintensities Are Associated with Perilesional Area Volume and Age
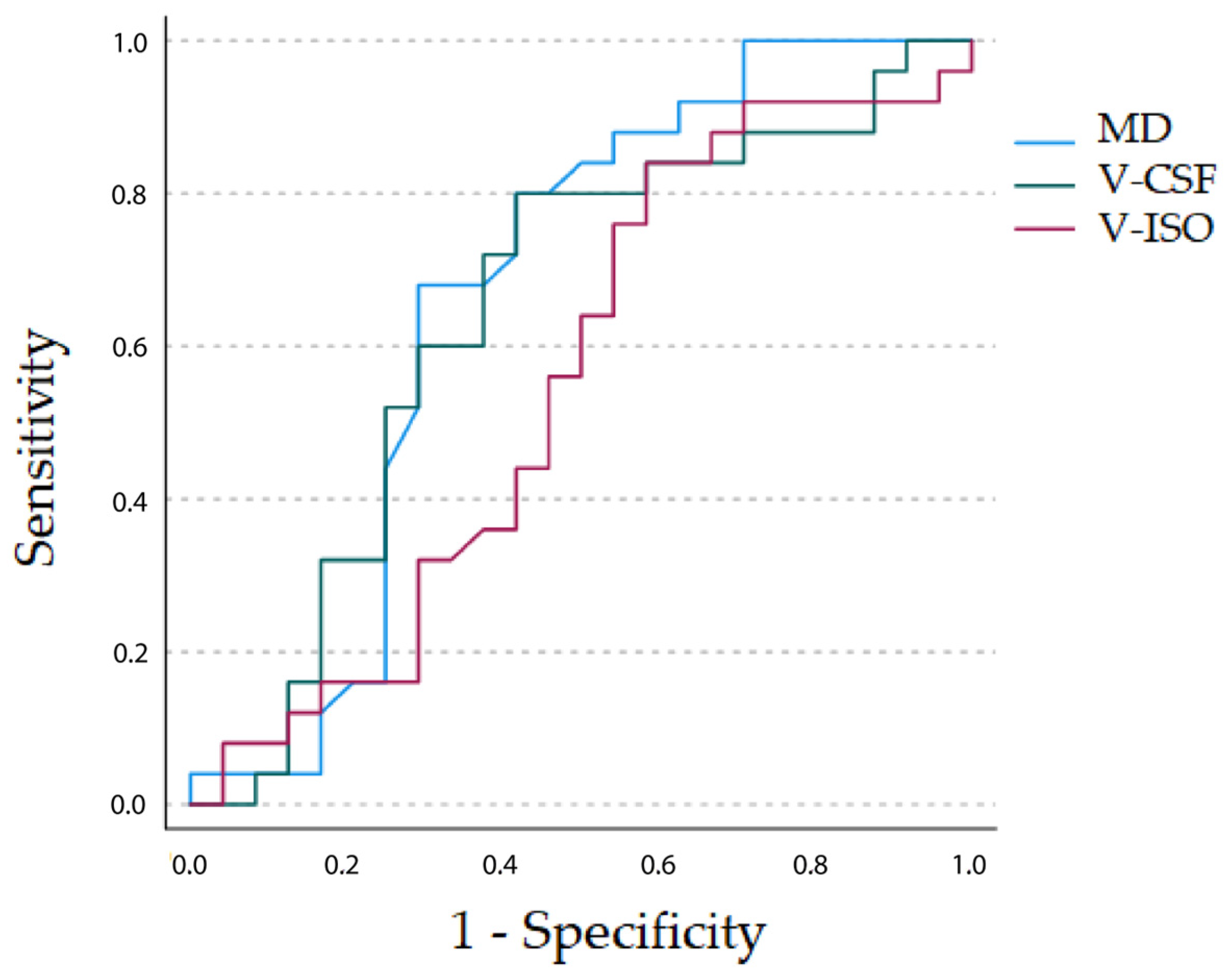
3.4. Comparative ROC Analysis of DTI MD, NODDI V-ISO and DMI V-CSF
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barajas, R.F.; Phillips, J.J.; Parvataneni, R.; Molinaro, A.; Essock-Burns, E.; Bourne, G.; Parsa, A.T.; Aghi, M.K.; McDermott, M.W.; Berger, M.S.; et al. Regional Variation in Histopathologic Features of Tumor Specimens from Treatment-Naive Glioblastoma Correlates with Anatomic and Physiologic MR Imaging. Neuro-Oncology 2012, 14, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Sahm, F.; Capper, D.; Jeibmann, A.; Habel, A.; Paulus, W.; Troost, D.; von Deimling, A. Addressing Diffuse Glioma as a Systemic Brain Disease with Single-Cell Analysis. Arch. Neurol. 2012, 69, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Pekmezci, M.; Perry, A. Neuropathology of Brain Metastases. Surg. Neurol. Int. 2013, 4, S245–S255. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; DeAngelis, L.M. Treatment of Brain Metastases. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 3475–3484. [Google Scholar] [CrossRef]
- Tan, A.C.; Ashley, D.M.; López, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of Glioblastoma: State of the Art and Future Directions. CA. Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef]
- Jiang, R.; Du, F.-Z.; He, C.; Gu, M.; Ke, Z.-W.; Li, J.-H. The Value of Diffusion Tensor Imaging in Differentiating High-Grade Gliomas from Brain Metastases: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e112550. [Google Scholar] [CrossRef]
- Tsougos, I.; Svolos, P.; Kousi, E.; Fountas, K.; Theodorou, K.; Fezoulidis, I.; Kapsalaki, E. Differentiation of Glioblastoma Multiforme from Metastatic Brain Tumor Using Proton Magnetic Resonance Spectroscopy, Diffusion and Perfusion Metrics at 3 T. Cancer Imaging Off. Publ. Int. Cancer Imaging Soc. 2012, 12, 423–436. [Google Scholar] [CrossRef]
- Hoefnagels, F.W.A.; De Witt Hamer, P.; Sanz-Arigita, E.; Idema, S.; Kuijer, J.P.A.; Pouwels, P.J.W.; Barkhof, F.; Vandertop, W.P. Differentiation of Edema and Glioma Infiltration: Proposal of a DTI-Based Probability Map. J. Neurooncol. 2014, 120, 187–198. [Google Scholar] [CrossRef]
- Wang, S.; Kim, S.; Chawla, S.; Wolf, R.L.; Knipp, D.E.; Vossough, A.; O’Rourke, D.M.; Judy, K.D.; Poptani, H.; Melhem, E.R. Differentiation between Glioblastomas, Solitary Brain Metastases, and Primary Cerebral Lymphomas Using Diffusion Tensor and Dynamic Susceptibility Contrast-Enhanced MR Imaging. AJNR Am. J. Neuroradiol. 2011, 32, 507–514. [Google Scholar] [CrossRef]
- Byrnes, T.J.D.; Barrick, T.R.; Bell, B.A.; Clark, C.A. Diffusion Tensor Imaging Discriminates between Glioblastoma and Cerebral Metastases in Vivo. NMR Biomed. 2011, 24, 54–60. [Google Scholar] [CrossRef]
- Zhang, H.; Schneider, T.; Wheeler-Kingshott, C.A.; Alexander, D.C. NODDI: Practical in Vivo Neurite Orientation Dispersion and Density Imaging of the Human Brain. NeuroImage 2012, 61, 1000–1016. [Google Scholar] [CrossRef] [PubMed]
- Novikov, D.S.; Veraart, J.; Jelescu, I.O.; Fieremans, E. Rotationally-Invariant Mapping of Scalar and Orientational Metrics of Neuronal Microstructure with Diffusion MRI. NeuroImage 2018, 174, 518–538. [Google Scholar] [CrossRef] [PubMed]
- Reisert, M.; Kellner, E.; Dhital, B.; Hennig, J.; Kiselev, V.G. Disentangling Micro from Mesostructure by Diffusion MRI: A Bayesian Approach. NeuroImage 2017, 147, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Rau, A.; Reisert, M.; Kellner, E.; Hosp, J.A.; Urbach, H.; Demerath, T. Increased Interstitial Fluid in Periventricular and Deep White Matter Hyperintensities in Patients with Suspected Idiopathic Normal Pressure Hydrocephalus. Sci. Rep. 2021, 11, 19552. [Google Scholar] [CrossRef]
- Demerath, T.; Donkels, C.; Reisert, M.; Heers, M.; Rau, A.; Schröter, N.; Schulze-Bonhage, A.; Reinacher, P.; Scheiwe, C.; Shah, M.J.; et al. Gray-White Matter Blurring of the Temporal Pole Associated With Hippocampal Sclerosis: A Microstructural Study Involving 3 T MRI and Ultrastructural Histopathology. Cereb. Cortex 2021, 32, 1882–1893. [Google Scholar] [CrossRef]
- Rau, A.; Schroeter, N.; Blazhenets, G.; Dressing, A.; Walter, L.I.; Kellner, E.; Bormann, T.; Mast, H.; Wagner, D.; Urbach, H.; et al. Widespread White Matter Oedema in Subacute COVID-19 Patients with Neurological Symptoms. Brain J. Neurol. 2022, 145, 3203–3213. [Google Scholar] [CrossRef]
- Rau, A.; Jost, W.H.; Demerath, T.; Kellner, E.; Reisert, M.; Urbach, H. Diffusion Microstructure Imaging in Progressive Supranuclear Palsy: Reduced Axonal Volumes in the Superior Cerebellar Peduncles, Dentato-Rubro-Thalamic Tracts, Ventromedial Thalami, and Frontomesial White Matter. Cereb. Cortex 2022, 32, 5628–5636. [Google Scholar] [CrossRef]
- Würtemberger, U.; Diebold, M.; Erny, D.; Hosp, J.A.; Schnell, O.; Reinacher, P.C.; Rau, A.; Kellner, E.; Reisert, M.; Urbach, H.; et al. Diffusion Microstructure Imaging to Analyze Perilesional T2 Signal Changes in Brain Metastases and Glioblastomas. Cancers 2022, 14, 1155. [Google Scholar] [CrossRef]
- Kadota, Y.; Hirai, T.; Azuma, M.; Hattori, Y.; Khant, Z.A.; Hori, M.; Saito, K.; Yokogami, K.; Takeshima, H. Differentiation between Glioblastoma and Solitary Brain Metastasis Using Neurite Orientation Dispersion and Density Imaging. J. Neuroradiol. 2020, 47, 197–202. [Google Scholar] [CrossRef]
- Mao, J.; Zeng, W.; Zhang, Q.; Yang, Z.; Yan, X.; Zhang, H.; Wang, M.; Yang, G.; Zhou, M.; Shen, J. Differentiation between High-Grade Gliomas and Solitary Brain Metastases: A Comparison of Five Diffusion-Weighted MRI Models. BMC Med. Imaging 2020, 20, 124. [Google Scholar] [CrossRef]
- Veraart, J.; Novikov, D.S.; Christiaens, D.; Ades-Aron, B.; Sijbers, J.; Fieremans, E. Denoising of Diffusion MRI Using Random Matrix Theory. NeuroImage 2016, 142, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Kellner, E.; Dhital, B.; Kiselev, V.G.; Reisert, M. Gibbs-Ringing Artifact Removal Based on Local Subvoxel-Shifts. Magn. Reson. Med. 2016, 76, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Daducci, A.; Canales-Rodríguez, E.J.; Zhang, H.; Dyrby, T.B.; Alexander, D.C.; Thiran, J.-P. Accelerated Microstructure Imaging via Convex Optimization (AMICO) from Diffusion MRI Data. NeuroImage 2015, 105, 32–44. [Google Scholar] [CrossRef]
- Behler, A.; Kassubek, J.; Müller, H.-P. Age-Related Alterations in DTI Metrics in the Human Brain-Consequences for Age Correction. Front. Aging Neurosci. 2021, 13, 682109. [Google Scholar] [CrossRef] [PubMed]
- Holly, K.S.; Fitz-Gerald, J.S.; Barker, B.J.; Murcia, D.; Daggett, R.; Ledbetter, C.; Gonzalez-Toledo, E.; Sun, H. Differentiation of High-Grade Glioma and Intracranial Metastasis Using Volumetric Diffusion Tensor Imaging Tractography. World Neurosurg. 2018, 120, e131–e141. [Google Scholar] [CrossRef]
- Lu, S.; Ahn, D.; Johnson, G.; Cha, S. Peritumoral Diffusion Tensor Imaging of High-Grade Gliomas and Metastatic Brain Tumors. Am. J. Neuroradiol. 2003, 24, 937–941. [Google Scholar]
- Pasternak, O.; Sochen, N.; Gur, Y.; Intrator, N.; Assaf, Y. Free Water Elimination and Mapping from Diffusion MRI. Magn. Reson. Med. 2009, 62, 717–730. [Google Scholar] [CrossRef]
- Kamiya, K.; Hori, M.; Aoki, S. NODDI in Clinical Research. J. Neurosci. Methods 2020, 346, 108908. [Google Scholar] [CrossRef]
- Roth, P.; Happold, C.; Weller, M. Corticosteroid Use in Neuro-Oncology: An Update. Neuro-Oncology Pr. 2015, 2, 6–12. [Google Scholar] [CrossRef]
- Soffietti, R.; Abacioglu, U.; Baumert, B.; Combs, S.E.; Kinhult, S.; Kros, J.M.; Marosi, C.; Metellus, P.; Radbruch, A.; Villa Freixa, S.S.; et al. Diagnosis and Treatment of Brain Metastases from Solid Tumors: Guidelines from the European Association of Neuro-Oncology (EANO). Neuro-Oncology 2017, 19, 162–174. [Google Scholar] [CrossRef]
- Haddad, A.F.; Young, J.S.; Morshed, R.A.; Berger, M.S. FLAIRectomy: Resecting beyond the Contrast Margin for Glioblastoma. Brain Sci. 2022, 12, 544. [Google Scholar] [CrossRef] [PubMed]
- Lasocki, A.; Gaillard, F. Non-Contrast-Enhancing Tumor: A New Frontier in Glioblastoma Research. AJNR Am. J. Neuroradiol. 2019, 40, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Eidel, O.; Burth, S.; Neumann, J.-O.; Kieslich, P.J.; Sahm, F.; Jungk, C.; Kickingereder, P.; Bickelhaupt, S.; Mundiyanapurath, S.; Bäumer, P.; et al. Tumor Infiltration in Enhancing and Non-Enhancing Parts of Glioblastoma: A Correlation with Histopathology. PLoS ONE 2017, 12, e0169292. [Google Scholar] [CrossRef]
- Vallatos, A.; Al-Mubarak, H.F.I.; Birch, J.L.; Galllagher, L.; Mullin, J.M.; Gilmour, L.; Holmes, W.M.; Chalmers, A.J. Quantitative Histopathologic Assessment of Perfusion MRI as a Marker of Glioblastoma Cell Infiltration in and beyond the Peritumoral Edema Region. J. Magn. Reson. Imaging JMRI 2019, 50, 529–540. [Google Scholar] [CrossRef]
- Rapp, M.; Baernreuther, J.; Turowski, B.; Steiger, H.-J.; Sabel, M.; Kamp, M.A. Recurrence Pattern Analysis of Primary Glioblastoma. World Neurosurg. 2017, 103, 733–740. [Google Scholar] [CrossRef]
- Lemercier, P.; Paz Maya, S.; Patrie, J.T.; Flors, L.; Leiva-Salinas, C. Gradient of Apparent Diffusion Coefficient Values in Peritumoral Edema Helps in Differentiation of Glioblastoma from Solitary Metastatic Lesions. AJR Am. J. Roentgenol. 2014, 203, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Sawlani, V.; Patel, M.D.; Davies, N.; Flintham, R.; Wesolowski, R.; Ughratdar, I.; Pohl, U.; Nagaraju, S.; Petrik, V.; Kay, A.; et al. Multiparametric MRI: Practical Approach and Pictorial Review of a Useful Tool in the Evaluation of Brain Tumours and Tumour-like Lesions. Insights Imaging 2020, 11, 84. [Google Scholar] [CrossRef]
| GBM | Metastasis | p-Value | |
|---|---|---|---|
| n | 24 | 25 | |
| Sex (m/f) | 13/11 | 13/12 | p = 0.826 |
| Age (years) (SD) | 65.5 (13.1) | 65.5 (12.1) | p = 0.995 |
| Perifocal T2 volume (ml) [IQR] | 19.1 [24.4] | 23.0 [41.5] | p = 0.729 |
| Previous corticosteroid therapy | 10/24 (41.7%) | 9/25 (36.0%) | p = 0.696 |
| MD [IQR] | 1.67 [4.10] | 1.86 [2.20] | p = 0.006 |
| Min, Max 25%, 75% | 1.09, 2.05 1.48, 1.89 | 1.52, 2.22 1.72, 1.94 | |
| V-ISO [IQR] | 3.13 [2.89] | 3.82 [1.34] | p = 0.060 |
| Min, Max 25%, 75% | 0.235, 6.12 1.67, 4.55 | 2.07, 6.65 3.23, 4.57 | |
| V-CSF [IQR] | 3.29 [1.74] | 4.35 [1.40] | p = 0.001 |
| Min, Max 25%, 75% | 1.1, 5.77 2.58, 4.32 | 1.98, 5.52 3.59, 4.99 |
| GBM | Metastasis | p-Value | |
|---|---|---|---|
| n | 14 | 16 | |
| Sex (m/f) | 7/7 | 7/9 | p = 0.822 |
| Age (years) (SD) | 63.1 (12.5) | 66.3 (12.1) | p = 0.786 |
| Perifocal T2 volume (ml) [IQR] | 19.6 [23.4] | 5.1 [36.7] | p = 0.729 |
| MD [IQR] | 1.64 [0.48] | 1.88 [0.18] | p = 0.008 |
| V-ISO [IQR] | 3.13 [3.32] | 3.77 [1.55] | p = 0.077 |
| V-CSF [IQR] | 3.24 [1.99] | 4.33 [1.36] | p = 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Würtemberger, U.; Rau, A.; Reisert, M.; Kellner, E.; Diebold, M.; Erny, D.; Reinacher, P.C.; Hosp, J.A.; Hohenhaus, M.; Urbach, H.; et al. Differentiation of Perilesional Edema in Glioblastomas and Brain Metastases: Comparison of Diffusion Tensor Imaging, Neurite Orientation Dispersion and Density Imaging and Diffusion Microstructure Imaging. Cancers 2023, 15, 129. https://doi.org/10.3390/cancers15010129
Würtemberger U, Rau A, Reisert M, Kellner E, Diebold M, Erny D, Reinacher PC, Hosp JA, Hohenhaus M, Urbach H, et al. Differentiation of Perilesional Edema in Glioblastomas and Brain Metastases: Comparison of Diffusion Tensor Imaging, Neurite Orientation Dispersion and Density Imaging and Diffusion Microstructure Imaging. Cancers. 2023; 15(1):129. https://doi.org/10.3390/cancers15010129
Chicago/Turabian StyleWürtemberger, Urs, Alexander Rau, Marco Reisert, Elias Kellner, Martin Diebold, Daniel Erny, Peter C. Reinacher, Jonas A. Hosp, Marc Hohenhaus, Horst Urbach, and et al. 2023. "Differentiation of Perilesional Edema in Glioblastomas and Brain Metastases: Comparison of Diffusion Tensor Imaging, Neurite Orientation Dispersion and Density Imaging and Diffusion Microstructure Imaging" Cancers 15, no. 1: 129. https://doi.org/10.3390/cancers15010129
APA StyleWürtemberger, U., Rau, A., Reisert, M., Kellner, E., Diebold, M., Erny, D., Reinacher, P. C., Hosp, J. A., Hohenhaus, M., Urbach, H., & Demerath, T. (2023). Differentiation of Perilesional Edema in Glioblastomas and Brain Metastases: Comparison of Diffusion Tensor Imaging, Neurite Orientation Dispersion and Density Imaging and Diffusion Microstructure Imaging. Cancers, 15(1), 129. https://doi.org/10.3390/cancers15010129






